Effects of the Taste Substances and Metal Cations in Green Tea Infusion on the Turbidity of EGCG–Mucin Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Determination of Turbidity Value
2.2.2. Study on the Complexation between Epicatechins and Mucin
2.2.3. Interaction Turbidity Value between Epicatechins
2.2.4. Interaction Turbidity Value between EGCG and Pas
2.2.5. Interaction Turbidity Value between EGCG and Metal Cations (MCs)
2.2.6. Interaction Turbidity Value between EGCG and Typical Taste Substances (TTSs)
2.2.7. Verification in Green Tea Infusion
Detection of Main Astringent Substances in Green Tea Infusion
Sensory Evaluation
2.3. Statistical Analysis
3. Results and Discussion
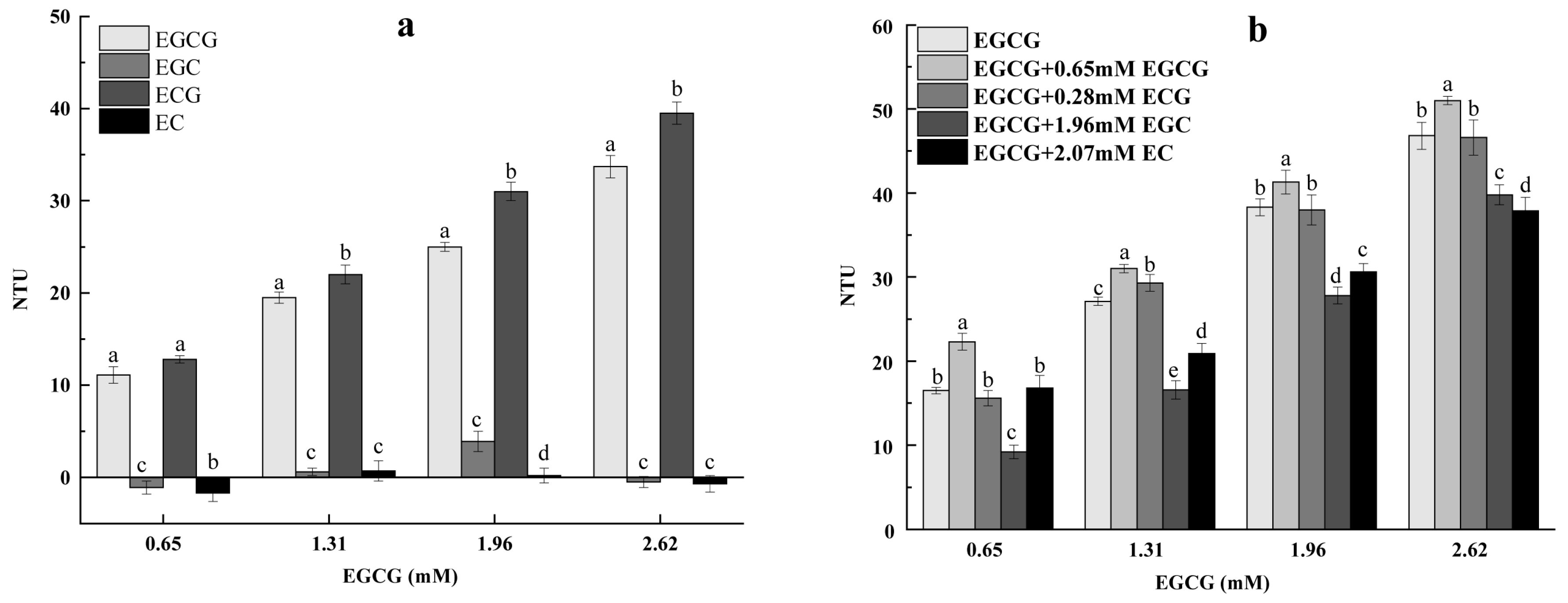
3.1. Effect of Epicatechins on the Turbidity of Mucin Solution and EGCG–Mucin Mixtures
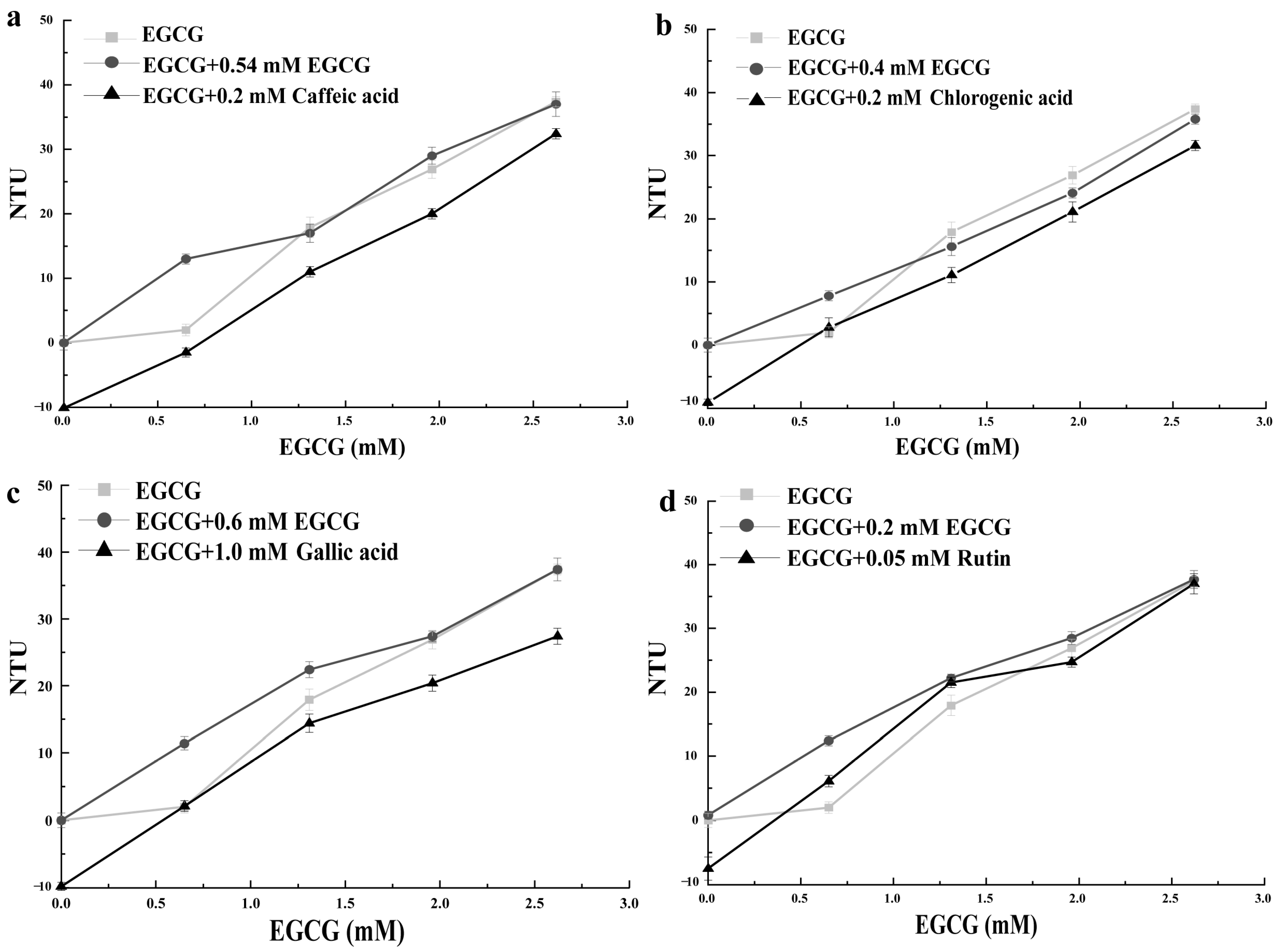
3.2. Effect of PAs on the Turbidity of Mucin Solution and EGCG–Mucin Mixtures
3.2.1. Caffeic Acid
3.2.2. Chlorogenic Acid
3.2.3. Gallic Acid
3.2.4. Rutin
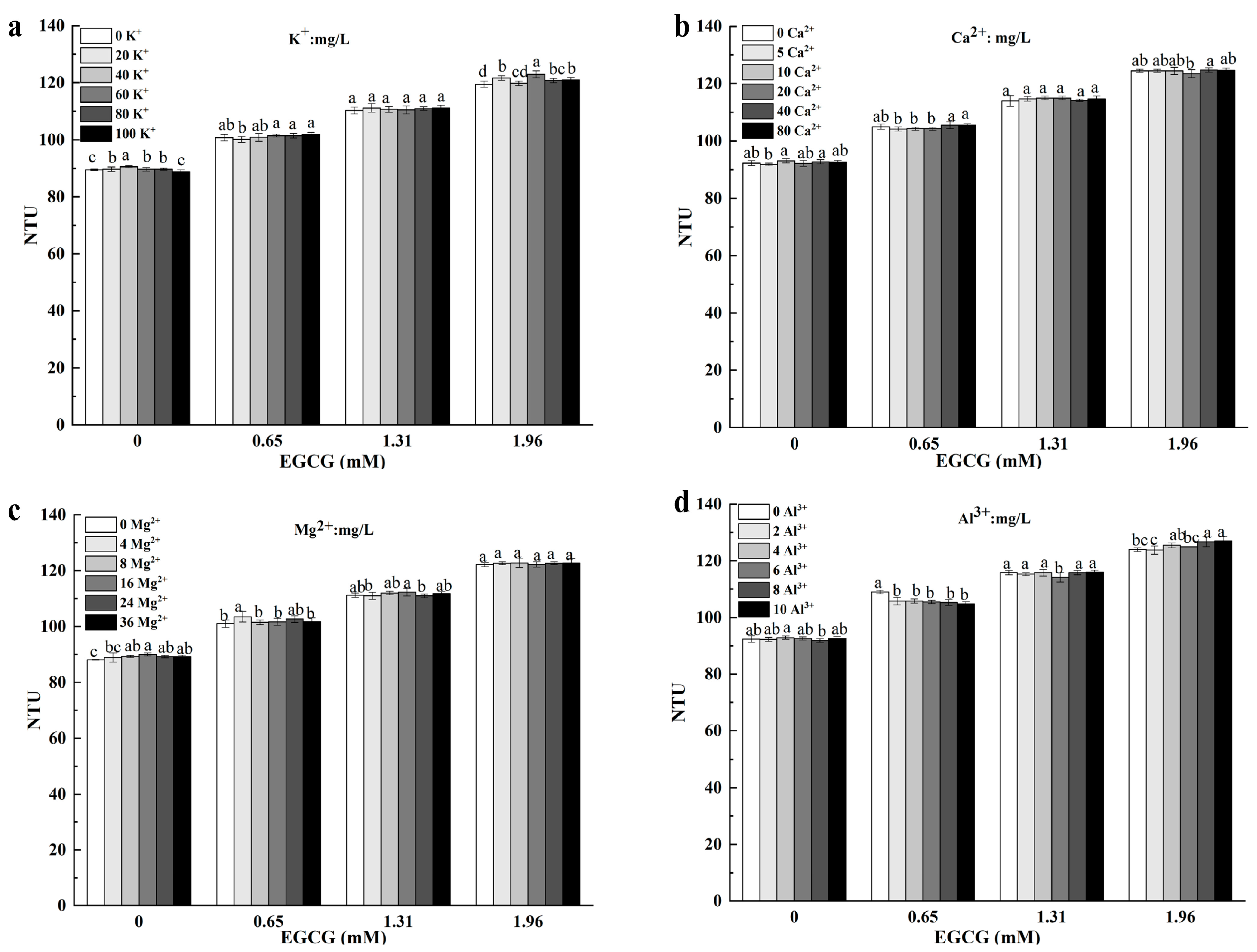
3.3. Effect of MCs on the Turbidity of Mucin Solution and EGCG–Mucin Mixtures
3.3.1. Potassium Ion (K+)
3.3.2. Calcium Ion (Ca2+)
3.3.3. Magnesium Ion (Mg2+)
3.3.4. Aluminum Ion (Al3+)
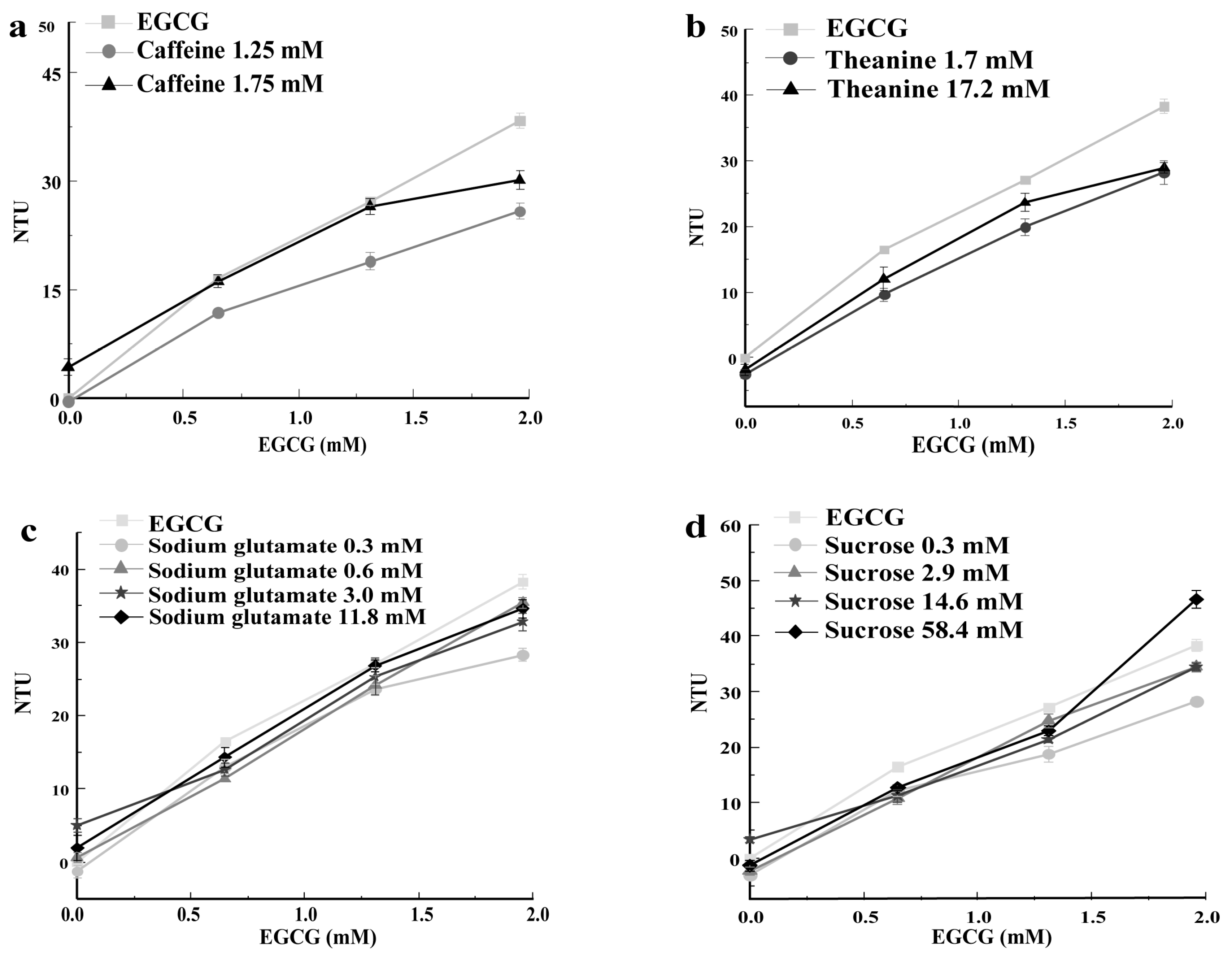
3.4. Effect of TTSs in Tea Infusion on the Turbidity of Mucin Solution and EGCG–Mucin Mixtures
3.4.1. Caffeine
3.4.2. Theanine
3.4.3. Sodium Glutamate
3.4.4. Sucrose
3.5. Astringency Intensity Evaluation of Green Tea Infusion by the Turbidity Method
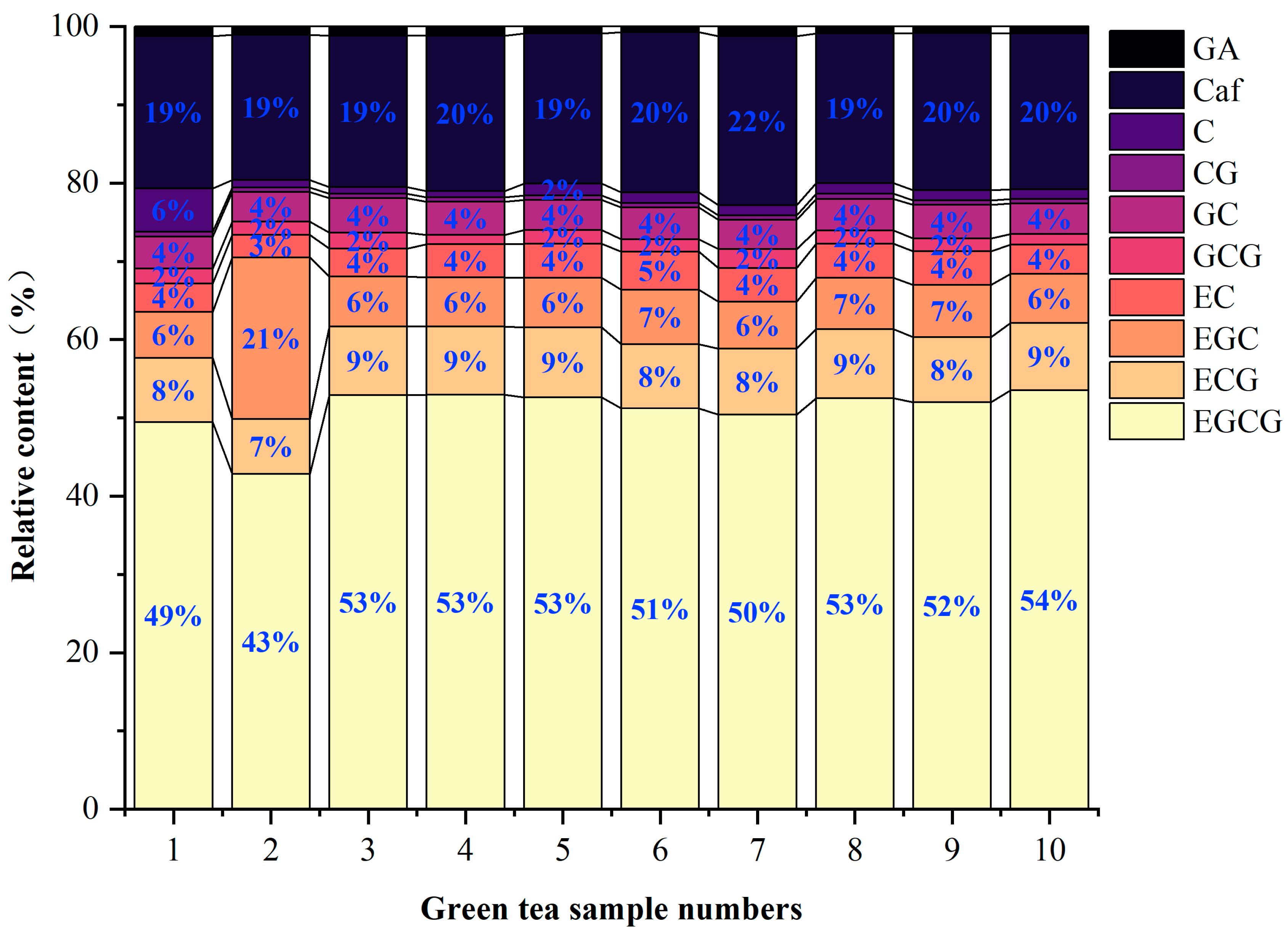
3.5.1. Analyses of the Main Astringent Substances in Green Tea Infusion
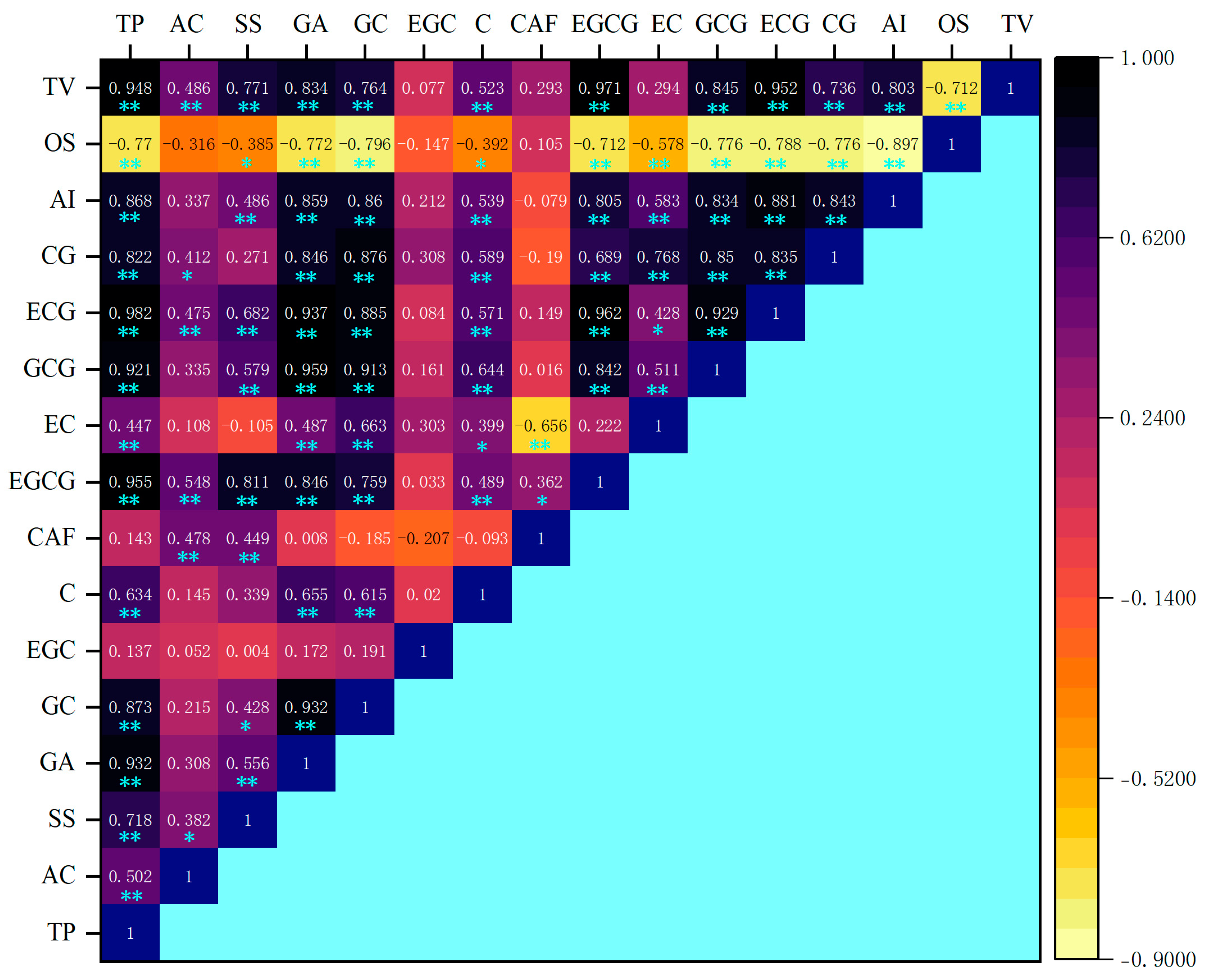
3.5.2. Correlation Analyses between Turbidity Value and Astringency Intensity of Green tea Infusion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, X. Tea Biochemistry; China Agriculture Press: Beijing, China, 2003. [Google Scholar]
- Zhang, Y. The Taste Characteristics of Typical Phenolic Acids and their Effects on the Bitterness and Astringency of Green Tea Infusion; Anhui Agricultural University: Hefei, China, 2019. [Google Scholar]
- Zhang, Y. Study on the Taste Characteristics of the Main Catechins in Green Tea Infusion; Chinese Academy of Agricultural Sciences: Beijing, China, 2016. [Google Scholar]
- Yu, P.; Yeo, S.L.; Low, M.Y.; Zhou, W. Identifying key non-volatile compounds in ready-to-drink green tea and their impact on taste profile. Food Chem. 2014, 155, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Chen, G.; Yin, J.; Chen, J.; Wang, F.; Xu, Y. Effects of phenolic acids and quercetin-3-O-rutinoside on the bitterness and astringency of green tea infusion. NPJ Sci. Food 2022, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, D.; Duan, Q.; Yang, M. Study on forming principle and masking technology of astringent taste of Chinese materia medica. Chin. Tradit. Herb. Drugs 2014, 45, 2716–2721. [Google Scholar] [CrossRef]
- Valentová, H.; Skrovánková, S.; Panovská, Z.; Pokorny, J. Time–intensity studies of astringent taste. Food Chem. 2002, 78, 29–37. [Google Scholar] [CrossRef]
- De-Freitas, V.; Mateus, N. Structural features of procyanidin interactions with salivary proteins. J. Agric. Food Chem. 2001, 49, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Freitas, V.D.; Mateus, N. Nephelometric study of salivary protein–tannin aggregates. J. Sci. Food Agric. 2002, 82, 113–119. [Google Scholar] [CrossRef]
- Ye, Q.; Chen, G.; Pan, W.; Cao, Q.; Xu, Y. A Predictive Model for Astringency Based on in vitro Interactions between Salivary Proteins and (-)Epigallocatechin Gallate. Food Chem. 2020, 340, 127845. [Google Scholar] [CrossRef] [PubMed]
- Guinard, J.X.; Zoumas-Morse, C.; Walchak, C. Relation between parotid saliva flow and composition and the perception of gustatory and trigeminal stimuli in foods. Physiol. Behav. 1997, 63, 109. [Google Scholar] [CrossRef]
- Mehansho, H.; Butler, L.G.; Carlson, D.M. Dietary Tannins and Salivary Proline-Rich Proteins: Interactions, Induction, and Defense Mechanisms. Annu. Rev. Nutr. 1987, 7, 423–440. [Google Scholar] [CrossRef]
- Canon, F.; Giuliani, A.; Paté, F.; Sarni-Manchado, P. Ability of a salivary intrinsically unstructured protein to bind different tannin targets revealed by mass spectrometry. Anal. Bioanal. Chem. 2010, 398, 815–822. [Google Scholar] [CrossRef]
- Mcrae, J.M.; Falconer, R.J.; Kennedy, J.A. Thermodynamics of Grape and Wine Tannin Interaction with Polyproline: Implications for Red Wine Astringency. J. Agric. Food Chem. 2010, 58, 12510–12518. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; López-Solís, R.; Pea-Neira, l.; Zamora-Marín, F. Tannin–protein interaction is more closely associated with astringency than tannin–protein precipitation: Experience with two oenological tannins and a gelatin. Int. J. Food Sci. Technol. 2010, 45, 2629–2636. [Google Scholar] [CrossRef]
- Aree, T. Understanding structures and thermodynamics of β-cyclodextrin encapsulation of chlorogenic, caffeic and quinic acids: Implications for enriching antioxidant capacity and masking bitterness in coffee. Food Chem. 2019, 293, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhang, X.; Xiao, Z.; Song, S.; Jia, C.; Yu, H.; Fang, L.; Xu, C. Characterization of taste-active compounds of various cherry wines and their correlation with sensory attributes. J. Chromatogr. B 2012, 902, 55–60. [Google Scholar] [CrossRef] [PubMed]
- He, W.; He, K.; Liu, X.; Ye, L.; Lin, X.; Ma, L.; Yang, P.; Wu, X. Modulating the allergenicity and functional properties of peanut protein by covalent conjugation with polyphenols. Food Chem. 2023, 415, 135733. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Teng, J.; Huang, L.; Wei, B.; Xia, N. Determination of the variations in the metabolic profile and sensory quality of Liupao tea during fermentation through UHPLC-HR-MS metabolomics. Food Chem. 2022, 404, 134773. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ma, B.; Xu, C.; Wang, J.; Wang, Z.; Huang, Y.; Ma, C. Impact of enzymatic fermentation on taste, chemical compositions and in vitro antioxidant activities in Chinese teas using E-tongue, HPLC and amino acid analyzer. LWT 2022, 163, 113549. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, Y.; Du, Q.; Chen, J.; Yuan, H.; Xu, Y. Effect of Ca2+ concentration on the tastes from the main chemicals in green tea infusions. Food Res. Int. 2014, 62, 941–946. [Google Scholar] [CrossRef]
- Liu, S. Study on the Fresh and Brisk Taste and Its Discriminant Model of Green Tea. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2014. [Google Scholar]
- Jin, X. The Research on Taste Compounds of Green Tea. Master’s Thesis, Southwest University, Chongqing, China, 2007. [Google Scholar]
- Guo, B.; Cheng, Q. Complexation properties of tea components with metal ions. J. Tea Sci. 1991, 11, 139–144. [Google Scholar]
- Hu, X.; Xu, Y.; Zhong, X.; Hong, Z.; Du, Q.; Yuan, H.; Yin, J. Effect of Ca2+ on Sediment Formation in the Tea Infusion under Different Fermentation Degree. J. Tea Sci. 2014, 34, 458–464. [Google Scholar] [CrossRef]
- Nahar, M.S.; Zhang, J. Impact of natural water chemistry on public drinking water in Japan. Environ. Earth Sci. 2013, 69, 127–140. [Google Scholar] [CrossRef]
- Yin, J.; Xu, Y.; Chen, J.; Shen, D.; Wang, F.; Chen, S.; Qian, X. Effects of Main Metal Ions on Quality of Cold Soluble Instant Green Tea. Food Sci. 2009, 30, 99–105. [Google Scholar]
- Tang, D.; Shen, S. Study on Catechins’ Buffer Abilities to OH− and Their Complex with Al3+. J. Tea Sci. 2003, 23, 66–70. [Google Scholar]
- Guo, Y.; Jiang, C.; Yang, Y.; Zhang, Y.; Wang, D.; Guo, Y. Effect of microwave combined with metal ions on the properties and structure of wheat embryo protein. Food Sci. 2024, 45, 1–11. [Google Scholar]
- Zhang, L.; Sahu, I.D.; Xu, M.; Wang, Y.; Hu, X. Effect of metal ions on the binding reaction of (−)-epigallocatechin gallate to β -lactoglobulin. Food Chem. 2017, 221, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Luo, Z.; Tong, H. Study on main taste compounds and quantitative descriptive analysis of taste in green tea infusion. Sci. Technol. Food Ind. 2012, 33, 343–346. [Google Scholar] [CrossRef]
- Brannan, G.D.; Setser, C.S.; Kemp, K.E. Interaction of astringency and taste characteristics1. J. Sens. Stud. 2001, 16, 179–197. [Google Scholar] [CrossRef]
- Liu, Z.; Ran, Q.; Li, Q.; Yang, T.; Dai, Y.; Zhang, T.; Fang, S.; Pan, K.; Long, L. Interaction between major catechins and umami amino acids in green tea based on electronic tongue technology. J. Food Sci. 2023, 88, 2339–2352. [Google Scholar] [CrossRef]
- Shu, K.; Kenji, K.; Hideki, M.; Andrea, H.; Thomas, H. Molecular and sensory studies on the umami taste of Japanese green tea. J. Agric. Food Chem. 2006, 54, 2688–2694. [Google Scholar]
| PAs/0.2 mM | Equal Astringency Intensity/Scores | EGCG/mM |
|---|---|---|
| Caffeic acid | 1.5 | 0.5 |
| Chlorogenic acid | 1.0 | 0.4 |
| Gallic acid | 1.8 | 0.6 |
| Rutin | 0.5 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Ye, Q.; Cao, Q.; Liu, Y.; Li, X.; Liu, Z.; Gong, Y.; Zhang, S.; Yin, J.; Xu, Y. Effects of the Taste Substances and Metal Cations in Green Tea Infusion on the Turbidity of EGCG–Mucin Mixtures. Foods 2024, 13, 1172. https://doi.org/10.3390/foods13081172
Xu L, Ye Q, Cao Q, Liu Y, Li X, Liu Z, Gong Y, Zhang S, Yin J, Xu Y. Effects of the Taste Substances and Metal Cations in Green Tea Infusion on the Turbidity of EGCG–Mucin Mixtures. Foods. 2024; 13(8):1172. https://doi.org/10.3390/foods13081172
Chicago/Turabian StyleXu, Longjie, Qingqing Ye, Qingqing Cao, Yuyi Liu, Xinghui Li, Zhengquan Liu, Yushun Gong, Sheng Zhang, Junfeng Yin, and Yongquan Xu. 2024. "Effects of the Taste Substances and Metal Cations in Green Tea Infusion on the Turbidity of EGCG–Mucin Mixtures" Foods 13, no. 8: 1172. https://doi.org/10.3390/foods13081172
APA StyleXu, L., Ye, Q., Cao, Q., Liu, Y., Li, X., Liu, Z., Gong, Y., Zhang, S., Yin, J., & Xu, Y. (2024). Effects of the Taste Substances and Metal Cations in Green Tea Infusion on the Turbidity of EGCG–Mucin Mixtures. Foods, 13(8), 1172. https://doi.org/10.3390/foods13081172








