A Polysaccharide from Ficus carica L. Exerts Immunomodulatory Activity in Both In Vitro and In Vivo Experimental Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Polysaccharide Extraction and Purification
2.3. Characterization of FCP
2.3.1. Determination of Basic Components in FCP
2.3.2. Molecular Weight of FCP
2.3.3. Monosaccharide Composition Analysis
2.3.4. UV Analysis
2.3.5. FT-IR Spectroscopy Analysis
2.3.6. SEM Analysis
2.3.7. Atomic Force Microscopy (AFM) Analysis
2.3.8. Methylation Analysis
2.3.9. Triple-Helix Construction Analysis
2.4. Immunomodulatory Activity of FCP In Vitro
2.4.1. Cell Culture, Grouping and Administration
2.4.2. Cell Viability Assessment
2.4.3. Pinocytic Test
2.4.4. Determination of NO, IL-6, and TNF-a Cytokine Production Level
2.5. Immunomodulatory Activity of FCP In Vivo
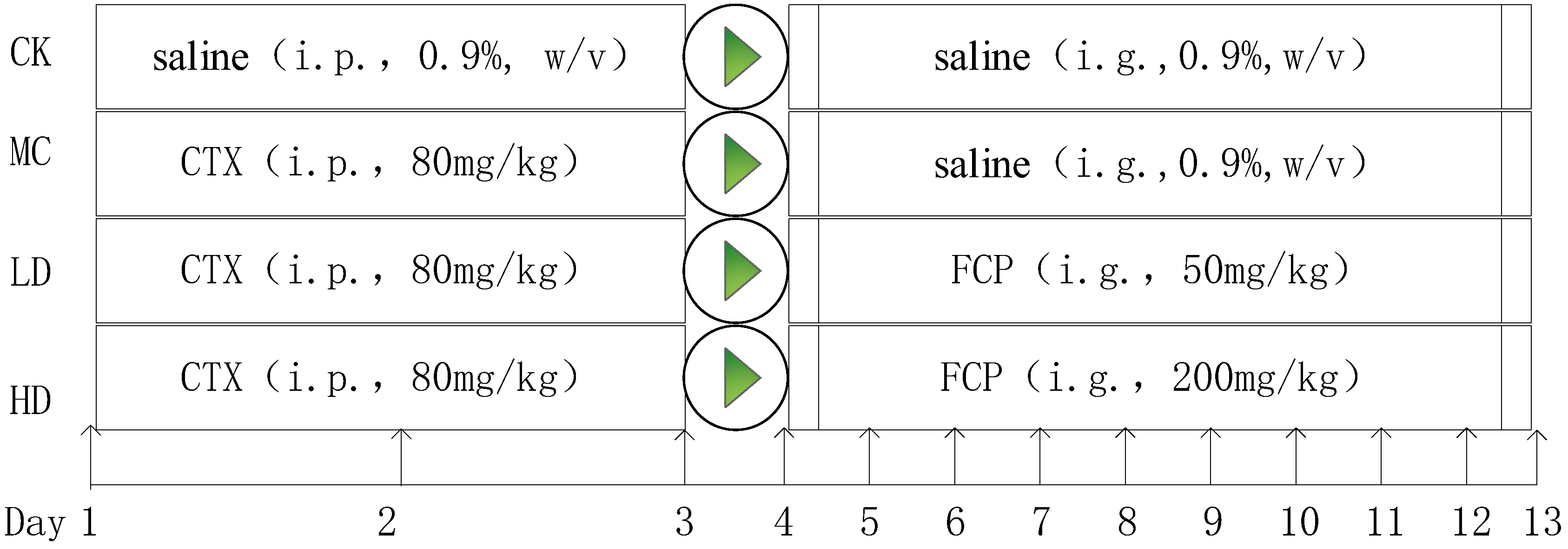
2.5.1. Animal Experiments
2.5.2. Determination of Serum Cytokines
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Compositions and Molecular Weight of FCP
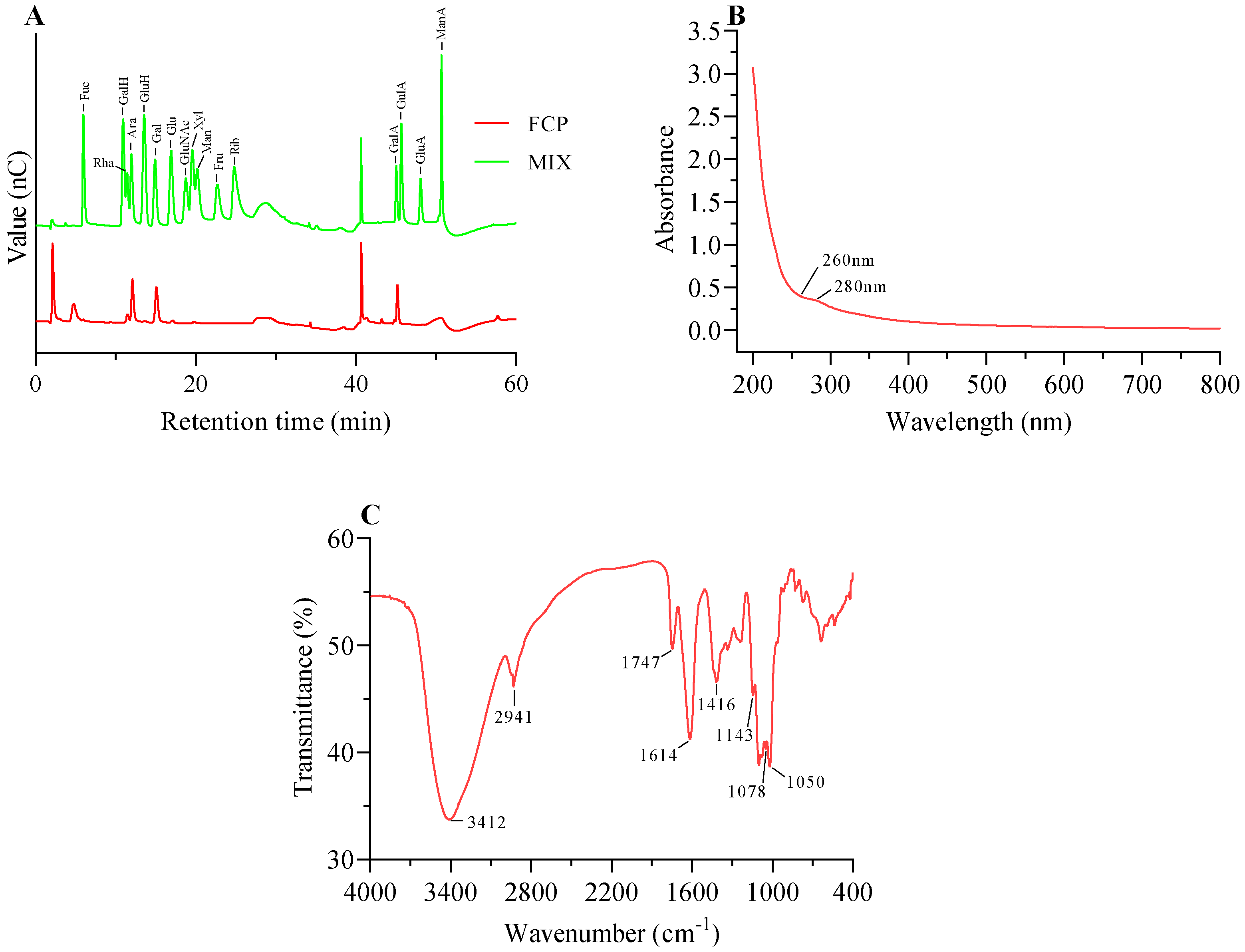
3.2. Monosaccharide Composition Analysis
3.3. Ultraviolet Spectrum Analysis
3.4. FT-IR Analysis
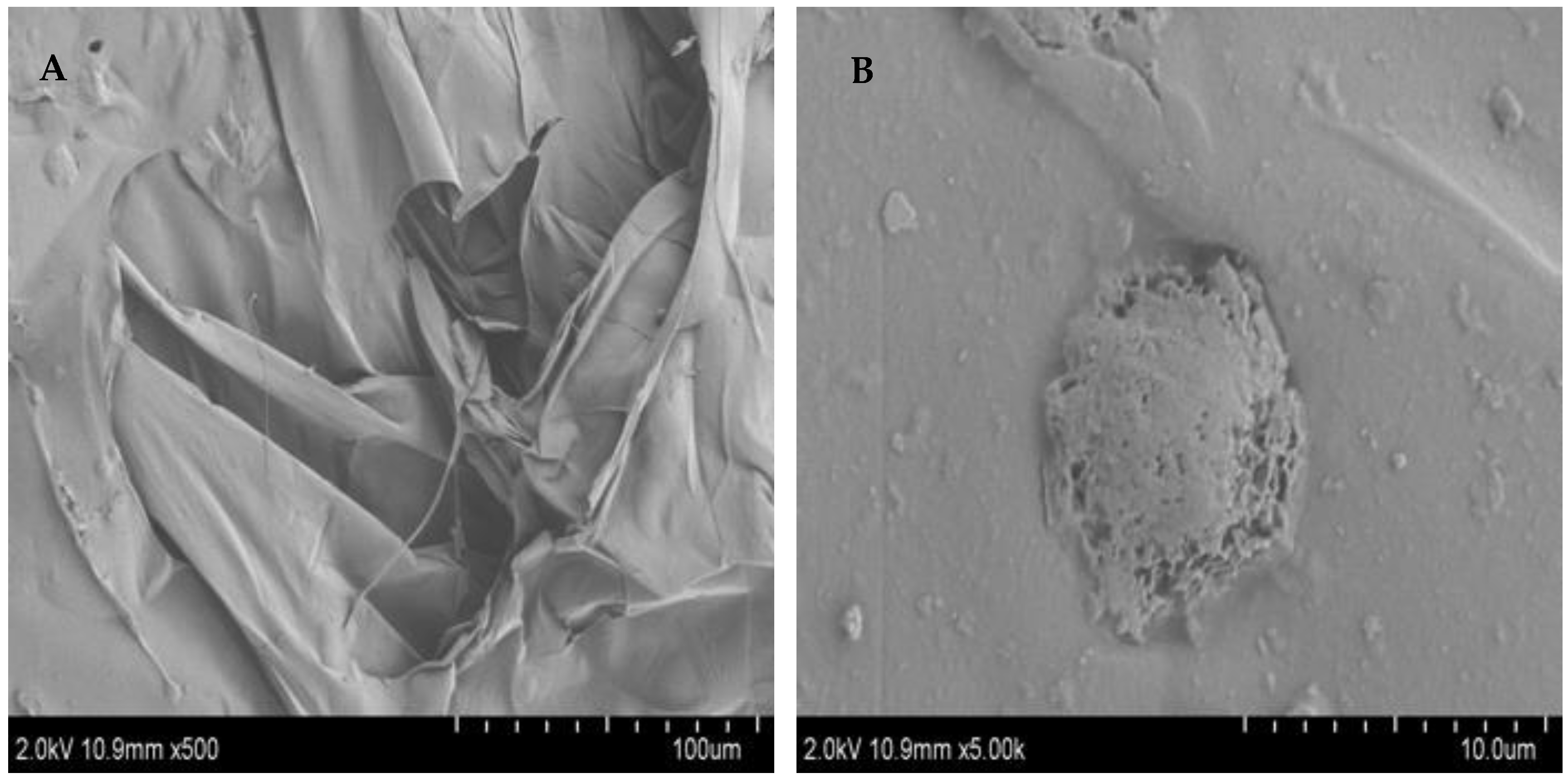
3.5. SEM Characterization of FCP
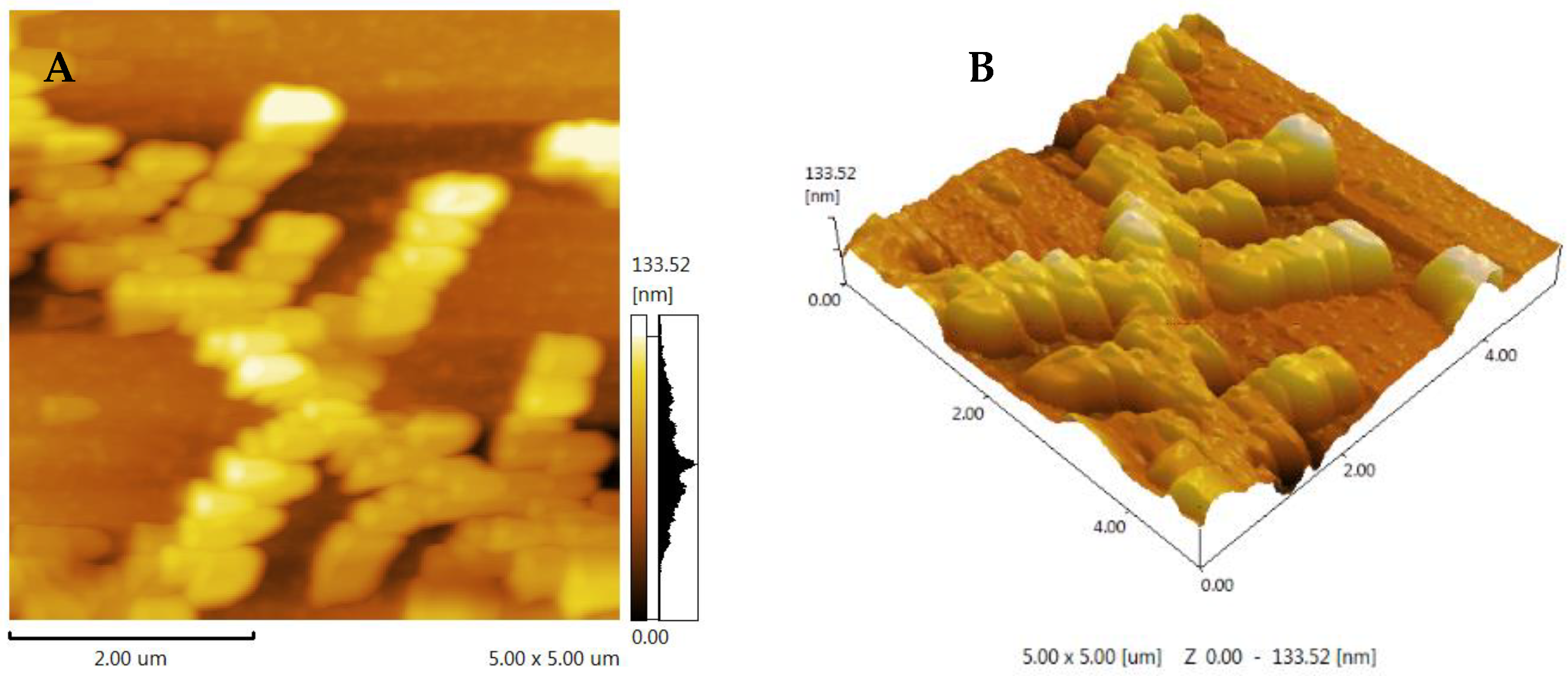
3.6. AFM Analysis of FCP
3.7. Methylation Analysis
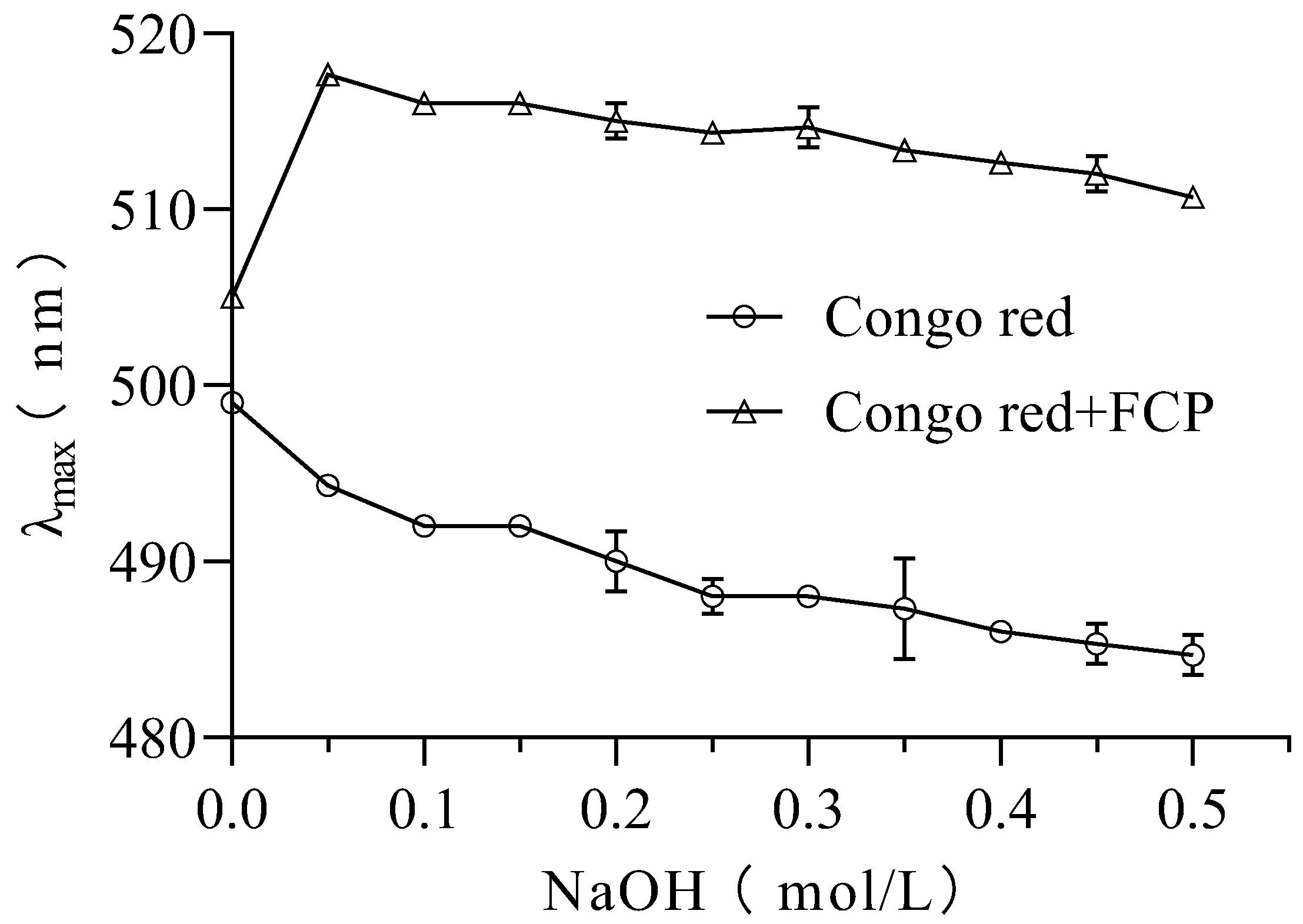
3.8. Congo Red Analysis
3.9. Immunomodulatory Activity In Vitro
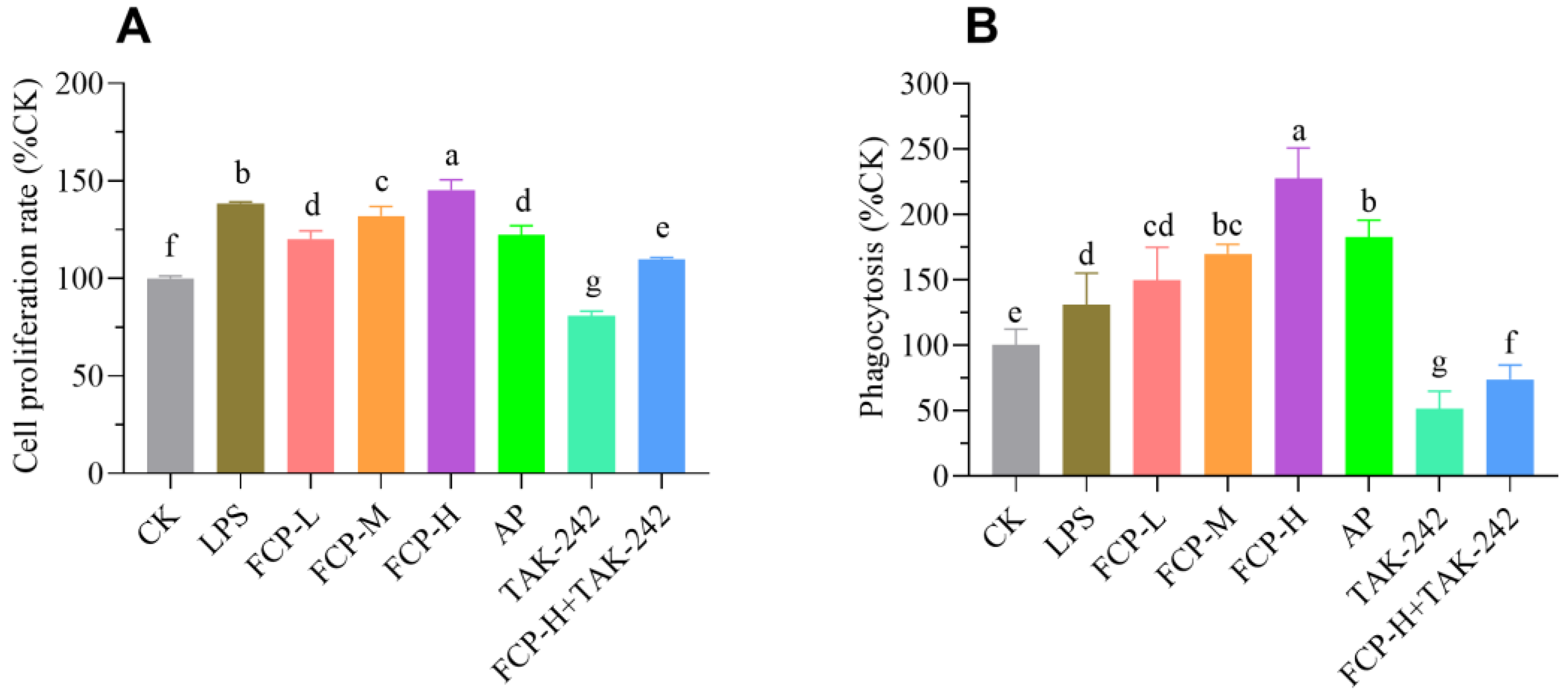
3.9.1. Assessment of Cell Proliferation
3.9.2. Assay of Phagocytosis
3.9.3. Determination of NO
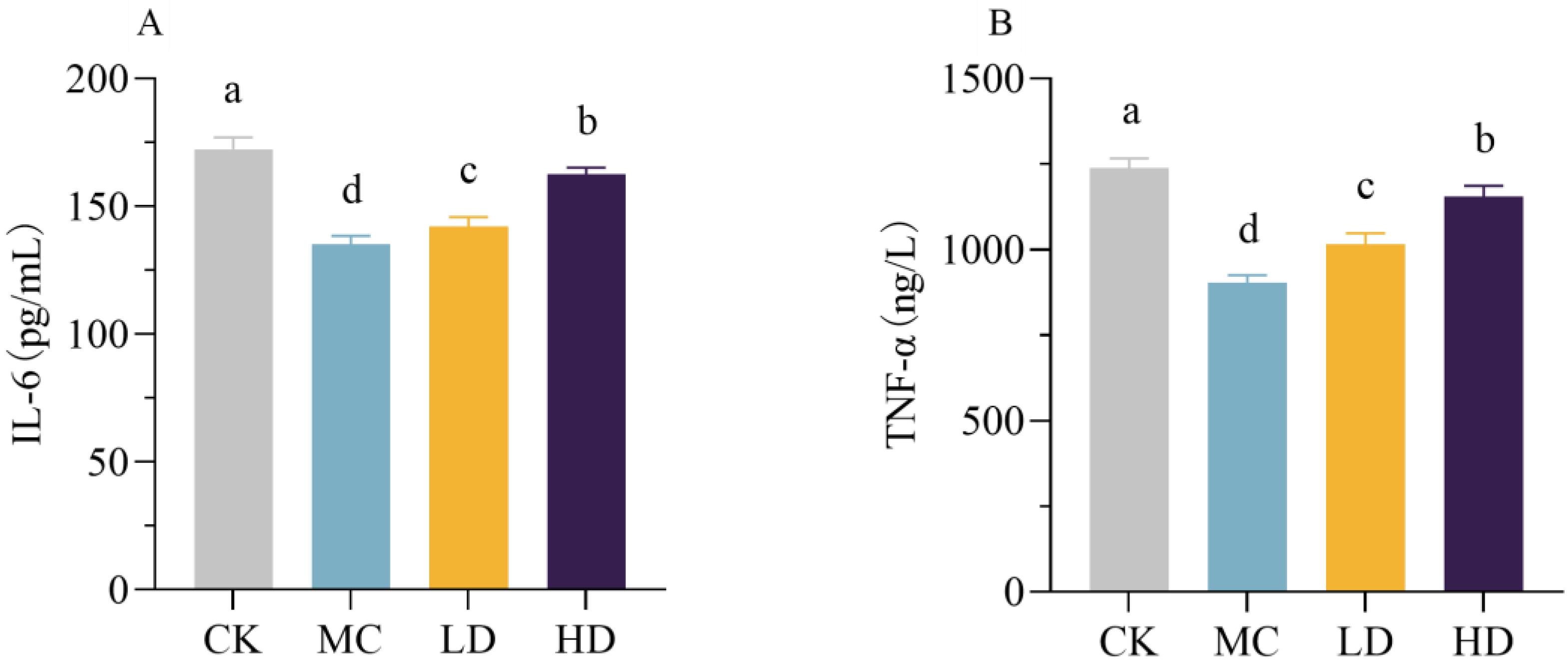
3.10. Immunomodulatory Activity In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giordano, C.; Maleci, L.; Agati, G.; Petruccelli, R. Ficus carica L. Leaf Anatomy: Trichomes and Solid Inclusions. Ann. Appl. Biol. 2020, 176, 47–54. [Google Scholar] [CrossRef]
- Li, J.; Cao, Q.; Fan, J.; Han, Y. Optimization of Conditions for Extraction of Polysaccharide from Ficus carica by Using Response Surface Methodology. Acta Agric. Jiangxi 2017, 29, 93–97. [Google Scholar]
- Uddin, M.S. A Review on Nutritional Values and Pharmacological Importance of Ficus carica. J. Curr. Res. Food Sci. 2021, 2, 7–11. [Google Scholar]
- Ayuso, M.; Carpena, M.; Taofiq, O.; Albuquerque, T.G.; Simal-Gandara, J.; Oliveira, M.B.P.; Prieto, M.A.; Ferreira, I.C.; Barros, L. Fig “Ficus carica L.” and Its by-Products: A Decade Evidence of Their Health-Promoting Benefits towards the Development of Novel Food Formulations. Trends Food Sci. Technol. 2022, 127, 1–13. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Aldebasi, Y.H. Ficus carica and Its Constituents Role in Management of Diseases. Asian J. Pharm. Clin. Res. 2017, 10, 49–53. [Google Scholar] [CrossRef]
- Al-Snafi, A. Nutritional and Pharmacological Importance of Ficus carica-A Review. IOSR J. Pharm. 2017, 7, 33–48. [Google Scholar] [CrossRef]
- Chakka, V.P.; Zhou, T. Carboxymethylation of Polysaccharides: Synthesis and Bioactivities. Int. J. Biol. Macromol. 2020, 165, 2425–2431. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Yang, W.; Huang, H. Preparation, Deproteinization and Comparison of Bioactive Polysaccharides. Trends Food Sci. 2021, 109, 564–568. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Abdelhamid, A.; Rihouey, C.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Optimized Extraction of Pectin-Like Polysaccharide from Suaeda fruticosa Leaves: Characterization, Antioxidant, Anti-Inflammatory and Analgesic Activities. Carbohyd. Polym. 2018, 185, 127–137. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Huang, G. Preparation and Antioxidant Activities of Important Traditional Plant Polysaccharides. Int. J. Biol. Macromol. 2018, 111, 780–786. [Google Scholar] [CrossRef]
- Ai, J.; Bao, B.; Battino, M.; Giampieri, F.; Chen, C.; You, L.; Cespedes-Acuña, C.L.; Ognyanov, M.; Tian, L.; Bai, W. Recent Advances on Bioactive Polysaccharides from Mulberry. Food Funct. 2021, 12, 5219–5235. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Yang, W.; Huang, G. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, J.; Li, M.; Zhu, S.; Guo, S.; Guo, H.; Wang, T.; Zhang, Y.; Zhang, J.; Wang, J. The Role of Se Content in Improving Anti-Tumor Activities and Its Potential Mechanism for Selenized Artemisia sphaerocephala Polysaccharides. Food Funct. 2021, 12, 2058–2074. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Chen, C.; Leng, A.J.; Qu, J.L. Advances in the Extraction, Purification, Structural Characteristics and Biological Activities of Eleutherococcus senticosus Polysaccharides: A Promising Medicinal and Edible Resource with Development Value. Front. Pharmacol. 2021, 12, 753007. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, J.; Zhu, J.; Huang, C.; Bi, S.; Song, L.; Hu, X.; Yu, R. Structural Characterization and Immunomodulatory Activity of a Novel Polysaccharide from Ficus carica. Food Funct. 2018, 9, 3930–3943. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xue, Z.; Fang, Y.; Liu, X.; Yang, Y.; Shi, G.; Feng, S.; Zhao, L.J.F. Structure–Immunomodulatory Activity Relationships of Hedysarum Polysaccharides Extracted by a Method Involving a Complex Enzyme Combined with Ultrasonication. Food Funct. 2019, 10, 1146–1158. [Google Scholar] [CrossRef]
- Wu, Y.-G.; Wang, K.-W.; Zhao, Z.-R.; Zhang, P.; Liu, H.; Zhou, G.-J.; Cheng, Y.; Wu, W.-J.; Cai, Y.-H.; Wu, B.-L. A Novel Polysaccharide from Dendrobium devonianum Serves as a TLR4 agonist for Activating Macrophages. Int. J. Biol. Macromol. 2019, 133, 564–574. [Google Scholar] [CrossRef]
- Chen, X.; Cai, B.; Wang, J.; Sheng, Z.; Yang, H.; Wang, D.; Chen, J.; Ning, Q. Mulberry Leaf-Derived Polysaccharide Modulates the Immune Response and Gut Microbiota Composition in Immunosuppressed Mice. J. Funct. Foods 2021, 83, 104545. [Google Scholar] [CrossRef]
- Tian, B.; Liu, R.; Xu, T.; Cai, M.; Mao, R.; Huang, L.; Yang, K.; Zeng, X.; Peilong, S. Modulating Effects of Hericium erinaceus Polysaccharides on the Immune Response by Regulating Gut Microbiota in Cyclophosphamide-Treated Mice. J. Sci. Food Agric. 2023, 103, 3050–3064. [Google Scholar] [CrossRef]
- Nie, X.-R.; Li, H.-Y.; Du, G.; Lin, S.; Hu, R.; Li, H.-Y.; Zhao, L.; Zhang, Q.; Chen, H.; Wu, D.-T. Structural Characteristics, Rheological Properties, and Biological Activities of Polysaccharides from Different Cultivars of Okra (Abelmoschus esculentus) Collected in China. Int. J. Biol. Macromol. 2019, 139, 459–467. [Google Scholar] [CrossRef]
- Kakar, M.U.; Kakar, I.U.; Mehboob, M.Z.; Zada, S.; Soomro, H.; Umair, M.; Iqbal, I.; Umer, M.; Shaheen, S.; Syed, S.F. A Review on Polysaccharides from Artemisia Sphaerocephala Krasch Seeds, their Extraction, Modification, Structure, and Applications. Carbohyd. Polym. 2021, 252, 117113. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Blumenkrantz, N.; Asboe-Hansen, G. New Method for Quantitative Determination of Uronic Acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Ming, K.; Ma, N.; Sun, J.; Wang, D.; Ding, M.; Ding, Y. Portulaca oleracea L. Polysaccharide Ameliorates Lipopolysaccharide-Induced Inflammatory Responses and Barrier Dysfunction in Porcine Intestinal Epithelial Monolayers. J. Funct. Foods 2022, 91, 104997. [Google Scholar] [CrossRef]
- Ahmadian, S.; Kenari, R.E.; Amiri, Z.R.; Sohbatzadeh, F.; Khodaparast, M.H.H. Effect of Ultrasound-Assisted Cold Plasma Pretreatment on Cell Wall Polysaccharides Distribution and Extraction of Phenolic Compounds from Hyssop (Hyssopus officinalis L.). Int. J. Biol. Macromol. 2023, 233, 123557. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, F.; Ding, Y.; Li, H.-Y.; Xiang, X.-R.; Ye, Q.; Zhang, J.; Zhao, L.; Qin, W.; Gan, R.-Y.; et al. Polysaccharides from Loquat (Eriobotrya japonica) Leaves: Impacts of Extraction Methods on Their Physicochemical Characteristics and Biological Activities. Int. J. Biol. Macromol. 2020, 146, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, J.; Xia, X.; Wong, I.N.; Chung, S.K.; Xu, B.; El-Seedi, H.R.; Wang, B.; Huang, R. Sulfated Heteropolysaccharides from Undaria Pinnatifida: Structural Characterization and Transcript-Metabolite Profiling of Immunostimulatory Effects on RAW264. 7 Cells. Food Chem. X 2022, 13, 100251. [Google Scholar] [CrossRef]
- Fu, Y.; Feng, K.-L.; Wei, S.-Y.; Xiang, X.-R.; Ding, Y.; Li, H.-Y.; Zhao, L.; Qin, W.; Gan, R.-Y.; Wu, D.-T. Comparison of Structural Characteristics and Bioactivities of Polysaccharides from Loquat Leaves Prepared by Different Drying Techniques. Int. J. Biol. Macromol. 2020, 145, 611–619. [Google Scholar] [CrossRef]
- Zongo, A.W.-S.; Zogona, D.; Zhang, Z.; Youssef, M.; Zhou, P.; Chen, Y.; Geng, F.; Chen, Y.; Li, J.; Li, B.J.F.; et al. Immunomodulatory activity of Senegalia macrostachya (Reichenb. ex DC.) Kyal. & Boatwr seed polysaccharide fraction through the activation of the MAPK Signaling Pathway in RAW264. 7 Macrophages. Food Funct. 2022, 13, 4664–4677. [Google Scholar]
- Ma, Z.; Sun, Q.; Chang, L.; Peng, J.; Zhang, M.; Ding, X.; Zhang, Q.; Liu, G.; Liu, X.; Lan, Y.J.F.C. A Natural Anti-Obesity Reagent Derived from Sea Buckthorn Polysaccharides: Structure Characterization and Anti-Obesity Evaluation In Vivo. Food Chem. 2022, 375, 131884. [Google Scholar] [CrossRef]
- Lin, X.; Liu, K.; Yin, S.; Qin, Y.; Shen, P.; Peng, Q.J.A. A Novel Pectic Polysaccharide of Jujube Pomace: Structural Analysis and Intracellular Antioxidant Activities. Antioxidants 2020, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mo, J.; Xiang, W.; Shi, X.; Guo, L.; Li, Y.; Bao, Y.; Zheng, L. Immunoregulatory Effects of Tetrastigma hemsleyanum Polysaccharide via TLR4-Mediated NF-κB and MAPK Signaling Pathways in Raw264. 7 Macrophages. Biomed. Pharmacother. 2023, 161, 114471. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; You, S.; Yelithao, K.; Palanisamy, S.; Prabhu, N.M.; Nan, M. Isolation, Structural Elucidation and Immuno-Stimulatory Properties of Polysaccharides from Cuminum cyminum. Carbohyd. Polym. 2020, 230, 115636. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wei, Y.; Liang, L.; Huang, L.; Yu, G.; Li, Q. A Novel Low-Molecular-Mass Pumpkin Polysaccharide: Structural Characterization, Antioxidant Activity, and Hypoglycemic Potential. Carbohyd. Polym. 2021, 251, 117090. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qi, J.; Ho, C.-T.; Li, B.; Mu, J.; Zhang, Y.; Hu, H.; Mo, W.; Chen, Z.; Xie, Y. Structural Characterization and Immunomodulatory Activity of a Water-Soluble Polysaccharide from Ganoderma leucocontextum Fruiting Bodies. Carbohyd. Polym. 2020, 249, 116874. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Chen, G. Extraction, Structural Analysis, Derivatization and Antioxidant Activity of Polysaccharide from Chinese Yam. Food Chem. 2021, 361, 130089. [Google Scholar] [CrossRef] [PubMed]
- El Nokab, M.E.H.; Van Der Wel, P.C. Use of solid-state NMR Spectroscopy for Investigating Polysaccharide-Based Hydrogels: A Review. Carbohyd. Polym. 2020, 240, 116276. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Huang, B.; Yang, B.; Ye, L.; Zeng, J.; Xiong, Z.; Chen, Y.; Guo, S.; Yang, Y.; Ma, W. Structural Elucidation of a Novel Polysaccharide from Ophiopogonis Radix and its Self-Assembly Mechanism in Aqueous Solution. Food Chem. 2023, 402, 134165. [Google Scholar] [CrossRef]
- Chen, R.; Li, H.; Li, S.; Jin, C.; Lu, J. Extraction Optimization, Preliminary Characterization and Immunological Activity of Polysaccharides from Figs. Int. J. Biol. Macromol. 2015, 72, 185–194. [Google Scholar] [CrossRef]
- Yin, X.; Zhuang, X.; Luo, W.; Liao, M.; Huang, L.; Cui, Q.; Huang, J.; Yan, C.; Jiang, Z.; Liu, Y. Andrographolide Promote the Growth and Immunity of Litopenaeus vannamei, and Protects Shrimps against Vibrio Alginolyticus by Regulating Inflammation and Apoptosis via a ROS-JNK Dependent Pathway. Front. Immunol. 2022, 13, 990297. [Google Scholar] [CrossRef]
- Park, W.S.; Lee, J.; Na, G.; Park, S.; Seo, S.-K.; Choi, J.S.; Jung, W.-K.; Choi, I.-W. Benzyl Isothiocyanate Attenuates Inflammasome Activation in Pseudomonas aeruginosa LPS-Stimulated THP-1 Cells and Exerts Regulation through the MAPKs/NF-κB Pathway. Int. J. Mol. Sci. 2022, 23, 1228. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zeng, Y.-J.; Chen, X.; Luo, S.-Y.; Pu, L.; Li, F.-Z.; Zong, M.-H.; Lou, W.-Y. A Novel Polysaccharide from the Roots of Millettia Speciosa Champ: Preparation, Structural Characterization and Immunomodulatory Activity. Int. J. Biol. Macromol. 2020, 145, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zeng, R.; Qi, J.; Ho, C.-T.; Li, B.; Chen, Z.; Chen, S.; Xiao, C.; Hu, H.; Cai, M. Immunoregulatory Activity of a Low-Molecular-Weight Heteropolysaccharide from Ganoderma leucocontextum Fruiting Bodies In Vitro and In Vivo. Food Chem. X 2022, 14, 100321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Song, Y.; Martínez-Cuesta, M.C.; Peláez, C.; Li, E.; Requena, T.; Wang, H.; Sun, Y. Immunological Activity and Gut Microbiota Modulation of Pectin from Kiwano (Cucumis metuliferus) Peels. Foods 2022, 11, 1632. [Google Scholar] [CrossRef]
- Meng, M.; Sun, Y.; Bai, Y.; Xu, J.; Sun, J.; Han, L.; Sun, H.; Han, R. A Polysaccharide from Pleurotus Citrinopileatus Mycelia Enhances the Immune Response in Cyclophosphamide-Induced Immunosuppressed Mice via p62/Keap1/Nrf2 Signal Transduction Pathway. Int. J. Biol. Macromol. 2023, 228, 165–177. [Google Scholar] [CrossRef]
- Xie, H.; Fang, J.; Farag, M.A.; Li, Z.; Sun, P.; Shao, P. Dendrobium officinale Leaf Polysaccharides Regulation of Immune Response and Gut Microbiota Composition in Cyclophosphamide-Treated Mice. Food Chem. X 2022, 13, 100235. [Google Scholar] [CrossRef]

| Parameters | FCP |
|---|---|
| Total carbohydrate (%) | 93.46 ± 2.27 |
| Uronic acid (%) | 60.40 ± 0.61 |
| Protein (%) | 1.59 ± 0.67 |
| Starch (%) | n.d. |
| Total phenolics (%) | n.d. |
| Mw (kDa) | 127.5 |
| Mw/Mn | 1.85 |
| RT | Methylated Sugar | Mass Fragments (m/z) | Type of Linkage |
|---|---|---|---|
| 10.508 | 2, 3, 5-Me3-Araf | 43, 71, 87, 101, 117, 129, 145, 161 | Araf-(1→ |
| 11.804 | 2, 3, 4-Me2-Arap | 43, 71, 87, 101, 117, 131, 161 | Arap-(1→ |
| 13.997 | 2, 3, 4-Me3-Rhap | 43, 59, 72, 89, 101, 115, 117, 131, 175 | Rhap-(1→ |
| 15.223 | 2, 3-Me2-Araf | 43, 71, 87, 99, 101, 117, 129, 161, 189 | →5)-Araf-(1→ |
| 15.473 | 2, 4-Me2-Rhap | 43, 58, 85, 89, 101, 117, 127, 131, 159, 201 | →3)-Rhap-(1→ |
| 17.908 | 2, 3, 4, 6-Me4-Glcp | 43, 71, 87, 101, 117, 129, 145, 161, 205 | Glcp-(1→ |
| 18.541 | 2, 3, 4, 6-Me4-Galp | 43, 71, 87, 101, 117, 129, 145, 161, 205 | Galp-(1→ |
| 19.129 | 2-Me1-Araf | 43, 58, 85, 99, 117, 127, 159, 201 | →3, 5)-Araf-(1→ |
| 21.08 | 2, 4, 6-Me3-Glcp | 43, 87, 99, 101, 117, 129, 161, 173, 233 | →3)-Glcp-(1→ |
| 21.414 | 2, 3, 6-Me3-Galp | 43, 87, 99, 101, 113, 117, 129, 131, 161, 173, 233 | →4)-Galp-(1→ |
| 21.835 | 2, 4, 6-Me3-Galp | 43, 87, 99, 101, 117, 129, 161, 173, 233 | →3)-Galp-(1→ |
| 23.048 | 2, 3, 4-Me3-Glcp | 43, 87, 99, 101, 117, 129, 161, 189, 233 | →6-Glcp-(1→ |
| 23.757 | 2, 3, 4-Me3-Galp | 43, 87, 99, 101, 117, 129, 161, 189, 233 | →6)-Galp-(1→ |
| 24.424 | 2, 6-Me2-Glcp | 43, 87, 97, 117, 159, 185 | →3, 4)-Glcp-(1→ |
| 25.57 | 2, 6-Me2-Galp | 43, 87, 99, 117, 129, 143, 159 | →3, 4)-Galp-(1→ |
| 26.721 | 2, 3-Me2-Glcp | 43, 71, 85, 87, 99, 101, 117, 127, 159, 161, 201 | →4, 6)-Glcp-(1→ |
| 27.134 | 2, 4-Me2-Glcp | 43, 87, 117, 129, 159, 189, 233 | →3, 6)-Glcp-(1→ |
| 28.613 | 2, 3-Me2-Galp | 43, 71, 85, 87, 99, 101, 117, 127, 159, 161, 201, 261 | →4, 6)-Galp-(1→ |
| 30.602 | 2, 4-Me2-Galp | 43, 87, 117, 129, 159, 189, 233 | →3, 6)-Galp-(1→ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Zhang, Q.-Q.; Lin, S.; Zhang, Q.; Yan, J.; Wu, D.-T.; Liu, S.-X.; Qin, W. A Polysaccharide from Ficus carica L. Exerts Immunomodulatory Activity in Both In Vitro and In Vivo Experimental Models. Foods 2024, 13, 195. https://doi.org/10.3390/foods13020195
Ye L, Zhang Q-Q, Lin S, Zhang Q, Yan J, Wu D-T, Liu S-X, Qin W. A Polysaccharide from Ficus carica L. Exerts Immunomodulatory Activity in Both In Vitro and In Vivo Experimental Models. Foods. 2024; 13(2):195. https://doi.org/10.3390/foods13020195
Chicago/Turabian StyleYe, Lin, Qin-Qiu Zhang, Shang Lin, Qing Zhang, Jing Yan, Ding-Tao Wu, Shu-Xiang Liu, and Wen Qin. 2024. "A Polysaccharide from Ficus carica L. Exerts Immunomodulatory Activity in Both In Vitro and In Vivo Experimental Models" Foods 13, no. 2: 195. https://doi.org/10.3390/foods13020195





