Dissipation, Residue and Human Dietary Risk Assessment of Pyraclostrobin and Cyazofamid in Grapes Using an HPLC-UV Detector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Extraction and Purification Process of the Samples
2.3. Instrumentation
2.4. Field Trial Tests
2.5. Deterministic Model
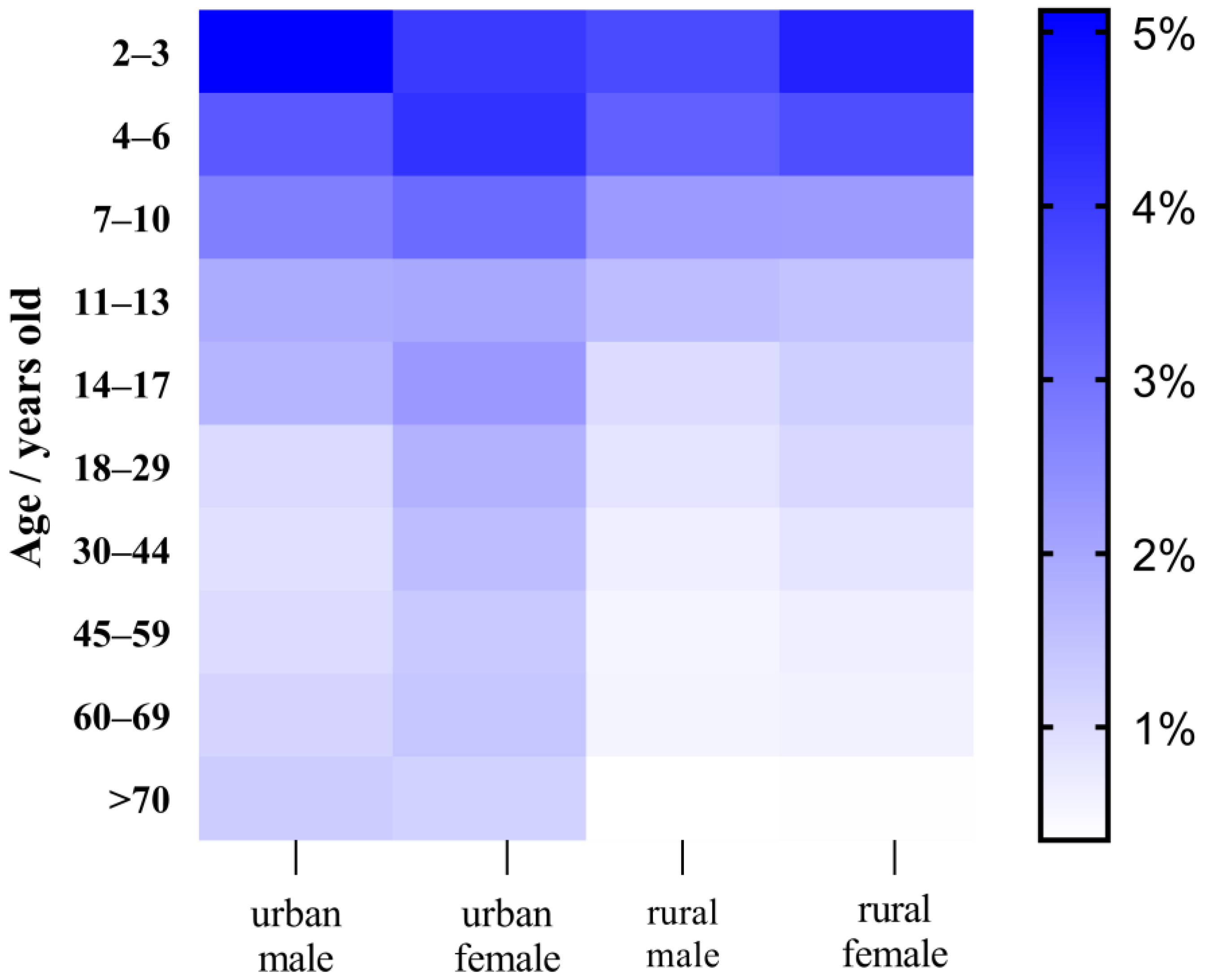
2.6. Cumulative Dietary Risk Assessment on Probabilistic Method
3. Results and Discussion
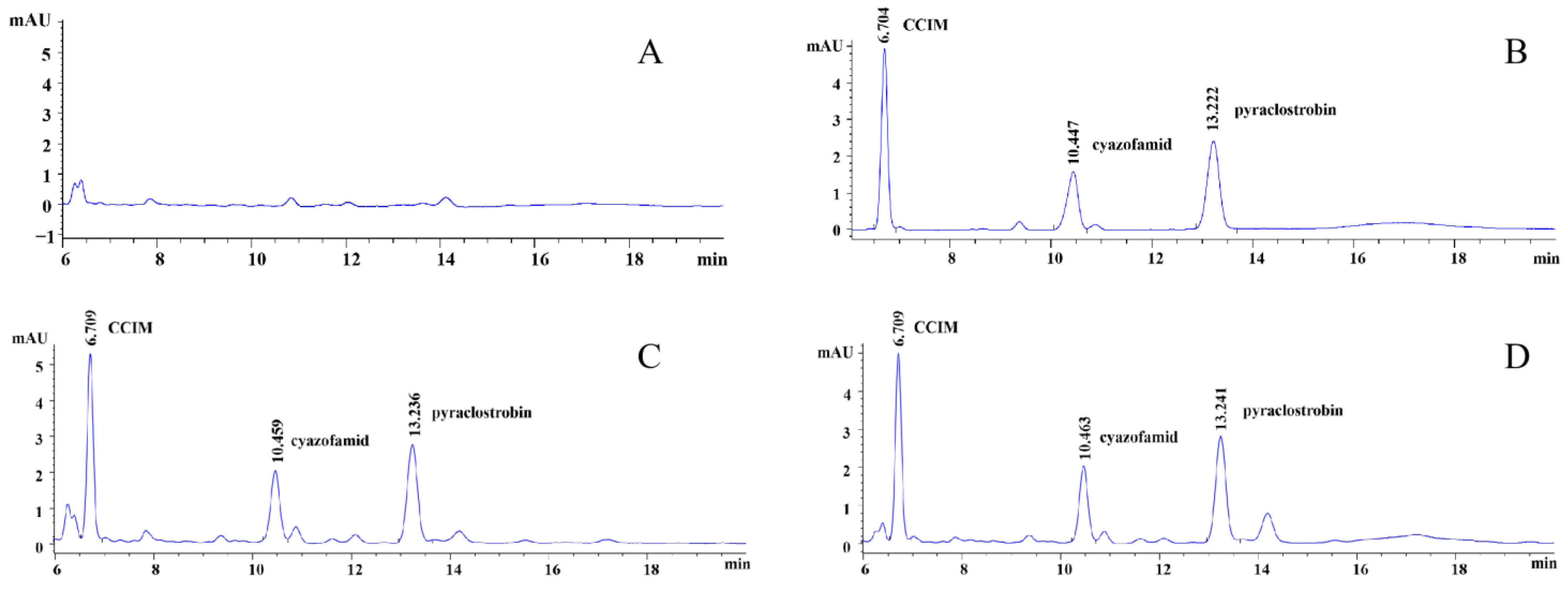
3.1. Instrument Results
3.2. Method Validation
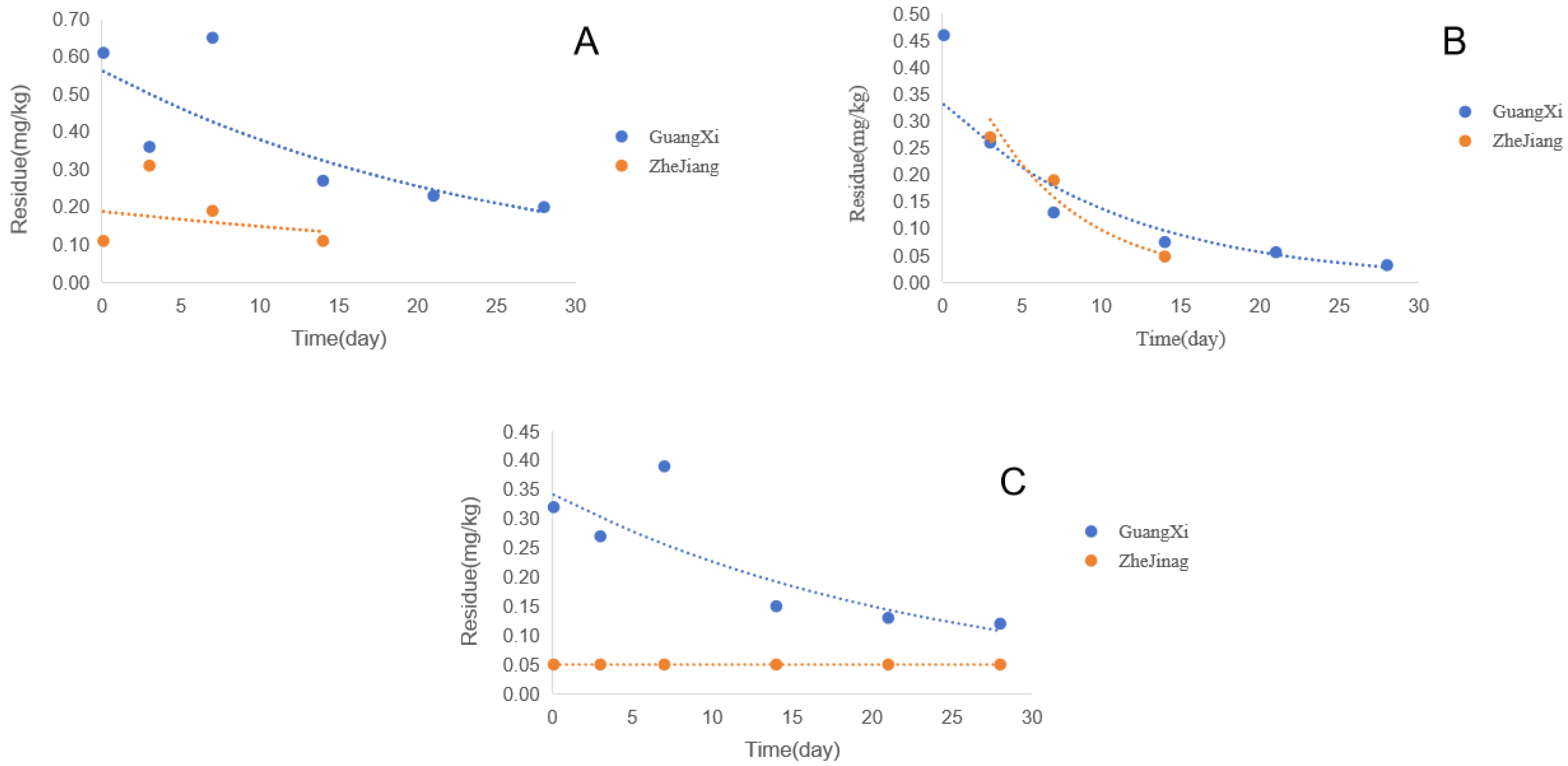
3.3. Dissipation Behavior
3.4. Final Residue Testing
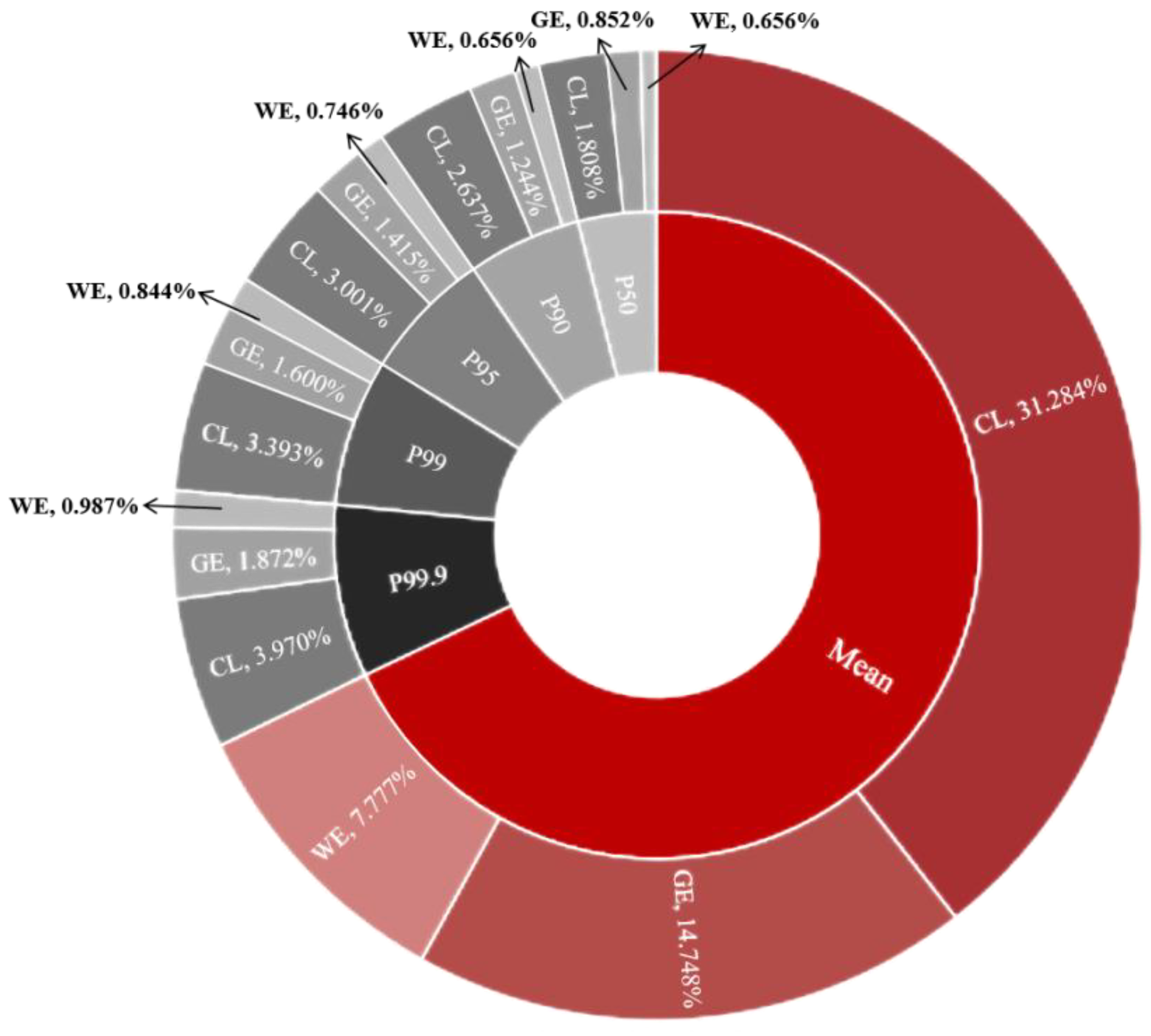
3.5. Acute Dietary Risk Assessment
3.6. Chronic Dietary Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, X.; Sun, S.; Liu, Z.; Yang, X. Drought analysis during the growth stages of grape in the main grape-growing regions in China. Theor. Appl. Climatol. 2022, 149, 1497–1507. [Google Scholar] [CrossRef]
- OIV (International Organizationof Vineand Wine). State of the World Vine and Wine Sector in 2022. Available online: https://www.oiv.int/sites/default/files/documents/2023_SWVWS_report_EN.pdf (accessed on 7 October 2023).
- Wang, Y.; Cao, X.; Han, Y.; Han, X.; Wang, Z.; Xue, T.; Ye, Q.; Zhang, L.; Duan, X.; Wang, H.; et al. Kaolin particle film protects grapevine cv. Cabernet Sauvignon against downy mildew by forming particle film at the leaf surface, directly acting on sporangia and inducing the defense of the plant. Front. Plant Sci. 2022, 12, 796545. [Google Scholar] [CrossRef]
- Wang, C.; Guo, L.; Yao, J.; Wang, A.; Gao, F.; Zhao, X.; Zeng, Z.; Wang, Y.; Sun, C.; Cui, H.; et al. Preparation, characterization and antifungal activity of pyraclostrobin solid nanodispersion by self-emulsifying technique. Pest Manag. Sci. 2019, 75, 2785–2793. [Google Scholar] [CrossRef] [PubMed]
- Tsialtas, J.T.; Theologidou, G.S.; Karaoglanidis, G.S. Effects of pyraclostrobin on leaf diseases, leaf physiology, yield and quality of durum wheat under Mediterranean conditions. Crop Prot. 2018, 113, 48–55. [Google Scholar] [CrossRef]
- Hou, K.; Lu, C.; Shi, B.; Xiao, Z.; Wang, X.; Zhang, J.; Cheng, C.; Ma, J.; Du, Z.; Li, B.; et al. Evaluation of agricultural soil health after applying pyraclostrobin in wheat/maize rotation field based on the response of soil microbes. Agric. Ecosyst. Environ. 2022, 340, 108186. [Google Scholar] [CrossRef]
- Li, M.; Xu, W.; Hu, D.; Song, B. Preparation and application of pyraclostrobin microcapsule formulations. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 578–585. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, Y.; Hu, J. Dissipation, residues and risk assessment of pyraclostrobin and picoxystrobin in cucumber under field conditions. J. Sci. Food Agric. 2020, 100, 5145–5151. [Google Scholar] [CrossRef]
- Mitani, S.; Araki, S.; Takii, Y.; Ohshima, T.; Matsuo, N.; Miyoshi, H. The Biochemical Mode of Action of the Novel Selective Fungicide Cyazofamid: Specific Inhibition of Mitochondrial Complex III in Phythium spinosum. Pestic. Biochem. Physiol. 2001, 71, 107–115. [Google Scholar] [CrossRef]
- Pang, N.; Dou, X.; Hu, J. Residue behaviours, dissipation kinetics and dietary risk assessment of pyaclostrobin, cyazofamid and its metabolite in grape. J. Sci. Food Agric. 2019, 99, 6167–6172. [Google Scholar] [CrossRef]
- Skandalis, N.; Dimopoulou, A.; Beri, D.; Tzima, A.; Malandraki, I.; Theologidis, I.; Bitivanos, S.; Varveri, C.; Klitsinaris, T.; Vassilakos, N. Effect of pyraclostrobin application on viral and bacterial diseases of tomato. Plant Dis. 2016, 100, 1321–1330. [Google Scholar] [CrossRef]
- Moura, A.C.M.; Lago, I.N.; Cardoso, C.F.; dos Reis Nascimento, A.; Pereira, I.; Vaz, B.G. Rapid monitoring of pesticides in tomatoes (Solanum lycopersicum L.) during pre-harvest intervals by paper spray ionization mass spectrometry. Food Chem. 2020, 310, 125938. [Google Scholar] [CrossRef] [PubMed]
- Amaro, A.C.E.; Ramos, A.R.P.; Macedo, A.C.; Ono, E.O.; Rodrigues, J.D. Effects of the fungicides azoxystrobin, pyraclostrobin and boscalid on the physiology of Japanese cucumber. Sci. Hortic. 2018, 228, 66–75. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, C.; Yu, J.; Zhang, C.; Wu, M.; He, H.; Zhu, Y.; Lou, F.; Wu, Y.; Wang, Y.; et al. Field investigations of dissipations and residues of cyazofamid in soil and tomato: Risk assessment of human exposure to cyazofamid via tomato intake. Environ. Sci. Pollut. Res. 2016, 24, 3483–3492. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, X.L.; Yang, W.C.; Yang, G.F. Comparative kinetics of Qi site inhibitors of cytochrome bc1 complex: Picomolar antimycin and micromolar cyazofamid. Chem. Biol. Drug Des. 2014, 83, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Xu, Z.; Gao, M.; Li, L.; Li, H.; Cheng, H.; Zhang, C.; Tian, G. The dissipation of cyazofamid and its main metabolite in soil response oppositely to biochar application. Chemosphere 2019, 218, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Mitani, S.; Araki, S.; Yamaguchi, T.; Takii, Y.; Ohshima, T.; Matsuo, N. Antifungal activity of the novel fungicide cyazofamid against Phytophthora infestans and other plant pathogenic fungi in vitro. Pestic. Biochem. Physiol. 2001, 70, 92–99. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Zhang, S.; Zhu, L.; Du, Z.; Wang, J. Acute and subchronic toxicity of pyraclostrobin in zebrafish (Danio rerio). Chemosphere 2017, 188, 510–516. [Google Scholar] [CrossRef]
- Cui, F.; Chai, T.; Liu, X.; Wang, C. Toxicity of three strobilurins (kresoxim-methyl, pyraclostrobin, and trifloxystrobin) on Daphnia magna. Environ. Toxicol. Chem. 2016, 36, 182–189. [Google Scholar] [CrossRef]
- Ma, J.; Cheng, C.; Du, Z.; Li, B.; Wang, J.; Wang, J.; Wang, Z.; Zhu, L. Toxicological effects of pyraclostrobin on the antioxidant defense system and DNA damage in earthworms (Eisenia fetida). Ecol. Indic. 2019, 101, 111–116. [Google Scholar] [CrossRef]
- Li, B.x.; Wang, W.c.; Zhang, X.p.; Zhang, D.x.; Ren, Y.p.; Gao, Y.; Mu, W.; Liu, F. Using Coordination Assembly as the Microencapsulation Strategy to Promote the Efficacy and Environmental Safety of Pyraclostrobin. Adv. Funct. Mater. 2017, 27, 1701841. [Google Scholar] [CrossRef]
- Li, H.; Cao, F.; Zhao, F.; Yang, Y.; Teng, M.; Wang, C.; Qiu, L. Developmental toxicity, oxidative stress and immunotoxicity induced by three strobilurins (pyraclostrobin, trifloxystrobin and picoxystrobin) in zebrafish embryos. Chemosphere 2018, 207, 781–790. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An International Database for Pesticide Risk Assessments and Management. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/564.htm (accessed on 13 October 2023).
- Navruz, F.Z.; Köstekçi, Ö.Ç.; Ince, S. Effect of Pyraclostrobin-Based Herbicide on DNA Damage and Reproductive Performance in Drosophila Melanogasters. J. Appl. Biol. Sci. 2023, 17, 266–275. [Google Scholar]
- Inoue, L.V.; Domingues, C.E.; Gregorc, A.; Silva-Zacarin, E.C.; Malaspina, O. Harmful effects of pyraclostrobin on the fat body and pericardial cells of foragers of africanized honey bee. Toxics 2022, 10, 530. [Google Scholar] [CrossRef]
- Farder-Gomes, C.F.; Grella, T.C.; Malaspina, O.; Nocelli, R.F.C. Exposure to sublethal concentrations of imidacloprid, pyraclostrobin, and glyphosate harm the behavior and fat body cells of the stingless bee Scaptotrigona postica. Sci. Total Environ. 2024, 907, 168072. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Pesticide residues in food—2019 Evaluation: Part II, Toxicological. In Food & Agriculture Organization; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Wu, M.; Bian, J.; Han, S.; Zhang, C.; Xu, W.; Tao, L.; Li, Z.; Zhang, Y. Characterization of hepatotoxic effects induced by pyraclostrobin in human HepG2 cells and zebrafish larvae. Chemosphere 2023, 340, 139732. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Mathieu, B.; Vandensande, Y.; Gallez, B. Impact of Exposure to Pyraclostrobin and to a Pyraclostrobin/Boscalid Mixture on the Mitochondrial Function of Human Hepatocytes. Molecules 2023, 28, 7013. [Google Scholar] [CrossRef] [PubMed]
- Çayır, A.; Coskun, M.; Coskun, M. Micronuclei, nucleoplasmic bridges, and nuclear buds induced in human lymphocytes by the fungicide signum and its active ingredients (boscalid and pyraclostrobin). Environ. Toxicol. 2014, 29, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Cobanoglu, H.; Coskun, B.; Cayir, A. Assessment of genotoxic effects of a fungicide product and its active substances on human peripheral blood mononuclear cells. Pestic. I Fitomedicina 2019, 34, 61–67. [Google Scholar] [CrossRef]
- European Food Safety Authority. Peer review of the pesticide risk assessment of the active substance cyazofamid. EFSA J. 2016, 14, e04503. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, N.; Zhang, S.; Wang, W.; Zou, Y.; Gu, Z. The dissipation of cyazofamid and its main metabolite CCIM during tomato growth and tomato paste making process. Food Addit. Contam. Part A 2019, 36, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, S.; Li, X.; He, L.; Zhu, J.; Mu, W.; Liu, F. Residue determination of pyraclostrobin, picoxystrobin and its metabolite in pepper fruit via UPLC-MS/MS under open field conditions. Ecotoxicol. Environ. Saf. 2019, 182, 109445. [Google Scholar] [CrossRef]
- Lee, H.; Kim, E.; Lee, J.-H.; Sung, J.H.; Choi, H.; Kim, J.-H. Analysis of cyazofamid and its metabolite in the environmental and crop samples using LC–MS/MS. Bull. Environ. Contam. Toxicol. 2014, 93, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Feng, X.; Pan, L.; Jing, J.; Zhang, H. Residue and risk assessment of fluopicolide and cyazofamid in grapes and soil using LC-MS/MS and modified QuEChERS. RSC Adv. 2018, 8, 35485–35495. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Lee, S.-H.; Kwak, S.-Y.; Nam, A.-J.; Kim, H.-J.; Kim, J.-E. Residue Monitoring and Risk Assessment of Cyazofamid and Its Metabolite in Korean Cabbage Under Greenhouse Conditions. Bull. Environ. Contam. Toxicol. 2020, 105, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Caldas, E.D. Approaches for cumulative dietary risk assessment of pesticides. Curr. Opin. Food Sci. 2023, 53, 101079. [Google Scholar] [CrossRef]
- Chen, R.; Xue, X.; Wang, G.; Wang, J. Determination and dietary intake risk assessment of 14 pesticide residues in apples of China. Food Chem. 2021, 351, 129266. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, R.; Su, Y.; Hu, J.; Liu, X. Fate, residues and dietary risk assessment of the fungicides epoxiconazole and pyraclostrobin in wheat in twelve different regions, China. Ecotoxicol. Environ. Saf. 2021, 207, 111236. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Men, X.; Li, R.; Liu, T.; Liang, H.; Fang, F.; Sun-Waterhouse, D.; Wang, Y. Residue behaviors and dietary risk of cyazofamid in turnip, onion and romaine lettuce assessed by a QuEChERS-LC-MS/MS method. Food Sci. Hum. Wellness 2023, 12, 1538–1544. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China NY/T 788-2018 Guidelines for the Testing of Pesticide Resides in Crops 2018. Available online: https://www.sdtdata.com/fx/fmoa/tsLibCard/169332.html (accessed on 1 June 2019).
- Li, C.; Chen, Z.; Qin, D.; Liu, R.; Li, L.; Li, W.; He, Y.; Yuan, L. Simultaneous determination of the herbicide bixlozone and its metabolites in plant and animal samples by liquid chromatography–tandem mass spectrometry. J. Sep. Sci. 2021, 44, 822–832. [Google Scholar] [CrossRef]
- Yang, M.; Luo, F.; Zhang, X.; Zhou, L.; Lou, Z.; Zhao, M.; Chen, Z. Dissipation and risk assessment of multiresidual fungicides in grapes under field conditions. J. Agric. Food Chem. 2019, 68, 1071–1078. [Google Scholar] [CrossRef]
- Hamilton, D.; Ambrus, A.; Dieterle, R.; Felsot, A.; Harris, C.; Petersen, B.; Racke, K.; Wong, S.S.; Gonzalez, R.; Tanaka, K.; et al. Pesticide residues in food—Acute dietary exposure. Pest Manag. Sci. 2004, 60, 311–339. [Google Scholar] [CrossRef]
- GB 2763-2021; National Health Commission of the People’s Republic of China; Ministry of Agriculture and Rural Affairs of the People’s Republic of China; State Administration for Market Regulation. National Food Safety Standard-Maximum Residue Limits for Pesticides in Food. Standards Press of China: Beijing, China, 2021. Available online: http://www.cnhfa.org.cn/fagui/show.php?itemid=602 (accessed on 27 July 2023).
- JMPR (The Joint FAO/WHO Meeting on Pesticide Residues). JMPR Report CYAZOFAMID, No.281. 2015. Available online: https://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report08/CYAZOFAMID.pdf (accessed on 1 October 2023).
- JMPR (The Joint FAO/WHO Meeting on Pesticide Residues). JMPR Report PYRACLOSTROBIN, No.229. 2014. Available online: https://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report08/PYRACLOSTROBIN.pdf (accessed on 1 October 2023).
- EFSA Panel on Plant Protection Products and Their Residues (PPR). Guidance on the use of probabilistic methodology for modelling dietary exposure to pesticide residues. EFSA J. 2012, 10, 2839. [Google Scholar] [CrossRef]
- Qiao, X. Some considerations on dietary exposure assessment of pesticide residues in food. Chin. J. Pestic. Sci. 2020, 22, 727–733. [Google Scholar]
- Van Klaveren, J.D.; Boon, P.E. Probabilistic risk assessment of dietary exposure to single and multiple pesticide residues or contaminants: Summary of the work performed within the SAFE FOODS project. Food Chem. Toxicol. 2009, 47, 2879–2882. [Google Scholar] [CrossRef] [PubMed]
- Boon, P.E.; Lignell, S.; van Klaveren, J.D.; Nij, E.T. Estimation of the Acute Dietary Exposure to Pesticides Using the Probabilistic Approach and the Point Estimate Methodology; RIKILT-Veiligheid & Gezondheid, Sweden. 2004. Available online: https://library.wur.nl/WebQuery/wurpubs/fulltext/28647 (accessed on 27 October 2023).
- Bosgra, S.; van der Voet, H.; Boon, P.E.; Slob, W. An integrated probabilistic framework for cumulative risk assessment of common mechanism chemicals in food: An example with organophosphorus pesticides. Regul. Toxicol. Pharmacol. 2009, 54, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, S.; Gao, Y.; Ma, Y.; Hu, J.; Liu, X. Dissipation behavior, residue distribution and dietary risk assessment of field-incurred boscalid and pyraclostrobin in grape and grape field soil via MWCNTs-based QuEChERS using an RRLC-QqQ-MS/MS technique. Food Chem. 2019, 274, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Farha, W.; Abd El-Aty, A.; Rahman, M.M.; Shin, H.-C.; Shim, J.-H. An overview on common aspects influencing the dissipation pattern of pesticides: A review. Environ. Monit. Assess. 2016, 188, 693. [Google Scholar] [CrossRef] [PubMed]
- Boobis, A.R.; Ossendorp, B.C.; Banasiak, U.; Hamey, P.Y.; Sebestyen, I.; Moretto, A. Cumulative risk assessment of pesticide residues in food. Toxicol. Lett. 2008, 180, 137–150. [Google Scholar] [CrossRef]
- Wang, Z.; Di, S.; Qi, P.; Xu, H.; Zhao, H.; Wang, X. Dissipation, accumulation and risk assessment of fungicides after repeated spraying on greenhouse strawberry. Sci. Total Environ. 2021, 758, 144067. [Google Scholar] [CrossRef]
- Jin, S.; He, Y.; Hu, J. Survey Report on the Nutrition and Health of Chinese Residents: Data Set on the Status of Nutrition and Health in 2002. In Dietary Nutrient Intake (Chapter 4); People’s Hygiene Press: Beijing, China, 2002. [Google Scholar]
- EFSA. Peer Review of the Pesticide Risk Assessment of the Active Substance BAS 750 F (Mefentrifluconazole). Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2018.5379 (accessed on 27 October 2023).
- Lefferts, L.Y. Pesticide residues variability and acute dietary risk assessment: A consumer perspective. Food Addit. Contam. 2000, 17, 511–517. [Google Scholar] [CrossRef]
- Solecki, R.; Davies, L.; Dellarco, V.; Dewhurst, I.; Van Raaij, M.; Tritscher, A. Guidance on setting of acute reference dose (ARfD) for pesticides. Food Chem. Toxicol. 2005, 43, 1569–1593. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, A.; Nili-Ahmadabadi, A.; Rahimi, A.; Vahidinia, A.; Taheri, M. Dissipation behavior and risk assessment of fungicide and insecticide residues in grape under open-field, storage and washing conditions. J. Clean. Prod. 2020, 270, 122287. [Google Scholar] [CrossRef]
- Polat, B. Reduction of some insecticide residues from grapes with washing treatments. Turk. J. Entomol. 2021, 45, 125–137. [Google Scholar] [CrossRef]
- Lozowicka, B.; Jankowska, M.; Hrynko, I.; Kaczynski, P. Removal of 16 pesticide residues from strawberries by washing with tap and ozone water, ultrasonic cleaning and boiling. Environ. Monit. Assess. 2016, 188, 51. [Google Scholar] [CrossRef]
- Sarangapani, C.; Scally, L.; Gulan, M.; Cullen, P. Dissipation of pesticide residues on grapes and strawberries using plasma-activated water. Food Bioprocess Technol. 2020, 13, 1728–1741. [Google Scholar] [CrossRef]
- Sadło, S.; Szpyrka, E.; Piechowicz, B.; Antos, P.; Józefczyk, R.; Balawejder, M. Reduction of captan, boscalid and pyraclostrobin residues on apples using water only, gaseous ozone, and ozone aqueous solution. Ozone Sci. Eng. 2017, 39, 97–103. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.; Mir, M.M.; Sofi, S.A.; Shah, M.A.; Sidiq, T.; Sunooj, K.V.; Hamdani, A.M.; Khaneghah, A.M. Current strategies for the reduction of pesticide residues in food products. J. Food Compos. Anal. 2022, 106, 104274. [Google Scholar] [CrossRef]
- Peng, W.; Zhao, L.; Liu, F.; Xue, J.; Li, H.; Shi, K. Effect of paste processing on residue levels of imidacloprid, pyraclostrobin, azoxystrobin and fipronil in winter jujube. Food Addit. Contam. Part A 2014, 31, 1562–1567. [Google Scholar] [CrossRef]
- El-Sharkawy, H.H.A.; Abo-El-Wafa, T.S.A.; Ibrahim, S.A.-A. Biological control agents improve the productivity and induce the resistance against downy mildew of grapevine. J. Plant Pathol. 2018, 100, 33–42. [Google Scholar] [CrossRef]
- Falk, S.P.; Pearson, R.C.; Gadoury, D.M.; Seem, R.C.; Sztejnberg, A. Fusarium proliferatum as a biocontrol agent against grape downy mildew. Phytopathology 1996, 86, 1010–1017. [Google Scholar] [CrossRef]
- Zang, C.; Lin, Q.; Xie, J.; Lin, Y.; Yu, S.; Zhao, K.; Liang, C. The biological control of the grapevine downy mildew disease using Ochrobactrum sp. Plant Prot. Sci. 2019, 56, 52–61. [Google Scholar] [CrossRef]
- English-Loeb, G.; Norton, A.P.; Gadoury, D.; Seem, R.; Wilcox, W. Biological control of grape powdery mildew using mycophagous mites. Plant Dis. 2007, 91, 421–429. [Google Scholar] [CrossRef]
- Khursheed, A.; Rather, M.A.; Jain, V.; Rasool, S.; Nazir, R.; Malik, N.A.; Majid, S.A. Plant based natural products as potential ecofriendly and safer biopesticides: A comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef]
- Rodgers, P.B. Potential of biopesticides in agriculture. Pestic. Sci. 1993, 39, 117–129. [Google Scholar] [CrossRef]
- Lengai, G.M.; Muthomi, J.W. Biopesticides and their role in sustainable agricultural production. J. Biosci. Med. 2018, 6, 7. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Liu, R.; Yuan, L. Dissipation, Residue and Human Dietary Risk Assessment of Pyraclostrobin and Cyazofamid in Grapes Using an HPLC-UV Detector. Foods 2024, 13, 314. https://doi.org/10.3390/foods13020314
Zhao P, Liu R, Yuan L. Dissipation, Residue and Human Dietary Risk Assessment of Pyraclostrobin and Cyazofamid in Grapes Using an HPLC-UV Detector. Foods. 2024; 13(2):314. https://doi.org/10.3390/foods13020314
Chicago/Turabian StyleZhao, Peiying, Rong Liu, and Longfei Yuan. 2024. "Dissipation, Residue and Human Dietary Risk Assessment of Pyraclostrobin and Cyazofamid in Grapes Using an HPLC-UV Detector" Foods 13, no. 2: 314. https://doi.org/10.3390/foods13020314
APA StyleZhao, P., Liu, R., & Yuan, L. (2024). Dissipation, Residue and Human Dietary Risk Assessment of Pyraclostrobin and Cyazofamid in Grapes Using an HPLC-UV Detector. Foods, 13(2), 314. https://doi.org/10.3390/foods13020314





