Biodiversity of Aspergillus Species and Their Mycotoxin Production Potential in Dry Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Locations
2.2. Sampling and Sample Preparation
2.3. Mycological Screening of Samples
2.4. Fungal Colony Counts
2.5. Fungal Isolation from Sample Cultures
2.6. Molecular Characterization of Fungal Isolates
2.6.1. DNA Extraction
2.6.2. Agarose Gel DNA Electrophoresis
2.6.3. PCR Amplification of Extracted Genomic DNA
2.6.4. Sequencing of PCR Products
2.6.5. Sequence Analysis
2.6.6. Detection of Aflatoxin-Producing Genes of Aspergilla
2.6.7. Optimization of Polymerase Chain Reaction
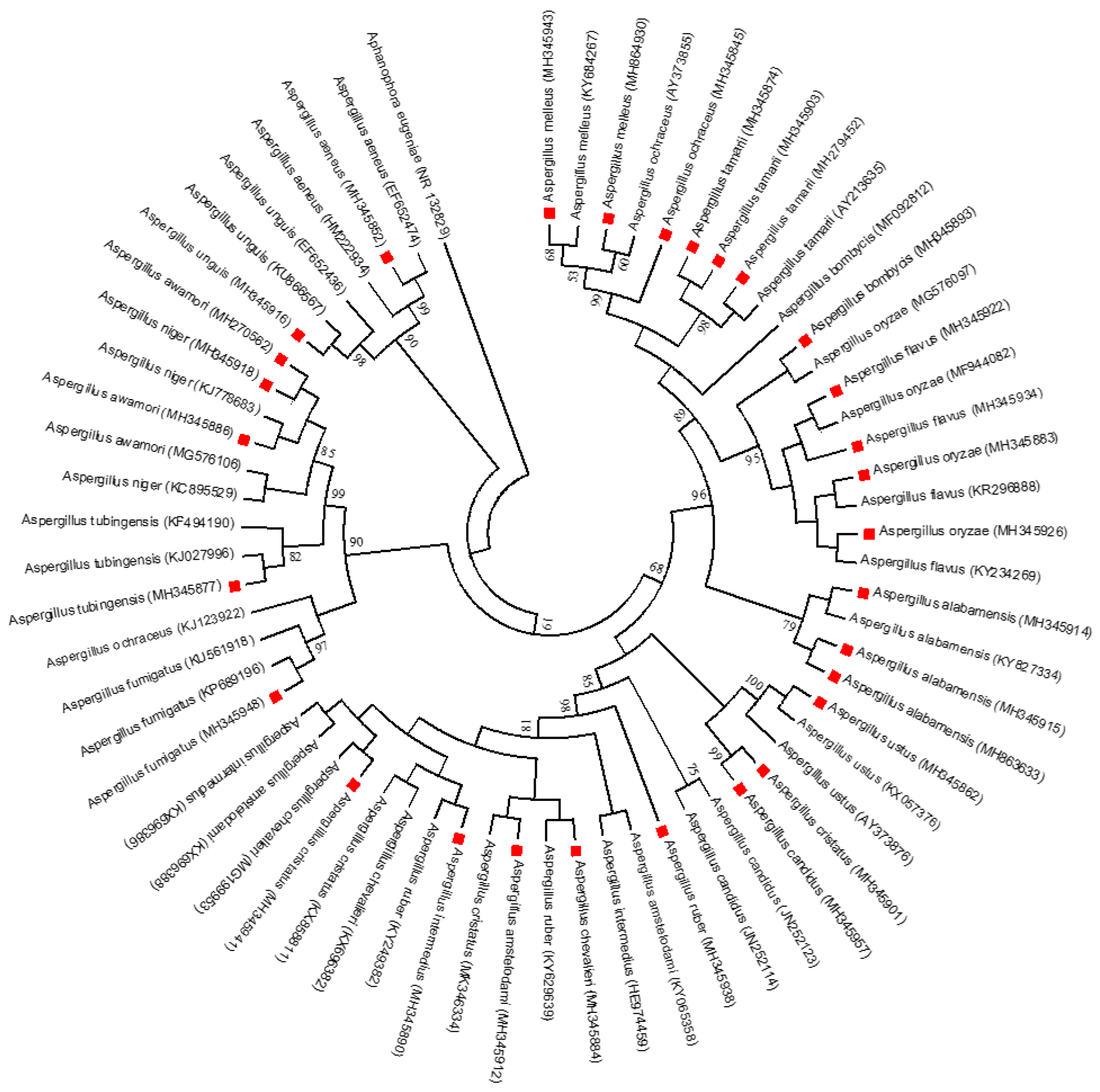
2.7. Phylogenetic Analysis
2.8. Statistical Analyses
3. Results and Discussion
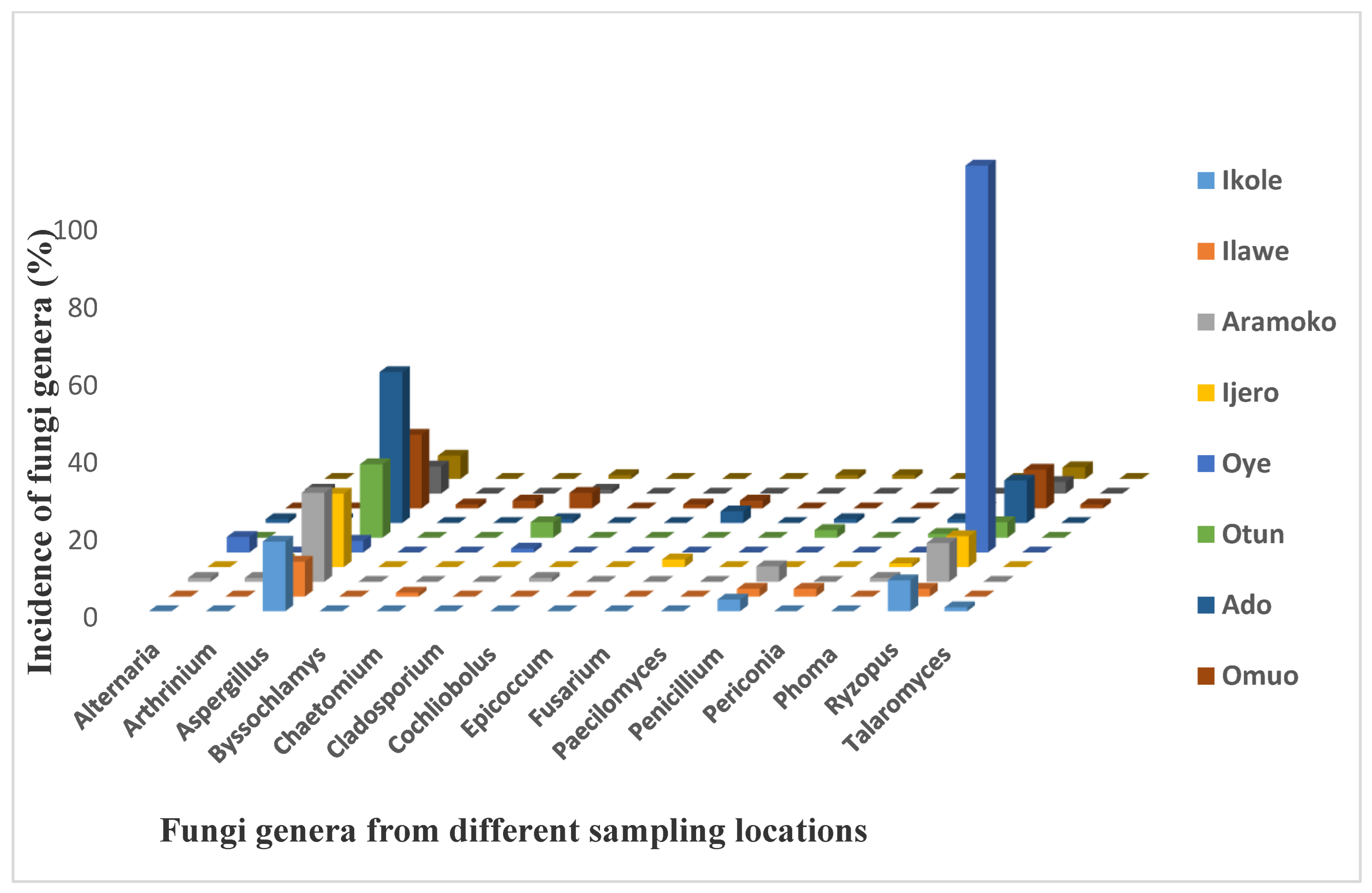
3.1. The Fungal Counts and Distribution of Fungal Isolates in Dry Meat
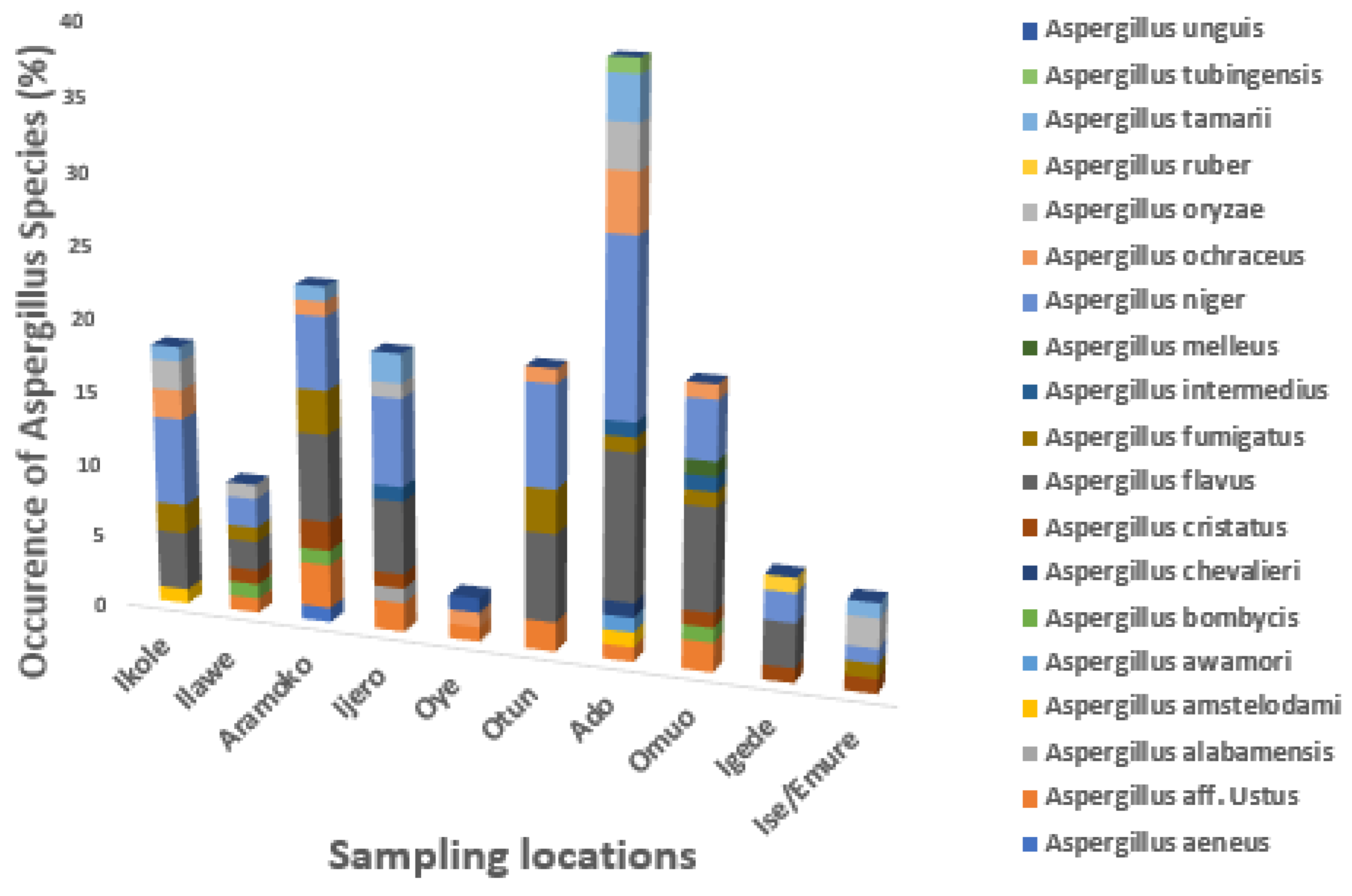
3.2. The Occurrence of Aspergillus Species in Dried Meat across Different Locations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lawrie, R.A.; Ledward, D. Lawrie’s Meat Science, 7th ed.; Woodhead Publishing: Cambridge, UK, 2014; p. 1. [Google Scholar]
- Semba, R.D.; Ramsing, R.; Rahman, N.; Kraemer, K.; Bloem, M.W. Legumes as a sustainable source of protein in human diets. Glob. Food Secur. 2021, 28, 100520. [Google Scholar] [CrossRef]
- Millward, D.J.; Garnett, T. Plenary Lecture 3 Food and the planet: Nutritional dilemmas of greenhouse gas emission reductions through reduced intakes of meat and dairy foods: Conference on ‘Over-and undernutrition: Challenges and approaches. Proc. Nutr. Soc. 2010, 69, 103–118. [Google Scholar] [CrossRef]
- Zhou, G.H.; Xu, X.L.; Liu, Y. Preservation technologies for fresh meat–A review. Meat Sci. 2010, 86, 119–128. [Google Scholar] [CrossRef]
- Egbunike, G.N.; Okubanjo, A.O. Effects of processing upon the quality of Nigerian meat products. Livest. Prod. Sci. 1999, 59, 155–163. [Google Scholar] [CrossRef]
- Fonkem, D.N.; Tanya, V.N.; Ebangi, A.L. Effects of season on the microbiological quality of Kilishi, a traditional Cameroonian dried beef product. Tropicultura 2010, 28, 10–15. [Google Scholar]
- Wiklund, E.; Malmfors, G.; Finstad, G.L.; Åhman, B.; Skuterud, L.; Adamczewski, J.; Garner, K. Meat quality and meat hygiene. In Reindeer and Caribou, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 353–382. [Google Scholar]
- Asefa, D.T.; Kure, C.F.; Gjerde, R.O.; Omer, M.K.; Langsrud, S.; Nesbakken, T.; Skaar, I. Fungal growth pattern, sources and factors of mould contamination in a dry-cured meat production facility. Int. J. Food Microbiol. 2010, 140, 131–135. [Google Scholar] [CrossRef]
- Ajiboye, E.A.; Alhassan, S.; Adedayo, R.M.; Kolawole, M.O.; Oladosu, O.T. Physicochemical properties and microorganisms isolated from dried meat obtained in Oja-Oba market in Ilorin, Nigeria. Pelagia Res. Libr. 2011, 2, 391–400. [Google Scholar]
- Oladejo, D.A.; Adebayo-Tayo, B.C. Moulds, proximate mineral composition and mycotoxin contamination of Banda (“kundi”/ “tinko”) sold in Ibadan, Oyo State, Nigeria. AU J. Technol. 2011, 15, 32–40. [Google Scholar]
- Cary, J.W.; Gilbert, M.K.; Lebar, M.D.; Majumdar, R.; Calvo, A.M. Aspergillus flavus secondary metabolites: More than just aflatoxins. Food Saf. 2018, 6, 7–32. [Google Scholar] [CrossRef]
- Njobeh, P.B.; Dutton, M.F.; Koch, S.H.; Chuturgoon, A.; Stoev, S.; Seifert, K. Contamination with storage fungi of human food from Cameroon. Int. J. Food Microbiol. 2009, 135, 193–198. [Google Scholar] [CrossRef]
- Adetunji, M.C.; Alika, O.P.; Awa, N.P.; Atanda, O.O.; Mwanza, M. Microbiological quality and risk assessment for aflatoxins in groundnuts and roasted cashew nuts meant for human consumption. J. Toxicol. 2018, 2018, 1308748. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Hall, T. Bio Edit; Ibis Therapeutics: Carlsbad, CA, USA, 2004. [Google Scholar]
- Altschul, S.F.; Koonin, E.V. Iterated profile searches with PSI-BLAST—A tool for discovery in protein databases. Trends Biochem. Sci. 1998, 23, 444–447. [Google Scholar] [CrossRef]
- Rashid, M.; Khalil, S.; Ayub, N.; Ahmed, W.; Khan, A.G. Categorization of Aspergillus flavus and Aspergillus parasiticus isolates of stored wheat grains in to aflatoxigenic and non-aflatoxigenic. Pak. J. Bot 2008, 40, 2177–2192. [Google Scholar]
- Gallo, A.; Tandon, M.; Alevizos, I.; Illei, G.G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 2012, 7, e30679. [Google Scholar] [CrossRef]
- Fakruddin, M.D.; Chowdhury, A.; Hossain, M.N.; Ahmed, M.M. Characterization of aflatoxin producing Aspergillus flavus from food and feed samples. Springer Plus 2015, 4, 159. [Google Scholar] [CrossRef]
- Abd-Elghany, S.M.; Sallam, K.I. Rapid determination of total aflatoxins and ochratoxins A in meat products by immuno-affinity fluorimetry. Food Chem. 2015, 179, 253–256. [Google Scholar] [CrossRef]
- Montso, P.K.; Ateba, C.N. Molecular detection of Clostridium species in beef obtained from retail shops in North West Province, South Africa. J. Food Nutr. Res. 2014, 2, 236–243. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Ferreira, V.; Barbosa, J.; Vendeiro, S.; Mota, A.; Silva, F.; Monteiro, M.J.; Hogg, T.; Gibbs, P.; Teixeira, P. Chemical and microbiological characterization of alheira: A typical Portuguese fermented sausage with particular reference to factors relating to food safety. Meat Sci. 2006, 73, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, S.A. Quality and safety assessment of sun-dried meat product (kundi) from Ibadan, Oyo state, Nigeria. Cogent Food Agric. 2016, 2, 1209074. [Google Scholar] [CrossRef]
- Castellari, C.; Quadrelli, A.M.; Laich, F. Surface mycobiota on Argentinean dry fermented sausages. Int. J. Food Microbiol. 2010, 142, 149–155. [Google Scholar] [CrossRef]
- Klich, M.A.; Mullaney, E.J.; Daly, C.B.; Cary, J.W. Molecular and physiological aspects of aflatoxin and sterigmatocystin biosynthesis by Aspergillus tamarii and A. ochraceoroseus. Appl. Microbiol. Biotechnol. 2000, 53, 605–609. [Google Scholar] [CrossRef]
- Oyetunji, T.O. Mycological evaluation of a ground cocoa-based beverage. Afr. J. Biotechnol. 2006, 5, 2073–2076. [Google Scholar]
- Borgemeister, C.; Adda, C.; Sétamou, M.; Hell, K.; Djomamou, B.; Markham, R.H.; Cardwell, K.F. Timing of harvest in maize: Effects on post-harvest losses due to insects and fungi in central Benin, with particular reference to Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae). Agric. Ecosyst. Environ. 1998, 69, 233–242. [Google Scholar] [CrossRef]
- Bankole, S.A.; Adebanjo, A. Mycotoxins in food in West Africa: Current situation and possibilities of controlling it. Afr. J. Biotechnol. 2003, 2, 254–263. [Google Scholar]
- Makun, H.A.; Dutton, M.F.; Njobeh, P.B.; Phoku, J.Z.; Yah, C.S. Incidence, phylogeny and mycotoxigenic potentials of fungi isolated from rice in Niger State, Nigeria. J. Food Saf. 2011, 31, 334–349. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D.; Pitt, J.I.; Hocking, A.D. Primary keys and miscellaneous fungi. In Fungi Food Spoilage; Springer: Boston, MA, USA, 2009; pp. 53–143. [Google Scholar] [CrossRef]
- Sweeney, M.J.; Dobson, A.D. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef]
- Mulunda, M.; Dzoma, B.; Nyirenda, M.; Bakunzi, F. Mycotoxins occurrence in selected staple food in main markets from Lubumbashi, Democratic Republic of Congo. J. Food Agric. Environ. 2013, 11, 51–54. [Google Scholar]
- Reiter, E.V.; Vouk, F.; Böhm, J.; Razzazi-Fazeli, E. Aflatoxins in rice–A limited survey of products marketed in Austria. Food Control 2010, 21, 988–991. [Google Scholar] [CrossRef]
- Davari, E.; Mohsenzadeh, M.; Mohammadi, G.; Rezaeian-Doloei, R. Characterization of aflatoxigenic Aspergillus flavus and A. parasiticus strain isolates from animal feedstuffs in northeastern Iran. Iran. J. Vet. Res. 2015, 16, 150. [Google Scholar] [PubMed]
- Woloshuk, C.P.; Foutz, K.R.; Brewer, J.F.; Bhatnagar, D.; Cleveland, T.E.; Payne, G. Molecular characterization of aflR, a regulatory locus for aflatoxin biosynthesis. Appl. Environ. Microbiol. 1994, 60, 2408–2414. [Google Scholar] [CrossRef]
- Bok, J.W.; Keller, N.P. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot. Cell 2004, 3, 527–535. [Google Scholar] [CrossRef]
- Perrin, R.M.; Fedorova, N.D.; Bok, J.W.; Cramer, R.A., Jr.; Wortman, J.R.; Kim, H.S.; Nierman, W.C.; Keller, N.P. Transcriptional regulation of chemical diversity in Aspergillus fumigatus by LaeA. PLoS Pathog. 2007, 3, e50. [Google Scholar] [CrossRef]
- Georgianna, D.R.; Hawkridge, A.M.; Muddiman, D.C.; Payne, G.A. Temperature-dependent regulation of proteins in Aspergillus flavus: Whole organism stable isotope labeling by amino acids. J. Proteome Res. 2008, 7, 2973–2979. [Google Scholar] [CrossRef]
- Ehrlich, K.C.; Montalbano, B.G.; Cary, J.W. Binding of the C6-zinc cluster protein, AFLR, to the promoters of aflatoxin pathway biosynthesis genes in Aspergillus parasiticus. Gene 1999, 230, 249–257. [Google Scholar] [CrossRef]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef]
- Obrian, G.R.; Georgianna, D.R.; Wilkinson, J.R.; Yu, J.; Abbas, H.K.; Bhatnagar, D.; Cleveland, T.E.; Nierman, W.; Payne, G.A. The effect of elevated temperature on gene transcription and aflatoxin biosynthesis. Mycologia 2007, 99, 232–239. [Google Scholar] [CrossRef]
- Wayne, L.G.; Brenner, D.J.; Colwell, R.R.; Grimont, P.A.; Kandler, O.; Krichevsky, M.I.; Moore, L.H.; Moore, W.E.; Murray, R.; Stackebrandt, E.S.; et al. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Evol. Microbiol. 1987, 37, 463–464. [Google Scholar] [CrossRef]
- Aremu, B.R.; Babalola, O.O. Construction of specific primers for rapid detection of South African exportable vegetable macergens. Int. J. Environ. Res. Public Health 2015, 12, 12356–12370. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, K.T.; Stackebrandt, E. Defining taxonomic ranks. Prokaryotes 2013, 1, 229–254. [Google Scholar]

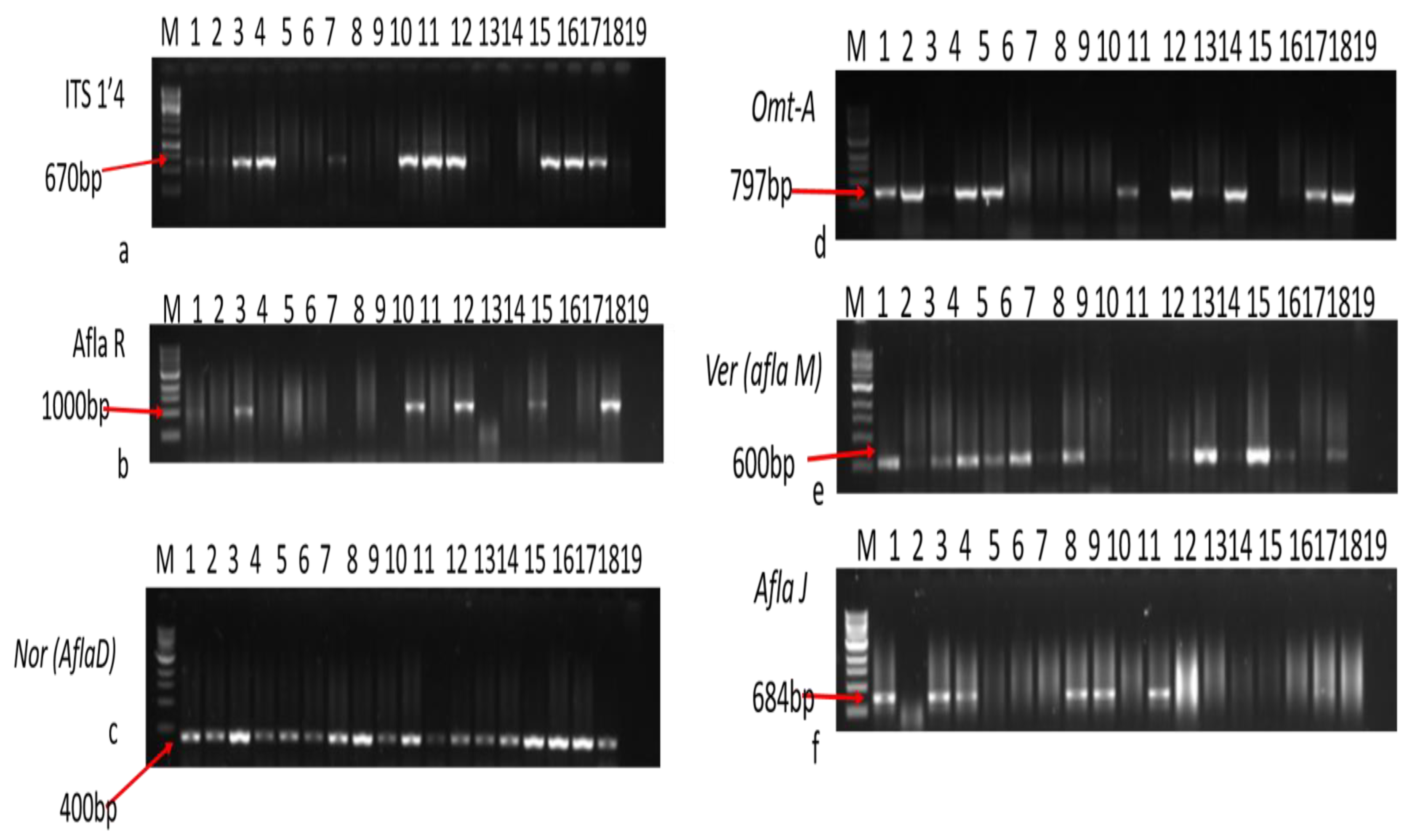
| Primer Code | Target Gene | Primer Sequences 5′–3′ | Product Size (bp) |
|---|---|---|---|
| Nor-F | aflD (nor-1) | ACCGCTACGCCGGCACTCTCGGCAC | 400 |
| Nor-R | GTTGGCCGCCAGCTTCGACACTCCG | ||
| Ver-F | aflM (ver-1) | GCCGCAGGCCGCGGAGAAAGTGGT | 600 |
| Ver-R | GGGGATATACTCCCGCGACACAGCC | ||
| Omt-F | aflP (omtA) | GTGGACGGACCTAGTCCGACATCAC | 797 |
| Omt-R | GTCGGCGCCACGCACTGGGTTGGGG | ||
| AflR-for | AflR | TATCTCCCCCCGGGCATCTCCCGG | 1000 |
| AflR-rev | CCGTCAGACAGCCACTGGACACGG | ||
| AflJ-for | aflS (aflJ) | TGAATCCGTACCCTTTGAGG | 684 |
| AflJ-rev | GGAATGGGATGGAGATGAGA |
| Location | CFU g−1 Range | Mean | No of Fungi Isolates |
|---|---|---|---|
| Ikole | 2.0 × 102–1.6 × 104 | 2.6 × 103 | 10 |
| Ilawe | 1.0 × 101–4.5 × 102 | 1.3 × 102 | 11 |
| Aramoko | 1.0 × 102–6.9 × 103 | 1.5 × 103 | 16 |
| Ijero | 2.0 × 102–3.2 × 102 | 1.5 × 102 | 13 |
| Oye | 5.0 × 101–4.1 × 102 | 2.9 × 102 | 6 |
| Otun | 1.0 × 101–1.9 × 102 | 5.4 × 101 | 11 |
| Ado | 2.0 × 101–2.1 × 103 | 3.2 × 102 | 19 |
| Omuo | 3.0 × 101–6.4 × 102 | 1.6 × 102 | 16 |
| Igede | 9.0 × 101–3.2 × 102 | 1.0 × 102 | 7 |
| Ise/Emure | 1.0 × 101–2.5 × 102 | 9.5 × 101 | 9 |
| S/No | Name of Isolates | Nor afLD | Ver afLM | Omt afLP | afLR | afLJ | AP | Accession Number | NCBI % Similarity |
|---|---|---|---|---|---|---|---|---|---|
| 1 | A. flavus | + | + | + | + | + | P | MH345936 | 99 |
| 2 | A. flavus | + | + | + | + | + | P | MH345870 | 99 |
| 3 | A. flavus | + | + | + | + | + | P | MH345846 | 100 |
| 4 | A. flavus | + | + | + | + | + | P | MH345934 | 100 |
| 5 | A. flavus | + | + | + | + | + | P | MH345922 | 100 |
| 6 | A. flavus | + | + | + | + | + | P | MH345895 | 99 |
| 7 | A. flavus | + | + | + | + | + | P | MH345879 | 99 |
| 8 | A. flavus | + | + | + | + | + | P | MH345959 | 99 |
| 9 | A. flavus | + | + | + | + | + | P | MH345842 | 99 |
| 10 | A. flavus | + | + | + | + | + | P | MH345932 | 99 |
| 11 | A. flavus | + | + | + | + | + | P | MH345881 | 100 |
| 12 | A. flavus | + | + | + | + | + | P | MH345952 | 100 |
| 13 | A. flavus | + | + | + | + | + | P | MH345913 | 99 |
| 14 | A. flavus | + | + | + | + | + | P | MH345910 | 100 |
| 15 | A. flavus | + | + | + | + | + | P | MH345946 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dada, T.A.; Ekwomadu, T.I.; Ngoma, L.; Mwanza, M. Biodiversity of Aspergillus Species and Their Mycotoxin Production Potential in Dry Meat. Foods 2024, 13, 3221. https://doi.org/10.3390/foods13203221
Dada TA, Ekwomadu TI, Ngoma L, Mwanza M. Biodiversity of Aspergillus Species and Their Mycotoxin Production Potential in Dry Meat. Foods. 2024; 13(20):3221. https://doi.org/10.3390/foods13203221
Chicago/Turabian StyleDada, Toluwase Adeseye, Theodora Ijeoma Ekwomadu, Lubanza Ngoma, and Mulunda Mwanza. 2024. "Biodiversity of Aspergillus Species and Their Mycotoxin Production Potential in Dry Meat" Foods 13, no. 20: 3221. https://doi.org/10.3390/foods13203221
APA StyleDada, T. A., Ekwomadu, T. I., Ngoma, L., & Mwanza, M. (2024). Biodiversity of Aspergillus Species and Their Mycotoxin Production Potential in Dry Meat. Foods, 13(20), 3221. https://doi.org/10.3390/foods13203221








