Classification of Sesame Oil Based on Processing-Originated Differences in the Volatile Organic Compound Profile by a Colorimetric Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sensor Fabrication
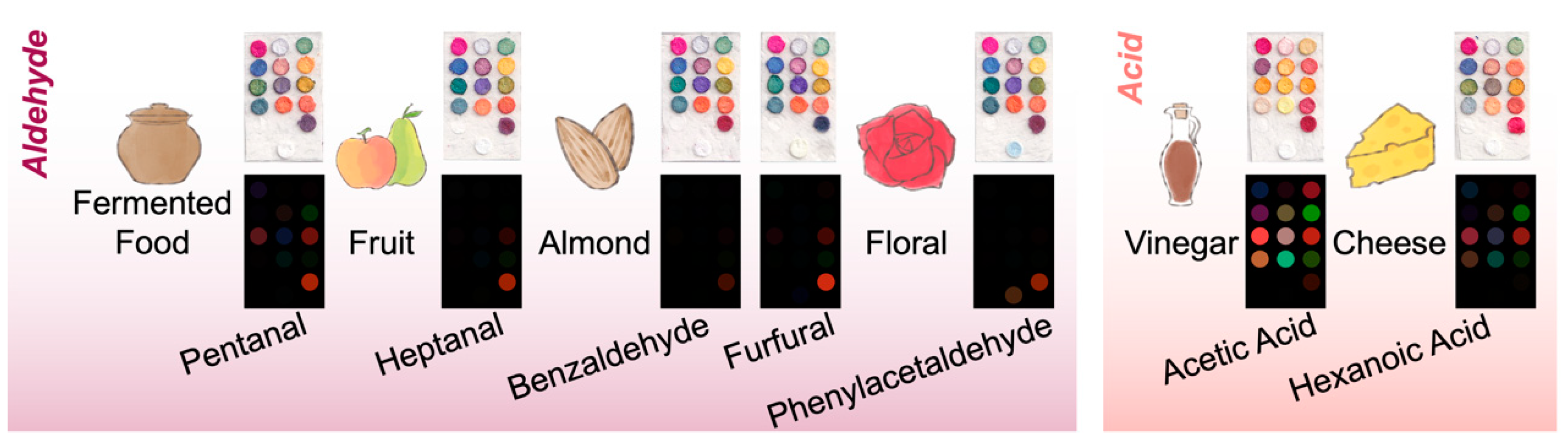
2.3. Sensor Validation with Chemical Standards
2.4. Edible Oil Samples
2.5. Headspace Solid-Phase Microextraction–Gas Chromatography (HS-SPME-GC)
2.6. Colorimetric Sensing of VOCs from Fragrant Sesame Oil
2.7. Data Analysis
2.8. Reversed-Phase Liquid Chromatography (RP-LC)
3. Results
3.1. Sensor Validation with Chemical Standards
3.2. Sensor Validation with Sesame Oil Standards
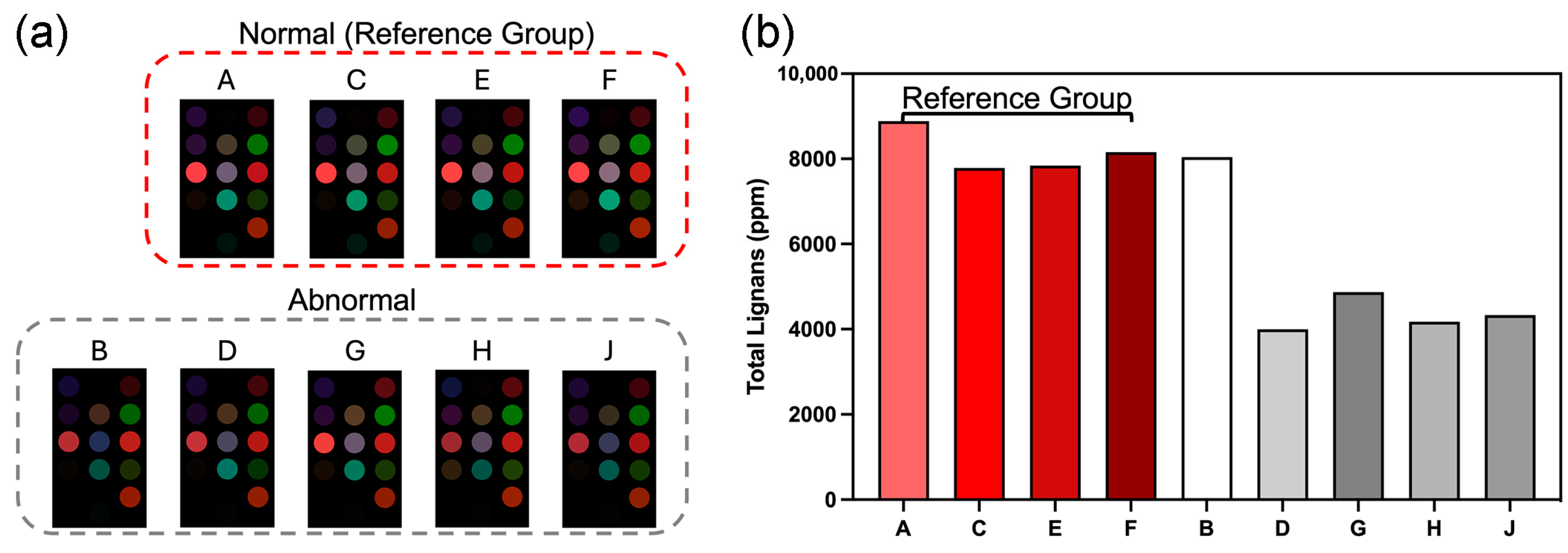
3.3. Commercial Fragrant Sesame Oil
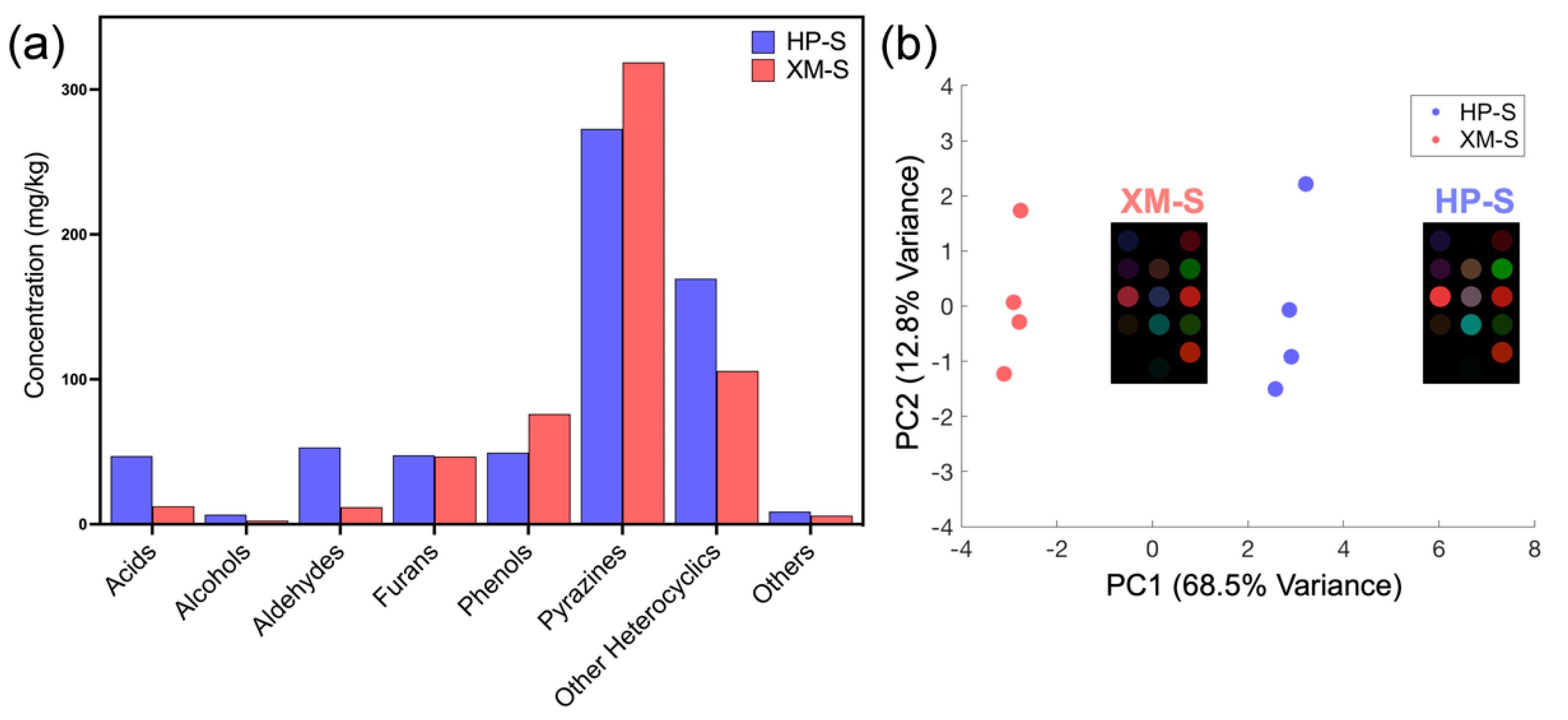
3.3.1. GC-MS Analysis
3.3.2. Colorimetric Sensing
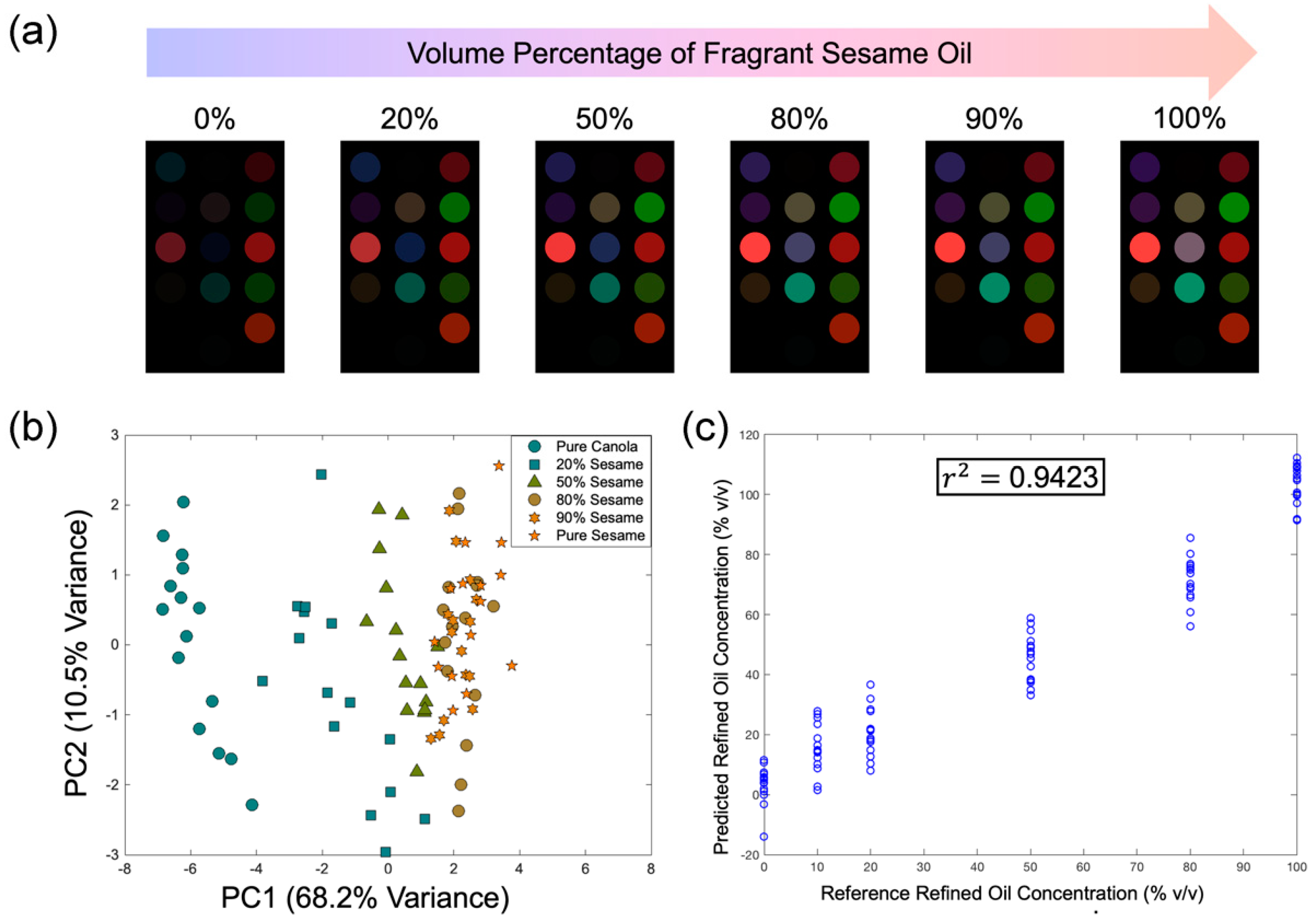
3.4. Colorimetric Detection of Refined Oil in Blended Sesame Oil
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dun, Q.; Yao, L.; Deng, Z.; Li, H.; Li, J.; Fan, Y.; Zhang, B. Effects of Hot and Cold-Pressed Processes on Volatile Compounds of Peanut Oil and Corresponding Analysis of Characteristic Flavor Components. LWT 2019, 112, 107648. [Google Scholar] [CrossRef]
- Hu, H.; Shi, A.; Liu, H.; Liu, L.; Fauconnier, M.L.; Wang, Q. Study on Key Aroma Compounds and Its Precursors of Peanut Oil Prepared with Normal- and High-Oleic Peanuts. Foods 2021, 10, 3036. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ji, J.; Tan, C.; Chen, D.; Luo, F.; Wang, Y.; Chen, X. Headspace Solid-Phase Microextraction Coupled to Gas Chromatography for the Analysis of Aldehydes in Edible Oils. Talanta 2014, 120, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.C.; Liu, J.J.; Jia, L.Z.; Wang, P.; Han, X. Speciation of VOCs in the Cooking Fumes from Five Edible Oils and Their Corresponding Health Risk Assessments. Atmos. Environ. 2019, 211, 6–17. [Google Scholar] [CrossRef]
- Bedigian, D.; Harlan, J.R. Evidence for Cultivation of Sesame in the Ancient World. Econ. Bot. 1986, 40, 137–154. [Google Scholar] [CrossRef]
- Namiki, M. The Chemistry and Physiological Functions of Sesame. Food Rev. Int. 1995, 11, 281–329. [Google Scholar] [CrossRef]
- Shi, L.K.; Liu, R.J.; Jin, Q.Z.; Wang, X.G. The Contents of Lignans in Sesame Seeds and Commercial Sesame Oils of China. J. Am. Oil Chem. Soc. 2017, 94, 1035–1044. [Google Scholar] [CrossRef]
- Shi, L.K.; Zheng, L.; Liu, R.; Chang, M.; Jin, Q.; Wang, X. Chemical Characterization, Oxidative Stability, and in Vitro Antioxidant Capacity of Sesame Oils Extracted by Supercritical and Subcritical Techniques and Conventional Methods: A Comparative Study Using Chemometrics. Eur. J. Lipid Sci. Technol. 2018, 120, 1700326. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, Y.; Li, P.; Xi, H.; Zhao, W.; Wang, D.; Mao, J.; Zhang, S.; Sun, S.; Xie, J. Characterization of Traditional Chinese Sesame Oil by Using Headspace Solid-Phase Microextraction/Gas Chromatography–Mass Spectrometry, Electronic Nose, Sensory Evaluation, and RapidOxy. Foods 2022, 11, 3555. [Google Scholar] [CrossRef]
- Dong, X.; Li, P.; Wei, F.; Jiang, M.; Zhao, Y.; Li, G.; Chen, H.; Zhao, Y. The Impact of Processing on the Profile of Volatile Compounds in Sesame Oil. Eur. J. Lipid Sci. Technol. 2012, 114, 277–286. [Google Scholar] [CrossRef]
- Devarajan, S.; Singh, R.; Chatterjee, B.; Zhang, B.; Ali, A. A Blend of Sesame Oil and Rice Bran Oil Lowers Blood Pressure and Improves the Lipid Profile in Mild-to-Moderate Hypertensive Patients. J. Clin. Lipidol. 2016, 10, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.F.; Ciou, J.Y.; Wu, C.L. Commercialized Sesame Oil Analysis: Quality Characterization and Oxidative Stability of Blended Sesame Oil. ACS Food Sci. Technol. 2021, 1, 1222–1227. [Google Scholar] [CrossRef]
- Chen, Z.; Fu, J.; Dou, X.; Deng, Z.; Wang, X.; Ma, F.; Yu, L.; Yun, Y.-H.; Li, P.; Zhang, L. Comprehensive Adulteration Detection of Sesame Oil Based on Characteristic Markers. Food Chem. X 2023, 18, 100745. [Google Scholar] [CrossRef] [PubMed]
- Gharby, S. Refining Vegetable Oils: Chemical and Physical Refining. Sci. World J. 2022, 2022, 6627013. [Google Scholar] [CrossRef]
- Yin, W.; Washington, M.; Ma, X.; Yang, X.; Lu, A.; Shi, R.; Zhao, R.; Wang, X. Consumer Acceptability and Sensory Profiling of Sesame Oils Obtained from Different Processes. Grain Oil Sci. Technol. 2020, 3, 39–48. [Google Scholar] [CrossRef]
- Banerjee, R.; Tudu, B.; Bandyopadhyay, R.; Bhattacharyya, N. A Review on Combined Odor and Taste Sensor Systems. J. Food Eng. 2016, 190, 10–21. [Google Scholar] [CrossRef]
- Blackman, J.; Rutledge, D.N.; Tesic, D.; Saliba, A.; Scollary, G.R. Examination of the Potential for Using Chemical Analysis as a Surrogate for Sensory Analysis. Anal. Chim. Acta 2010, 660, 2–7. [Google Scholar] [CrossRef]
- Shen, N.; Moizuddin, S.; Wilson, L.; Duvick, S.; White, P.; Pollak, L. Relationship of Electronic Nose Analyses and Sensory Evaluation of Vegetable Oils during Storage. J. Am. Oil Chem. Soc. 2001, 78, 937–940. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Micholt, E.; Scheerlinck, N.; Vandendriessche, T.; Hertog, M.L.; Ferguson, I.; Lammertyn, J. Real Time Aroma Reconstruction Using Odour Primaries. Sens. Actuators B Chem. 2016, 227, 561–572. [Google Scholar] [CrossRef]
- Persaud, K.; Dodd, G. Analysis of Discrimination Mechanisms in the Mammalian Olfactory System Using a Model Nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef]
- Gardner, J.W.; Bartlett, P.N. A Brief History of Electronic Noses. Sens. Actuators B Chem. 1994, 18, 210–211. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Cai, P.; Luo, Y.; Cui, Z.; Loh, X.J.; Chen, X. Artificial Sense Technology: Emulating and Extending Biological Senses. ACS Nano 2021, 15, 18671–18678. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, X.; Liu, L.; Zhang, R. A Novel Method for Qualitative Analysis of Edible Oil Oxidation Using an Electronic Nose. Food Chem. 2016, 202, 229–235. [Google Scholar] [CrossRef]
- Dong, H.; Ling, Z.; Zhang, X.; Zhang, X.; Ramaswamy, S.; Xu, F. Smart Colorimetric Sensing Films with High Mechanical Strength and Hydrophobic Properties for Visual Monitoring of Shrimp and Pork Freshness. Sens. Actuators B Chem. 2020, 309, 127752. [Google Scholar] [CrossRef]
- Guo, L.; Wang, T.; Wu, Z.; Wang, J.; Wang, M.; Cui, Z.; Ji, S.; Cai, J.; Xu, C.; Chen, X. Portable Food-Freshness Prediction Platform Based on Colorimetric Barcode Combinatorics and Deep Convolutional Neural Networks. Adv. Mater. 2020, 32, 2004805. [Google Scholar] [CrossRef]
- Hu, X.G.; Li, X.; Park, S.H.; Kim, Y.-H.; Yang, S.I. Nondestructive Monitoring of Kiwi Ripening Process Using Colorimetric Ethylene Sensor. Bull. Korean Chem. Soc. 2016, 37, 759–762. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yang, Y.J.; Kim, J.S.; Choi, D.S.; Park, S.H.; Jin, S.Y.; Park, J.S. Non-Destructive Monitoring of Apple Ripeness Using an Aldehyde Sensitive Colorimetric Sensor. Food Chem. 2018, 267, 149–156. [Google Scholar] [CrossRef]
- Xie, M.; Jia, M.; Zhao, H.; Zhang, L. Visual Determination of Oxidation of Edible Oil by a Nanofiber Mat Prepared from Polyvinyl Alcohol and Schiff’s Reagent. Microchim. Acta 2020, 187, 597. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, T.; Jiang, J.; Wang, Y.; Zhang, X.; Xia, B.; Dong, W. Visual Detection of Edible Oil Oxidation by Using Chitin-Based Colorimetric Sensor for Aldehydes. Colloids Surf. Physicochem. Eng. Asp. 2021, 628, 127303. [Google Scholar] [CrossRef]
- Huang, L.; Wang, M.; Liu, H. Identification of Adulterated Extra Virgin Olive Oil by Colorimetric Sensor Array. Food Anal. Methods 2022, 15, 647–657. [Google Scholar] [CrossRef]
- Mehdizadeh, S.A.; Noshad, M.; Chaharlangi, M.; Ampatzidis, Y. Development of an Innovative Optoelectronic Nose for Detecting Adulteration in Quince Seed Oil. Foods 2023, 12, 4350. [Google Scholar] [CrossRef] [PubMed]
- Mariana, I.; Nicoleta, U.; Sorin-Ştefan, B.; Gheorghe, V.; Mirela, D. Actual Methods for Obtaining Vegetable Oil from Oilseeds. In Proceedings of the 2nd International Conference of Thermal Equipment, Renewable Energy and Rural Development, Băile Olăneşti, Romania, 20–22 July 2013. [Google Scholar]
- Negash, Y.A.; Amare, D.E.; Bitew, B.D.; Dagne, H. Assessment of Quality of Edible Vegetable Oils Accessed in Gondar City, Northwest Ethiopia. BMC Res. Notes 2019, 12, 793. [Google Scholar] [CrossRef] [PubMed]
- Roncero, J.M.; Álvarez-Ortí, M.; Pardo-Giménez, A.; Rabadán, A.; Pardo, J.E. Influence of Pressure Extraction Systems on the Performance, Quality and Composition of Virgin Almond Oil and Defatted Flours. Foods 2021, 10, 1049. [Google Scholar] [CrossRef]
- Chabiri, S.; Hati, S.; Dimari, G.; Ogugbuaja, V. Comparative Quality Assessment of Branded and Unbranded Edible Vegetable Oils in Nigeria. Pac. J. Sci. Technol. 2009, 10, 927–934. [Google Scholar]
- Hasan, M.K.; Hossain, M.B.; Dhar, P.K.; Haque, M.R. Comparative Study on Cholesterol Content and Physicochemical Properties of Some Branded and Unbranded Commercial Edible Oils in Khulna, Bangladesh. J. Chem. Health Risks 2019, 9, 321–329. [Google Scholar] [CrossRef]
- Duffy, E.; Cauven, E.; Morrin, A. Colorimetric Sensing of Volatile Organic Compounds Produced from Heated Cooking Oils. ACS Omega 2021, 6, 7394–7401. [Google Scholar] [CrossRef]
- Li, Z.; Fang, M.; LaGasse, M.K.; Askim, J.R.; Suslick, K.S. Colorimetric Recognition of Aldehydes and Ketones. Angew. Chem. 2017, 129, 9992–9995. [Google Scholar] [CrossRef]
- Maga, J.A.; Sizer, C.E.; Myhre, D. Pyrazines in Foods. Crit. Rev. Food Sci. Nutr. 1973, 4, 39–115. [Google Scholar] [CrossRef]
- Taylor, J.K. Quality Assurance of Chemical Measurements; Routledge: Abingdon, UK, 2018; ISBN 0-203-74161-7. [Google Scholar]
- Zambelli, A.; León, A.; Garcés, R. 2—Mutagenesis in Sunflower. In Sunflower; Martínez-Force, E., Dunford, N.T., Salas, J.J., Eds.; AOCS Press: Urbana, IL, USA, 2015; pp. 27–52. ISBN 978-1-893997-94-3. [Google Scholar]
- Hubbe, M.A.; Ayoub, A.; Daystar, J.S.; Venditti, R.A.; Pawlak, J.J. Enhanced Absorbent Products Incorporating Cellulose and Its Derivatives: A Review. BioResources 2013, 8, 6556–6629. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Feng, T.; Luo, J.; Sameen, D.E.; Hossen, M.A.; Dai, J.; Li, S.; Qin, W. Development and Characterization of Aldehyde-Sensitive Cellulose/Chitosan/Beeswax Colorimetric Papers for Monitoring Kiwifruit Maturity. Int. J. Biol. Macromol. 2021, 187, 566–574. [Google Scholar] [CrossRef]
- Potyrailo, R.A.; Golubkov, S.P.; Borsuk, P.S.; Talanchuk, P.M.; Novosselov, E.F. pH Indicator Based Ammonia Gas Sensor: Studies of Spectral Performance under Variable Conditions of Temperature and Humidity. Analyst 1994, 119, 443–448. [Google Scholar] [CrossRef]
- Kochhar, S. Oxidative Pathways to the Formation of Off-Flavours. In Food Taints and Off-Flavours; Springer: Boston, MA, USA, 1996; pp. 168–225. [Google Scholar]
- Morales, M.; Rios, J.; Aparicio, R. Changes in the Volatile Composition of Virgin Olive Oil during Oxidation: Flavors and off-Flavors. J. Agric. Food Chem. 1997, 45, 2666–2673. [Google Scholar] [CrossRef]
- Shao, X.; Li, H.; Wang, N.; Zhang, Q. Comparison of Different Classification Methods for Analyzing Electronic Nose Data to Characterize Sesame Oils and Blends. Sensors 2015, 15, 26726–26742. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Shi, H.-M.; Elejalde, U.; Chen, X. Classification of Sesame Oil Based on Processing-Originated Differences in the Volatile Organic Compound Profile by a Colorimetric Sensor. Foods 2024, 13, 3230. https://doi.org/10.3390/foods13203230
Liu T, Shi H-M, Elejalde U, Chen X. Classification of Sesame Oil Based on Processing-Originated Differences in the Volatile Organic Compound Profile by a Colorimetric Sensor. Foods. 2024; 13(20):3230. https://doi.org/10.3390/foods13203230
Chicago/Turabian StyleLiu, Tianyi, Hai-Ming Shi, Untzizu Elejalde, and Xiaodong Chen. 2024. "Classification of Sesame Oil Based on Processing-Originated Differences in the Volatile Organic Compound Profile by a Colorimetric Sensor" Foods 13, no. 20: 3230. https://doi.org/10.3390/foods13203230





