Structural and Physicochemical Characterization of Resistant Starch from Sixteen Banana Cultivars across Three Genome Groups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Banana Materials
2.2. Preparation of Banana Flour
2.3. Isolation and Measurement of BRS
2.4. In Vitro Digestion of Starch and Determination of pGI
2.5. Normal Light and Polarizing Microscopy
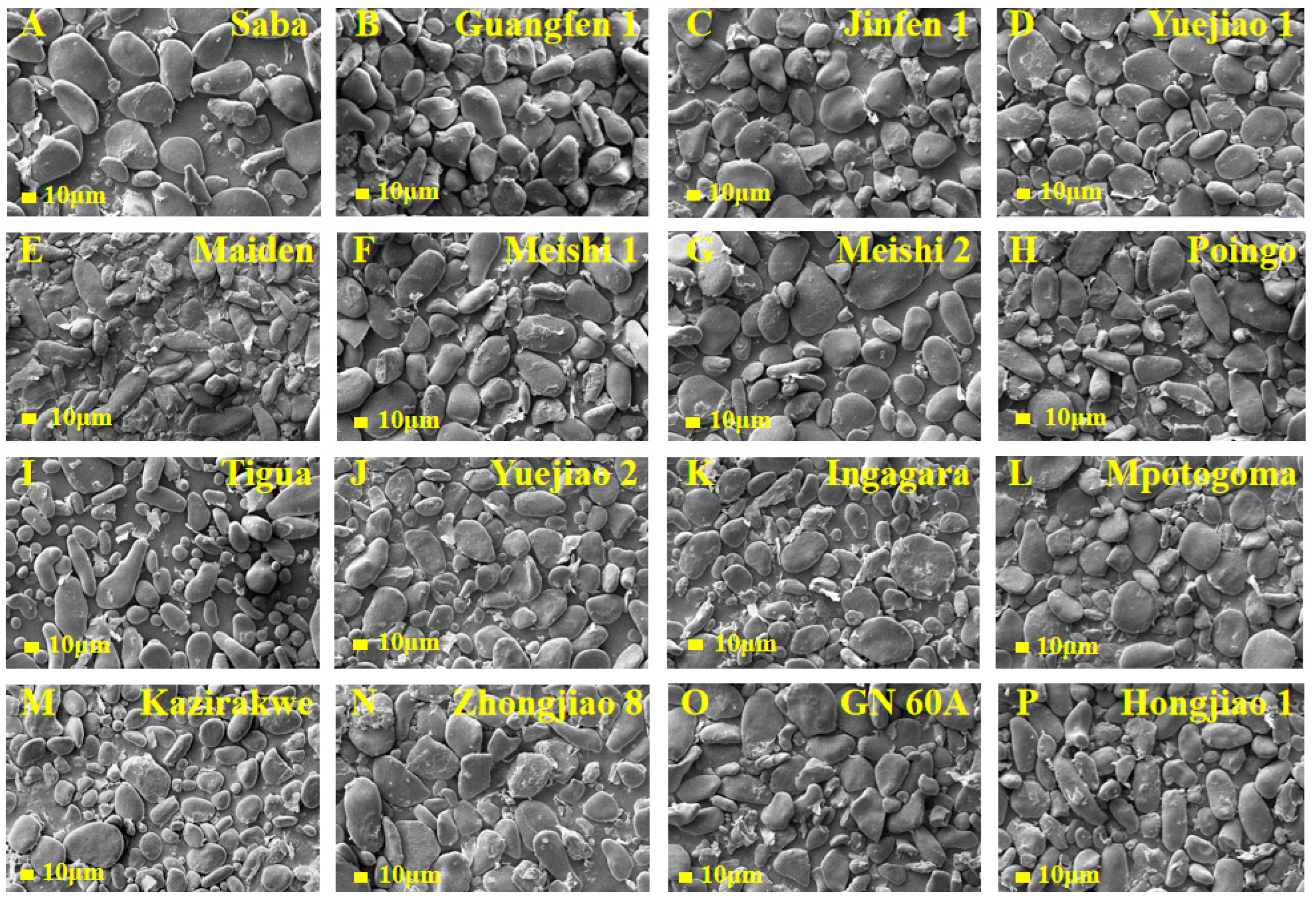
2.6. Scanning Electron Microscopy (SEM)
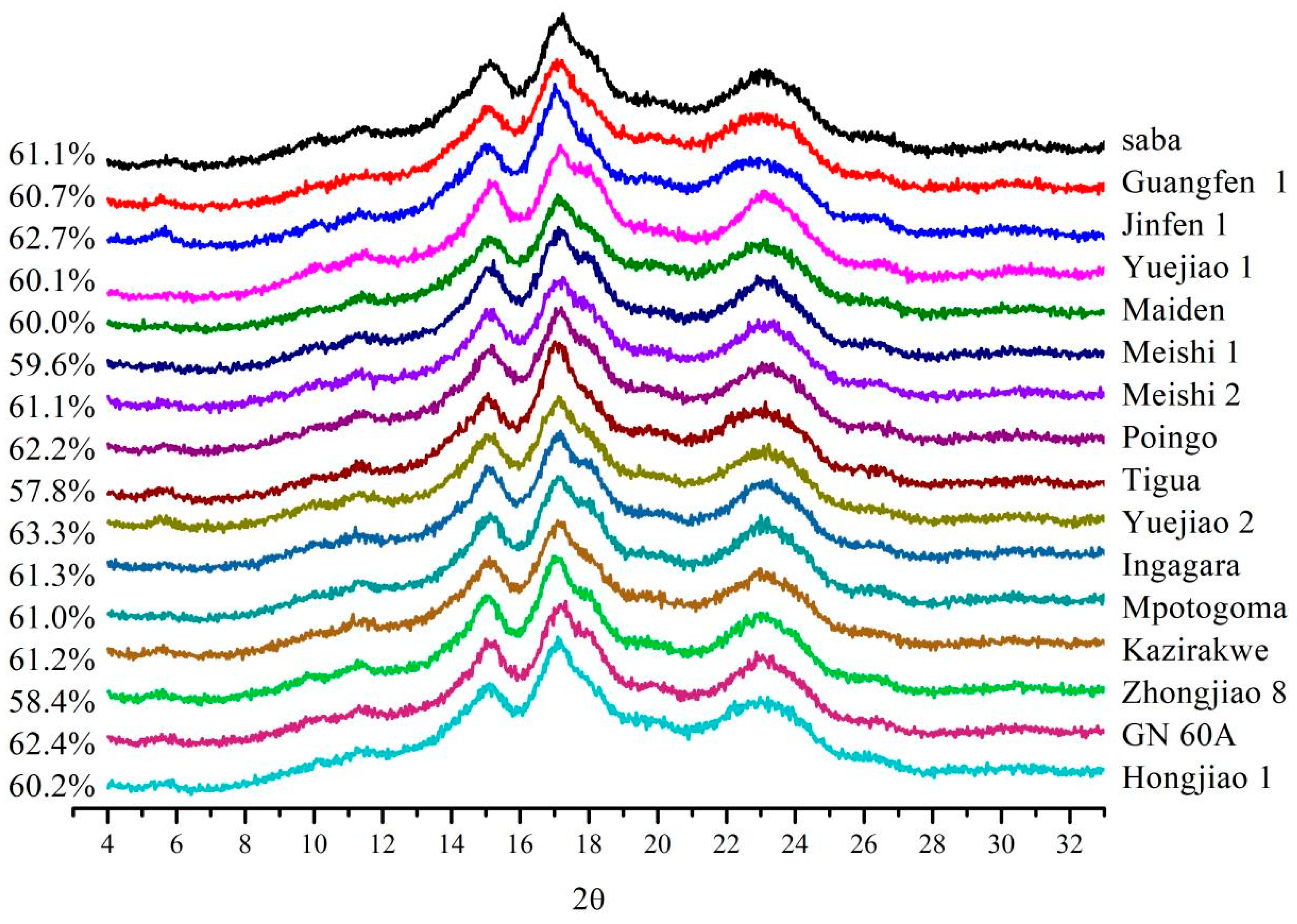
2.7. X-ray Diffraction (XRD)
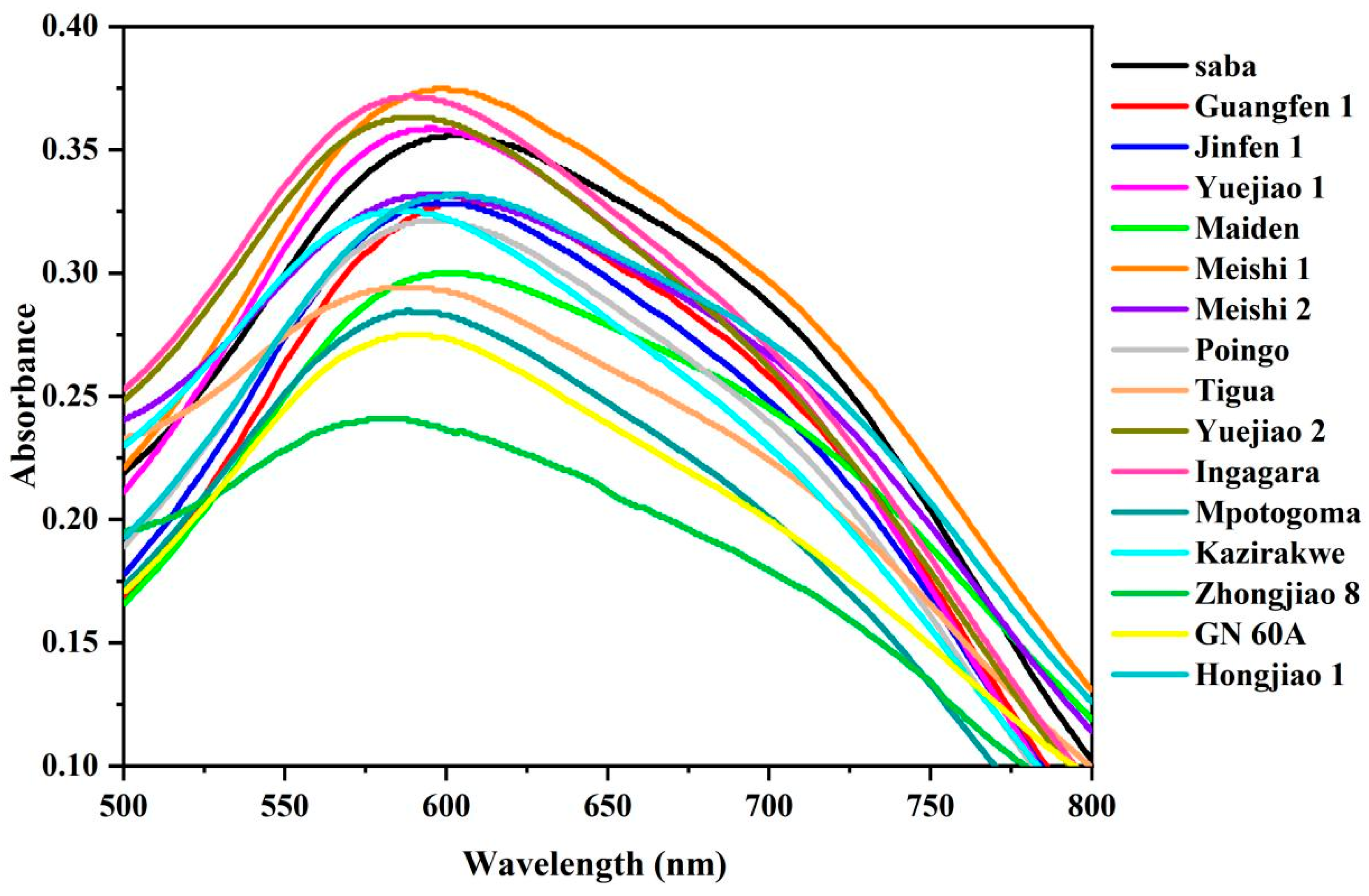
2.8. Starch-Iodine Absorption Spectrum Determination
2.9. Differential Scanning Calorimetry (DSC) Analysis
2.10. Brabender Viscosity Measurement
2.11. Statistical Analysis
3. Results and Discussion
3.1. Banana Starch Content
3.2. Comparison of the Predicted Glycemic Index (pGI) of BRS
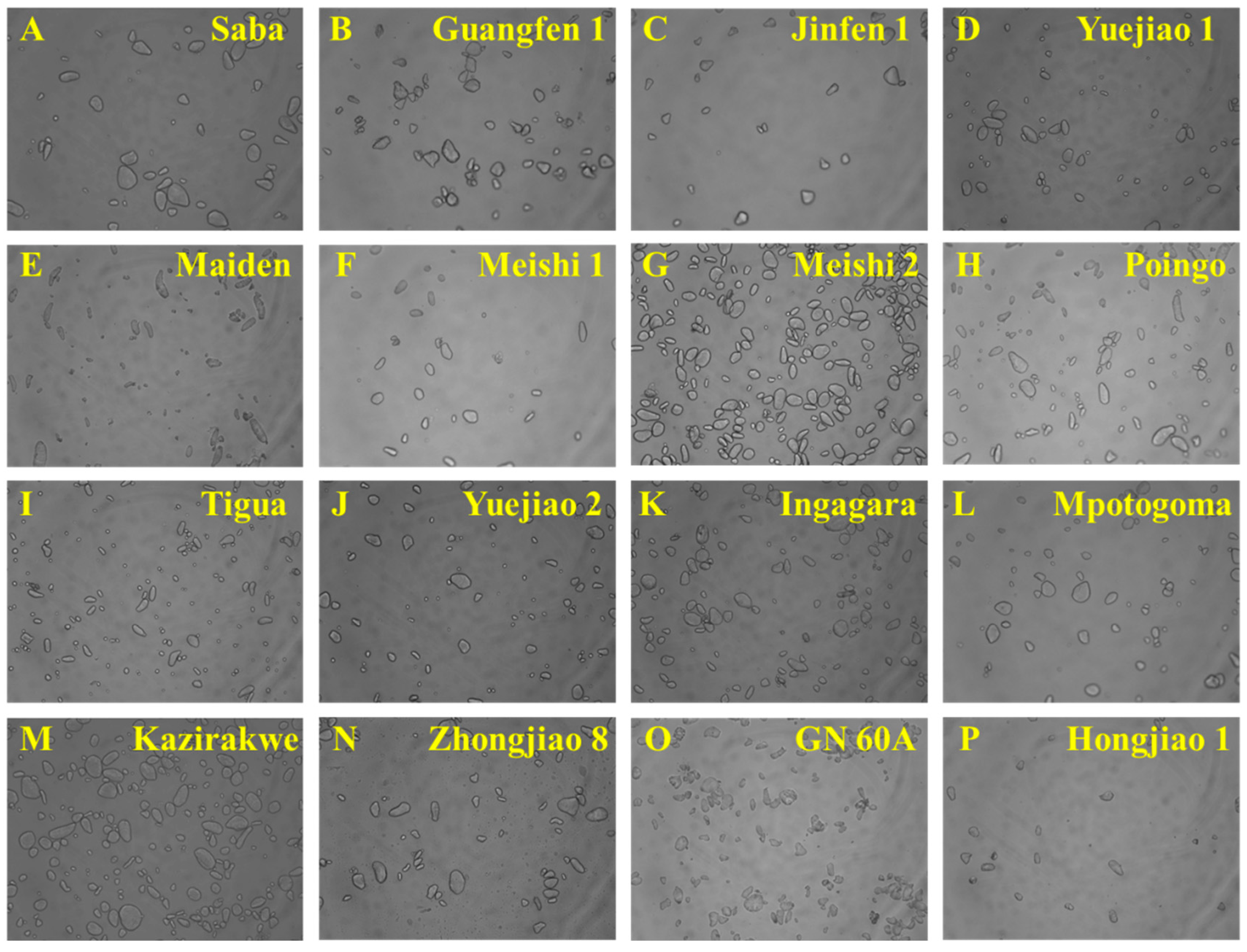
3.3. Granule Morphology
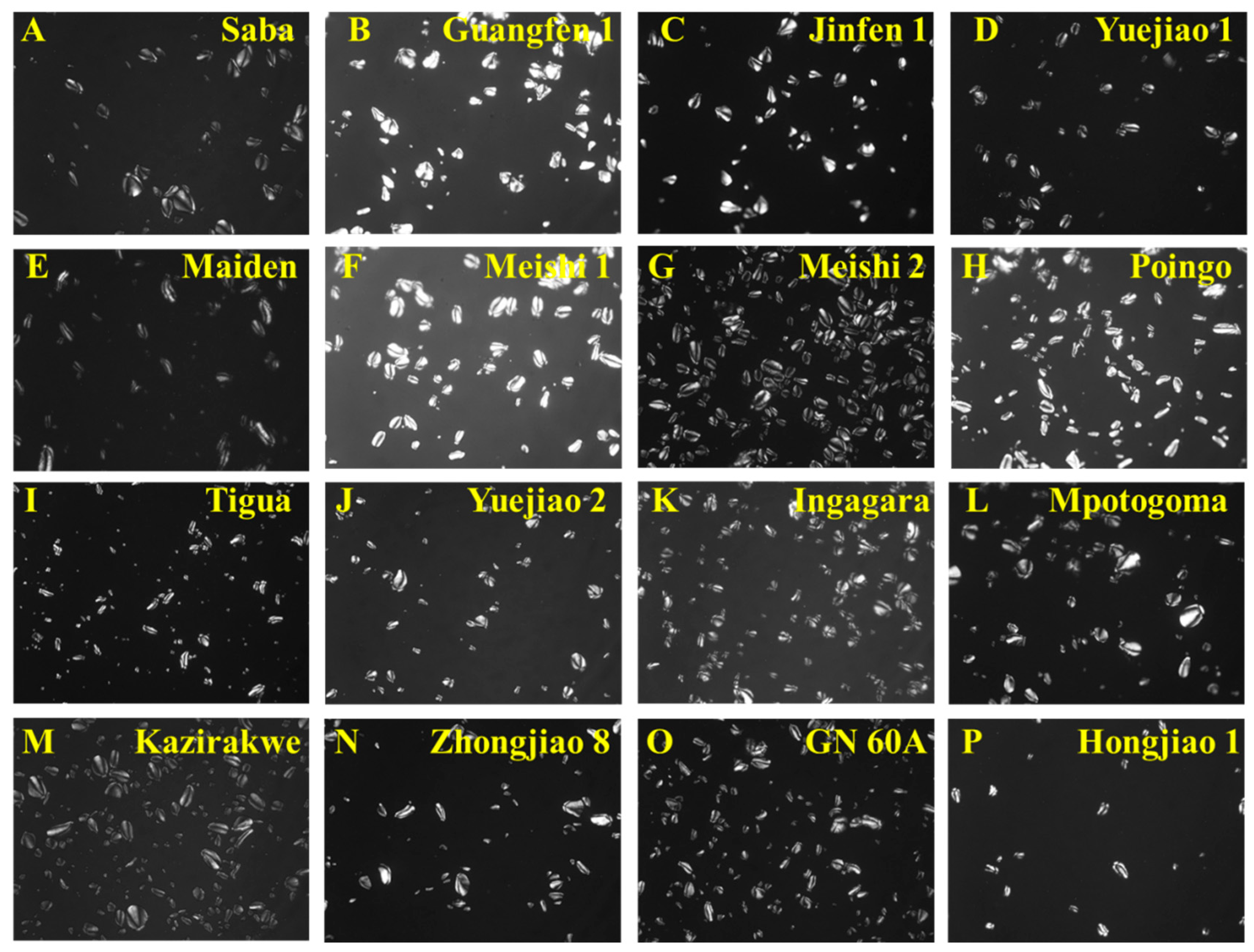
3.4. Crystalline Structure
3.5. Starch-Iodine Absorption Spectra
3.6. Thermal Properties
3.7. Pasting Properties
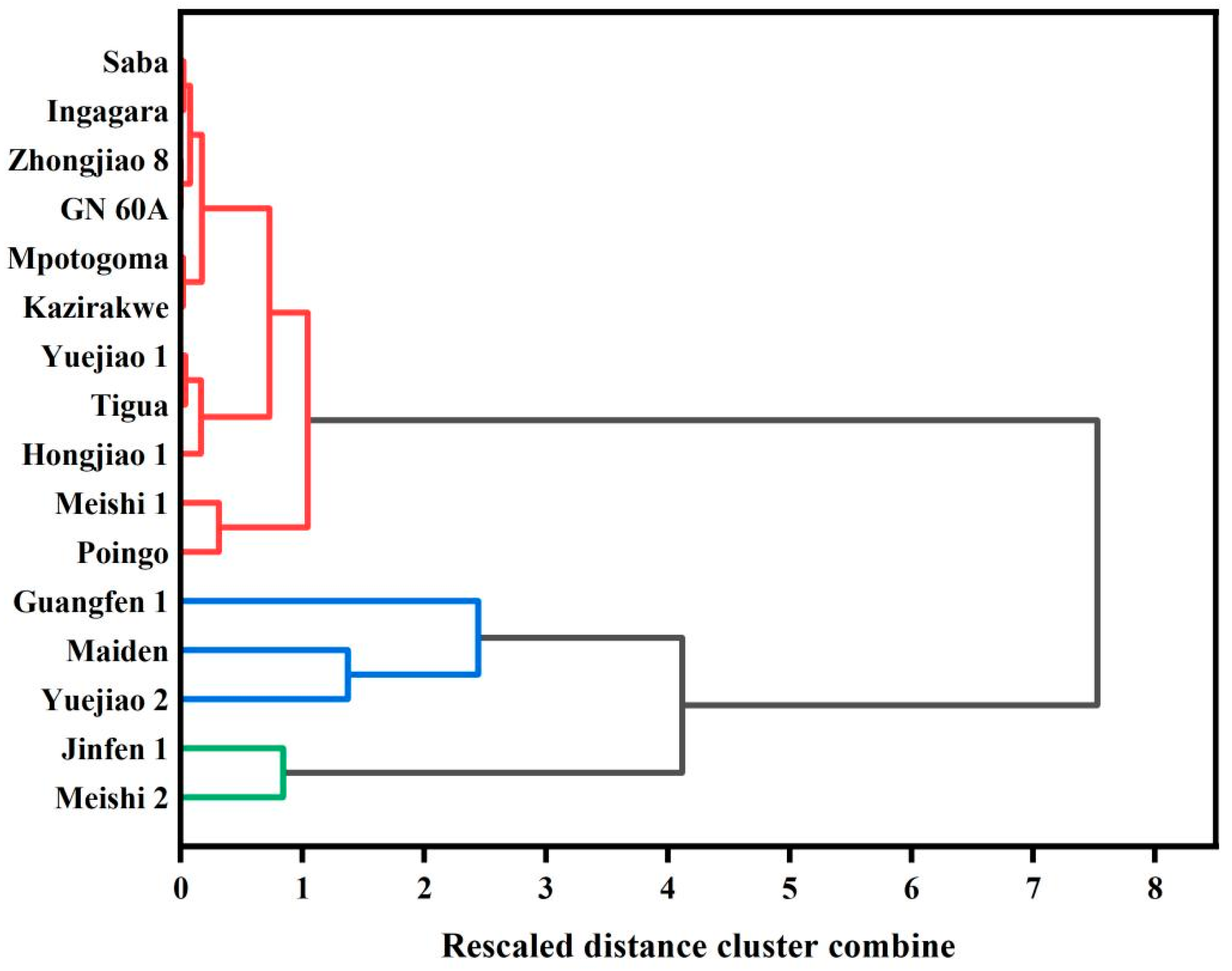
3.8. Clustered Heat Map Analysis on BRS Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soltani, M.; Alimardani, R.; Omid, M. Evaluating banana ripening status from measuring dielectric properties. J. Food Eng. 2011, 105, 625–631. [Google Scholar] [CrossRef]
- Sheikh, B.Y.; Sarker, M.M.R.; Kamarudin, M.N.A.; Ismail, A. Prophetic medicine as potential functional food elements in the intervention of cancer: A review. Biomed. Pharmacother. 2017, 95, 614–648. [Google Scholar] [CrossRef]
- Kumar, P.S.; Saravanan, A.; Sheeba, N.; Uma, S. Structural, functional characterization and physicochemical properties of green banana flour from dessert and plantain bananas (Musa spp.). Lwt 2019, 116, 108524. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y.; Tan, S.; Wang, J. Effects of Banana Resistant Starch on the Biochemical Indexes and Intestinal Flora of Obese Rats Induced by a High-Fat Diet and Their Correlation Analysis. Front. Bioeng. Biotechnol. 2021, 9, 575724. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.J.; Sánchez-Zapata, E.; Pérez-Álvarez, J.A. Resistant starch as functional ingredient: A review. Food Res. Int. 2010, 43, 931–942. [Google Scholar] [CrossRef]
- Dhull, S.B.; Malik, T.; Kaur, R.; Kumar, P.; Kaushal, N.; Singh, A. Banana Starch: Properties Illustration and Food Applications—A Review. Starch Stärke 2020, 73, 2000085. [Google Scholar] [CrossRef]
- Cordoba, L.d.P.; da Silva, R.G.; Gomes, D.d.S.; Schnitzler, E.; Waszczynskyj, N. Brazilian green banana. J. Therm. Anal. Calorim. 2018, 134, 2065–2073. [Google Scholar] [CrossRef]
- Salazar, D.; Arancibia, M.; Lalaleo, D.; Rodríguez-Maecker, R.; López-Caballero, M.E.; Montero, M.P. Physico-chemical properties and filmogenic aptitude for edible packaging of Ecuadorian discard green banana flours (Musa acuminanta AAA). Food Hydrocoll. 2022, 122, 107048. [Google Scholar] [CrossRef]
- Wang, J.S.; Wang, A.B.; Ma, W.H.; Xu, B.Y.; Zang, X.P.; Tan, L.; Jin, Z.Q.; Li, J.Y. Comparison of physicochemical properties and in vitro digestibility of starches from seven banana cultivars in China. Int. J. Biol. Macromol. 2019, 121, 279–284. [Google Scholar] [CrossRef]
- Marta, H.; Cahyana, Y.; Djali, M.; Arcot, J.; Tensiska, T. A comparative study on the physicochemical and pasting properties of starch and flour from different banana (Musa spp.) cultivars grown in Indonesia. Int. J. Food Prop. 2019, 22, 1562–1575. [Google Scholar] [CrossRef]
- Tan, S.M.; Wang, J.; Chen, P.S.; Zhu, X.H. Effects of banana maturity on the physicochemical properties of resistant starch. Sci. Technol. Food Ind. 2017, 38, 23–28. [Google Scholar] [CrossRef]
- Gao, H.; Huang, S.; Dong, T.; Yang, Q.; Yi, G. Analysis of resistant starch degradation in postharvest ripening of two banana cultivars: Focus on starch structure and amylases. Postharvest Biol. Technol. 2016, 119, 1–8. [Google Scholar] [CrossRef]
- Yanfeng, C. Comparison of Resistant Starch of Green Banana by Using Different Methods. Food Ferment. Ind. 2007, 33, 153–157. [Google Scholar]
- AOAC. Official Methods of Analysis, 22nd ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2002. [Google Scholar]
- Woolnough, J.W.; Bird, A.R.; Monro, J.A.; Brennan, C.S. The effect of a brief salivary α-amylase exposure during chewing on subsequent in vitro starch digestion curve profiles. Int. J. Mol. Sci. 2010, 11, 2780–2790. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.S.; Tudorica, C.M. Evaluation of potential mechanisms by which dietary fibre additions reduce the predicted glycaemic index of fresh pastas. Int. J. Food Sci. Technol. 2008, 43, 2151–2162. [Google Scholar] [CrossRef]
- Cleven, R.; Van den Berg, C.; Van Der Plas, L. Crystal structure of hydrated potato starch. Starch-Stärke 1978, 30, 223–228. [Google Scholar] [CrossRef]
- Klucinec, J.D.; Thompson, D.B. Fractionation of high-amylose maize starches by differential alcohol precipitation and chromatography of the fractions. Cereal Chem. 1998, 75, 887–896. [Google Scholar] [CrossRef]
- Aparicio-Saguilán, A.; Flores-Huicochea, E.; Tovar, J.; García-Suárez, F.; Gutiérrez-Meraz, F.; Bello-Pérez, L.A. Resistant starch-rich powders prepared by autoclaving of native and lintnerized banana starch: Partial characterization. Starch Stärke 2005, 57, 405–412. [Google Scholar] [CrossRef]
- Zhang, P.; Whistler, R.L.; BeMiller, J.N.; Hamaker, B.R. Banana starch: Production, physicochemical properties, and digestibility—A review. Carbohydr. Polym. 2005, 59, 443–458. [Google Scholar] [CrossRef]
- Espinosa-Solis, V.; Sanchez-Ambriz, S.L.; Hamaker, B.R.; Bello-Pérez, L.A. Fine structural characteristics related to digestion properties of acid-treated fruit starches. Starch Stärke 2011, 63, 717–727. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C.; Smith, S.M. Starch degradation. Annu. Rev. Plant Biol. 2005, 56, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, S.C.; Kossmann, J.; Smith, A.M. Starch: Its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Capriles, V.; Arêas, J. Approaches to reduce the glycemic response of gluten-free products: In vivo and in vitro studies. Food Funct. 2016, 7, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S. Dietary glycemic index and obesity. J. Nutr. 2000, 130, 280S–283S. [Google Scholar] [CrossRef]
- Agama-Acevedo, E.; Islas-Hernández, J.J.; Pacheco-Vargas, G.; Osorio-Díaz, P.; Bello-Pérez, L.A. Starch digestibility and glycemic index of cookies partially substituted with unripe banana flour. LWT Food Sci. Technol. 2012, 46, 177–182. [Google Scholar] [CrossRef]
- Zhang, G.; Ao, Z.; Hamaker, B.R. Slow digestion property of native cereal starches. Biomacromolecules 2006, 7, 3252–3258. [Google Scholar] [CrossRef]
- Han, J.-A.; BeMiller, J.N. Preparation and physical characteristics of slowly digesting modified food starches. Carbohydr. Polym. 2007, 67, 366–374. [Google Scholar] [CrossRef]
- Yang, M.; Chang, L.; Jiang, F.; Zhao, N.; Zheng, P.; Simbo, J.; Yu, X.; Du, S.K. Structural, physicochemical and rheological properties of starches isolated from banana varieties (Musa spp.). Food Chem X 2022, 16, 100473. [Google Scholar] [CrossRef]
- Bi, Y.; Zhang, Y.; Jiang, H.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Molecular structure and digestibility of banana flour and starch. Food Hydrocoll. 2017, 72, 219–227. [Google Scholar] [CrossRef]
- Soares, C.A.; Peroni-Okita, F.H.; Cardoso, M.B.; Shitakubo, R.; Lajolo, F.M.; Cordenunsi, B.R. Plantain and banana starches: Granule structural characteristics explain the differences in their starch degradation patterns. J. Agric. Food Chem. 2011, 59, 6672–6681. [Google Scholar] [CrossRef] [PubMed]
- Bello-Pérez, L.A.; Agama-Acevedo, E.; Sáyago-Ayerdi, S.G.; Moreno-Damian, E.; Figueroa, J.D.C. Some Structural, Physicochemical and Functional Studies of Banana Starches Isolated from two Varieties Growing in Guerrero, México. Starch Stärke 2000, 52, 68–73. [Google Scholar] [CrossRef]
- Cahyana, Y.; Titipanillah, R.; Mardawati, E.; Sukarminah, E.; Rialita, T.; Andoyo, R.; Djali, M.; Hanidah, I.I.; Setiasih, I.S.; Handarini, K. Non-starch contents affect the susceptibility of banana starch and flour to ozonation. J. Food Sci. Technol. 2018, 55, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, H.H.; Chen, P.S. Structural and physicochemical properties of banana resistant starch from four cultivars. Int. J. Food Prop. 2016, 20, 1338–1347. [Google Scholar] [CrossRef]
- Vatanasuchart, N.; Niyomwit, B.; Wongkrajang, K. Resistant starch content, in vitro starch digestibility and physico-chemical properties of flour and starch from Thai bananas. Maejo Int. J. Sci. Technol. 2012, 6, 259. [Google Scholar]
- Chen, L.; Zhao, Y.; Zhang, P.F.; Li, X.X.; Li, L. Structure of Potato Starches with Different Varieties. J. South China Univ. Technol. 2013, 41, 133–138. [Google Scholar]
- Zhou, D.T.; Ma, Z.; Xu, J.B.; Cui, W.X.; Hu, X.Z. Variations in structural and physicochemical properties of ultrasonicautoclaved pea resistant starch during in vitro digestion. Food Ferment. Ind. 2019, 45, 85–90. [Google Scholar] [CrossRef]
- Sievert, D. Enzyme-resistant starch. I. Characterization and evaluation by enzymatic, thermoanalytical, and microscopic methods. Cereal Chem. 1989, 66, 342–347. [Google Scholar]
- Zhang, S.; Li, Z.; Lin, L.; Zhang, L.; Wei, C. Starch components, starch properties and appearance quality of opaque kernels from rice mutants. Molecules 2019, 24, 4580. [Google Scholar] [CrossRef]
- Zhang, L.C. Study on the Influencing Factors and the Physicochemical Properties of Resistant Starch for Potato (Solanum tuberosum L.) Tuber. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2014. [Google Scholar]
- Zhao, M.X. Study on Preparation of Resistant Starch and Composite Films by High Hydrostatic Pressure Synergistic Enzymatic Hydrolysis. Master’s Thesis, Inner Mongolia University of Technology, Hohhot, China, 2018. [Google Scholar]
- Teng, B.; Zhang, Y.; Du, S.; Wu, J.; Li, Z.; Luo, Z.; Yang, J. Crystalline, thermal and swelling properties of starches from single-segment substitution lines with different Wx alleles in rice (Oryza sativa L.). J. Sci. Food Agric. 2017, 97, 108–114. [Google Scholar] [CrossRef]
- Chavez-Salazar, A.; Bello-Perez, L.A.; Agama-Acevedo, E.; Castellanos-Galeano, F.J.; Alvarez-Barreto, C.I.; Pacheco-Vargas, G. Isolation and partial characterization of starch from banana cultivars grown in Colombia. Int. J. Biol. Macromol. 2017, 98, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Noosuk, P.; Hill, S.E.; Pradipasena, P.; Mitchell, J.R. Structure-Viscosity Relationships for Thai Rice Starches. Starch Stärke 2003, 55, 337–344. [Google Scholar] [CrossRef]
- Kong, L.; Kong, M.Z.; Yu, J.X.; Lv, Y.P. Digestibility and physicochemical properties of quinoa starch. Food Sci. Technol. 2019, 44, 285–290. [Google Scholar] [CrossRef]
- Zhao, K.; Gu, G. Research advances in starch digestibility analysis. Food Sci. 2007, 9, 146–149. [Google Scholar]

| Cultivar Name | Cultivated Group | Sub-Type | Genome |
|---|---|---|---|
| Saba | Saba | Saba | ABB |
| Guangfen 1 | Pisang Awak | Pisang Awak | ABB |
| Jinfen 1 | Pisang Awak | Pisang Awak | ABB |
| Yuejiao 1 | Plantain | French | AAB |
| Maiden | Plantain | French | AAB |
| Meishi 1 | Plantain | French Horn | AAB |
| Meishi 2 | Plantain | French Horn | AAB |
| Poingo | Maoli-Popoulu | Maoli-Popoulu | AAB |
| Tigua | Iholena | Iholena | AAB |
| Yuejiao 2 | EAHB | Mbidde | AAA |
| Ingagara | EAHB | Nakitembe | AAA |
| Mpotogoma | EAHB | Musakala | AAA |
| Kazirakwe | EAHB | Nakabululu | AAA |
| Zhongjiao 8 | Cavendish | Giant Cavendish | AAA |
| GN 60A | Cavendish | Dwarf Cavendish | AAA |
| Hongjiao 1 | Red and Green | Red | AAA |
| Sample | BRS (g/100 g) | NRS (g/100 g) | Total Starch (g/100 g) | BRS/Total Starch (%) | pGI | ||||
|---|---|---|---|---|---|---|---|---|---|
| D1 | D7 | D1 | D7 | D1 | D7 | D1 | D7 | D1 | |
| Saba | 48.79 ± 5.70 fg | 1.43 ± 1.56 e | 5.76 ± 0.15 fg | 7.88 ± 1.25 j | 54.50 ± 5.81 fg | 9.28 ± 1.74 h | 89.3 ± 2.54 bd | 13.19 ± 17.71 ef | 14.68 ± 0.27 c |
| Guangfen 1 | 68.69 ± 4.30 cd | 23.92 ± 0.89 b | 6.41 ± 3.47 fg | 34.05 ± 0.21 bc | 75.09 ± 4.72 ce | 57.96 ± 1.10 b | 91.47 ± 1.91 ac | 41.24 ± 0.95 bc | 11.72 ± 0.23 hi |
| Jinfen 1 | 90.92 ± 3.19 ab | 38.91 ± 2.97 a | 6.21 ± 0.80 fg | 36.41 ± 2.13 ab | 97.25 ± 3.43 ab | 75.32 ± 3.03 a | 93.48 ± 0.65 ab | 51.62 ± 3.35 ab | 15.32 ± 0.26 b |
| Yuejiao 1 | 65.93 ± 5.90 ce | 0.00 ± 0.00 e | 4.46 ± 0.33 g | 31.56 ± 2.27 cd | 70.52 ± 7.71 cf | 31.56 ± 2.27 d | 93.50 ± 1.29 ab | 0.00 ± 0.00 f | 12.19 ± 0.13 gh |
| Maiden | 90.10 ± 8.03 ab | 15.24 ± 3.73 c | 8.90 ± 0.47 ef | 11.25 ± 2.57 ij | 98.94 ± 9.03 a | 26.49 ± 2.51 ef | 91.02 ± 1.07 ac | 57.10 ± 12.42 a | 11.44 ± 0.35 i |
| Meishi 1 | 59.09 ± 2.80 dg | 9.39 ± 4.43 cd | 27.63 ± 0.26 a | 28.76 ± 0.88 de | 86.73 ± 3.50 ac | 38.16 ± 3.75 c | 68.08 ± 1.38 g | 23.78 ± 10.74 de | 12.40 ± 0.09 fg |
| Meishi 2 | 96.74 ± 3.43 a | 28.42 ± 5.67 b | 3.64 ± 0.40 g | 31.75 ± 2.89 cd | 100.38 ± 3.15 a | 60.17 ± 2.78 b | 96.35 ± 1.47 a | 46.90 ± 8.62 ab | 9.71 ± 0.30 j |
| Poingo | 50.33 ± 15.62 eg | 12.40 ± 3.00 c | 19.35 ± 0.20 bc | 26.94 ± 0.93 ef | 69.69 ± 17.07 df | 39.34 ± 2.17 c | 70.16 ± 10.33 g | 31.19 ± 7.49 cd | 12.72 ± 0.27 ef |
| Tigua | 62.50 ± 2.27 cf | 0.00 ± 0.00 e | 22.41 ± 2.51 b | 40.16 ± 2.61 a | 84.92 ± 2.43 ad | 40.16 ± 2.61 c | 73.58 ± 1.18 fg | 0.00 ± 0.00 f | 11.61 ± 0.14 i |
| Yuejiao 2 | 77.35 ± 2.44 bc | 4.01 ± 0.35 de | 15.98 ± 3.40 cd | 25.71 ± 0.69 ef | 93.33 ± 2.00 ab | 29.73 ± 0.93 de | 82.86 ± 1.37 de | 13.50 ± 1.05 ef | 11.60 ± 0.23 i |
| Ingagara | 47.22 ± 3.49 fg | 0.00 ± 0.00 e | 13.13 ± 2.37 de | 17.87 ± 0.23 g | 60.34 ± 3.57 eg | 17.87 ± 0.23 g | 78.17 ± 1.85 ef | 0.00 ± 0.00 f | 14.15 ± 0.08 d |
| Mpotogoma | 44.72 ± 4.05 g | 0.00 ± 0.00 e | 2.03 ± 2.13 g | 16.81 ± 0.45 gh | 46.75 ± 4.31 g | 16.81 ± 0.45 g | 95.58 ± 1.08 ab | 0.00 ± 0.00 f | 11.58 ± 0.25 i |
| Kazirakwe | 42.39 ± 8.98 g | 0.00 ± 0.00 e | 14.55 ± 0.99 d | 18.17 ± 0.61 g | 56.87 ± 9.31 fg | 18.17 ± 0.61 g | 73.67 ± 6.54 fg | 0.00 ± 0.00 f | 16.47 ± 0.06 a |
| Zhongjiao 8 | 52.43 ± 4.20 dg | 0.00 ± 0.00 e | 21.13 ± 0.92 b | 39.25 ± 0.55 a | 73.73 ± 3.76 ce | 39.25 ± 0.55 c | 70.99 ± 3.05 g | 0.00 ± 0.00 f | 12.58 ± 0.06 fg |
| GN 60A | 52.52 ± 3.61 dg | 0.00 ± 0.00 e | 9.41 ± 2.14 ef | 14.03 ± 0.64 hi | 61.93 ± 4.26 eg | 14.03 ± 0.64 gh | 84.81 ± 1.23 ce | 0.00 ± 0.00 f | 12.45 ± 0.08 fg |
| Hongjiao 1 | 57.37 ± 4.98 dg | 0.00 ± 0.00 e | 23.23 ± 1.20 ab | 23.64 ± 0.14 f | 80.61 ± 5.90 bd | 23.64 ± 0.14 f | 71.06 ± 1.88 g | 0.00 ± 0.00 f | 13.20 ± 0.12 e |
| Cluster | Cultivar | Genotype |
|---|---|---|
| C1 | Meishi 2, Jinfen 1 | AAB, ABB |
| C2 | A-Maiden, Yuejiao 2 | AAB, AAA |
| B-Guangfen 1 | ABB | |
| C3 | A-Meishi 1, Poingo | AAB, AAB |
| B-Hongjiao 1, Tigua, Yuejiao 1 | AAA, AAB, AAB | |
| C-Kazirakwe, Mpotogoma, Ingagara, Saba, GN 60A, Zhongjiao 8 | AAA, AAA, AAA, ABB, AAA, AAA |
| Sample | To (°C) | Tp (°C) | Tc (°C) | ΔT (°C) (Tc − To) |
|---|---|---|---|---|
| Saba | 54.5 ± 16.1 | 71.7 ± 13.4 | 76.7 ± 11.5 | 22.2 ± 16.2 |
| Guangfen 1 | 61.8 ± 9.7 | 79.8 ± 2.1 | 84.2 ± 2.1 | 22.4 ± 8.0 |
| Jinfen 1 | 61.5 ± 11.6 | 81.8 ± 0.6 | 85.4 ± 1.1 | 30.0 ± 12.7 |
| Yuejiao 1 | 56.6 ± 17.8 | 78.6 ± 5.3 | 83. 9 ± 5.2 | 27.2 ± 13.3 |
| Maiden | 59.9 ± 1.3 | 73.5 ± 1.6 | 77.1 ± 1.6 | 17.1 ± 2.8 |
| Meishi 1 | 59.6 ± 17.8 | 76.1 ± 6.0 | 82.1 ± 6.1 | 22.5 ± 12.3 |
| Meishi 2 | 66.3 ± 10.5 | 81.1 ± 2.6 | 86.8 ± 2.4 | 20.5 ± 8.2 |
| Poingo | 58.2 ± 8.1 | 76.0 ± 2.6 | 81.7 ± 2.0 | 23.5 ± 7.3 |
| Tigua | 48.1 ± 8.6 | 78.2 ± 0.8 | 81.7 ± 0.7 | 33.6 ± 9.1 |
| Yuejiao 2 | 48.4 ± 9.0 | 78.8 ± 3.8 | 79.7 ± 7.8 | 31.4 ± 8.7 |
| Ingagara | 53.5 ± 4.3 | 80.5 ± 5.0 | 84.6 ± 4.5 | 31.1 ± 2.2 |
| Mpotogoma | 64.1 ± 14.3 | 72.2 ± 13.2 | 85.6 ± 3.5 | 21.4 ± 10.9 |
| Kazirakwe | 68.6 ± 2.6 | 76.1 ± 0.9 | 80.5 ± 0.8 | 11.9 ± 3.1 |
| Zhongjiao 8 | 49.9 ± 1.7 | 75.6 ± 4.7 | 81.9 ± 5.1 | 32.1 ± 6.2 |
| GN 60A | 56.7 ± 7.5 | 82.4 ± 4.4 | 87.0 ± 3.3 | 30.4 ± 4.2 |
| Hongjiao 1 | 58.5 ± 9.9 | 73.6 ± 1.6 | 79.0 ± 1.9 | 20.5 ± 8.3 |
| Sample | A (°C) | B (BU) | C (CU) | D (DU) | E (BU) | F (BU) | B-D (BU) | E-D (BU) |
|---|---|---|---|---|---|---|---|---|
| Saba | 78.1 | 239 | 237 | 211 | 261 | 253 | 28 | 50 |
| Guangfen 1 | 77.5 | 275 | 275 | 250 | 326 | 331 | 25 | 76 |
| Jinfen 1 | 77.9 | 261 | 253 | 247 | 311 | 315 | 14 | 64 |
| Yuejiao 1 | 79.2 | 273 | 270 | 217 | 281 | 285 | 56 | 64 |
| Maiden | 75.8 | 62 | 60 | 57 | 68 | 71 | 5 | 11 |
| Meishij 1 | 78.9 | 250 | 244 | 183 | 235 | 236 | 67 | 52 |
| Meishij 2 | 79.4 | 264 | 263 | 176 | 224 | 236 | 88 | 48 |
| Poingo | 76.7 | 184 | 140 | 175 | 214 | 211 | 9 | 39 |
| Tigua | 74.2 | 328 | 315 | 267 | 360 | 358 | 61 | 93 |
| Yuejiao 2 | 75.7 | 289 | 284 | 258 | 318 | 318 | 31 | 60 |
| Ingagara | 79.2 | 140 | 127 | 130 | 160 | 160 | 10 | 30 |
| Mpotogoma | 77.5 | 222 | 215 | 199 | 245 | 256 | 23 | 46 |
| Kazirakwe | 73.7 | 294 | 289 | 251 | 302 | 305 | 43 | 51 |
| Zhongjiao 8 | 78.8 | 131 | 118 | 123 | 159 | 161 | 8 | 36 |
| GN 60A | 79.1 | 251 | 226 | 231 | 292 | 299 | 20 | 61 |
| Hongjiao 1 | 72.7 | 182 | 182 | 156 | 201 | 202 | 26 | 45 |
| Style | Name | Genotype |
|---|---|---|
| 1 | Saba, Mpotogoma, Kazirakwe | ABB, AAA, AAA |
| 2 | Maiden | AAB |
| 3 | Meishi 1, Poinga, Hongjiao 1, Ingagara, Zhongjiao 8 | AAB, AAA, AAA, AAA, AAA |
| 4 | Guangfen 1, Yuejiao 1, GN 60A, Yuejiao 2, Jinfen 1, Meishi 2, Tigua | ABB, AAB, AAA, AAA, ABB, AAB, AAB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, M.; Tu, S.; Fu, J.; Wang, J.; Sheng, O. Structural and Physicochemical Characterization of Resistant Starch from Sixteen Banana Cultivars across Three Genome Groups. Foods 2024, 13, 3277. https://doi.org/10.3390/foods13203277
Liang M, Tu S, Fu J, Wang J, Sheng O. Structural and Physicochemical Characterization of Resistant Starch from Sixteen Banana Cultivars across Three Genome Groups. Foods. 2024; 13(20):3277. https://doi.org/10.3390/foods13203277
Chicago/Turabian StyleLiang, Minhong, Shiyun Tu, Jinfeng Fu, Juan Wang, and Ou Sheng. 2024. "Structural and Physicochemical Characterization of Resistant Starch from Sixteen Banana Cultivars across Three Genome Groups" Foods 13, no. 20: 3277. https://doi.org/10.3390/foods13203277
APA StyleLiang, M., Tu, S., Fu, J., Wang, J., & Sheng, O. (2024). Structural and Physicochemical Characterization of Resistant Starch from Sixteen Banana Cultivars across Three Genome Groups. Foods, 13(20), 3277. https://doi.org/10.3390/foods13203277






