NaCl Stress Stimulates Phenolics Biosynthesis and Antioxidant System Enhancement of Quinoa Germinated after Magnetic Field Pretreatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Materials’ Treatment Methods
2.3. Determination of Sprout Length, Germination Percentage, Single Plant Weight, Moisture Content, and Fresh/Dry Weight
2.4. Determination of Starch, Reducing Sugar, Soluble Protein, Free Amino Acid, Crude Fat, and Ash Content
2.5. Determination of Phenolic and Flavonoid Content
2.6. Determination of Key Enzyme Activity in the Phenolic Synthesis Pathway
2.7. Determination of Key Enzyme Gene Expression in Phenolic Synthesis Pathway
2.8. Determination of Antioxidation Capacity
2.9. Determination of Antioxidative Enzyme Activity
2.10. Statistical Analysis
3. Results
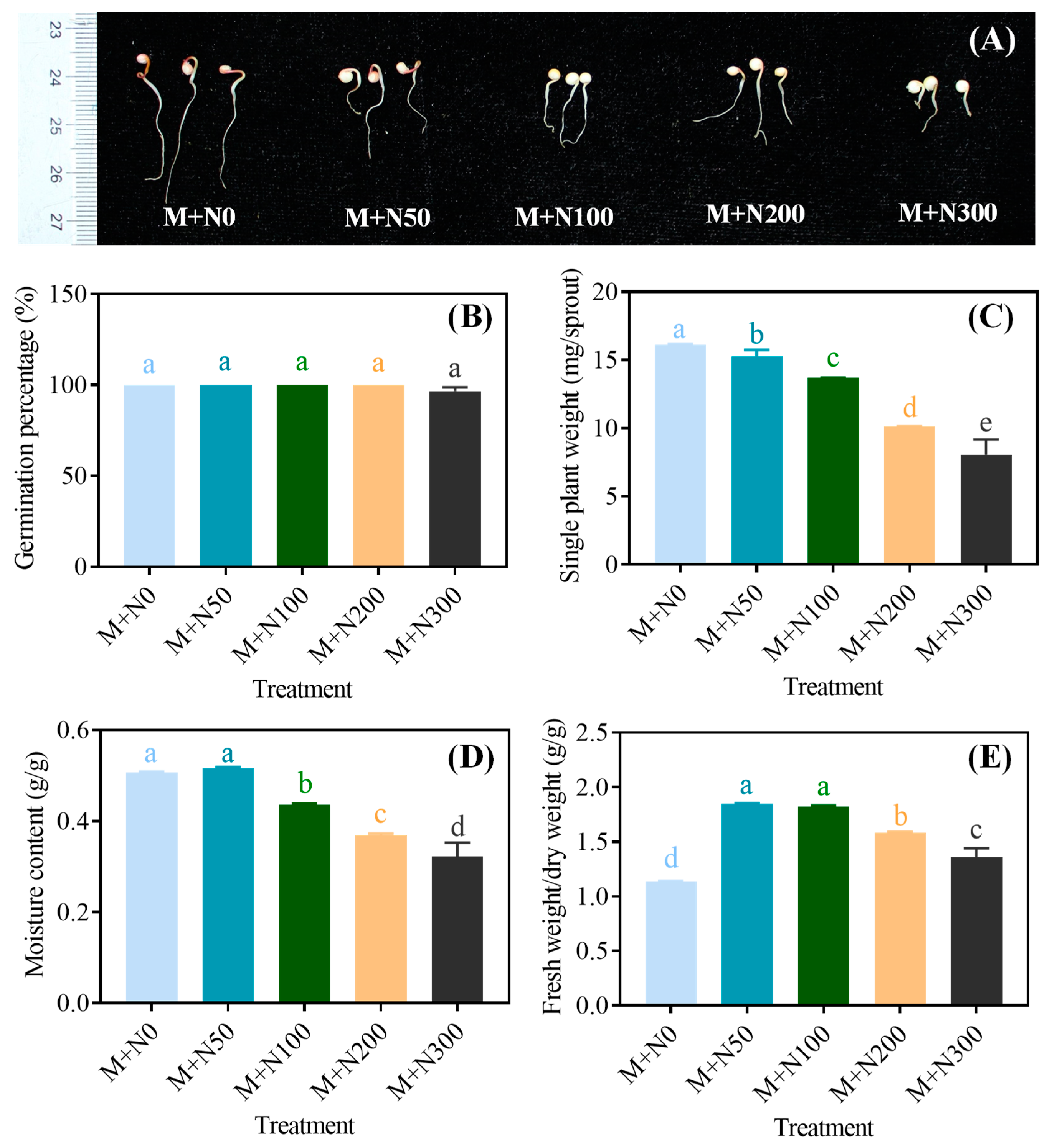
3.1. Effect of NaCl Stress on the Morphology of Quinoa Sprouts after Magnetic Field Pretreatment
3.2. Effect of NaCl Stress on the Basic Nutrient Content of Quinoa Sprouts after Magnetic Field Pretreatment
3.3. Effect of NaCl Stress on the Phenolic Content of Quinoa Sprouts after Magnetic Field Pretreatment
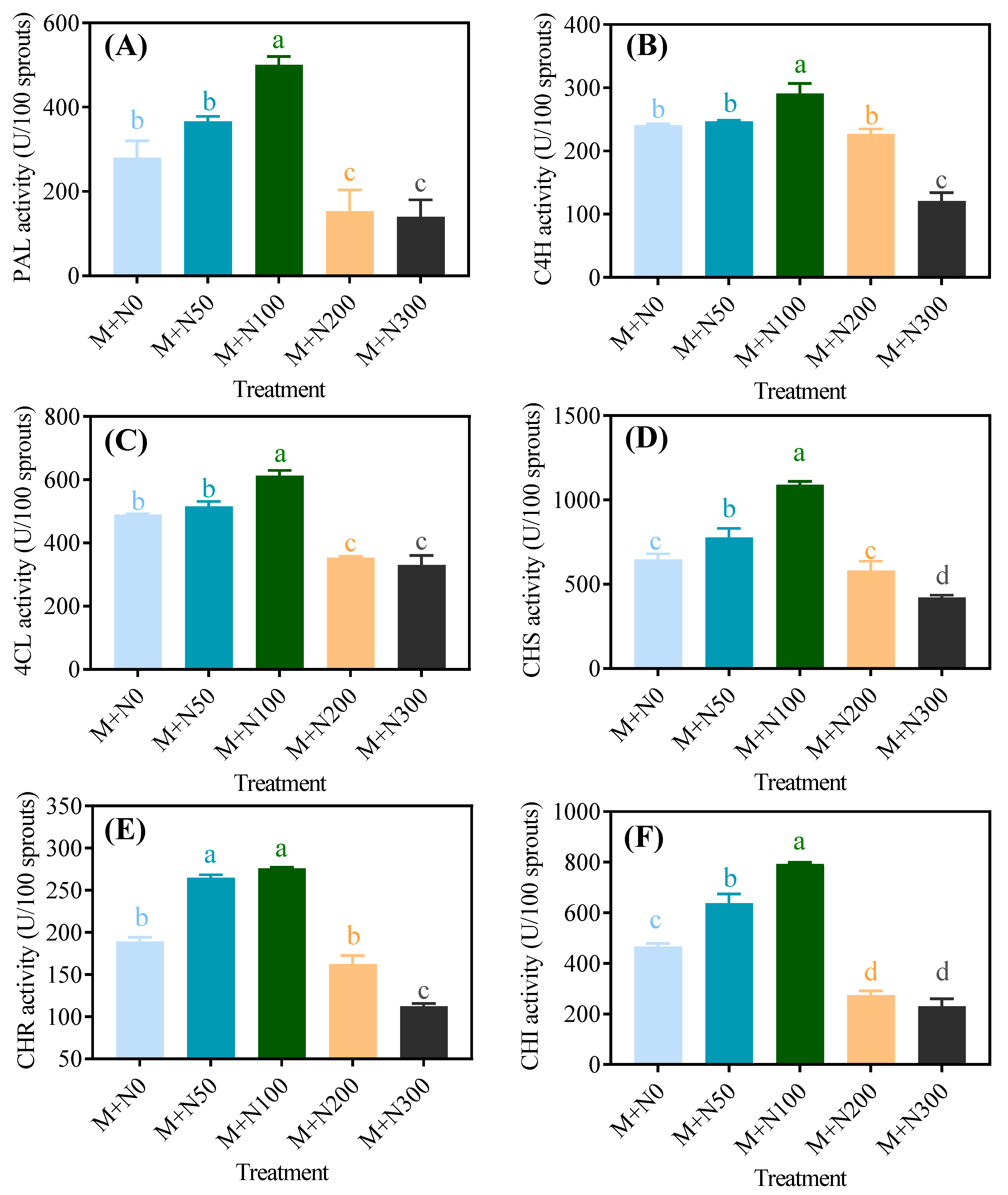
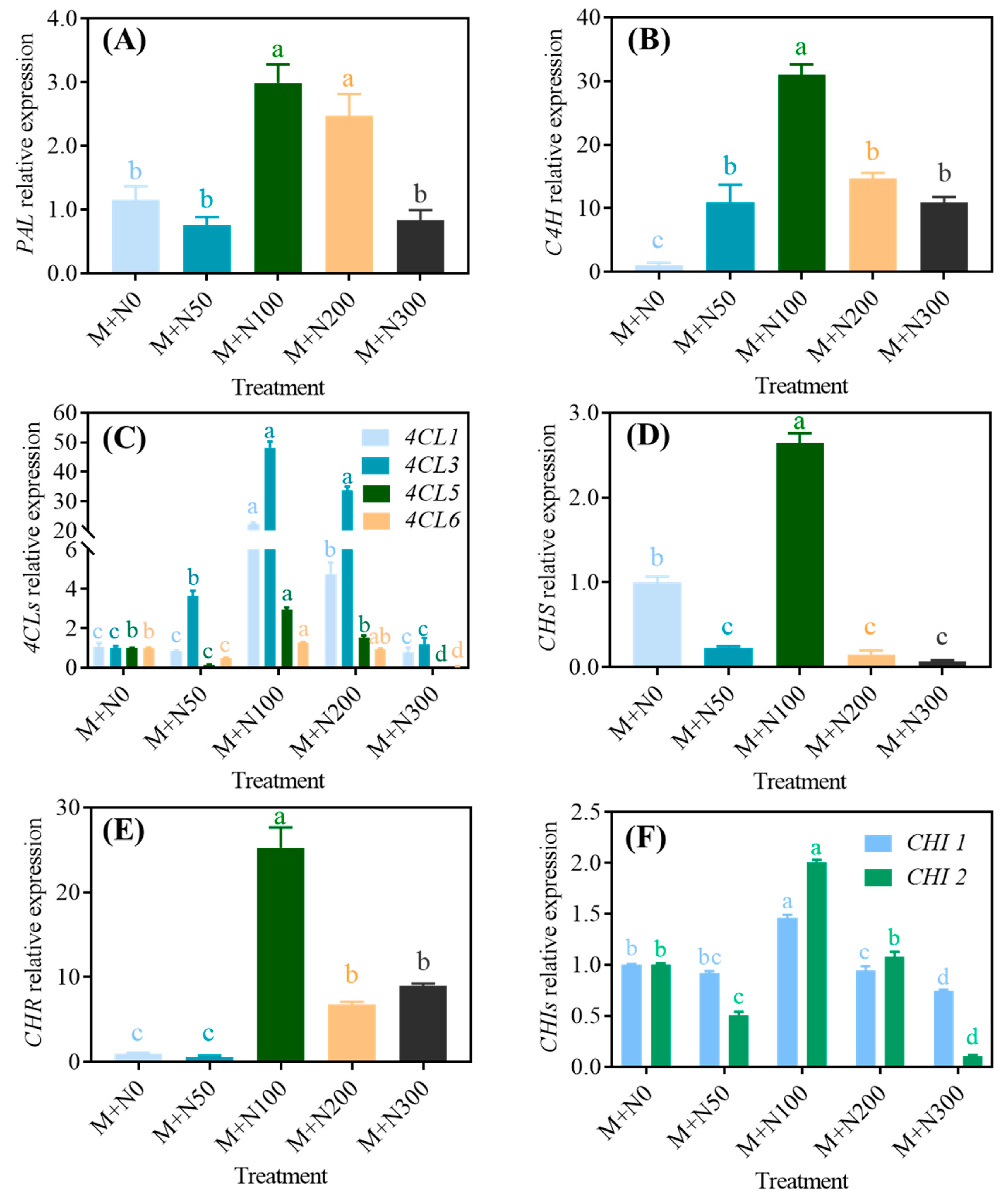
3.4. Effect of NaCl Stress on the Activity and the Gene Expression of Phenolic Synthase in Quinoa Sprouts after Magnetic Field Pretreatment
3.4.1. Enzyme Activity
3.4.2. Gene Expression
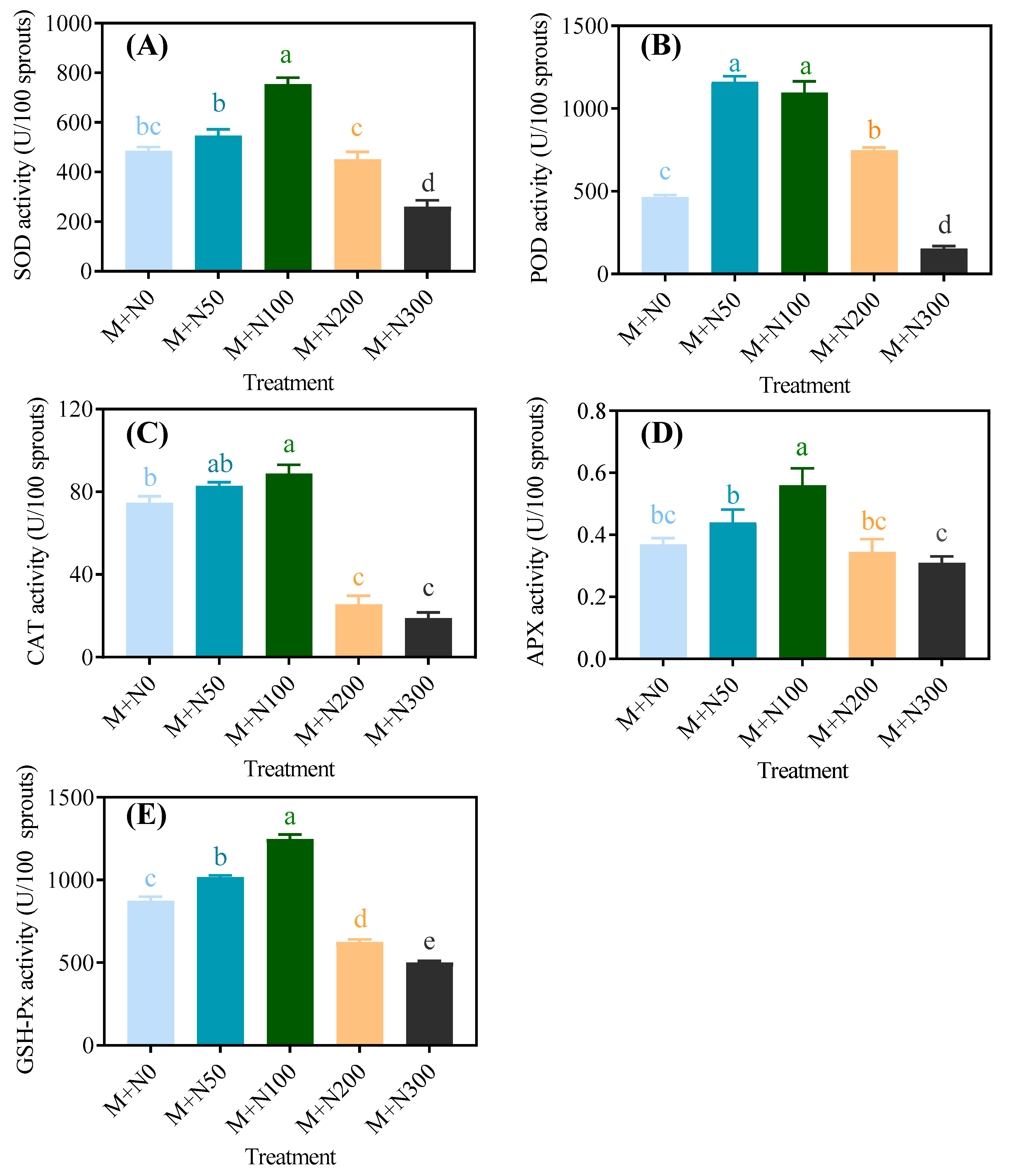
3.5. Effect of NaCl Stress on the Antioxidative System of Quinoa Sprouts after Magnetic Field Pretreatment
3.5.1. Antioxidative Capacity
3.5.2. Antioxidative Enzyme Activity
3.6. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhargava, A.; Srivastava, S. Quinoa: Botany, Production and Uses; CABI: Oxford, UK, 2013. [Google Scholar]
- Bhinder, S.; Kumari, S.; Singh, B.; Kaur, A.; Singh, N. Impact of germination on phenolic composition, antioxidant properties, antinutritional factors, mineral content and Maillard reaction products of malted quinoa flour. Food Chem. 2021, 346, 128915. [Google Scholar] [CrossRef] [PubMed]
- Abugoch, L.E. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. Adv. Nutr. 2009, 58, 1043–4526. [Google Scholar]
- Nkhata, S.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jin, Z.; Simsek, S.; Hall, C.; Rao, J.; Chen, B. Effect of germination on the chemical composition, thermal, pasting, and moisture sorption properties of flours from chickpea, lentil, and yellow pea. Food Chem. 2019, 295, 579–587. [Google Scholar] [CrossRef]
- Wang, S.F.; Zhang, X.J.; Fan, Y.H.; Wang, Y.T.; Yang, R.Q.; Wu, J.R.; Xu, J.H.; Tu, K. Effect of magnetic field pretreatment on germination characteristics, phenolic biosynthesis, and antioxidant capacity of quinoa. Plant Physiol. Biochem. 2024, 212, 108734. [Google Scholar] [CrossRef]
- Stoleru, V.; Slabu, C.; Vitanescu, M.; Peres, C.; Cojocaru, A.; Covasa, M.; Mihalache, G. Tolerance of three quinoa cultivars (Chenopodium quinoa willd.) to salinity and alkalinity stress during germination stage. Agronomy 2019, 9, 287. [Google Scholar] [CrossRef]
- Hinojosa, L.; González, J.; Barrios-Masias, F.; Fuentes, F.; Murphy, K. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, P.; Chen, Z.J.; Gu, Z.X.; Yang, R.Q. NaCl stress on physio-biochemical metabolism and antioxidant capacity in germinated hulless barley (Hordeum vulgare L.). J. Sci. Food Agric. 2019, 99, 1755–1764. [Google Scholar] [CrossRef]
- Hussin, S.A.; Ali, S.H.; Lotfy, M.E.; Abd EI-Samad, E.H.; Eid, M.A.; Abd-Elkader, A.M.; Eisa, S.S. Morpho-physiological mechanisms of two different quinoa ecotypes to resist salt stress. BMC Plant Biol. 2023, 23, 374. [Google Scholar] [CrossRef]
- Slatni, T.; Ben Slimene, I.; Harzalli, Z.; Taamalli, W.; Smaoui, A.; Abdelly, C.; Elkahoui, S. Enhancing quinoa (Chenopodium quinoa) growth in saline environments through salt-tolerant rhizobacteria from halophyte biotope. Physiol. Plant. 2024, 176, e14466. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Skalicky, M.; Brestic, M.; Pavla, V. Cross-talk between nitric oxide, hydrogen peroxide and calcium in salt-stressed Chenopodium quinoa Willd. at seed germination stage. Plant Physiol. Biochem. 2020, 154, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Mouttaqi, A.E.; Sabraoui, T.; Belcaid, M.; Ibourki, M.; Mnaouer, I.; Lazaar, K.; Sehbaoui, F.; Ait Elhaj, R.; Khaldi, M.; Rafik, S.; et al. Agro-morphological and biochemical responses of quinoa (Chenopodium quinoa Willd. var: ICBA-Q5) to organic amendments under various salinity conditions. Front. Plant Sci. 2023, 14, 1143170. [Google Scholar] [CrossRef]
- Kim, N.S.; Kwon, S.J.; Cuong, D.M.; Jeon, J.; Park, J.S.; Park, S.U. Accumulation of phenylpropanoids in tartary buckwheat (Fagopyrum tataricum) under salt Stress. Agronomy 2019, 9, 739. [Google Scholar] [CrossRef]
- Crizel, R.L.; Perin, E.C.; Siebeneichler, T.J.; Borowski, J.M.; Messias, R.S.; Rombaldi, C.V.; Galli, V. Abscisic acid and stress induced by salt: Effect on the phenylpropanoid, L-ascorbic acid and abscisic acid metabolism of strawberry fruits. Plant Physiol. Biochem. 2020, 152, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Le, L.Q.; Gong, X.X.; An, Q.; Xiang, D.B.; Zou, L.; Peng, L.X.; Wu, X.Y.; Tan, M.L.; Nie, Z.L.; Qi, W.Q.; et al. Quinoa sprouts as potential vegetable source: Nutrient composition and functional contents of different quinoa sprout varieties. Food Chem. 2021, 357, 129752. [Google Scholar] [CrossRef]
- Świeca, M.; Dziki, D. Improvement in sprouted wheat flour functionality: Effect of time, temperature and elicitation. Int. J. Food Sci. Technol. 2015, 50, 2135–2142. [Google Scholar] [CrossRef]
- Piao, M.Y.; Lee, H.J.; Yong, H.I.; Beak, S.H.; Kim, H.J.; Jo, C.; Baik, M. Comparison of reducing sugar content, sensory traits, and fatty acids and volatile compound profiles of the longissimus thoracis among Korean cattle, Holsteins, and Angus steers. Asian Austral. J. Anim. 2019, 32, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fountoulakis, M.; Lahm, H.W. Hydrolysis and amino acid composition analysis of proteins. J. Chromatogr. A 1998, 826, 109–134. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Ti, H.H.; Zhang, R.F.; Zhang, M.W.; Wei, Z.C.; Chi, J.W.; Deng, Y.Y.; Zhang, Y. Effect of extrusion on phytochemical profiles in milled fractions of black rice. Food Chem. 2015, 178, 186–194. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Assis, J.S.; Maldonado, R.; Muoz, T.; Escribano, M.I.; Merodio, C. Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit. Postharvest Biol. Technol. 2001, 23, 33–39. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, Y.; Bi, Y.; Li, C.; Deng, H.; Hu, L.; Dong, B. Effect of postharvest acibenzolar-S-methyl dipping on phenylpropanoid pathway metabolism in muskmelon (Cucumis melo L.) fruits. Sci. Hortic. 2014, 168, 113–119. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Wang, M.; Sun, M.M.; Gu, Z.X.; Yang, R.Q. GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress. Food Chem. 2019, 270, 593–601. [Google Scholar] [CrossRef]
- Jin, M.; Jiao, J.; Zhao, Q.; Ban, Q.; Gao, M.; Suo, J.; Zhu, Q.; Rao, J. Dose effect of exogenous abscisic acid on controlling lignification of postharvest kiwifruit (Actinidia chinensis cv. hongyang). Food Control 2021, 124, 107911. [Google Scholar] [CrossRef]
- Yang, K.J.; Zhang, Y.F.; Zhu, L.H.; Li, Z.T.; Deng, B.L. Omethoate treatment mitigates high salt stress inhibited maize seed germination. Pestic. Biochem. Physiol. 2018, 144, 79–82. [Google Scholar] [CrossRef]
- Xiao, F.; Zhou, H.P. Plant salt response: Perception, signaling, and tolerance. Front. Plant Sci. 2022, 13, 1053699. [Google Scholar] [CrossRef]
- He, H.; Zhou, W.; Lü, H.; Liang, B. Growth, leaf morphological and physiological adaptability of leaf beet (Beta vulgaris var. cicla) to salt stress: A soil culture experiment. Agronomy 2022, 12, 1393. [Google Scholar] [CrossRef]
- Luo, X.F.; Dai, Y.J.; Zheng, C.; Yang, Y.Z.; Chen, W.; Wang, Q.C.; Umashankar, C.; Du, J.B.; Liu, W.G.; Shu, K. The ABI4-RbohD/VTC2 regulatory module promotes Reactive Oxygen Species (ROS) accumulation to decrease seed germination under salinity stress. New Phytol. 2020, 229, 950–962. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Almeida, L.M.; Dinis, T.C.P. Dietary polyphenols: A novel strategy to modulate microbiota-gut-brain axis. Trends Food Sci. Technol. 2018, 78, 224–233. [Google Scholar] [CrossRef]
- Bhinder, S.; Singh, N.; Kaur, A. Impact of germination on nutraceutical, functional and gluten free muffin making properties of Tartary buckwheat (Fagopyrum tataricum). Food Hydrocoll. 2022, 124, 107268. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ulhassan, Z.; Qi, W.; Lu, H.; AbdElgawad, H.; Minkina, T.; Sushkova, S.; Rajput, V.D.; El-Keblawy, A.; Josko, I.; et al. Association of jasmonic acid priming with multiple defense mechanisms in wheat plants under high salt stress. Front. Plant Sci. 2022, 13, 886862. [Google Scholar] [CrossRef]
- Gan, R.Y.; Lui, W.Y.; Wu, K.; Chan, C.L.; Dai, S.H.; Sui, Z.Q.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Z.; Gao, Y.; Huang, X.; Zou, Y.; Yang, T. Effects of germination on the nutritional properties, phenolic profiles, and antioxidant activities of buckwheat. J. Food Sci. 2015, 80, 1111–1119. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Mota, T.R.; Salatta, F.V.; Sinzker, R.C.; Končitíková, R.; Kopečný, D.; dos Santos, W.D. Cell wall remodeling under salt stress: Insights into changes in polysaccharides, feruloylation, lignification, and phenolic metabolism in maize. Plant Cell Environ. 2020, 43, 2172–2191. [Google Scholar] [CrossRef]
- Ariel Carciochi, R.; Galván-D’Alessandro, L.; Vandendriessche, P.; Chollet, S. Effect of germination and fermentation process on the antioxidant compounds of quinoa seeds. Plant Foods Hum. Nutr. 2016, 71, 361–367. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Xia, H.; Ni, Z.Y.; Pan, D.M. Effects of exogenous melatonin on antioxidant capacity in Actinidia seedlings under salt stress. IOP Conf. Ser. Earth Environ. Sci. 2017, 94, 012024. [Google Scholar] [CrossRef]
- Rossatto, T.; do Amaral, M.N.; Benitez, L.C.; Vighi, I.L.; Braga, E.J.B.; de Magalhães Júnior, A.M.; Maia, M.A.C.; da Silva Pinto, L. Gene expression and activity of antioxidant enzymes in rice plants, cv. BRS AG, under saline stress. Physiol. Mol. Biol. Plants 2017, 23, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Farhangi-Abriz, S.; Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf. 2017, 137, 64–70. [Google Scholar] [CrossRef] [PubMed]




| Gene | Access No. | F(5′-3′) | R(5′-3′) | Length (bp) |
|---|---|---|---|---|
| PAL | XM_021904443.1 | TCGGTAGAGCTCGCTGAGT | AATACTCCAGCGTTCAAAAATCTTA | 188 |
| C4H | XM_021861074.1 | ATTGATCACATTCTGGAAGCACAAG | TAGCTCGTCCCTTAGCTTCCT | 188 |
| 4CL1 | XM_021868660.1 | AAGACCACAAATAATCTCACCCAA | TTCCCAGAATCAGAGTCAATCAA | 114 |
| 4CL3 | XM_021900806.1 | TCCAAGGTGGACGACTTAATCT | CTCGGTCTTCATCGGTAAAACTA | 140 |
| 4CL5 | XM_021896032.1 | TTGGCTATGTAGATGATGACGATG | TGCTGGTGGAACCTGGAAG | 89 |
| 4CL6 | XM_021909854.1 | CGGTGCTGCCCCTTTAACTA | TCAGTCATGCCATAACCCTGAA | 164 |
| CHS | XM_021906739.1 | AGTTTAAGCGCATGTGTGACAA | TCCCATGTAAGTACACATGTTAGGA | 125 |
| CHR | XM_021892157 | ACGAGCAATCCACCTTACAACT | AGTGCCTAAGCCGATGACG | 105 |
| CHI1 | XM_021900156.1 | ACCTCATGGATCTCTTACGATAGG | TACAGCCTCCGACAAGTTCT | 161 |
| CHI2 | XM_021882243.1 | AATGGAAGGGTAAATCAGGAAAG | CCACAGGTGCTGCGGTAAG | 75 |
| Actin-1 | XM_021904392.1 | ATGTTCCCTGGTATCGCTGA | TGATCTTCATGCTGCTGGGG | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, X.; Wang, Y.; Wu, J.; Lee, Y.-W.; Xu, J.; Yang, R. NaCl Stress Stimulates Phenolics Biosynthesis and Antioxidant System Enhancement of Quinoa Germinated after Magnetic Field Pretreatment. Foods 2024, 13, 3278. https://doi.org/10.3390/foods13203278
Wang S, Zhang X, Wang Y, Wu J, Lee Y-W, Xu J, Yang R. NaCl Stress Stimulates Phenolics Biosynthesis and Antioxidant System Enhancement of Quinoa Germinated after Magnetic Field Pretreatment. Foods. 2024; 13(20):3278. https://doi.org/10.3390/foods13203278
Chicago/Turabian StyleWang, Shufang, Xuejiao Zhang, Yiting Wang, Jirong Wu, Yin-Won Lee, Jianhong Xu, and Runqiang Yang. 2024. "NaCl Stress Stimulates Phenolics Biosynthesis and Antioxidant System Enhancement of Quinoa Germinated after Magnetic Field Pretreatment" Foods 13, no. 20: 3278. https://doi.org/10.3390/foods13203278
APA StyleWang, S., Zhang, X., Wang, Y., Wu, J., Lee, Y.-W., Xu, J., & Yang, R. (2024). NaCl Stress Stimulates Phenolics Biosynthesis and Antioxidant System Enhancement of Quinoa Germinated after Magnetic Field Pretreatment. Foods, 13(20), 3278. https://doi.org/10.3390/foods13203278







