A Critical Analysis of the Opportunities and Challenges of Phage Application in Seafood Quality Control
Abstract
:1. Introduction
2. Overview of Phage
3. Phage Production
3.1. Phage Isolation
3.2. Phage Cultivation
3.3. Phage Purification
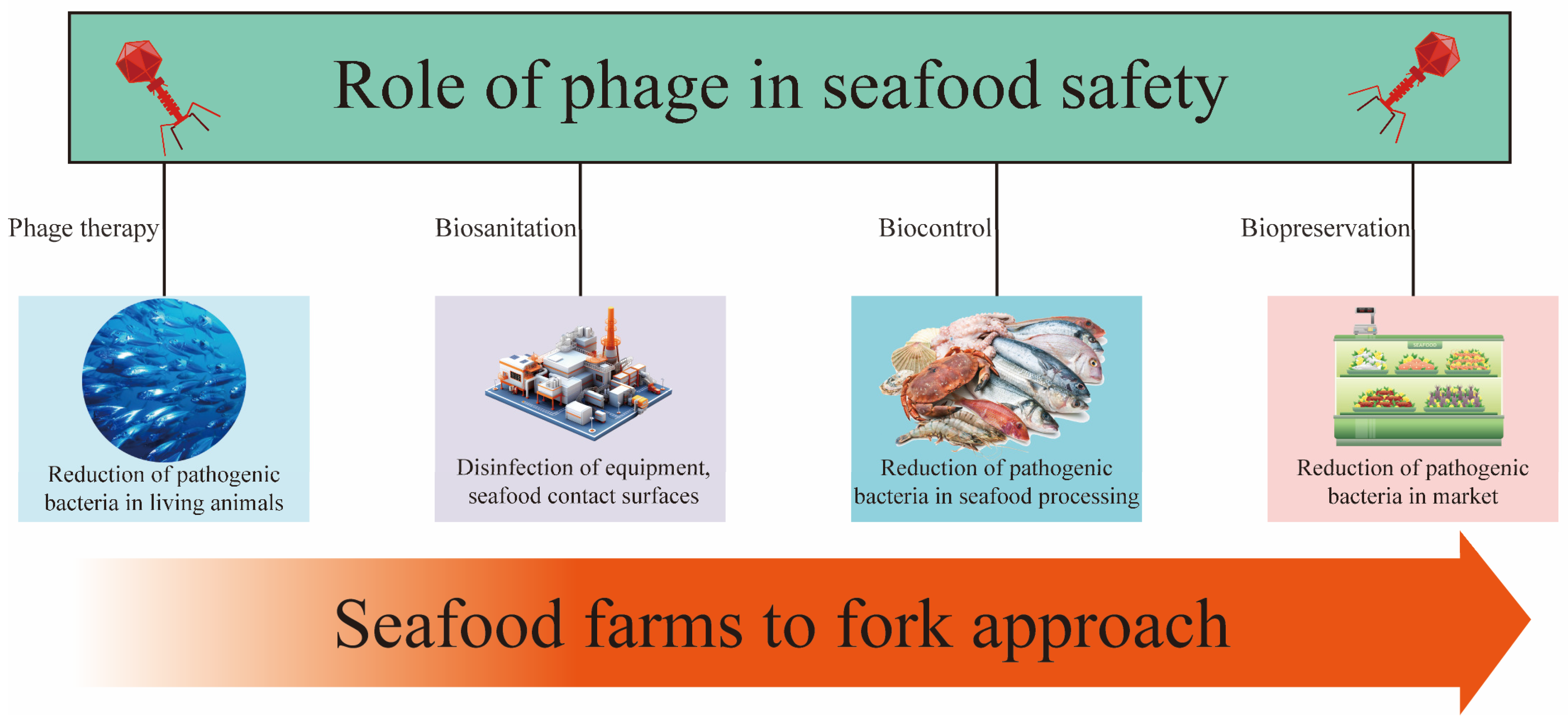
4. Applications of Phage in Seafood
4.1. Biocontrol
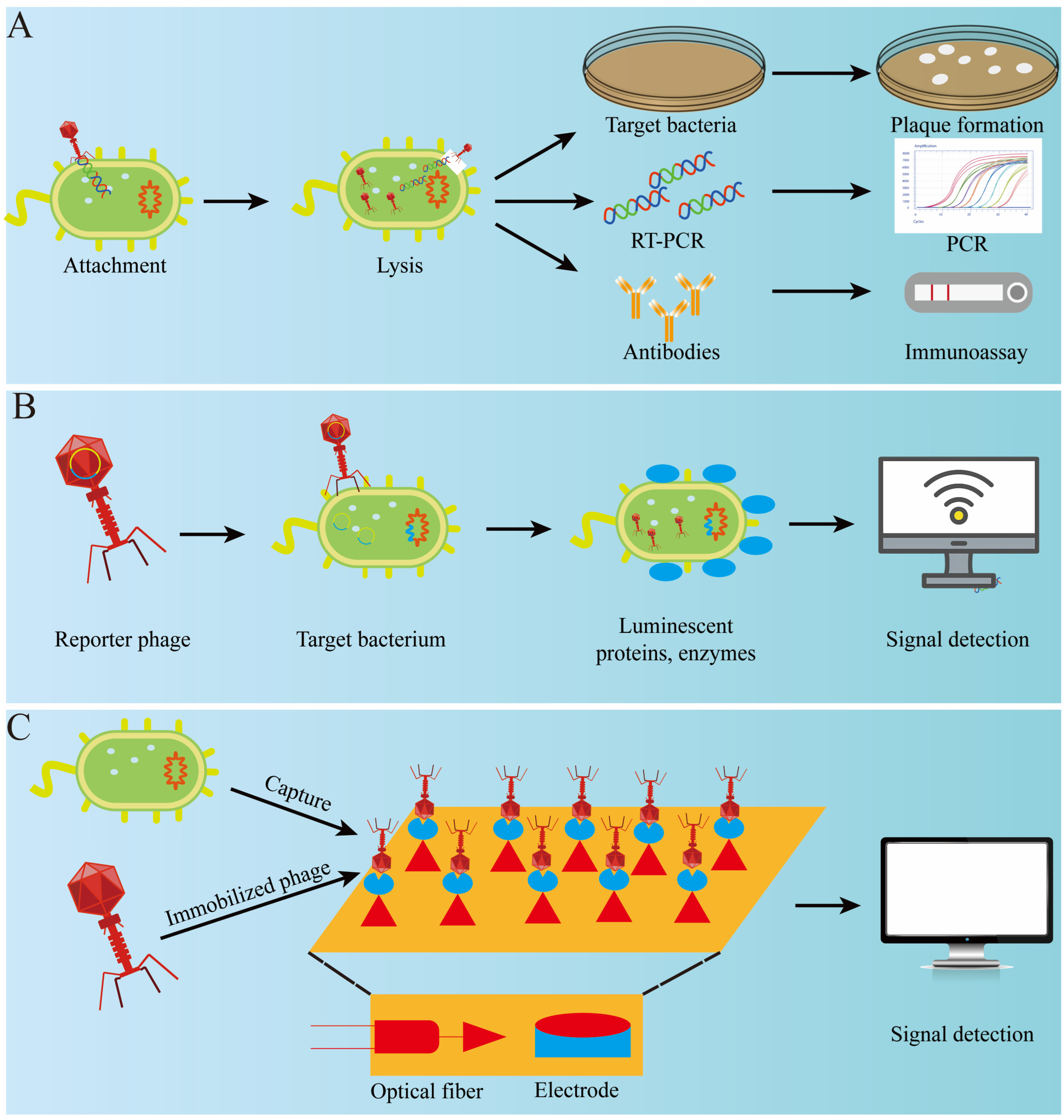
4.2. Detection
4.2.1. Phage Amplification Detection
4.2.2. Phage-Based Immunological Detection
4.2.3. Reporter Phage Detection
4.2.4. Phage-Based Optical Detection
4.2.5. Phage-Based Biosensor Detection
5. Limitations of Phage Application
5.1. Application Stability
5.2. Complete Application Security
5.3. Public Acceptance
5.4. Resistance to Bacteriophage
6. Outlook of Phage Applications
6.1. Phage Cocktail
6.2. Designing New Phages
6.3. Bacteriophages Encapsulation
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Zhao, Z.; Wang, X.; Xie, J. Hydrogen sulfide in seafood: Formation, hazards, and control. Trends Food Sci. Technol. 2024, 148, 104512. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Z.; Feng, L.; Qu, D.; Zhu, J. Identification of microbial communities and multi-species biofilms contamination in seafood processing environments with different hygiene conditions. Food Microbiol. 2024, 122, 104553. [Google Scholar] [CrossRef] [PubMed]
- Littman, R.A.; Fiorenza, E.A.; Wenger, A.S.; Berry, K.L.; van de Water, J.A.; Nguyen, L.; Aung, S.T.; Parker, D.M.; Rader, D.N.; Harvell, C.D.; et al. Coastal urbanization influences human pathogens and microdebris contamination in seafood. Sci. Total Environ. 2020, 736, 139081. [Google Scholar] [CrossRef] [PubMed]
- Sireesha, T.; Gowda, N.A.N.; Kambhampati, V. Ultrasonication in seafood processing and preservation: A comprehensive review. Appl. Food Res. 2022, 2, 100208. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Shiekh, K.A.; Benjakul, S. Pros and cons of cold plasma technology as an alternative non-thermal processing technology in seafood industry. Trends Food Sci. Technol. 2021, 111, 617–627. [Google Scholar] [CrossRef]
- Rode, T.M.; Rotabakk, B.T. Extending shelf life of desalted cod by high pressure processing. Innov. Food Sci. Emerg. Technol. 2021, 69, 102476. [Google Scholar] [CrossRef]
- Aponte, M.; Anastasio, A.; Marrone, R.; Mercogliano, R.; Peruzy, M.F.; Murru, N. Impact of gaseous ozone coupled to passive refrigeration system to maximize shelf-life and quality of four different fresh fish products. LWT 2018, 93, 412–419. [Google Scholar] [CrossRef]
- Vikram, A.; Callahan, M.T.; Woolston, J.W.; Sharma, M.; Sulakvelidze, A. Phage biocontrol for reducing bacterial foodborne pathogens in produce and other foods. Curr. Opin. Biotechnol. 2022, 78, 102805. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, M.; Kim, B.S. Phage biocontrol of zoonotic food-borne pathogen Vibrio parahaemolyticus for seafood safety. Food Control 2023, 144, 109334. [Google Scholar] [CrossRef]
- Salmond, G.P.; Fineran, P.C. A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef]
- Nikolich, M.P.; Filippov, A.A. Bacteriophage therapy: Developments and directions. Antibiotics 2020, 9, 135. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Bao, H. Phage inactivation of foodborne Shigella on ready-to-eat spiced chicken. Poult. Sci. 2013, 92, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Guglielmotti, D.M.; Mercanti, D.J.; Reinheimer, J.A.; Quiberoni, A.D.L. Efficiency of physical and chemical treatments on the inactivation of dairy bacteriophages. Front. Microbiol. 2012, 2, 282. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host receptors for bacteriophage adsorption. FEMS Microbiol. Lett. 2016, 363, fnw002. [Google Scholar] [CrossRef] [PubMed]
- Rakhuba, D.; Kolomiets, E.; Dey, E.S.; Novik, G. Bacteriophage receptors, mechanisms of phage adsorption and penetration into host cell. Pol. J. Microbiol. 2010, 59, 145. [Google Scholar] [CrossRef]
- Dennehy, J.J.; Abedon, S.T. Adsorption: Phage acquisition of bacteria. In Bacteriophages: Biology, Technology, Therapy; Springer: Berlin/Heidelberg, Germany, 2021; pp. 93–117. [Google Scholar]
- Hu, B.; Margolin, W.; Molineux, I.J.; Liu, J. Structural remodeling of bacteriophage T4 and host membranes during infection initiation. Proc. Natl. Acad. Sci. USA 2015, 112, E4919–E4928. [Google Scholar] [CrossRef]
- Cahill, J.; Young, R. Phage lysis: Multiple genes for multiple barriers. Adv. Virus Res. 2019, 103, 33–70. [Google Scholar]
- Gill, J.J.; Hyman, P. Phage choice, isolation, and preparation for phage therapy. Curr. Pharm. Biotechnol. 2010, 11, 2–14. [Google Scholar] [CrossRef]
- Hyman, P. Phages for phage therapy: Isolation, characterization, and host range breadth. Pharmaceuticals 2019, 12, 35. [Google Scholar] [CrossRef]
- Agboluaje, M.; Sauvageau, D. Bacteriophage production in bioreactors. In Bacteriophage Therapy: From Lab to Clinical Practice; Humana Press: New York, NY, USA, 2018; pp. 173–193. [Google Scholar]
- Nabergoj, D.; Modic, P.; Podgornik, A. Effect of bacterial growth rate on bacteriophage population growth rate. Microbiologyopen 2018, 7, e00558. [Google Scholar] [CrossRef]
- Jurač, K.; Nabergoj, D.; Podgornik, A. Bacteriophage production processes. Appl. Microbiol. Biotechnol. 2019, 103, 685–694. [Google Scholar] [CrossRef]
- João, J.; Lampreia, J.; Prazeres, D.M.F.; Azevedo, A.M. Manufacturing of bacteriophages for therapeutic applications. Biotechnol. Adv. 2021, 49, 107758. [Google Scholar] [CrossRef] [PubMed]
- Lunan, K.D.; Sinsheimer, R.L. A study of the nucleic acid of bacteriophage T7. Virology 1956, 2, 455–462. [Google Scholar] [CrossRef]
- Tanir, T.; Orellana, M.; Escalante, A.; de Souza, C.M.; Koeris, M.S. Manufacturing bacteriophages (Part 1 of 2): Cell line development, upstream, and downstream considerations. Pharmaceuticals 2021, 14, 934. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, F.; Shi, J.; Malik, D.J. High Throughput Manufacturing of Bacteriophages Using Continuous Stirred Tank Bioreactors Connected in Series to Ensure Optimum Host Bacteria Physiology for Phage Production. Viruses 2018, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Branston, S.; Stanley, E.; Keshavarz-Moore, E.; Ward, J. Precipitation of filamentous bacteriophages for their selective recovery in primary purification. Biotechnol. Prog. 2012, 28, 129–136. [Google Scholar] [CrossRef]
- Dong, D.; Sutaria, S.; Hwangbo, J.Y.; Chen, P. A simple and rapid method to isolate purer M13 phage by isoelectric precipitation. Appl. Microbiol. Biotechnol. 2013, 97, 8023–8029. [Google Scholar] [CrossRef]
- Monjezi, R.; Tey, B.T.; Sieo, C.C.; Tan, W.S. Purification of bacteriophage M13 by anion exchange chromatography. J. Chromatogr. B 2010, 878, 1855–1859. [Google Scholar] [CrossRef]
- Zakharova, M.Y.; Kozyr, A.V.; Ignatova, A.N.; Vinnikov, I.A.; Shemyakin, I.G.; Kolesnikov, A.V. Purification of filamentous bacteriophage for phage display using size-exclusion chromatography. Biotechniques 2005, 38, 194–198. [Google Scholar] [CrossRef]
- Castro-Mejía, J.L.; Muhammed, M.K.; Kot, W.; Neve, H.; Franz, C.M.A.P.; Hansen, L.H.; Vogensen, F.K.; Nielsen, D.S. Optimizing protocols for extraction of bacteriophages prior to metagenomic analyses of phage communities in the human gut. Microbiome 2015, 3, 64. [Google Scholar] [CrossRef]
- González-Mora, A.; Ruiz-Ruiz, F.; Benavides, J.; Willson, R.C.; Rito-Palomares, M. Recovery and primary purification of bacteriophage M13 using aqueous two-phase systems. J. Chem. Technol. Biotechnol. 2017, 92, 2808–2816. [Google Scholar] [CrossRef]
- Clavijo, V.; Torres-Acosta, M.A.; Vives-Flórez, M.J.; Rito-Palomares, M. Aqueous two-phase systems for the recovery and purification of phage therapy products: Recovery of salmonella bacteriophage ϕSan23 as a case study. Sep. Purif. Technol. 2019, 211, 322–329. [Google Scholar] [CrossRef]
- Hsu, T.-K.; Shih, H.-Y.; Huang, H.-J.; Hsu, J.C.-K.; Wang, H.-C.; Chen, Y.-Y.; Chen, L.-L. Isolation and characterization of the novel phage BP14 for lysing Vibrio parahaemolyticus and reducing virulence proteins. Aquaculture 2024, 581, 740484. [Google Scholar] [CrossRef]
- You, H.J.; Lee, J.H.; Oh, M.; Hong, S.Y.; Kim, D.; Noh, J.; Kim, M.; Kim, B.S. Tackling Vibrio parahaemolyticus in ready-to-eat raw fish flesh slices using lytic phage VPT02 isolated from market oyster. Food Res. Int. 2021, 150, 110779. [Google Scholar] [CrossRef]
- Ren, H.; Li, Z.; Xu, Y.; Wang, L.; Li, X. Protective effectiveness of feeding phage cocktails in controlling Vibrio parahaemolyticus infection of sea cucumber Apostichopus japonicus. Aquaculture 2019, 503, 322–329. [Google Scholar] [CrossRef]
- Kim, B.H.; Ashrafudoulla; Shaila, S.; Park, H.J.; Sul, J.D.; Park, S.H.; Ha, S.-D. Isolation, characterization, and application of bacteriophage on Vibrio parahaemolyticus biofilm to control seafood contamination. Int. J. Antimicrob. Agents 2024, 64, 107194. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.T.; Kim, H.B.; Choi, S.H.; Lee, J.H. Characterization of bacteriophage VVP001 and its application for the inhibition of Vibrio vulnificus causing seafood-borne diseases. Food Microbiol. 2021, 94, 103630. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Cong, C.; Wang, L.; Li, X.; Li, J.; Yang, H.; Li, S.; Xu, Y. Protective effectiveness of feeding phage cocktails in controlling Vibrio harveyi infection of turbot Scophthalmus maximus. Aquaculture 2021, 535, 736390. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, M.; Wang, Y.; Yang, Z.; Ye, M.; Wu, L.; Bao, H.; Pang, M.; Zhou, Y.; Wang, R.; et al. Broad host range phage vB-LmoM-SH3-3 reduces the risk of Listeria contamination in two types of ready-to-eat food. Food Control 2020, 108, 106830. [Google Scholar] [CrossRef]
- Axelsson, L.; Bjerke, G.A.; McLeod, A.; Berget, I.; Holck, A.L. Growth behavior of Listeria monocytogenes in a traditional Norwegian fermented fish product (Rakfisk), and its inhibition through bacteriophage addition. Foods 2020, 9, 119. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, Y.; Wang, L.; Yao, L.; Li, F.; Zhai, Y.; Zhang, Y. Biocontrol of Salmonella Typhimurium in Raw Salmon Fillets and Scallop Adductors by Using Bacteriophage SLMP1. J. Food Prot. 2018, 81, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Moreirinha, C.; Lewicka, M.; Almeida, P.; Clemente, C.; Cunha, Â.; Delgadillo, I.; Romalde, J.L.; Nunes, M.L.; Almeida, A. Bacteriophages with potential to inactivate Salmonella Typhimurium: Use of single phage suspensions and phage cocktails. Virus Res. 2016, 220, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Amal, M.N.A.; Yasin, I.S.M.; Saad, M.Z.; Nasruddin, N.S.; Al-Saari, N.; Mino, S.; Sawabe, T. Vibriosis in cultured marine fishes: A review. Aquaculture 2019, 512, 734289. [Google Scholar] [CrossRef]
- Molina-Quiroz, R.C.; Silva-Valenzuela, C.A. Interactions of Vibrio phages and their hosts in aquatic environments. Curr. Opin. Microbiol. 2023, 74, 102308. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, D.; Li, D.; Wang, H.; Zhao, Y.; Sun, X. The prevalence, virulence, and antibiotic resistance of Vibrio parahemolyticus in aquatic products and their breeding environment in Shanghai. Food Ferment. Ind. 2023, 49, 250–257. [Google Scholar]
- Nikapitiya, C.; Chandrarathna, H.; Dananjaya, S.; De Zoysa, M.; Lee, J. Isolation and characterization of phage (ETP-1) specific to multidrug resistant pathogenic Edwardsiella tarda and its in vivo biocontrol efficacy in zebrafish (Danio rerio). Biologicals 2020, 63, 14–23. [Google Scholar] [CrossRef]
- Chen, L.; Fan, J.; Yan, T.; Liu, Q.; Yuan, S.; Zhang, H.; Yang, J.; Deng, D.; Huang, S.; Ma, Y. Isolation and characterization of specific phages to prepare a cocktail preventing Vibrio sp. Va-F3 infections in shrimp (Litopenaeus vannamei). Front. Microbiol. 2019, 10, 2337. [Google Scholar] [CrossRef]
- Ahmadi, H.; Anany, H.; Walkling-Ribeiro, M.; Griffiths, M.W. Biocontrol of Shigella flexneri in Ground Beef and Vibrio cholerae in Seafood with Bacteriophage-Assisted High Hydrostatic Pressure (HHP) Treatment. Food Bioprocess Technol. 2015, 8, 1160–1167. [Google Scholar] [CrossRef]
- Pelon, W.; Luftig, R.B.; Johnston, K.H. Vibrio vulnificus Load Reduction in Oysters after Combined Exposure to Vibrio vulnificus–Specific Bacteriophage and to an Oyster Extract Component. J. Food Prot. 2005, 68, 1188–1191. [Google Scholar] [CrossRef]
- Gandhi, M.; Chikindas, M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef]
- Baños, A.; García-López, J.D.; Núñez, C.; Martínez-Bueno, M.; Maqueda, M.; Valdivia, E. Biocontrol of Listeria monocytogenes in fish by enterocin AS-48 and Listeria lytic bacteriophage P100. LWT—Food Sci. Technol. 2016, 66, 672–677. [Google Scholar] [CrossRef]
- Gündüz, H.; Öztürk, F. Prevalence of Listeria spp. in seafood samples and control of Listeria monocytogenes with Using LISTEX™ P100 bacteriophage applications in smoked rainbow trout. J. Agric. Sci. 2021, 27, 493–499. [Google Scholar] [CrossRef]
- Pereira, C.; Moreirinha, C.; Rocha, R.J.; Calado, R.; Romalde, J.L.; Nunes, M.L.; Almeida, A. Application of bacteriophages during depuration reduces the load of Salmonella Typhimurium in cockles. Food Res. Int. 2016, 90, 73–84. [Google Scholar] [CrossRef]
- Yang, Z.-Q.; Tao, X.-Y.; Zhang, H.; Rao, S.-Q.; Gao, L.; Pan, Z.-M.; Jiao, X.-A. Isolation and characterization of virulent phages infecting Shewanella baltica and Shewanella putrefaciens, and their application for biopreservation of chilled channel catfish (Ictalurus punctatus). Int. J. Food Microbiol. 2019, 292, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.H. Properties of Bacteriophage of Shewanella Putrefaciens and Its Application on Fresh Keeping Effects; Ocean University of China: Qingdao, China, 2012. [Google Scholar]
- Masák, J.; Čejková, A.; Schreiberová, O.; Řezanka, T. Pseudomonas biofilms: Possibilities of their control. FEMS Microbiol. Ecol. 2014, 89, 1–14. [Google Scholar] [CrossRef]
- Chu, L.L. Properties of Bacteriophage of Pseudomonas and Its Application on Spoilage Control; Wuhan University of Light Industry: Wuhan, China, 2018. [Google Scholar] [CrossRef]
- Pang, Z.Q.; Li, M.; Li, L.; Shen, Z.H. Isolation of Bacteriophages from Specific Spoilage Bacteria in Refrigerated Crisp Grass Carp and Its Investigation on Inhibition of Spoilage. In Abstract Collection of the 5th National Symposium on Microbial Resources and the Operational Services of the National Microbial Resource Platform; Chinese Academy of Agricultural Sciences: Beijing, China, 2013. [Google Scholar]
- Ding, Y.; Huang, C.; Chen, M.; Wang, J.; Shao, Y.; Wang, X. Rapid and simultaneous detection of viable S. aureus and its penicillin susceptibility by phage amplification techniques in different food matrices. LWT 2023, 176, 114526. [Google Scholar] [CrossRef]
- Stambach, N.R.; Carr, S.A.; Cox, C.R.; Voorhees, K.J. Rapid detection of Listeria by bacteriophage amplification and SERS-lateral flow immunochromatography. Viruses 2015, 7, 6631–6641. [Google Scholar] [CrossRef]
- Meile, S.; Kilcher, S.; Loessner, M.J.; Dunne, M. Reporter phage-based detection of bacterial pathogens: Design guidelines and recent developments. Viruses 2020, 12, 944. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Kim, S.; Ryu, S. Sensitive detection of viable Escherichia coli O157:H7 from foods using a luciferase-reporter phage phiV10lux. Int. J. Food Microbiol. 2017, 254, 11–17. [Google Scholar] [CrossRef]
- Miyanaga, K.; Hijikata, T.; Furukawa, C.; Unno, H.; Tanji, Y. Detection of Escherichia coli in the sewage influent by fluorescent labeled T4 phage. Biochem. Eng. J. 2006, 29, 119–124. [Google Scholar] [CrossRef]
- Roach, D.R.; Donovan, D.M. Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage 2015, 5, e1062590. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, L. Template Reporter Bacteriophage Platform and Multiple Bacterial Detection Assays Based Thereon. U.S. Patent 7,244,612, 17 July 2007. [Google Scholar]
- Zhou, Y.; Li, Z.; Huang, J.; Wu, Y.; Mao, X.; Tan, Y.; Liu, H.; Ma, D.; Li, X.; Wang, X. Development of a phage-based electrochemical biosensor for detection of Escherichia coli O157:H7 GXEC-N07. Bioelectrochemistry 2023, 150, 108345. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Wang, Y.; Wu, J.; Wang, X.; Xue, F.; Ren, J.; Dai, J.; Tang, F. Sterilizing effect of phage cocktail against Shiga toxin-producing Escherichia coli O157:H7 in foods. Food Biosci. 2023, 51, 102282. [Google Scholar] [CrossRef]
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage therapy for antibiotic-resistant bacterial infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Xu, Y.; Chen, Y.; Zhang, W.; Liu, T.; Chen, G.; Wang, K. Phage-based delivery systems: Engineering, applications, and challenges in nanomedicines. J. Nanobiotechnology 2024, 22, 365. [Google Scholar] [CrossRef]
- Hibstu, Z.; Belew, H.; Akelew, Y.; Mengist, H.M. Phage therapy: A different approach to fight bacterial infections. Biol. Targets Ther. 2022, ume 16, 173–186. [Google Scholar] [CrossRef]
- Stacey, H.J.; De Soir, S.; Jones, J.D.J.A. The safety and efficacy of phage therapy: A systematic review of clinical and safety trials. Antibiotics 2022, 11, 1340. [Google Scholar] [CrossRef]
- North, O.I.; Brown, E.D. Phage–antibiotic combinations: A promising approach to constrain resistance evolution in bacteria. Ann. New York Acad. Sci. 2021, 1496, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Meile, S.; Du, J.; Dunne, M.; Kilcher, S.; Loessner, M.J. Engineering therapeutic phages for enhanced antibacterial efficacy. Curr. Opin. Virol. 2022, 52, 182–191. [Google Scholar] [CrossRef]
- Hassan, A.Y.; Lin, J.T.; Ricker, N.; Anany, H. The age of phage: Friend or foe in the new dawn of therapeutic and biocontrol applications? Pharmaceuticals 2021, 14, 199. [Google Scholar] [CrossRef]
- Khan, A.; Rao, T.S.; Joshi, H.M. Phage therapy in the COVID-19 era: Advantages over antibiotics. Curr. Res. Microb. Sci. 2022, 3, 100115. [Google Scholar] [CrossRef] [PubMed]
- Ssekatawa, K.; Byarugaba, D.K.; Kato, C.D.; Wampande, E.M.; Ejobi, F.; Tweyongyere, R.; Nakavuma, J.L. A review of phage mediated antibacterial applications. Alex. J. Med. 2021, 57, 1–20. [Google Scholar] [CrossRef]
- Melo, L.D.; Oliveira, H.; Pires, D.P.; Dabrowska, K.; Azeredo, J. Phage therapy efficacy: A review of the last 10 years of preclinical studies. Crit. Rev. Microbiol. 2020, 46, 78–99. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.S.; Ibrahim, S.; Zaharinie, T.; Tang, S.S. Bacteriophage encapsulation—Trends and potential applications in aquaculture. Aquaculture 2024, 594, 741398. [Google Scholar] [CrossRef]
- García-Anaya, M.C.; Sepúlveda, D.R.; Rios-Velasco, C.; Zamudio-Flores, P.B.; Sáenz-Mendoza, A.I.; Acosta-Muñiz, C.H. The role of food compounds and emerging technologies on phage stability. Innov. Food Sci. Emerg. Technol. 2020, 64, 102436. [Google Scholar] [CrossRef]
- Olson, M.R.; Axler, R.P.; Hicks, R.E. Effects of freezing and storage temperature on MS2 viability. J. Virol. Methods 2004, 122, 147–152. [Google Scholar] [CrossRef]
- Tey, B.T.; Ooi, S.T.; Yong, K.C.; Ng, M.Y.; Ling, T.C.; Tan, W.S. Production of fusion m13 phage bearing the di-sulphide constrained peptide sequence (C-WSFFSNI-C) that interacts with hepatitis B core antigen. Afr. J. Biotechnol. 2009, 8, 268. [Google Scholar]
- Duarte, J.; Pereira, C.; Moreirinha, C.; Salvio, R.; Lopes, A.; Wang, D.; Almeida, A. New insights on phage efficacy to control Aeromonas salmonicida in aquaculture systems: An in vitro preliminary study. Aquaculture 2018, 495, 970–982. [Google Scholar] [CrossRef]
- Richards, K.; Malik, D.J. Bacteriophage encapsulation in pH-responsive core-shell capsules as an animal feed additive. Viruses 2021, 13, 1131. [Google Scholar] [CrossRef]
- Colavecchio, A.; Cadieux, B.; Lo, A.; Goodridge, L.D. Bacteriophages contribute to the spread of antibiotic resistance genes among foodborne pathogens of the Enterobacteriaceae family—A review. Front. Microbiol. 2017, 8, 1108. [Google Scholar] [CrossRef]
- Kenzaka, T.; Tani, K.; Sakotani, A.; Yamaguchi, N.; Nasu, M. High-frequency phage-mediated gene transfer among Escherichia coli cells, determined at the single-cell level. Appl. Environ. Microbiol. 2007, 73, 3291–3299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; LeJeune, J.T. Transduction of blaCMY-2, tet (A), and tet (B) from Salmonella enterica subspecies enterica serovar Heidelberg to S. Typhimurium. Vet. Microbiol. 2008, 129, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Enault, F.; Briet, A.; Bouteille, L.; Roux, S.; Sullivan, M.B.; Petit, M.-A. Phages rarely encode antibiotic resistance genes: A cautionary tale for virome analyses. ISME J. 2017, 11, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage applications for food production and processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef]
- Górski, A.; Weber-Dabrowska, B. The potential role of endogenous bacteriophages in controlling invading pathogens. Cell. Mol. Life Sci. 2005, 62, 511–519. [Google Scholar] [CrossRef]
- McCallin, S.; Alam Sarker, S.; Barretto, C.; Sultana, S.; Berger, B.; Huq, S.; Krause, L.; Bibiloni, R.; Schmitt, B.; Reuteler, G.; et al. Safety analysis of a Russian phage cocktail: From metagenomic analysis to oral application in healthy human subjects. Virology 2013, 443, 187–196. [Google Scholar] [CrossRef]
- Carlton, R.; Noordman, W.H.; Biswas, B.; de Meester, E.D.; Loessner, M.J. Bacteriophage P100 for control of Listeria monocytogenes in foods: Genome sequence, bioinformatic analyses, oral toxicity study, and application. Regul. Toxicol. Pharmacol. 2005, 43, 301–312. [Google Scholar] [CrossRef]
- Georjon, H.; Bernheim, A. The highly diverse antiphage defence systems of bacteria. Nat. Rev. Microbiol. 2023, 21, 686–700. [Google Scholar] [CrossRef]
- Murtazalieva, K.; Mu, A.; Petrovskaya, A.; Finn, R.D. The growing repertoire of phage anti-defence systems. Trends Microbiol. 2024. [Google Scholar] [CrossRef]
- Srikant, S.; Guegler, C.K.; Laub, M.T. The evolution of a counter-defense mechanism in a virus constrains its host range. eLife 2022, 11, e79549. [Google Scholar] [CrossRef]
- Cui, H.; Xu, Y.; Cong, C.; Li, C.; Li, X.; Li, S.; Li, J.; Wang, L. Evaluation of the preventive effect of phage cocktails on turbot ascites and its influence on main physiological indicators. Aquaculture 2022, 547, 737539. [Google Scholar] [CrossRef]
- Dunne, M.; Rupf, B.; Tala, M.; Qabrati, X.; Ernst, P.; Shen, Y.; Sumrall, E.; Heeb, L.; Plückthun, A.; Loessner, M.J.; et al. Reprogramming Bacteriophage Host Range through Structure-Guided Design of Chimeric Receptor Binding Proteins. Cell Rep. 2019, 29, 1336–1350.e4. [Google Scholar] [CrossRef] [PubMed]
- Kilcher, S.; Loessner, M.J. Engineering Bacteriophages as Versatile Biologics. Trends Microbiol. 2019, 27, 355–367. [Google Scholar] [CrossRef]
- Choińska-Pulit, A.; Mituła, P.; Śliwka, P.; Łaba, W.; Skaradzińska, A. Bacteriophage encapsulation: Trends and potential applications. Trends Food Sci. Technol. 2015, 45, 212–221. [Google Scholar] [CrossRef]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef]
- Silva Batalha, L.; Gontijo, M.T.P.; Teixeira, A.V.N.d.C.; Boggione, D.M.G.; Lopez, M.E.S.; Eller, M.R.; Mendonça, R.C.S. Encapsulation in alginate-polymers improves stability and allows controlled release of the UFV-AREG1 bacteriophage. Food Res. Int. 2021, 139, 109947. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.-S.; Han, J. Maltodextrin-trehalose miscible system-based bacteriophage encapsulation: Studies of plasticizing effect on encapsulated phage activity and food application as an antimicrobial agent. Food Control 2023, 146, 109550. [Google Scholar] [CrossRef]

| Target Organism | Phages | Seafood | Temp | Results and Observation | Reference |
|---|---|---|---|---|---|
| Vibrio parahaemolyticus | BP14 | Shrimp | 25 °C | A bacteriophage strain BP14, suitable for use in aquaculture, was isolated. | [35] |
| VPT02 | Brine shrimp, RTE raw fish flesh slices | 5 °C or 25 °C | The survival rate of brine shrimp increased from 16.7% to 46.7%, and the number of V. parahaemolyticus in RTE raw fish flesh slices was reduced by up to 3.9 log. | [36] | |
| PVP1 and PVP2 | Sea cucumbers | 18–20 °C | Phage feeding treatment increased the survival of sea cucumbers infected with V. parahaemolyticus VP-ABTNL, and there is no effect on the normal growth of sea cucumbers. | [37] | |
| CAU_VPP01 | Squid and mackerel | 4 °C | At MOI 10, phage CAU_VPP01 effectively reduced biofilm proliferation and biomass volume, thickness, and roughness at 4 °C. | [38] | |
| VPG01 | Paralichthys olivaceus | 25 °C | When a cutting board and a seafood item were treated with VPG01, the pathogen load was significantly decreased. | [9] | |
| Vibrio vulnificus | VVP001 | Abalone | 4 °C | VVP001 steadily inhibited V. vulnificus MO6-24/O up to 8 h. | [39] |
| Vibrio harveyi | VB_VhaP_Vh-5 and VB_VhaP_Vh-8 | turbot Scophthalmus maximus | 18 °C | Phage feeding could improve the survival rate of turbot infected by V. harveyi VH5 and had no effect on the normal growth of turbot. | [40] |
| Listeria monocytogenes | vB-LmoM-SH3-3 | Raw salmon | 4 °C | The application of phage SH3-3 in ready-to-eat salmon may reduce Listeria counts by 4.54 log within 72 h. | [41] |
| Listex™ P100 | Rakfisk | 7 °C or 8 °C | An average of 0.9 log reduction was observed throughout the fermentation period. | [42] | |
| Salmonella | SLMP1 | Raw Salmon Fillets and Scallop | 4 °C, 15 °C or 25 °C | The Salmonella counts of both inoculum levels on samples could be reduced below the detection limit or maintained at a low level by phage SLMP1 during storage at 4 °C. | [43] |
| phSE-2 and phSE-5 | Cockle | 16 °C | The application of single phage suspensions of phSE-2 and phSE-5 and phage cocktail phSE-2/phSE-5 can be successfully employed to inactivate Salmonella spp. in cockles during depuration. | [44] |
| Problem | Limitations | Solutions | Reference |
|---|---|---|---|
| Public acceptance | The use of phages is still affected by ignorance, fear, and doubts. | To improve the dissemination of knowledge about phages. | [70] |
| Stability | Most of the phages tend to lose their stability at cold storage. | Phage can be encapsulated using natural materials to maintain phage stability. | [71] |
| Safety | Phages can carry and transmit drug resistance genes. | Ensure that a virulent phage is used before application. | [72,73] |
| Resistance | Bacteria may rapidly develop resistance (even when in cocktails). | Continuous isolation and purification of novel, safe, and highly virulent phages. | [74,75,76] |
| Efficacy | Need of high multiplicity of infection (MOI) due to lower burst size of the phage. | Phage can be coupled with other bactericidal technologies. | [77,78,79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Guo, Z.; Xie, J. A Critical Analysis of the Opportunities and Challenges of Phage Application in Seafood Quality Control. Foods 2024, 13, 3282. https://doi.org/10.3390/foods13203282
Yan J, Guo Z, Xie J. A Critical Analysis of the Opportunities and Challenges of Phage Application in Seafood Quality Control. Foods. 2024; 13(20):3282. https://doi.org/10.3390/foods13203282
Chicago/Turabian StyleYan, Jun, Zhenghao Guo, and Jing Xie. 2024. "A Critical Analysis of the Opportunities and Challenges of Phage Application in Seafood Quality Control" Foods 13, no. 20: 3282. https://doi.org/10.3390/foods13203282
APA StyleYan, J., Guo, Z., & Xie, J. (2024). A Critical Analysis of the Opportunities and Challenges of Phage Application in Seafood Quality Control. Foods, 13(20), 3282. https://doi.org/10.3390/foods13203282







