Investigating the Antibacterial Effect of a Novel Gallic Acid-Based Green Sanitizer Formulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Preparation
2.2. Chemical Stock Preparation
2.3. Culturability Assay
2.4. Bacterial Cell Viability Assays
2.4.1. ATP Quantification
2.4.2. Membrane Integrity Assay
2.4.3. Calibration of Viability Assays
2.4.4. Cell Viability Analysis: Testing the Antimicrobial Formulations
2.4.5. Cell Viability Assessment by Flow Cytometry
2.4.6. Bacterial Viability Assay with Propidium Monoazide qPCR (PMAxx-vqPCR)
2.5. Estimation of ROS Production in Abiotic System
2.6. Statistical Analysis
3. Results
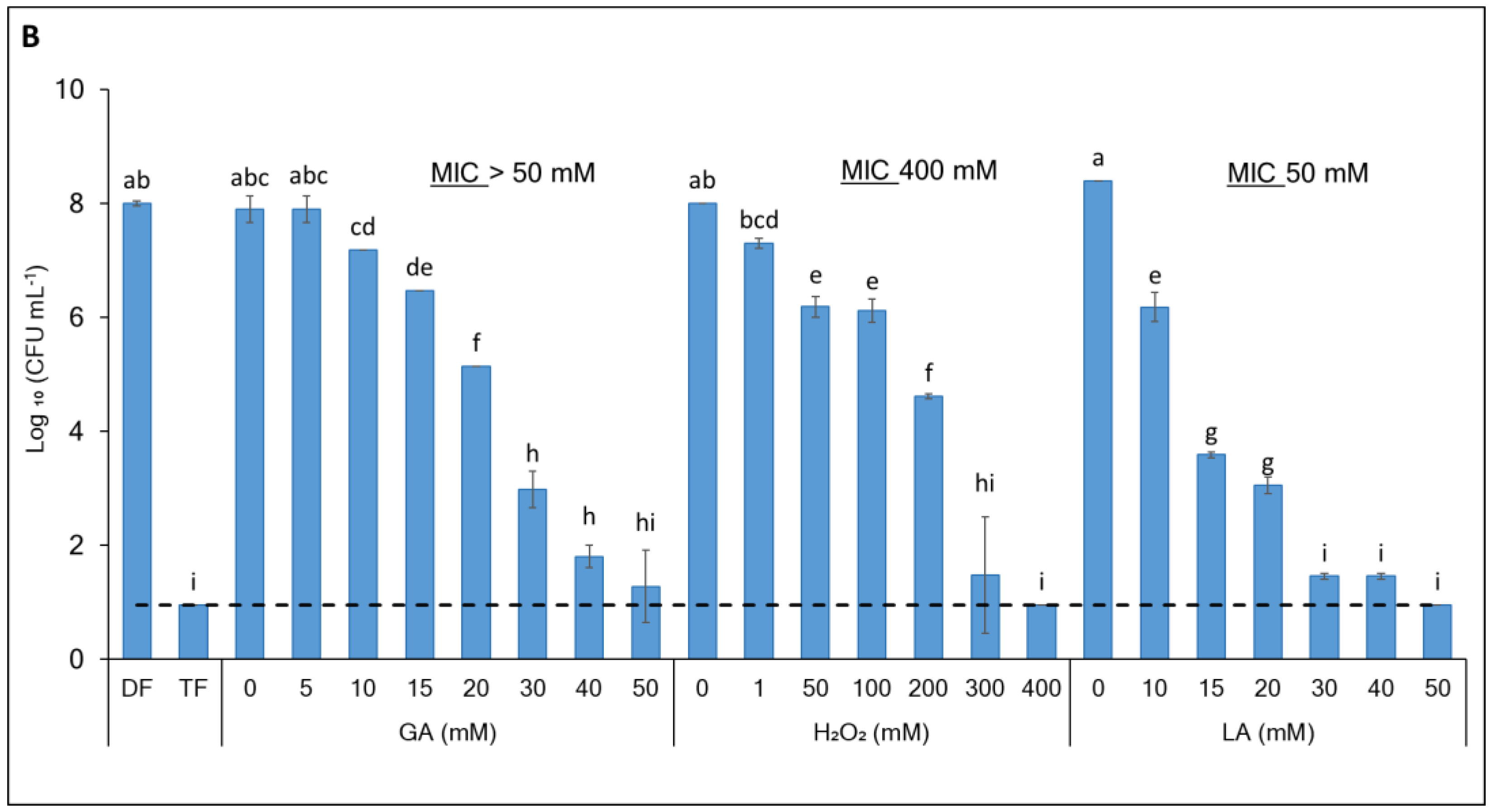
3.1. Enhancing the Antibacterial Effect of GA-Based Sanitizers Through Generating Synergistic Formulations
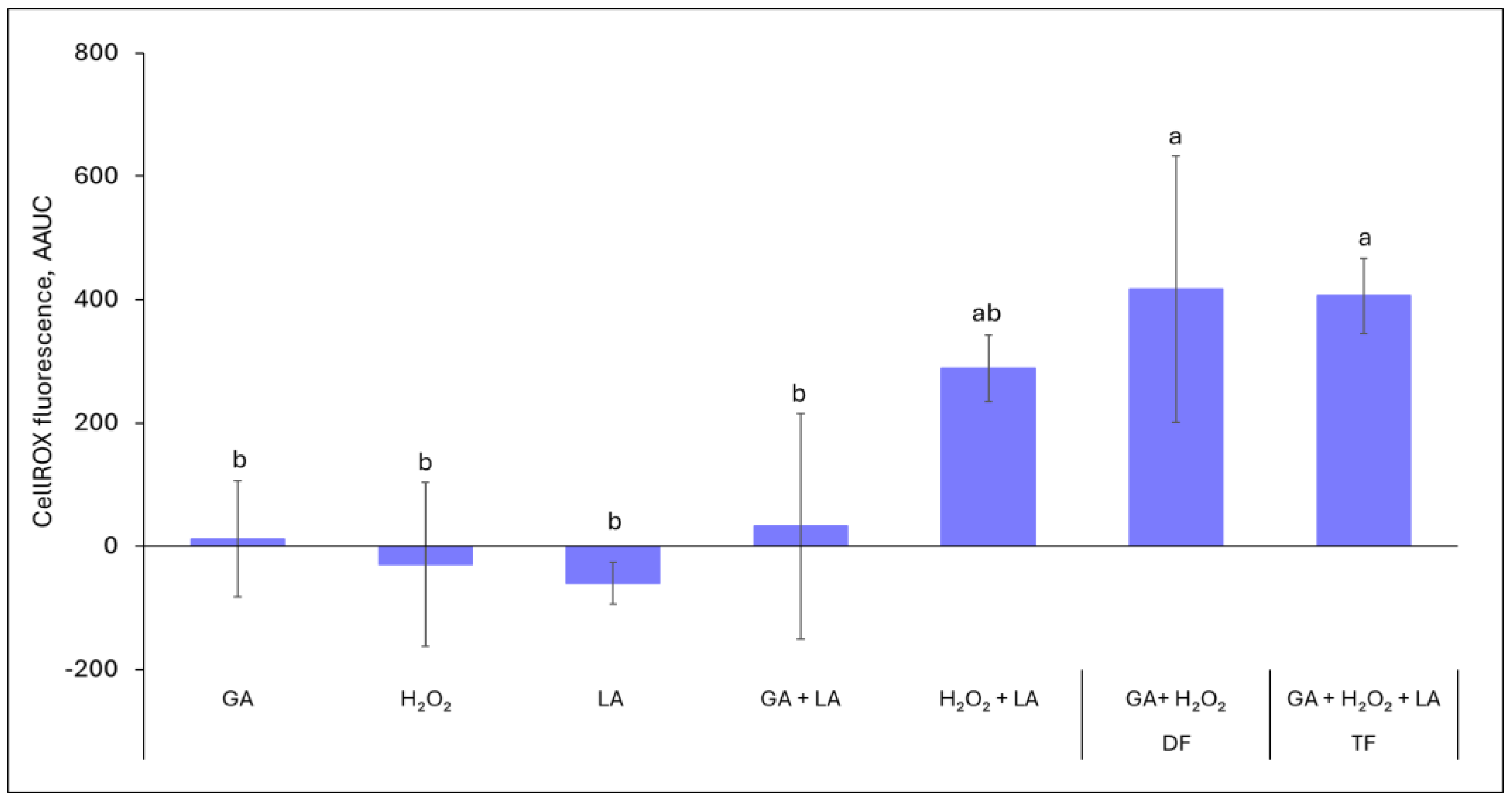
3.2. ROS Generation by Formulation Ingredients in Abiotic Systems
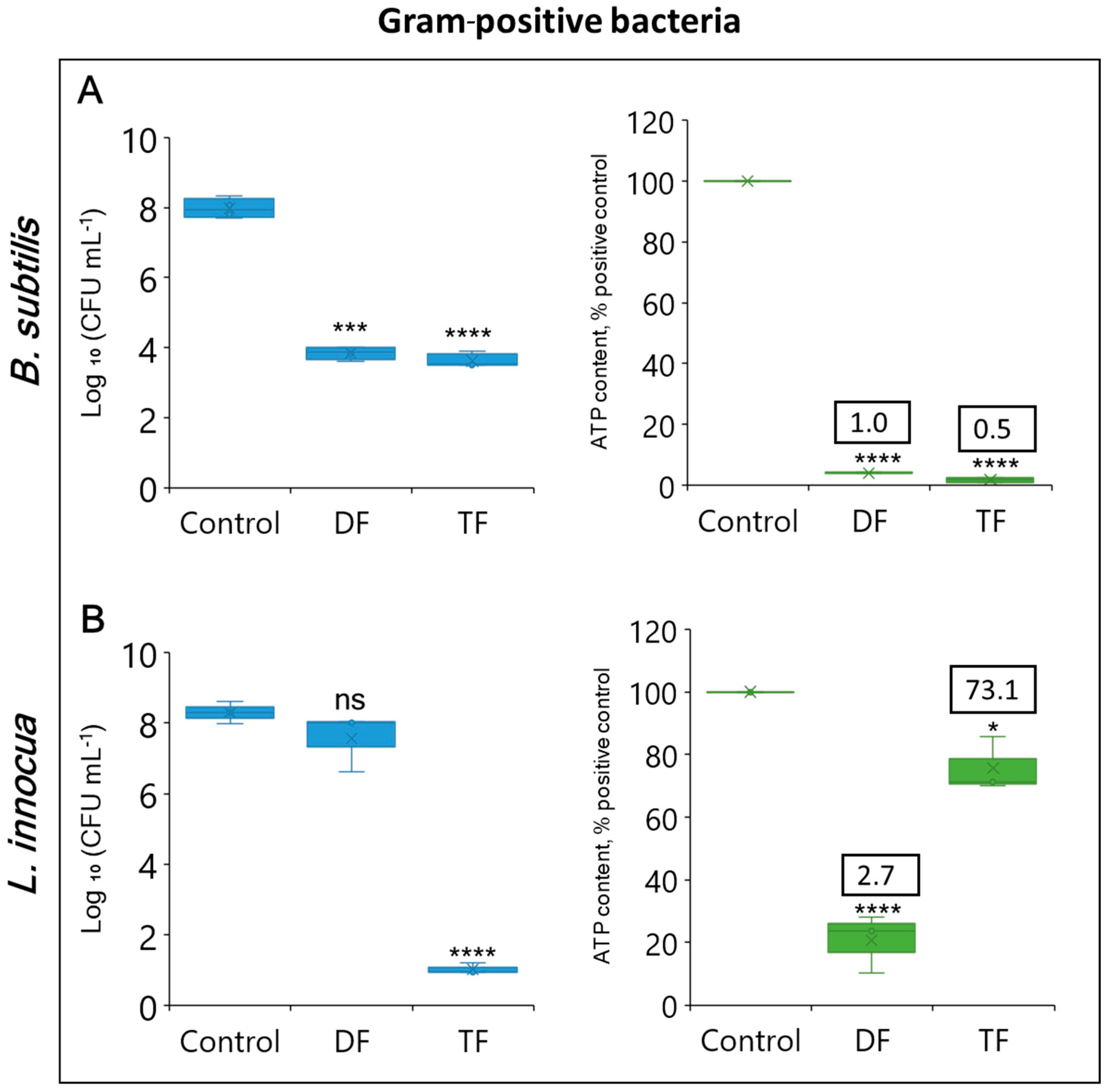
3.3. Cell Viability Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aiyedun, S.O.; Onarinde, B.A.; Swainson, M.; Dixon, R.A. Foodborne outbreaks of microbial infection from fresh produce in Europe and North America: A systematic review of data from this millennium. Int. J. Food Sci. Technol. 2020, 56, 2215–2223. [Google Scholar] [CrossRef]
- Turner, K.; Moua, C.N.; Hajmeer, M.; Barnes, A.; Needham, M. Overview of leafy greens–related food safety incidents with a California link: 1996 to 2016. J. Food Prot. 2019, 82, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Ukuku, D.O.; Pilizota, V.; Sapers, G.M. Influence of washing treatment on native microflora and Escherichia coli population of inoculated cantaloupes. J. Food Saf. 2007, 21, 31–47. [Google Scholar] [CrossRef]
- Keskinen, L.A.; Burke, A.; Annous, B.A. Efficacy of chlorine, acidic electrolyzed water and aqueous chlorine dioxide solutions to decontaminate Escherichia coli O157: H7 from lettuce leaves. Int. J. Food Microbiol. 2009, 132, 134–140. [Google Scholar] [CrossRef]
- Villanueva, C.M.; Cantor, K.P.; Cordier, S.; Jaakkola, J.J.; King, W.D.; Lynch, C.F.; Porru, S.; Kogevinas, M. Disinfection byproducts and bladder cancer: A pooled analysis. Epidemiology 2004, 15, 357–367. [Google Scholar] [CrossRef]
- Ölmez, H.; Kretzschmar, U. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Artés, F.; Allende, A. Minimal processing of fresh fruit, vegetables, and juices. In Emerging Technologies for Food Processing, 2nd ed.; Sun, D.-W., Ed.; Academic Press/Elsevier: Cambridge, MA, USA, 2014; pp. 583–597. [Google Scholar]
- Krasner, S.W.; Weinberg, H.S.; Richardson, S.D.; Pastor, S.J.; Chinn, R.; Sclimenti, M.J.; Onstad, G.D.; Thruston, A.D. Occurrence of a new generation of disinfection byproducts. Environ. Sci. Technol. 2006, 40, 7175–7185. [Google Scholar] [CrossRef]
- Hua, G.; Reckhow, D.A. Comparison of disinfection byproduct formation from chlorine and alternative disinfectants. Water Res. 2007, 41, 1667–1678. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future antimicrobials: Natural and functionalized phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Basu, A. Phenolic compounds potential health benefits and toxicity. In Utilisation of Bioactive Compounds from Agricultural and Food Production Waste; CRC Press: Boca Raton, FL, USA, 2017; pp. 27–59. [Google Scholar]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef] [PubMed]
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food safety through natural antimicrobials. Antibiotics 2019, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Seo, Y.H.; Oh, S.W. Antibacterial activities of polyphenols against foodborne pathogens and their application as antibacterial agents. Food. Sci. Biotechnol. 2022, 31, 985–997. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simoes, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Shao, D.; Li, J.; Li, J.; Tang, R.; Liu, L.; Shi, J.; Huang, Q.; Yang, H. Inhibition of gallic acid on the growth and biofilm formation of Escherichia coli and Streptococcus mutans. J. Food Sci. 2015, 80, M1299–M1305. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Guan, H.; Zhao, X.; Xie, Q.; Xie, Z.; Cai, F.; Dang, R.; Li, M.; Wang, C. Dietary gallic acid as an antioxidant: A review of its food industry applications, health benefits, bioavailability, nano-delivery systems, and drug interactions. Food Res. Int. 2024, 180, 114068. [Google Scholar] [CrossRef]
- Gomes, I.; Malheiro, J.; Mergulhão, F.; Maillard, J.Y.; Simões, M. Comparison of the efficacy of natural-based and synthetic biocides to disinfect silicone and stainless steel surfaces. FEMS Pathog. Dis. 2016, 74, ftw014. [Google Scholar] [CrossRef]
- Lambert, R.; Johnston, M.; Hanlon, G.; Denyer, S.P. Theory of antimicrobial combinations: Biocide mixtures–synergy or addition? J. Appl. Microbiol. 2003, 94, 747–759. [Google Scholar] [CrossRef]
- Denyer, S.P.; Hugo, W.B.; Harding, V.D. Synergy in preservative combinations. Int. J. Pharm. 1985, 25, 245–253. [Google Scholar] [CrossRef]
- Denyer, S.P. Mechanisms of action of antibacterial biocides. Int. Biodeterior. Biodegrad. 1995, 36, 227–245. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Liu, W.; Guo, T.; Gu, R.; Kong, J. Potential application and bactericidal mechanism of lactic acid–hydrogen peroxide consortium. Appl. Biochem. Biotechnol. 2019, 189, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Santiesteban-López, A.; Palou, E.; López-Malo, A. Susceptibility of foodborne bacteria to binary combinations of antimicrobials at selected aw and pH. J. Appl. Microbiol. 2007, 102, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Sokovic, M.; Ciric, A.; Koukoulitsa, C.; Bilia, A.R.; Skaltsa, H. Antimicrobial properties of Quercus ilex L. proanthocyanidin dimers and simple phenolics: Evaluation of their synergistic activity with conventional antimicrobials and prediction of their pharmacokinetic profile. J. Agric. Food Chem. 2011, 59, 6412–6422. [Google Scholar] [CrossRef]
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 2017, 12, e0172273. [Google Scholar] [CrossRef]
- de Oliveira, E.F.; Cossu, A.; Tikekar, R.V.; Nitin, N. Enhanced antimicrobial activity based on a synergistic combination of sublethal levels of stresses induced by UV-A light and organic acids. Appl. Environ. Microbiol. 2017, 83, e00383-17. [Google Scholar] [CrossRef]
- Ferro, S.; Amorico, T.; Deo, P. Role of food sanitising treatments in inducing the ‘viable but nonculturable’ state of microorganisms. Food Control 2018, 91, 321–329. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ghosh, A.R. Assessment of bacterial viability: A comprehensive review on recent advances and challenges. Microbiology 2019, 165, 593–610. [Google Scholar] [CrossRef]
- Truchado, P.; Gil, M.I.; Larrosa, M.; Allende, A. Detection and quantification methods for viable but non-culturable (VBNC) cells in process wash water of fresh-cut produce: Industrial validation. Front. Microbiol. 2020, 11, 673. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Pan, H.; Yang, D.; Rao, L.; Zhao, L.; Wang, Y.; Liao, X. Induction, detection, formation, and resuscitation of viable but non-culturable state microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 149–183. [Google Scholar] [CrossRef]
- Wideman, N.E.; Oliver, J.D.; Crandall, P.G.; Jarvis, N.A. Detection and potential virulence of viable but non-culturable (VBNC) Listeria monocytogenes: A review. Microorganisms 2021, 9, 194. [Google Scholar] [CrossRef]
- del Mar Lleò, M.; Benedetti, D.; Tafi, M.C.; Signoretto, C.; Canepari, P. Inhibition of the resuscitation from the viable but non-culturable state in Enterococcus faecalis. Environ. Microbiol. 2007, 9, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhong, J.; Wei, C.; Lin, C.W.; Ding, T. Current perspectives on viable but non-culturable state in foodborne pathogens. Front. Microbiol. 2017, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Cappelier, J.M.; Besnard, V.; Roche, S.M.; Velge, P.; Federighi, M. Avirulent viable but non culturable cells of Listeria monocytogenes need the presence of an embryo to be recovered in egg yolk and regain virulence after recovery. Vet. Res. 2007, 38, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef]
- Oliver, J.D. The viable but nonculturable state in bacteria. J. Microbiol. 2005, 43, 93–100. [Google Scholar]
- Montanari, C.; Tabanelli, G.; Barbieri, F.; Mora, D.; Duncan, R.; Gardini, F.; Arioli, S. Listeria monocytogenes sensitivity to antimicrobial treatments depends on cell origin. Sci. Rep. 2021, 11, 21263. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, E.W.; Shemesh, M.; Rodov, V. Enhancing antibacterial activity of gallic acid by synergistic interaction with GRAS compounds, as potential basis for “green” sanitizing agents. In Proceedings of the Boston Bacterial Meeting, Boston, MA, USA, 10–11 June 2021; p. 43. [Google Scholar]
- Zhou, B.; Luo, Y.; Nou, X.; Mwangi, E.; Poverenov, E.; Rodov, V.; Demokritou, P.; Fonseca, J.M. Effects of a novel combination of gallic acid, hydrogen peroxide and lactic acid on pathogen inactivation and shelf-life of baby spinach. Food Control 2023, 143, 109284. [Google Scholar] [CrossRef]
- Hu, M.; Gurtler, J.B. Selection of surrogate bacteria for use in food safety challenge studies: A review. J. Food Prot. 2017, 80, 1506–1536. [Google Scholar] [CrossRef]
- Tsror, L.; Hélias, V.; Mordechai-Lebiush, S.; Erlich, O.; Hazanovsky, M.; Chalupowicz, L.; Reuven, M.; Dror, O.; Valinsky, L.; Laurent, A.; et al. Characterization of Pectobacterium brasiliense strains from potato and vegetables in Israel. Plant Pathol. 2021, 70, 2179–2187. [Google Scholar] [CrossRef]
- Cossu, A.; Ercan, D.; Wang, Q.; Peer, W.A.; Nitin, N.; Tikekar, R.V. Antimicrobial effect of synergistic interaction between UV-A light and gallic acid against Escherichia coli O157:H7 in fresh produce wash water and biofilm. Innov. Food Sci. Emerg. Technol. 2016, 37, 44–52. [Google Scholar] [CrossRef]
- Maturin, L.; Peeler, J.T. Chapter 3: Aerobic plate count. In Bacteriological Analytical Manual (BAM); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2001; pp. 1–12. Available online: https://www.fda.gov/media/178943/download?attachment (accessed on 30 September 2024).
- Stein, C.; Makarewicz, O.; Bohnert, J.A.; Pfeifer, Y.; Kesselmeier, M.; Hagel, S.; Pletz, M.W. Three dimensional checkerboard synergy analysis of colistin, meropenem, tigecycline against multidrug-resistant clinical Klebsiella pneumonia isolates. PLoS ONE 2015, 10, e0126479. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, K.; Hattori, N.; La Duc, M.T.; Kern, R. ATP as a biomarker of viable microorganisms in clean-room facilities. J. Microbiol. Methods 2003, 52, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, P.; Schmidt-Emrich, S.; Maniura-Weber, K.; Ren, Q. Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 2015, 15, 36. [Google Scholar] [CrossRef]
- Emerson, J.B.; Adams, R.I.; Roman, C.M.B.; Brooks, B.; Coil, D.A.; Dahlhausen, K.; Ganz, H.H.; Hartmann, E.M.; Hsu, T.; Justice, N.B. Schrodinger’s microbes: Tools for distinguishing the living from the dead in microbial ecosystems. Microbiome 2017, 5, 86. [Google Scholar] [CrossRef]
- Lomakina, G.Y.; Modestova, Y.A.; Ugarova, N. Bioluminescence assay for cell viability. Biochemistry 2015, 80, 701–713. [Google Scholar] [CrossRef]
- Jacob, J. Evidence of a viable but nonculturable (VBNC) phase in B. abortus S19 under oxidative stress (H2O2, -Fe2+, bleach) and under non-oxidative inhibitory conditions (isopropanol, erythritol, selenite). Microorganisms 2024, 12, 491. [Google Scholar] [CrossRef]
- Gao, R.; Liao, X.; Zhao, X.; Liu, D.; Ding, T. The diagnostic tools for viable but nonculturable pathogens in the food industry: Current status and future prospects. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2146–2175. [Google Scholar] [CrossRef]
- Truchado, P.; Gil, M.I.; Allende, A. Peroxyacetic acid and chlorine dioxide unlike chlorine induce viable but non-culturable (VBNC) stage of Listeria monocytogenes and Escherichia coli O157:H7 in wash water. Food Microbiol. 2021, 100, 103866. [Google Scholar] [CrossRef] [PubMed]
- Codony, F.; Agusti, G.; Allue-Guardia, A. Cell membrane integrity and distinguishing between metabolically active and inactive cells as a means of improving viability PCR. Mol. Cell. Probes 2015, 29, 190–192. [Google Scholar] [CrossRef]
- Lançoni, R.; de Arruda, R.P.; Alves, M.B.R.; Oliveira, L.Z.; dos Santos, G.D.C.; Lemes, K.M.; Florez-Rodriguez, S.A.; Celeghini, E.C. Validation of the CellRox Deep Red® fluorescent probe to oxidative stress assessment in equine spermatozoa. Anim. Reprod. 2018, 14, 427–441. [Google Scholar] [CrossRef]
- Mandavilli, B.S.; Aggeler, R.J.; Chambers, K.M. Tools to measure cell health and cytotoxicity using high content imaging and analysis. In High Content Screening: A Powerful Approach to Systems Cell Biology and Phenotypic Drug Discovery; Johnston, P., Trask, O., Eds.; Humana Press: New York, NY, USA, 2018; pp. 33–46. [Google Scholar]
- Dogra, N.; Choudhary, R.; Kohli, P.; Haddock, J.D.; Makwana, S.; Horev, B.; Vinokur, Y.; Droby, S.; Rodov, V. Polydiacetylene nanovesicles as carriers of natural phenylpropanoids for creating antimicrobial food-contact surfaces. J. Agric. Food Chem. 2015, 63, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Fluorescence Response of CellROX Reagents to Various Reactive Oxygen Species (ROS). Available online: https://www.researchgate.net/file.PostFileLoader.html?id=528d91e5d11b8b49328b4706&assetKey=AS%3A272177469820935%401441903574158 (accessed on 21 July 2024).
- Barcelo, J.M.; Guieb, M.; Ventura, A.; Nacino, A.; Pinasen, H.; Viernes, L.; Yodong, T.; Estrada, B.L.; Valdez, D.; Binwag, T. Antibacterial, prooxidative and genotoxic activities of gallic acid and its copper and iron complexes against Escherichia coli. Asia Pac. J. Multidiscip. Res. 2014, 2, 45–56. [Google Scholar]
- Nakamura, K.; Yamada, Y.; Ikai, H.; Kanno, T.; Sasaki, K.; Niwano, Y. Bactericidal action of photoirradiated gallic acid via reactive oxygen species formation. J. Agric. Food Chem. 2012, 60, 10048–10054. [Google Scholar] [CrossRef]
- Wang, Q.; Leong, W.F.; Elias, R.J.; Tikekar, R.V. UV-C irradiated gallic acid exhibits enhanced antimicrobial activity via generation of reactive oxidative species and quinone. Food Chem. 2019, 287, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Abdelshafy, A.M.; Neetoo, H.; Al-Asmari, F. Antimicrobial activity of hydrogen peroxide for application in food safety and COVID-19 mitigation: An updated review. J. Food Prot. 2024, 87, 100306. [Google Scholar] [CrossRef]
- Wang, Q.; de Oliveira, E.F.; Alborzi, S.; Bastarrachea, L.J.; Tikekar, R.V. On mechanism behind UV-A light enhanced antibacterial activity of gallic acid and propyl gallate against Escherichia coli O157:H7. Sci. Rep. 2017, 7, 8325. [Google Scholar] [CrossRef]
- Nakamura, K.; Ishiyama, K.; Sheng, H.; Ikai, H.; Kanno, T.; Niwano, Y. Bactericidal activity and mechanism of photoirradiated polyphenols against Gram-positive and -negative bacteria. J. Agric. Food Chem. 2015, 63, 7707–7713. [Google Scholar] [CrossRef]
- Ghosh, S.; Korza, G.; Maciejewski, M.; Setlow, P. Analysis of metabolism in dormant spores of Bacillus species by 31P nuclear magnetic resonance analysis of low-molecular-weight compounds. J. Bacteriol. 2015, 197, 992–1001. [Google Scholar] [CrossRef]
- Liao, X.; Liu, D.; Ding, T. Nonthermal plasma induces the viable-but-nonculturable state in Staphylococcus aureus via metabolic suppression and the oxidative stress response. Appl. Environ. Microbiol. 2020, 86, e02216-19. [Google Scholar] [CrossRef]
- Bucur, F.I.; Grigore-Gurgu, L.; Crauwels, P.; Riedel, C.U.; Nicolau, A.I. Resistance of Listeria monocytogenes to stress conditions encountered in food and food processing environments. Front. Microbiol. 2018, 9, 2700. [Google Scholar] [CrossRef]
- Wang, H.; Feng, M.; Anwar, T.M.; Chai, W.; Ed-Dra, A.; Kang, X.; Rantsiou, K.; Kehrenberg, C.; Yue, M.; Li, Y. Change in antimicrobial susceptibility of Listeria spp. in response to stress conditions. Front. Sustain. Food Syst. 2023, 7, 1179835. [Google Scholar] [CrossRef]
- Raengpradub, S.; Wiedmann, M.; Boor, K.J. Comparative analysis of the σB-dependent stress responses in Listeria monocytogenes and Listeria innocua strains exposed to selected stress conditions. Appl. Environ. Microbiol. 2008, 74, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Shelef, L.A. Antimicrobial effects of lactates: A review. J. Food Prot. 1994, 57, 445–450. [Google Scholar] [CrossRef]
- Đurđević-Milošević, D.; Petrović, A.; Kalaba, V.; Stijepić, M.; Jovanović, G. Selected aspects of the antibacterial use of lactic acid in food processing. Eng. Proc. 2024, 67, 2. [Google Scholar] [CrossRef]
- Amrutha, B.; Sundar, K.; Shetty, P.H. Effect of organic acids on biofilm formation and quorum signaling of pathogens from fresh fruits and vegetables. Microb. Pathog. 2017, 111, 156–162. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, H. Effect of organic acids, hydrogen peroxide and mild heat on inactivation of Escherichia coli O157:H7 on baby spinach. Food Control 2011, 22, 1178–1183. [Google Scholar] [CrossRef]
- Valiolahi, M.; Najafi, M.A.; Eskandani, M.A.; Rahnama, M. Effects of organic acid alone and in combination with H2O2 and NaCl on Escherichia coli O157: H7: An evaluation of antioxidant retention and overall acceptability in Basil leaves (Ocimum basilicum). Int. J. Food Microbiol. 2019, 292, 56–63. [Google Scholar] [CrossRef]
- Lehrke, G.; Hernaez, L.; Mugliaroli, S.L.; Von Staszewski, M.; Jagus, R.J. Sensitization of Listeria innocua to inorganic and organic acids by natural antimicrobials. LWT-Food Sci. Technol. 2011, 44, 984–991. [Google Scholar] [CrossRef]
- Schottroff, F.; Frohling, A.; Zunabovic-Pichler, M.; Krottenthaler, A.; Schluter, O.; Jager, H. Sublethal injury and viable but non-culturable (VBNC) state in microorganisms during preservation of food and biological materials by non-thermal processes. Front. Microbiol. 2018, 9, 2773. [Google Scholar] [CrossRef]
- Besnard, V.; Federighi, M.; Declerq, E.; Jugiau, F.; Cappelier, J.M. Environmental and physico-chemical factors induce VBNC state in Listeria monocytogenes. Vet. Res. 2002, 33, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Gurresch, A.; Gerner, W.; Pin, C.; Wagner, M.; Hein, I. Evidence of metabolically active but non-culturable Listeria monocytogenes in long-term growth at 10 °C. Res. Microbiol. 2016, 167, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, E.; O’Byrne, C.; Oliver, J.D. Effect of weak acids on Listeria monocytogenes survival: Evidence for a viable but nonculturable state in response to low pH. Food Control 2009, 20, 1141–1144. [Google Scholar] [CrossRef]
- Highmore, C.J.; Warner, J.C.; Rothwell, S.D.; Wilks, S.A.; Keevil, C.W. Viable-but-nonculturable Listeria monocytogenes and Salmonella enterica serovar Thompson induced by chlorine stress remain infectious. mBio 2018, 9, e00540-18. [Google Scholar] [CrossRef]
- Kramer, B.; Muranyi, P. Effect of pulsed light on structural and physiological properties of Listeria innocua and Escherichia coli. J. Appl. Microbiol. 2014, 116, 596–611. [Google Scholar] [CrossRef]
- Trinh, N.T.T.; Dumas, E.; Thanh, M.L.; Degraeve, P.; Amara, C.B.; Gharsallaoui, A.; Oulahal, N. Effect of a Vietnamese Cinnamomum cassia essential oil and its major component trans-cinnamaldehyde on the cell viability, membrane integrity, membrane fluidity, and proton motive force of Listeria innocua. Can. J. Microbiol. 2015, 61, 263–271. [Google Scholar] [CrossRef]
- Lindback, T.; Rottenberg, M.E.; Roche, S.M.; Rorvik, L.M. The ability to enter into an avirulent viable but non-culturable (VBNC) form is widespread among Listeria monocytogenes isolates from salmon, patients and environment. Vet. Res. 2010, 41, 8. [Google Scholar] [CrossRef]
- Robben, C.; Witte, A.K.; Schoder, D.; Stessl, B.; Rossmanith, P.; Mester, P. A fast and easy ATP-based approach enables MIC testing for non-resuscitating VBNC pathogens. Front. Microbiol. 2019, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Białobrzewski, I. Mixed species biofilms of Lactobacillus plantarum and Listeria innocua show facilitated entrance to the VBNC state during chlorine-induced stress. J. Food Saf. 2019, 39, e12651. [Google Scholar] [CrossRef]
- Yang, D.; Wang, W.; Zhao, L.; Rao, L.; Liao, X. Resuscitation of viable but nonculturable bacteria promoted by ATP-mediated NAD+ synthesis. J. Adv. Res. 2024, 60, 27–39. [Google Scholar] [CrossRef]
- Mains, D.R.; Eallonardo, S.J.; Freitag, N.E. Identification of Listeria monocytogenes genes contributing to oxidative stress resistance under conditions relevant to host infection. Infect. Immun. 2021, 89, e00700-20. [Google Scholar] [CrossRef] [PubMed]
- Pine, L.; Malcolm, G.; Brooks, J.; Daneshvar, M. Physiological studies on the growth and utilization of sugars by Listeria species. Can. J. Microbiol. 1989, 35, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Lungu, B.; Ricke, S.; Johnson, M. Growth, survival, proliferation and pathogenesis of Listeria monocytogenes under low oxygen or anaerobic conditions: A review. Anaerobe 2009, 15, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Omwenga, E.O.; Hensel, A.; Pereira, S.; Shitandi, A.A.; Goycoolea, F.M. Antiquorum sensing, antibiofilm formation and cytotoxicity activity of commonly used medicinal plants by inhabitants of Borabu sub-county, Nyamira County, Kenya. PLoS ONE 2017, 12, e0185722. [Google Scholar] [CrossRef] [PubMed]
- Kiymaci, M.E.; Altanlar, N.; Gumustas, M.; Ozkan, S.A.; Akin, A. Quorum sensing signals and related virulence inhibition of Pseudomonas aeruginosa by a potential probiotic strain’s organic acid. Microb. Pathog. 2018, 121, 190–197. [Google Scholar] [CrossRef]
- Cortes, B.W.; Naditz, A.L.; Anast, J.M.; Schmitz-Esser, S. Transcriptome sequencing of Listeria monocytogenes reveals major gene expression changes in response to lactic acid stress exposure but a less pronounced response to oxidative stress. Front. Microbiol. 2020, 10, 3110. [Google Scholar] [CrossRef]
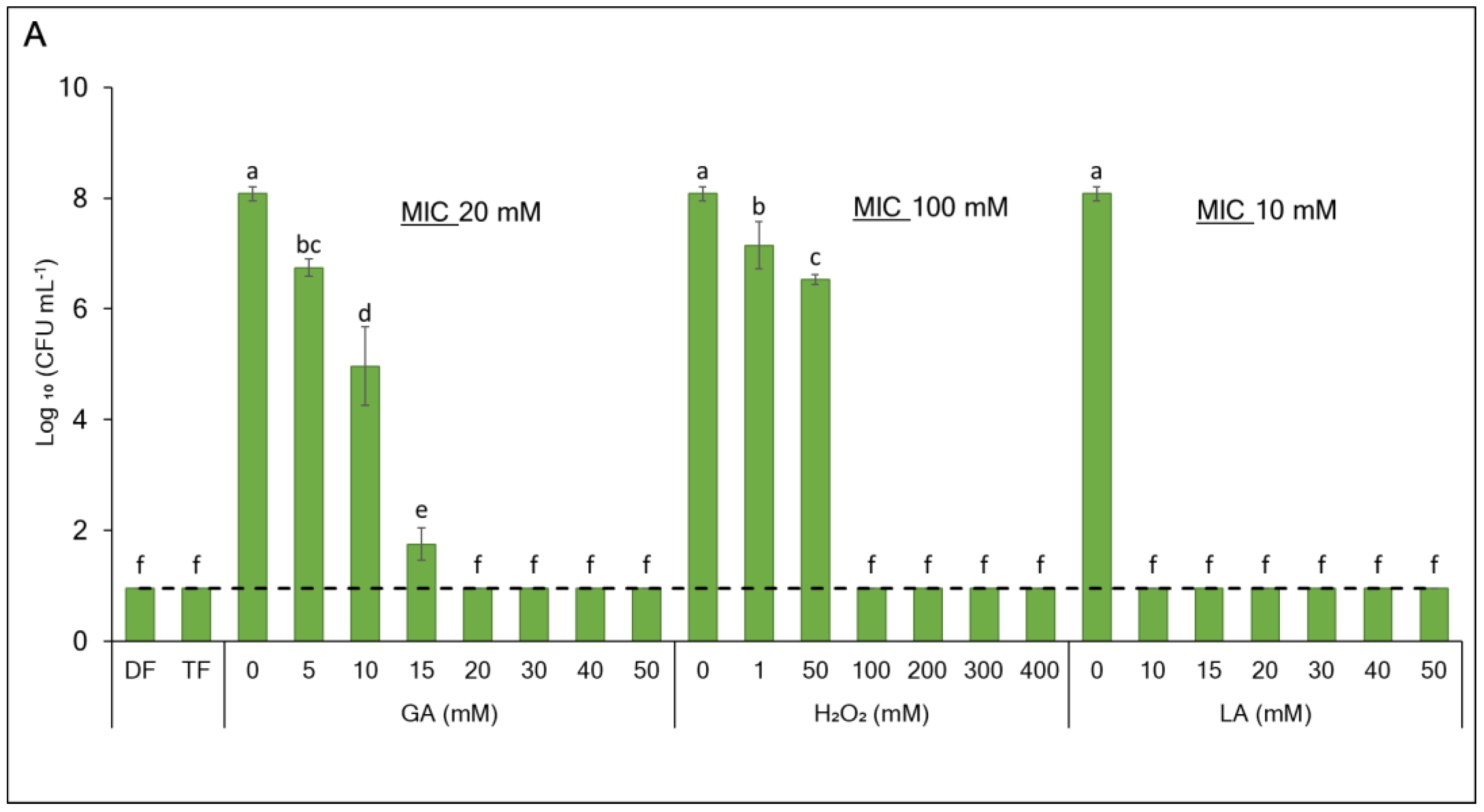




| Species | Formulation | GA | H2O2 | LA | FICI | Interaction | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MICalone | MICcomb. | MICalone | MICcomb. | MICalone | MICcomb. | ||||||
| E. coli | DF | 20 | 8 | 100 | 1 | 10 | 0 | 0.41 | Synergy | ||
| TF | 20 | 8 | 100 | 1 | 10 | 20 | 3.4 | Indifferent | |||
| L. innocua | DF | 50 | n.a. | 400 | n.a. | 50 | n.a. | n.a. | n.a. | ||
| TF | 50 | 8 | 400 | 1 | 50 | 20 | 0.56 | Synergy | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwangi, E.W.; Shemesh, M.; Rodov, V. Investigating the Antibacterial Effect of a Novel Gallic Acid-Based Green Sanitizer Formulation. Foods 2024, 13, 3322. https://doi.org/10.3390/foods13203322
Mwangi EW, Shemesh M, Rodov V. Investigating the Antibacterial Effect of a Novel Gallic Acid-Based Green Sanitizer Formulation. Foods. 2024; 13(20):3322. https://doi.org/10.3390/foods13203322
Chicago/Turabian StyleMwangi, Esther W., Moshe Shemesh, and Victor Rodov. 2024. "Investigating the Antibacterial Effect of a Novel Gallic Acid-Based Green Sanitizer Formulation" Foods 13, no. 20: 3322. https://doi.org/10.3390/foods13203322
APA StyleMwangi, E. W., Shemesh, M., & Rodov, V. (2024). Investigating the Antibacterial Effect of a Novel Gallic Acid-Based Green Sanitizer Formulation. Foods, 13(20), 3322. https://doi.org/10.3390/foods13203322







