Comparative Analysis of Quantitative Methods for Campylobacter spp. Quantification: ISO 10272-2:2017, Tempo® and Real-Time PCR in Refrigerated and Frozen Turkey Cuts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Taking Samples and Defining the Sampling Number
2.2. Sample Processing
2.3. Assay Quality Control
2.4. Campylobacter spp. Enumeration Using the Reference Method
2.4.1. Enumeration of the Plates After Incubation
2.4.2. Confirmation of Typical Colonies: Oxidase Test
2.4.3. Confirmation of Typical Colonies: Motility and Morphology Tests
2.4.4. Confirmation of Typical Colonies: Aerobiosis Test
2.5. Campylobacter spp. Enumeration with the TEMPO® Method Using the TEMPO® CAM Kit
2.6. Enumeration of Campylobacter spp. Using the Real-Time PCR Method (BIOTECON®)
2.6.1. Preparation of the Standard rtPCR Curve for Quantification
- ▪
- Pre-incubation: 1 cycle;
- ▪
- Step 1: 37 °C/4 min;
- ▪
- Step 2: 95 °C/5 min;
- ▪
- Amplification: 50 cycles;
- ▪
- Step 1: 95 °C/5 s;
- ▪
- Step 2: 60 °C/60 s—fluorescence detection.
2.6.2. Evaluation of Results After DNA Amplification
2.7. Statistical Analyses
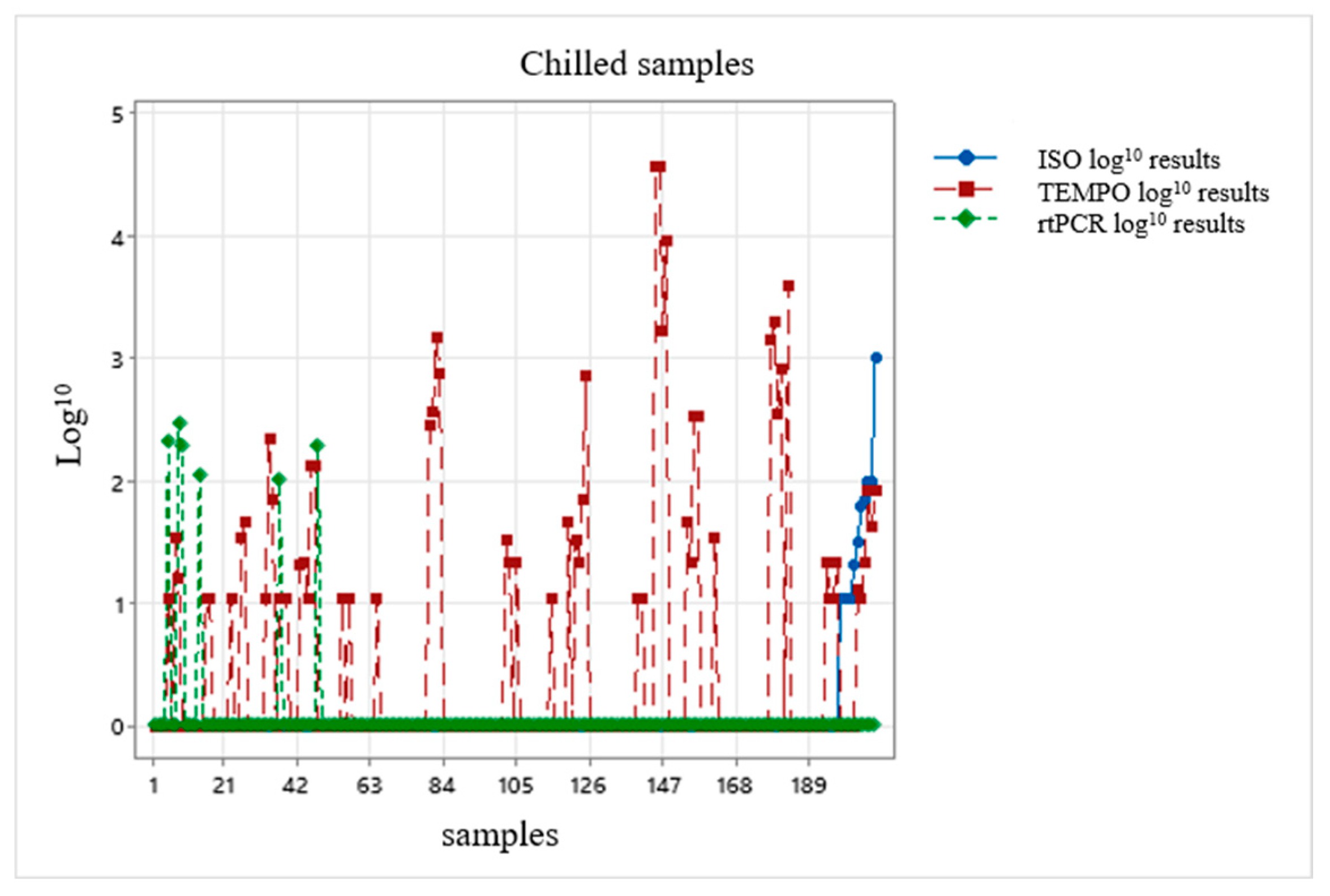
3. Results
3.1. Data Evaluation Using the Kruskal–Wallis Test
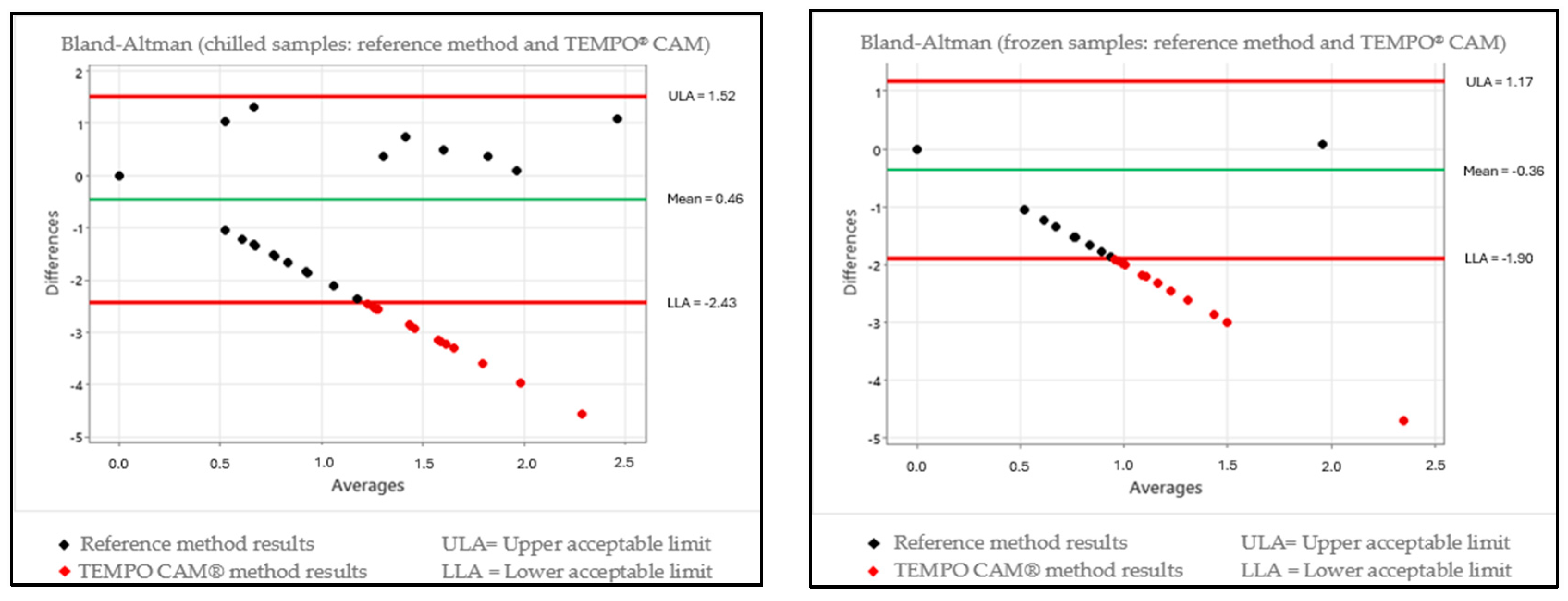
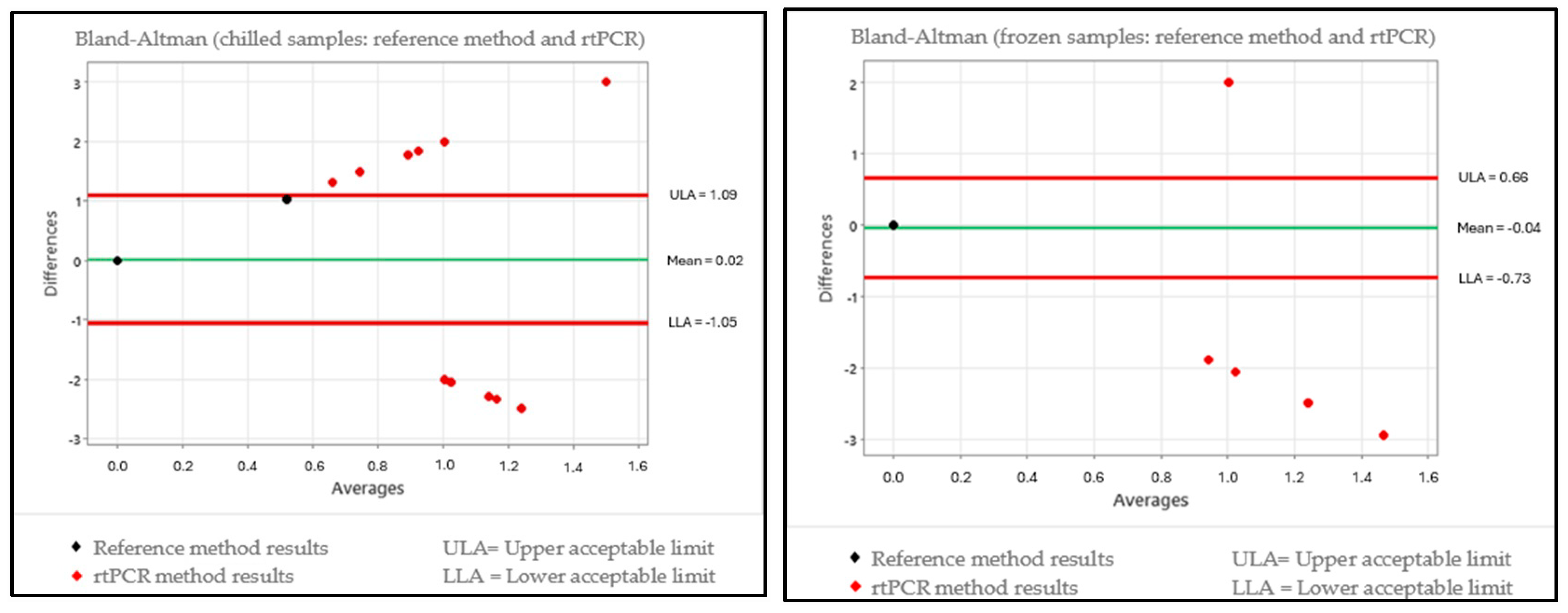
3.2. Data Evaluation Using the Bland–Altman Test
3.3. Comparison of the Kruskal–Wallis and Bland–Altman Test Data
3.4. Evaluation of Data for Calculation of Correction Factor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.J.; Rezende, C.; Feistel, J.; Moreira, N.; Oliveira, A. Campylobacter se sua ocorrência em abatedouros de aves. Enciclopédia Biosf. 2013, 9. Available online: https://conhecer.org.br/ojs/index.php/biosfera/article/view/3380 (accessed on 23 March 2023).
- EFSA and ECDC—European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, 6971. [Google Scholar] [CrossRef]
- Alaboudi, A.R.; Akram, R.; Malkawi, I.M.; Ismail, M.; Osaili, T.M.; Abu-Basha, E.A.; Guitian, J. Prevalence, antibiotic resistance and genotypes of Campylobacter jejuni and Campylobacter coli isolated from chickens in Irbid governorate, Jordan. Int. J. Food Microbiol. 2020, 327, 108656. [Google Scholar] [CrossRef]
- Tack, D.M.; Ray, L.; Griffin, P.M.; Cieslak, P.R.; Dunn, J.; Rissman, T.; Jervis, R.; Lathrop, S.; Muse, A.; Duwell, M.; et al. Preliminary incidence and trends of infections with pathogens transmitted commonly through food—Foodborne diseases active surveillance network, 10 U.S. Sites, 2016–2019. Morb. Mortal. Wkly. Rep. 2020, 69, 509–515. Available online: https://www.cdc.gov/mmwr/volumes/69/wr/pdfs/mm6917a1-H.pdf (accessed on 16 March 2023). [CrossRef]
- Zaidi, M.B.; Calva, J.J.; Estrada-Garcia, M.T.; Leon, V.; Vasquez, G.; Figueroa, G.; Lopes, E.; Contreras, J.; Abbott, J.; Zhao, S.; et al. Integrated food chain surveillance system for Salmonella sin Mexico. Emerg. Infect. Dis. 2008, 14, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Kassem, I.I.; Shen, Z.; Lin, J.; Rajashekara, G.; Zhang, Q. Campylobacter in poultry: Ecology and potential interventions. Avian Dis. 2015, 59, 185–1120. [Google Scholar] [CrossRef]
- Crespo, M.D.; Kathariou, S.; Grimes, J.L.; Cox, N.A.; Buhr, R.J.; Frye, J.G.; Miller, W.G.; Jackson, C.R.; Smith, D.P. Routes of transmission of Salmonella and Campylobacter in breeder turkeys. J. Appl. Poult. Res. 2016, 35, 591–609. [Google Scholar] [CrossRef]
- Silva, H.O.; Vidal, A.M.C. Pathogenic bacteria in turkey meat: A review. Rev. Bras. Hig. E Sanidade Anim. 2017, 11, 338–353. [Google Scholar] [CrossRef]
- EFSA and ECDC—European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017, 15, 5077. [CrossRef]
- Skarp, C.P.A.; Hänninen, L.; Rautelin, H.I.H. Campilobacterioses: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, A.; Hebel, M.; Mittler, M.; Hurck, C.; Kustwan, K.; Heitkönig, B.; Bitschinski, D.; Kreyenschmidt, J.; Lobato, L. Influence of different production systems on the quality and shelf life of poultry meat: A case study in the German sector. J. Food Qual. 2019, 2019, 3718057. [Google Scholar] [CrossRef]
- Hammad, H.H.M.; Meihu, M.; Arine, W.H.; Abdeen, E.E.; Guofeng, J.; Yongguo, J.; Warda, S.A.; Mamoun, A.H. Effect of freeze and re-freeze on chemical composition of beef and poultry meat at storage period 4.5 months. J. Food Process. Technol. 2019, 10, 1000791. [Google Scholar] [CrossRef]
- Gouvêa, D.M.; Mendonça RC, S.; Lopez ME, S.; Batalha, L.S. Absorbent food pads containing bacteriophages for potential antimicrobial use in refrigerated food products. LWT-Food Sci. Technol. 2016, 67, 159–166. [Google Scholar] [CrossRef]
- Belluco, S.; Barco, L.; Roccato, A.; Ricci, A. Escherichia coli and Enterobacteriaceae counts on poultry carcasses along the slaughterline: A systematic review and meta-analysis. Food Control 2016, 60, 269–280. [Google Scholar] [CrossRef]
- Brasil—Ministério da Agricultura, Pecuária e Abastecimento. Instrução Normativa Nº 20, de 21 de outubro de 2016. Controles de Salmonella spp. nos estabelecimentos de abate de aves registrados no SIF. Diário Oficial da União, Brasília, DF, 25 October 2016.
- Stella, S.; Tirloni, E.; Bernardi, C.; Grilli, G. Evaluation of effect of chilling steps during slaughtering on the Campylobacter sp. Counts on broiler carcasses. Poult. Sci. 2021, 100, 100866. [Google Scholar] [CrossRef]
- Maziero, M.T.; de Oliveira TC, R.M. Effect of refrigeration and frozen storage on the Campylobacter jejuni recovery from naturaly contaminated broiler carcasses. Braz. J. Microbiol. 2010, 41, 501–505. [Google Scholar] [CrossRef]
- EFSA and ECDC—European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, 5926. [Google Scholar] [CrossRef]
- Seliwiorstow, T.; Bare, J.; Berkvens, D.; Van Damme, I.; Uyttendaele, M.; De Zutter, L. Identification of risk factors for Campylobacter contamination levels on broiler carcasses during the slaughter process. Int. J. Food Microbiol. 2016, 226, 26–32. [Google Scholar] [CrossRef]
- Warriss, P.D.; Wilkins, L.J.; Brown, S.N.; Phillips, A.J.; Allen, V. Defaecation and weight of the gastrointestinal tract contents after feed and water withdrawal in broilers. Br. Poult. Sci. 2004, 45, 61–66. [Google Scholar] [CrossRef]
- Franchin, P.R.; Battistella PM, D.; Vieira, C.R. Evaluation of multi-sequencial interventions with water to reduce microbial loading as applied to chicken carcasses during slaughtering—A review. World’s Poult. Sci. J. 2010, 66, 203–213. [Google Scholar] [CrossRef]
- Santos, D.A.; Amaral, G.V.; Sartori, F.; Simas, J.V. The importance of hygienic and sanitary conditions in slaughterhouses: A literature review. Res. Soc. Dev. 2021, 10, e22610111455. [Google Scholar] [CrossRef]
- Oliveira, N.T.; Campos RM, L. Utilização das ferramentas de gestão de qualidade em frigorífico de abate de bovinos para exportação. Nutritime 2015, 12, 4016–4029. [Google Scholar]
- Borin, I.C.; Martins, V.; Taketani, N.F. The implementation of rapid microbiological methods in the pharmaceutical industry. Rev. Ens. Pioneiros 2021, 5, 20–34. [Google Scholar] [CrossRef]
- Pacholewicz, E.; Buhlera, C.; Wulstena, I.; Kraushaara, B.; Luub, H.Q.; Iwobic, A.N.; Huberc, I.; Stingl, K. Internal sample process control improves cultivation-independent quantification of thermotolerant. Campylobacter. Food Microbiol. 2018, 78, 53–61. [Google Scholar] [CrossRef]
- Ricke, S.C.; Feyel, K.M.; Chaney, W.E.; Shi, Z.; Pavlidis, H.; Yang, Y. Developments in rapid detection methods for the detection of foodborne Campylobacter in the United States. Front. Microbiol. 2019, 9, 3280. [Google Scholar] [CrossRef]
- Facciolà, A.; Riso, R.; Avventuroso, E.; Visalli, G.; Delia, S.A.; Laganà, P. Campylobacter: From microbiology to prevention. J. Prev. Med. Hyg. 2017, 58, E79–E92. [Google Scholar]
- Humphrey, T.; O’Brien, S.; Madsen, M. Campylobacters as zoonotic pathogens: A food production perspective. Int. J. Food Microbiol. 2007, 117, 237–257. [Google Scholar] [CrossRef]
- Silva, J.; Leite, D.; Fernandes, M.; Mena, C.; Gibbs, P.A.; Teixeira, P. Campylobacter sas a foodborne pathogen: A review. Front. Microbiol. 2011, 2, 200. [Google Scholar] [CrossRef]
- Papic, B.; Pate, M.; Henigman, U.; Zajc, U.; Gruntar, I.; Biasizzo, M.; Ocepek, M.; Kusar, D. New approaches on quantification of Campylobacter jejuni in poultry samples: The use of digital PCR and real-time PCR against the ISO standard plate count method. Front. Microbiol. 2017, 8, 331. [Google Scholar] [CrossRef]
- Krüger, N.J.; Buhler, C.; Iwobi, A.N.; Huber, I.; Ellerbroek, L.; Appel, B.; Stingl, K. “Limits of control”—Crucial parameters for a reliable quantification of viable Campylobacter by real-time PCR. PLoS ONE 2014, 9, e88108. [Google Scholar] [CrossRef] [PubMed]
- Eberle, K.N.; Kiess, A.S. Phenotypic and genotypic methods for typing Campylobacter jejuni and Campylobacter coli in poultry. Poult. Sci. 2012, 91, 255–264. [Google Scholar] [CrossRef]
- Al, S.; Incili, G.K.; Akcay, A.; Koluman, A. Development and evaluation of a novel Campylobacter senrichment medium. J. Microbiol. Methods 2019, 157, 117–122. [Google Scholar] [CrossRef]
- EC. Commission Regulation (EU) 2017/1495 of 23 August 2017 amending Regulation (EC) No 2073/2005 as Regards Campylobacter in Broiler Carcases (Text with EEA Relevance.) (OJ L 218 24.08.2017, p. 1, ELI). Available online: http://data.europa.eu/eli/reg/2017/1495/oj (accessed on 10 January 2022).
- ISO 10272-2:2017; Microbiology of the Food Chain—Horizontal Method for Detection and Enumeration of Campylobacter spp.—Part 2: Colony-Count Technique. ISO: Geneva, Switzerland, 2017.
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Coordenação-Geral de Controle e Avaliação—CGCOA. Memorando Nº 06, de 12 de Janeiro de 2018. Exportação para União Europeia (UE). Bovinos. Aves. Equinos. Ovinos. Pescado. Gelatina. Análises Laboratoriais. Regulamento (CE) n° 2073/2005, de 15 de Novembro de 2005. Regulamento (UE) n° 2017/1495, de 23 de Agosto de 2017. Este Memorando Cancela e Substitui o Memorando n° 199/2017/CGCOA/DIPOA. Brasília: Editora do Ministério da Agricultura e Pecuária. 2018. Available online: https://www.gov.br/agricultura/pt-br/assuntos/sanidade-animal-e-vegetal/saude-animal/programas-de-saude-animal/pnsa (accessed on 10 September 2024).
- Yossa, N.; Smiley, J.; Huang, M.C.J.; Yin, L.; Bell, R.; Tallent, S.; Brown, E.; Hammack, T. Comparison of TEMPO® BC with spiral plating methods for the enumeration of Bacillus cereus in cosmetic products either naturally preserved or preserved with phenoxyethanol. J. AOAC Int. 2019, 102, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Torlak, E.; Akan, I.M.; Gökmen, M. Comparisomn of TEMPO® EC and TBX medium for the enumeration of Escherichia coli in cheese. Lett. Appl. Microbiol. 2008, 47, 566–570. [Google Scholar] [CrossRef]
- Owen, M.; Willis, C.; Lamph, D. Evaluation of the TEMPO® most probable number technique for the enumeration of Enterobacteriaceae in food and dairy products. J. Appl. Microbiol. 2010, 109, 1810–1816. [Google Scholar] [CrossRef]
- Yörük, N.G. Most probable number technique in Escherichia coli count using ISO 16649-3, ISO 7251, and rapid test enumeration device (TEMPO EC) methods in milk and dairy products. J. Food Saf. 2018, 38, e12502. [Google Scholar] [CrossRef]
- Torlak, E.; Akan, I.M. Evaluation of TEMPO STA for the enumeration of coagulase-positive Staphylococci in cheese. Food Sci. Technol. Res. 2012, 18, 645–650. [Google Scholar] [CrossRef]
- Reis, L.P.; Menezes, L.D.M.; Lima, G.K.; Santos, E.L.S.; Dorneles, E.M.S.; Assis, D.C.S.; Lage, A.P.; Cançado, S.V.; Figueiredo, T.C. Detection of Campylobacter sin chilled and frozen broiler carcasses comparing immunoassay, PCR and real time PCR methods. Cienc. Rural 2018, 48, e20161034. [Google Scholar] [CrossRef]
- Josefsen, M.H.; Löfström, C.; Hansen, T.B.; Christensen, L.S.; Olsen, J.E.; Hoorfar, J. Rapid quantification of viable Campylobacter bacteria on chicken carcasses, using real-time PCR and propidium monoazide treatment, as a tool for quantitative risk assessment. Appl. Environ. Microbiol. 2010, 76, 5097–5104. [Google Scholar] [CrossRef]
- Dhanasekaran, S.; Doherty, T.M.; Kenneth, J. Comparison of different standards for real-time PCR-based absolute quantification. J. Immunol. Methods 2010, 354, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.M.; Long, F.; Suo, B. Molecular methods for the detection and characterization of foodborne pathogens. Pure Appl. Chem. 2010, 82, 69–79. [Google Scholar] [CrossRef]
- Cannon, R.M. Sense and sensitivity—Designing surveys based on an imperfect test. Prev. Vetetinary Med. 2001, 49, 141–163. [Google Scholar] [CrossRef]
- Szosland-Fałtyn, A.; Bartodziejska, B.; Krolasik, J.; Paziak-Domańska, B.; Korsak, D.; Chmiela, M. The prevalence of Campylobacter sin Polish poultry meat. Pol. J. Microbiol. 2018, 67, 117–120. [Google Scholar] [CrossRef]
- Huang, H.; Brooks, B.W.; Lowman, R.; Carrillo, C.D. Campylobacter species in animal, food and environmental sources and relevant testing programs in Canada. Can. J. Microbiol. 2015, 61, 701–721. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, O.; Erol, I. Prevalence of thermophilic Campylobacter sin turkey meat and antibiotic resistance of C. jejuni isolates. J. Food Saf. 2012, 32, 452–458. [Google Scholar] [CrossRef]
- Rahimi, E.; Ameri, M. Antimicrobial resistance patterns of Campylobacter sisolated from raw chicken, turkey, quail, partridge, and ostrich meat in Iran. Food Control 2011, 22, 1165–1170. [Google Scholar] [CrossRef]
- Rahimi, E.; Momtaz, H.; Bonyadian, M. PCR detection of Campylobacter sp. from turkey carcasses during processing plant in Iran. Food Control 2010, 21, 692–694. [Google Scholar] [CrossRef]
- Atanassova, V.; Reich, F.; Beckmann, L.; Klein, G. Prevalence of Campylobacter sin turkey meat from a slaughterhouse and in turkey meat retail products. FEMS Immunol. Med. Microbiol. 2007, 49, 141–145. [Google Scholar] [CrossRef]
- Whyte, P.; McGill, M.K.; Kelly, M.L.; Cowley, M.D.; Fanning, S.; Acke, M.E.; Lawlor, M.A.; Moran, M.L.; Scates, M.P.; Carroll, D.C.; et al. A Comparative Study of Thermophilic Campylobacter Isolates of Clinical, Food and Pet Origin Research. Safefood. 2006. Available online: https://www.safefood.net/research-reports/comparative-study-campylobacter (accessed on 20 November 2022).
- Rasschaert, G.; De Zutter, L.; Herman, L.; Heyndrickx, M. Campylobacter contamination of broilers: The role of transport and slaughterhouse. Int. J. Food Microbiol. 2020, 322, 108564. [Google Scholar] [CrossRef]
- Horrocks, S.M.; Anderson, R.C.; Nisbet, D.J.; Ricke, S.C. Incidence and ecology of Campylobacter jejuni and coli in animals. Anaerobe 2009, 15, 18–25. [Google Scholar] [CrossRef] [PubMed]
- ISO 6887-1:2017; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilution. 2nd ed. ISO: Geneva, Switzerland, 2017.
- Igwaran, A.; Okoh, A.I. Human campylobacteriosis: A public health concern of global importance. Heliyon 2019, 5, e02814. [Google Scholar] [CrossRef] [PubMed]
- ISO 16140-2:2016; Microbiology of the Food Chain—Method Validation—Part 2: Protocol for the Validation of Alternative (Proprietary) Methods against a Reference Method, 2016-First Edition. ISO: Geneva, Switzerland, 2016.
- Crowley, E.S.; Bird, P.M.; Torontali, M.K.; Agin, J.R.; Goins, D.G.; Johnson, R. TEMPO® TVC for the enumeration of aerobic mesophilic flora in foods: Collaborative study. J. AOAC Int. 2009, 92, 165–174. [Google Scholar] [CrossRef] [PubMed]
- NF Validation. Validation of Alternative Analytical Methods Certificate Number: BIO 12--/43-04/20. 1–47. Available online: https://nf-validation.afnor.org/en/wp-content/uploads/sites/2/2020/06/Synt-BIO-12-43-04-20_en.pdf (accessed on 21 March 2023).
- Crowley, E.; Bird, P.; Torontali, M.; Goetz, K.; Agin, J.; Goins, D.; Johnson, R. TEMPO® EC for the enumeration of Escherichia coli in foods: Collaborative study. J. AOAC Int. 2010, 93, 576–586. [Google Scholar] [CrossRef]
- Cirolini, A.; Baseggio, A.M.; Miotto, M.; Ramos, R.J.; Cattani, C.S.d.O.; Vieira, C.R.W. Evaluation of the PetrifilmTM and TEMPO® systems and the conventional method for counting microorganisms in pasteurized milk. Food Sci. Technol. 2013, 33, 784–789. [Google Scholar] [CrossRef]
- APHA—American Public Health Association. Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; APHA: Washington, DC, USA, 2001; Chapter 6. [Google Scholar]
- Franco, B.D.G.M.; Landgraf, M.; Destro, M.T. Capítulo 10—Métodos de Análise. In Microbiologia dos Alimentos; Atheneu: São Paulo, Brazil, 2008; pp. 168–169. [Google Scholar]
- Perdoncini, G.; Argello, Y.M.S.; Lima, L.M.; Furian, T.Q.; Borges, K.A.; Rodrigues, L.B.; dos Santos, L.R.; Borsoi, A.; Isolan, L.W.; Gomes, M.J.P.; et al. Detection and quantification of Campylobacter in poultry slaughterhouses using conventional microbiological technique, most probable number, and real-time PCR. Foodborne Pathog. Dis. 2022, 19, 143–150. [Google Scholar] [CrossRef]
- Lazou, T.P.; Gelasakis, A.I.; Chaintoutis, S.C.; Iossifidou, E.G.; Dovas, C.I. Method-dependent implications in foodborne pathogen quantification: The case of Campylobacter coli survival on meat as comparatively assessed by colony count and viability PCR. Front. Microbiol. 2021, 12, 604933. [Google Scholar] [CrossRef]
- Castañeda-Gulla, K.; Sattlegger, E.; Mutukumira, A.N. Persistent contamination on Salmonella, Campylobacter, Escherichia coli, and Staphylococcus aureus at a broiler farm in New Zealand. Can. J. Microbiol. 2020, 66, 171–185. [Google Scholar] [CrossRef]
- Irwin, P.L.; Nguyen LH, T.; Chen, C.Y. The relationship between purely stochastic sampling error and the number of technical replicates used to estimate concentration at an extreme dilution. Anal. Bioanal. Chem. 2010, 398, 895–903. [Google Scholar] [CrossRef]
- Repérant, E.; Laisney, M.J.; Nagard, B.; Quesne, S.; Rouxel, S.; Le Gall, F.; Chemaly, M.; Denis, M. Influence of enrichment and isolation media on the detection of Campylobacter sIn naturally contaminated chicken samples. J. Microbiol. Methods 2016, 128, 42–47. [Google Scholar] [CrossRef]
- Bovill, R.A.; Mackey, B.M. Resuscitation of ‘non-culturable’ cells from aged cultures of Campylobacter jejuni. Microbiology 1997, 143, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Magistrado, P.A.; Garcia, M.M.; Raymundo, A.K. Isolation and polymerase chain reaction-based detection of Campylobacter jejuni and Campylobacter coli from poultry in the Philippines. Int. J. Food Microbiol. 2001, 70, 197–206. [Google Scholar] [CrossRef]
- Seliwiorstow, T.; Duarte, A.; Baré, J.; Botteldoorn, N.; Dierick, K.; Uyttendaele, M.; De Zutter, L. Comparison of Sample Types and Analytical Methods for the Detection of Highly Campylobacter-Colonized Broiler Flocks at Different Stages in the Poultry Meat Production Chain. Foodborne Pathog. Dis. 2015, 12, 399–405. [Google Scholar] [CrossRef]
- Blodget, R.J.; Garthright, W.E. Several MPN models for serial dilutions with suppressed growth at low dilutions. Food Microbiol. 1998, 15, 91–99. [Google Scholar] [CrossRef]
- Zits, U.; Domig, K.J.; Hoehl, A.; Weiss, H.; Wilrich, P.T.; Kneifel, W. Evaluation three applications of a semi-automated most-probable-number method for the assessment of microbiological parameters in dairy products. Accredit. Qual. Assur. 2011, 16, 299–309. [Google Scholar] [CrossRef]
- Bhaduri, S.; Cottrell, B. Survival of cold-stressed Campylobacter jejuni on ground chickens and chicken skin during frozen storage. Appl. Environ. Microbiol. 2004, 70, 7103–7109. [Google Scholar] [CrossRef]
- ABPA—Associação Brasileira de Proteína Animal. Annual Report 2021. 2021, pp. 1–75. Available online: https://abpa-br.org/wp-content/uploads/2023/04/Relatorio-Anual-2023.pdf (accessed on 9 September 2024).
- Bortoli, W.; Bortoli, E.d.S.; Dalmina, K.A.; Melo, F.D.; Costa UM, d.a.; Ferraz, S.M. Ocurrence of Campylobacter sin chilled chicken carcasses slaughtered in the west region of Santa Catarina, Brazil. Acta Sci. Vet. 2017, 45, 6. [Google Scholar] [CrossRef]
- Jasson, V.; Jacxsens, L.; Luning, P.; Rajkovic, A.; Uyttendaele, M. Alternative microbial methods: An overview and selection criteria. Food Microbiol. 2010, 27, 710–730. [Google Scholar] [CrossRef]
- Tavolaro, P.; Ferrati, A.R.; Destro, M.T.; Landgraf, M.; Franco, B.D.G.M. Performance of two ready-to-use systems for enumeration of aerobic mesophilic microorganisms in frozen goat milk. Braz. J. Microbiol. 2005, 36, 295–300. [Google Scholar] [CrossRef]

| Reference | Country | Average Value of Prevalence in Turkeys (%) |
|---|---|---|
| [48] | Poland | 38.0 |
| [10] | Europe | 65.3 |
| [49] | Canada | 38.2 |
| [50] | Turkey | 45.6 |
| [51] | Iran | 28.8 |
| [52] | Iran | 32.8 |
| [53] | Germany | 29.2 |
| [54] | Ireland | 37.5 |
| Component | Volume a |
|---|---|
| Foodproof Campylobacter Master Mix | 18 μL |
| Foodproof Campylobacter Enzyme Solution | 1 μL |
| Foodproof Campylobacter Internal Control | 1 μL |
| FAM | ROX | Cy5 | HEX | Result |
|---|---|---|---|---|
| + | - | - | + or - | Campylobacter spp. detected |
| + | + | - | + or - | Positive for Campylobacter spp. and C. jejuni |
| + | - | + | + or - | Positive for Campylobacter spp. and C. coli |
| + | + | + | + or - | Positive for Campylobacter spp., C. jejuni and C. coli |
| - | - | - | + | Negative for Campylobacter spp., C. jejuni and C. coli |
| - | - | - | - | Result invalid |
| Campylobacter spp. Count log10 CFU/g (Mean) | |||
|---|---|---|---|
| Material | ISO 10272-2 Method | TEMPO® CAM Method | rtPCR Method (Biotecon®) |
| Chilled turkey wings | 0.09 | 0.51 | 0.14 |
| Frozen turkey wings | 0.00 | 0.38 | 0.04 |
| Chilled turkey drumsticks | 0.10 | 0.54 | 0.04 |
| Frozen turkey drumsticks | 0.04 | 0.39 | 0.09 |
| Chilled turkey breast | 0.07 | 0.54 | 0.04 |
| Frozen turkey breast | 0.01 | 0.37 | 0.07 |
| Quantification Results (Mean ± SD) | |||
|---|---|---|---|
| ISO 10272-2 | TEMPO® CAM | rtPCR Biotecon® | |
| log10 CFU/g | log10 CFU/g | log10 CFU/g | |
| Chilled cuts | 0.17 ± 0.53 | 1.09 ± 1.15 | 0.13 ± 0.53 |
| Frozen cuts | 0.02 ± 0.20 | 0.75 ± 0.99 | 0.09 ± 0.46 |
| Chilled and frozen cuts | 0.09 ± 0.41 | 0.91 ± 1.18 | 0.11 ± 0.49 |
| Condition of Sample | Comparison of Methods | Kruskal–Wallis Test | Bland–Altman Test |
|---|---|---|---|
| Chilled | ISO vs. Tempo | Statistically different | Outside expected range |
| ISO vs. rtPCR | Statistically equal | Outside expected range | |
| Frozen | ISO vs. Tempo | Statistically different | Outside expected range |
| ISO vs. rtPCR | Statistically equal | Within expected range |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Führ, C.A.; Giombelli, A.; Cerutti, M.F.; Bergmann, G.P.; Kindlein, L. Comparative Analysis of Quantitative Methods for Campylobacter spp. Quantification: ISO 10272-2:2017, Tempo® and Real-Time PCR in Refrigerated and Frozen Turkey Cuts. Foods 2024, 13, 3359. https://doi.org/10.3390/foods13213359
Führ CA, Giombelli A, Cerutti MF, Bergmann GP, Kindlein L. Comparative Analysis of Quantitative Methods for Campylobacter spp. Quantification: ISO 10272-2:2017, Tempo® and Real-Time PCR in Refrigerated and Frozen Turkey Cuts. Foods. 2024; 13(21):3359. https://doi.org/10.3390/foods13213359
Chicago/Turabian StyleFühr, Carlos Alberto, Audecir Giombelli, Marisete Fochesatto Cerutti, Guiomar Pedro Bergmann, and Liris Kindlein. 2024. "Comparative Analysis of Quantitative Methods for Campylobacter spp. Quantification: ISO 10272-2:2017, Tempo® and Real-Time PCR in Refrigerated and Frozen Turkey Cuts" Foods 13, no. 21: 3359. https://doi.org/10.3390/foods13213359
APA StyleFühr, C. A., Giombelli, A., Cerutti, M. F., Bergmann, G. P., & Kindlein, L. (2024). Comparative Analysis of Quantitative Methods for Campylobacter spp. Quantification: ISO 10272-2:2017, Tempo® and Real-Time PCR in Refrigerated and Frozen Turkey Cuts. Foods, 13(21), 3359. https://doi.org/10.3390/foods13213359





