Evaluating the Human Risks of Consumption of Foods of Bovine Origin with Ivermectin Residues in Ecuador
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Chemical Analysis
2.3. Food Consumption Survey
2.4. Risk to Consumer Health
3. Results and Discussion
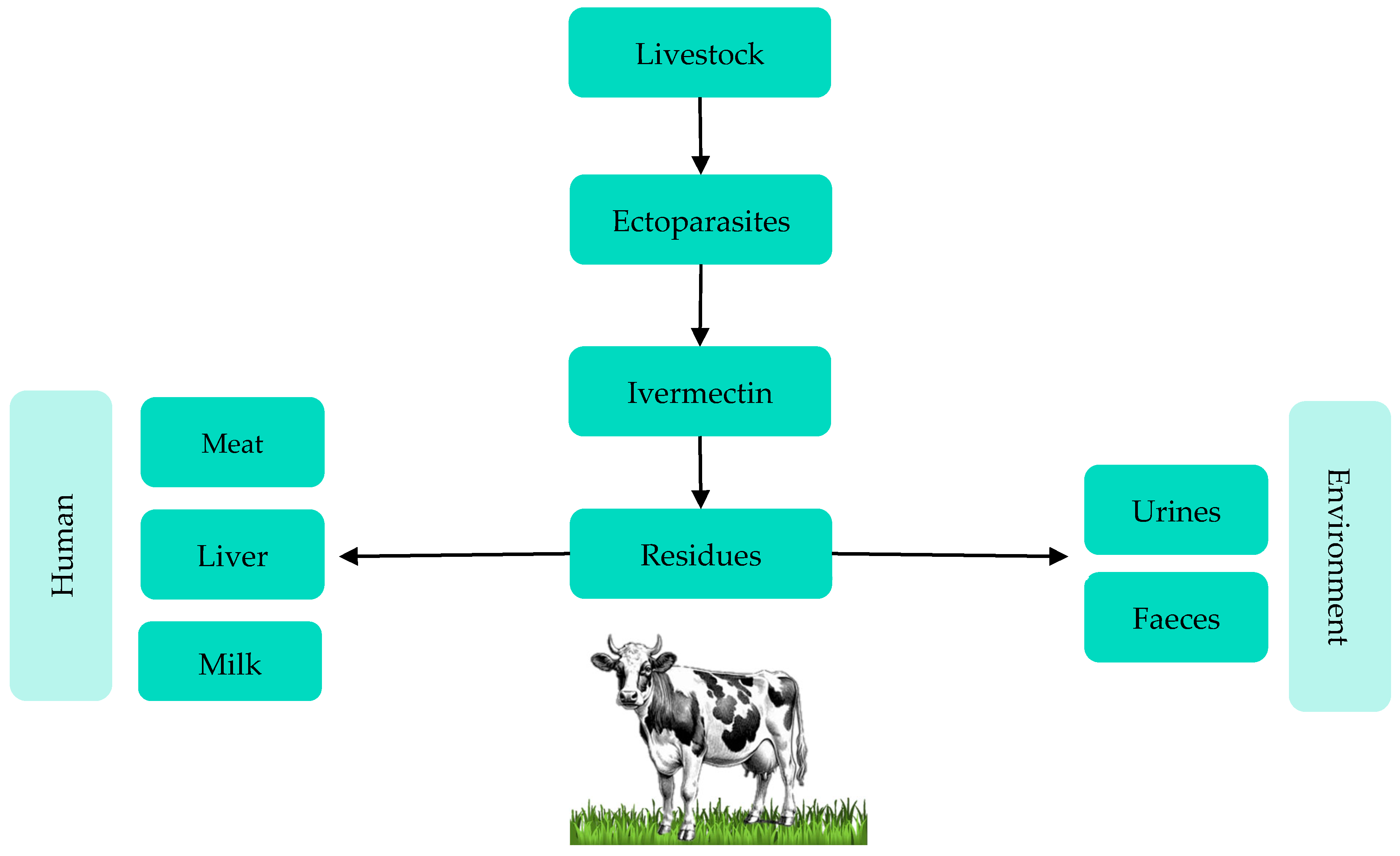
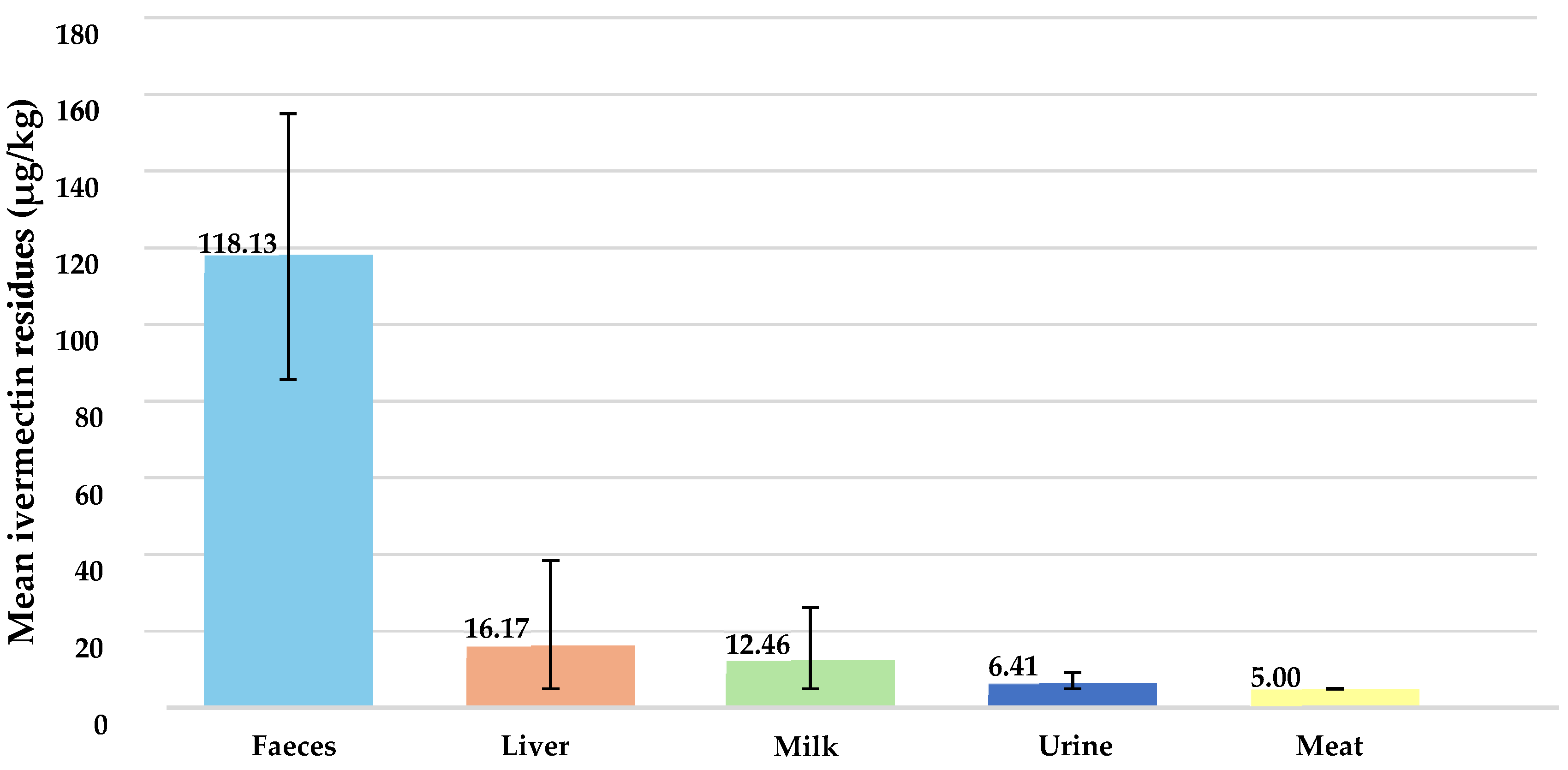
3.1. Ivermectin Residues
3.2. Consumption of Foods of Bovine Origin
3.3. Risk Assessment for Consumers of Ivermectin-Contaminated Foods of Bovine Origin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; CIRAD; ILRI. Livestock Sector Investment and Policy Toolkit (LSIPT)—Making Responsible Decisions; FAO: Rome, Italy, 2020. [Google Scholar]
- Boateng, E.F.; Nasiru, M.M.; Agyemang, M. Meat: Valuable Animal-Derived Nutritional Food. A Review. Asian Food Sci. J. 2020, 15, 9–19. [Google Scholar] [CrossRef]
- Woźniak, D.; Cichy, W.; Dobrzyńska, M.; Przysławski, J.; Drzymała-Czyż, S. Reasonableness of Enriching Cow’s Milk with Vitamins and Minerals. Foods 2022, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- FAO. Dairy Market Review: Overview of Global Market Developments in 2023; FAO: Rome, Italy, 2024; Available online: http://www.nmpf.org/files/DMReport_March31.pdf (accessed on 5 June 2024).
- FAO. Meat Market Review: Overview of Global Market Developments in 2023; FAO: Rome, Italy, 2024. [Google Scholar]
- INEC. Encuesta de Superficie y Producción Agropecuaria Continua; Instituto Nacional de Estadística y Censos: Quito, Ecuador, 2024. [Google Scholar]
- Agrocalidad. Categorías de Población de Ganado Bovino de Ecuador. 2018. Available online: https://www.agricultura.gob.ec/wp-content/uploads/2019/09/ANEXO-1.pdf (accessed on 28 March 2024).
- Rodríguez-Hidalgo, R.; Pérez-Otáñez, X.; Garcés-Carrera, S.; Vanwambeke, S.O.; Madder, M.; Benítez-Ortiz, W. The current status of resistance to alpha-cypermethrin, ivermectin, and amitraz of the cattle tick (Rhipicephalus microplus) in Ecuador. PLoS ONE 2017, 12, e0174652. [Google Scholar] [CrossRef]
- D’Auria, M.; Guarnaccio, A.; Racioppi, R.; Stoia, S.; Emanuele, L. Photodegradation of Drugs and Crop Protection Products. In Photochemistry of Heterocycles; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Pandit, M.A.; Tarkeshwar. Ivermectin: An Anthelminthic and Insecticide. In Chemistry and Biological Activities of Ivermectin; Wiley: Hoboken, NJ, USA, 2023; pp. 163–197. [Google Scholar] [CrossRef]
- Pecenka, J.R.; Lundgren, J.G. Effects of herd management and the use of ivermectin on dung arthropod communities in grasslands. Basic Appl. Ecol. 2019, 40, 19–29. [Google Scholar] [CrossRef]
- Mancini, L.; Lacchetti, I.; Chiudioni, F.; Cristiano, W.; Kevin, D.D.; Marcheggiani, S.; Carere, M.; Bindi, L.; Borrello, S. Need for a sustainable use of medicinal products: Environmental impacts of ivermectin. Ann. Ist. Super. Sanita 2020, 56, 492–496. [Google Scholar] [CrossRef]
- Soares, V.M.; Pereira, J.G.; Barreto, F.; Jank, L.; Rau, R.B.; Ribeiro, C.B.D.; dos Santos Castilhos, T.; Tomaszewski, C.A.; Hillesheim, D.R.; Mondadori, R.G.; et al. Residues of Veterinary Drugs in Animal Products Commercialized in the Border Region of Brazil, Argentina, and Uruguay. J. Food Prot. 2022, 85, 980–986. [Google Scholar] [CrossRef]
- FAO/WHO. Evaluation of Certain Veterinary Drug Residues in Food: Ninety-Fourth Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series No. 1041; WHO: Geneva, Switzerland, 2022; pp. 301–302. [Google Scholar] [CrossRef]
- FAO/WHO. Maximum Residue Limits (MRLs) and Risk Management Recommendations (RMRs) for Residues of Veterinary Drugs in Foods; World Health Organization—Technical Report Series; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Dedavid e Silva, L.A.; Ali, A.; Termignoni, C.; Vaz Júnior, I.d.S. Vaccination against Rhipicephalus microplus: An alternative to chemical control? Cienc. Rural 2024, 54, e20230161. [Google Scholar] [CrossRef]
- Nyokabi, S.; Luning, P.A.; de Boer, I.J.; Korir, L.; Muunda, E.; Bebe, B.O.; Lindahl, J.; Bett, B.; Oosting, S.J. Milk quality and hygiene: Knowledge, attitudes and practices of smallholder dairy farmers in central Kenya. Food Control 2021, 130, 108303. [Google Scholar] [CrossRef]
- Paucar-Quishpe, V.; Pérez-Otáñez, X.; Rodríguez-Hidalgo, R.; Cepeda-Bastidas, D.; Pérez-Escalante, C.; Grijalva-Olmedo, J.; Enríquez, S.; Arciniegas-Ortega, S.; Sandoval-Trávez, L. An economic evaluation of cattle tick acaricide-resistances and the financial losses in subtropical dairy farms of Ecuador: A farm system approach. PLoS ONE 2023, 18, e0287104. [Google Scholar] [CrossRef]
- CIL. En Ecuador, dos de cada tres litros de leche comercializan de modo informal; Centro De La Industria Láctea Del Ecuador: Quito, Ecuador, 2023; Available online: https://www.cil-ecuador.org/post/en-ecuador-dos-de-cada-tres-litros-de-leche-comercializan-de-modo-informal#:~:text=2min.-,EnEcuador%2Cdosdecadatreslitrosdelechecomercializan,5%27500.000litrospordía (accessed on 14 August 2024).
- Choco Andino Pichincha. Reserva de biósfera del Chocó Andino De Pichincha. Available online: https://www.chocoandinopichincha.com/ (accessed on 24 October 2022).
- Ministerio de Ambiente y Agua. Plan de Manejo Parque Nacional Cayambe Coca; Ministerio de Ambiente y Agua: Quito, Ecuador, 2022. [CrossRef]
- Benavides, B. Análisis de los Sistemas Productivos Agropecuarios Ganaderos en el Noroccidente de Pichincha; Repositorio Digital-Universidad Central del Ecuador: Quito, Ecuador, 2022. [Google Scholar]
- PDOT Quijos. Plan de Desarrollo y Ordenamiento Territorial del Cantón Quijos; Dirección de Planificación y Ordenamiento Territorial, GAD Municipal Quijos: Quito, Ecuador, 2024; pp. 1–394. [Google Scholar]
- Paucar, V.; Ron-Román, J.; Benítez-Ortiz, W.; Celi, M.; Berkvens, D.; Saegerman, C.; Ron-Garrido, L. Bayesian Estimation of the Prevalence and Test Characteristics (Sensitivity and Specificity) of Two Serological Tests (RB and SAT-EDTA) for the Diagnosis of Bovine Brucellosis in Small and Medium Cattle Holders in Ecuador. Microorganisms 2021, 9, 1815. [Google Scholar] [CrossRef]
- Agrocalidad. Reporte Vacunacion Fiebre Aftosa; Agencia de Regulación y Control Fito y Zoosanitario: Quito, Ecuador, 2023. [Google Scholar]
- INEN Acreditación de laboratorios de ensayo y calibración según. NTE INEN- ISO/IEC 17025:2018; INEN: Quito, Ecuador, 2018.
- INEC Censo De Población Y Vivienda (PV 2010). 2010. Available online: https://www.ecuadorencifras.gob.ec/censo-de-poblacion-y-vivienda/ (accessed on 31 May 2024).
- Carrasco Cabrera, L.; Medina Pastor, P.; EFSA. The 2020 European Union report on pesticide residues in food. EFSA J. 2022, 20, e07215. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Instructions for Electronic Submission of Data on Chemical Contaminants in Food and the Diet. Global Environment Monitoring System—Food Contamination Monitoring and Assessment Programme (GEMS/Food); Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2023. [Google Scholar]
- Hubal, E.A.C.; de Wet, T.; Du Toit, L.; Firestone, M.P.; Ruchirawat, M.; van Engelen, J.; Vickers, C. Identifying important life stages for monitoring and assessing risks from exposures to environmental contaminants: Results of a World Health Organization review. Regul. Toxicol. Pharmacol. 2014, 69, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Hagan, J.; Shaw, J.; Duncan, P. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents, 4th ed.; American Academy of Pediatrics: Itasca, Illinois, USA, 2017. [Google Scholar] [CrossRef]
- FAO/WHO. Joint FAO/WHO Expert Meeting on Dietary Exposure Assessment Methodologies for Residues of Veterinary Drugs: Final Report Including Report of Stakeholder Meeting; World Health Organization: Geneva, Switzerland, 2012; Available online: https://www.fao.org/fileadmin/user_upload/agns/pdf/jecfa/Dietary_Exposure_Assessment_Methodologies_for_Residues_of_Veterinary_Drugs.pdf (accessed on 22 July 2024).
- Novaes, S.F.D.; Schreiner, L.L.; Silva, I.P.E.; Franco, R.M. Residues of veterinary drugs in milk in Brazil. Ciência Rural 2017, 47, e20170215. [Google Scholar] [CrossRef][Green Version]
- EMA. Opinion of the Committee for Medicinal Products for Veterinary Use on the establishment of maximum residue limits; procedure no: EU/09/170/SCM; name of the substance: Octenidine dihydrochloride (INN). Vet. Med. Prod. Data Manag. Opin. 2013, 44, 1–3. [Google Scholar]
- Health Canada. List of Maximum Residue Limits (MRLs) for Veterinary Drugs in Foods. In Food and Drugs Act; Health Canada: Ottawa, ON, Canada, 2024. [Google Scholar]
- Paucar, V.; Pérez-Otáñez, X.; Rodríguez-Hidalgo, R.; Perez, C.; Cepeda-Bastidas, D.; Grijalva, J.; Enríquez, S.; Arciniegas-Ortega, S.; Vanwambeke, S.O.; Ron-Garrido, L.; et al. The Associated Decision and Management Factors on Cattle Tick Level of Infestation in Two Tropical Areas of Ecuador. Pathogens 2022, 11, 403. [Google Scholar] [CrossRef]
- Brito, S.N. Determinación de residuos de antibióticos en carne de ganado bovino por el método de ELISA en el Centro de Faenamiento de la Empresa Pública Metropolitana de rastro Quito- La Ecuatoriana; Repositorio Digital-Universidad Central del Ecuador: Quito, Ecuador, 2017; p. 168. [Google Scholar]
- Puga-Torres, B.; Aragón Vásquez, E.; Ron, L.; Álvarez, V.; Bonilla, S.; Guzmán, A.; Lara, D.; De la Torre, D. Milk Quality Parameters of Raw Milk in Ecuador between 2010 and 2020: A Systematic Literature Review and Meta-Analysis. Foods 2022, 11, 3351. [Google Scholar] [CrossRef]
- Puga-Torres, B.; Aragón, E.; Contreras, A.; Escobar, D.; Guevara, K.; Herrera, L.; López, N.; Luje, D.; Martínez, M.; Sánchez, L.; et al. Analysis of quality and antibiotic residues in raw milk marketed informally in the Province of Pichincha–Ecuador. Food Agric. Immunol. 2024, 35, 2291321. [Google Scholar] [CrossRef]
- de la Cueva, F.; Naranjo, A.; Torres, B.P.; Aragón, E. Presence of heavy metals in raw bovine milk from Machachi, Ecuador. Granja 2021, 33, 21–30. [Google Scholar] [CrossRef]
- Freire, N. Evaluacion cuantitativa de residuos en carne de ganado de engorde, post aplicacion pour-on, del producto fipronil mas ivermectina. Univ. Cent. Ecuad. 2017, 14, 1–49. [Google Scholar]
- Balseca, P. Determinacion cuantitativa de residuos en leche de epronomectina usado como mosquicida en vacas lecheras; Universidad Central del Ecuador: Quito, Ecuador, 2017; pp. 1–44. [Google Scholar]
- Junco, M.; Iglesias, L.E.; Sagués, M.F.; Guerrero, I.; Zegbi, S.; Saumell, C.A. Effect of macrocyclic lactones on nontarget coprophilic organisms: A review. Parasitol. Res. 2021, 120, 773–783. [Google Scholar] [CrossRef]
- Finch, D.; Schofield, H.; Floate, K.D.; Kubasiewicz, L.M.; Mathews, F. Implications of Endectocide Residues on the Survival of Aphodiine Dung Beetles: A Meta-Analysis. Environ. Toxicol. Chem. 2020, 39, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Herd, R.P.; Sams, R.A.; Ashcraft, S.M. Persistence of ivermectin in plasma and faeces following treatment of cows with ivermectin sustained-release, pour-on or injectable formulations. Int. J. Parasitol. 1996, 26, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Suárez, V.H.; Lifschitz, A.L.; Sallovitz, J.M.; Lanusse, C.E. Effects of faecal residues of moxidectin and doramectin on the activity of arthropods in cattle dung. Ecotoxicol. Environ. Saf. 2009, 72, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Lumaret, J.P.; Galante, E.; Lumbreras, C.; Mena, J.; Bertrand, M.; Bernal, J.L.; Cooper, J.F.; Kadiri, N.; Crowe, D. Field Effects of Ivermectin Residues on Dung Beetles. J. Appl. Ecol. 1993, 30, 428–436. [Google Scholar] [CrossRef]
- Kövecses, J.; Marcogliese, D.J. Avermectins: Potential Environmental Risks and Impacts on Freshwater Ecosystems in Quebec; Scientific and Technical Report ST-233E; Environment Canada, Quebec Region, Environmental Conservation, St. Lawrence Centre: Montréal, QC, Canada, 2005. [Google Scholar]
- Wohde, M.; Blanckenhorn, W.U.; Floate, K.D.; Lahr, J.; Lumaret, J.P.; Römbke, J.; Scheffczyk, A.; Tixier, T.; Düring, R.A. Analysis and dissipation of the antiparasitic agent ivermectin in cattle dung under different field conditions. Environ. Toxicol. Chem. 2016, 35, 1924–1933. [Google Scholar] [CrossRef]
- Mendoza, M.; El Comercio. El 36% de la carne sale de camales clandestinos. 2017. Available online: https://www.elcomercio.com/actualidad/quito/carne-camales-clandestinos-quito-normativa.html (accessed on 7 June 2024).
- Castillo, M.J.; Carpio, C.E. Demand for High-Quality Beef Attributes in Developing Countries: The Case of Ecuador. J. Agric. Appl. Econ. 2019, 51, 568–590. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M.; Meat and Dairy Production. OurWorldInData.org. Available online: https://ourworldindata.org/meat-production (accessed on 7 June 2024).
- Baquerizo, V.; Córdova, V. Impacto económico del sector lácteo: Un estudio de los gastos publicitarios y las ventas en tiempos de pandemia. 593 Digit. Publ. CEIT 2022, 7, 310–321. [Google Scholar] [CrossRef]
- CIL. El sector lácteo ecuatoriano se reactiva con miras positivas para el 2022; Centro de Industria Láctea del Ecuador: Quito, Ecuador, 2022; Available online: https://www.cil-ecuador.org/post/el-sector-lácteo-ecuatoriano-se-reactiva-con-miras-positivas-para-el-2022 (accessed on 21 October 2023).
- Bermeo, F. Seguridad alimentaria; Responsabilidad de los Gobiernos Autónomos Descentralizados Provinciales. Flacsoandes 2015, 13, 1–4. [Google Scholar]
- CIL. Entre 2022 y 2023 el consumo de lácteos en Ecuador cayó un 12%; Centro De La Industria Láctea Del Ecuador: Quito, Ecuador, 2024. [Google Scholar]
- JECFA. Toxicological Evaluation of Certain Veterinary Drug Residues in Food- Eighty-first meeting of the Joint FAO/WHO Expert Committee on Food Additives; WHO: Geneva, Switzerland, 2016; Volume 18. [Google Scholar]
- Lankas, G.R.; Minsker, D.H.; Robertson, R.T. Effects of ivermectin on reproduction and neonatal toxicity in rats. Food Chem. Toxicol. 1989, 27, 523–529. [Google Scholar] [CrossRef]
- Hoang, R.; Temple, C.; Correia, M.S.; Clemons, J.; Hendrickson, R.G. Characteristics of ivermectin toxicity in patients taking veterinary and human formulations for the prevention and treatment of COVID-19. Clin. Toxicol. 2022, 60, 1350–1355. [Google Scholar] [CrossRef]
- Vragović, N.; Bažulić, D.; Njari, B. Risk assessment of streptomycin and tetracycline residues in meat and milk on Croatian market. Food Chem. Toxicol. 2011, 49, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Agrocalidad. Centros de faenamiento habilitados como MABIO utilizarán logo para diferenciar sus productos; Agencia de Regulación y Control Fito y Zoosanitario: Quito, Ecuador, 2020; Available online: https://actoresproductivos.com/agrocalidad-presenta-oficialmente-el-logotipo-mabio// (accessed on 9 June 2024).
- USP 42, Official Monographs, Ivermectin; United Stated Pharmacopeial Convention: Rockville, MD, USA, 2020; pp. 2555–2556.
- Nuñez, M.; Palma, M.; Araneda, M.; Pérez, R. Validation of an analytical method and determination of ivermectin residues in sheep tissues. Rev. Cient. 2007, 17, 6. [Google Scholar]
 provinces of Pichincha (right) and Napo (left);
provinces of Pichincha (right) and Napo (left);  Quito (country capital);
Quito (country capital);  Area 1 (northwest of Pichincha);
Area 1 (northwest of Pichincha);  Area 2 (Quijos River Valley);
Area 2 (Quijos River Valley);  populated areas where surveys were conducted;
populated areas where surveys were conducted;  location of slaughterhouses; and
location of slaughterhouses; and  main routes of the milk collection trucks.
main routes of the milk collection trucks.
 provinces of Pichincha (right) and Napo (left);
provinces of Pichincha (right) and Napo (left);  Quito (country capital);
Quito (country capital);  Area 1 (northwest of Pichincha);
Area 1 (northwest of Pichincha);  Area 2 (Quijos River Valley);
Area 2 (Quijos River Valley);  populated areas where surveys were conducted;
populated areas where surveys were conducted;  location of slaughterhouses; and
location of slaughterhouses; and  main routes of the milk collection trucks.
main routes of the milk collection trucks.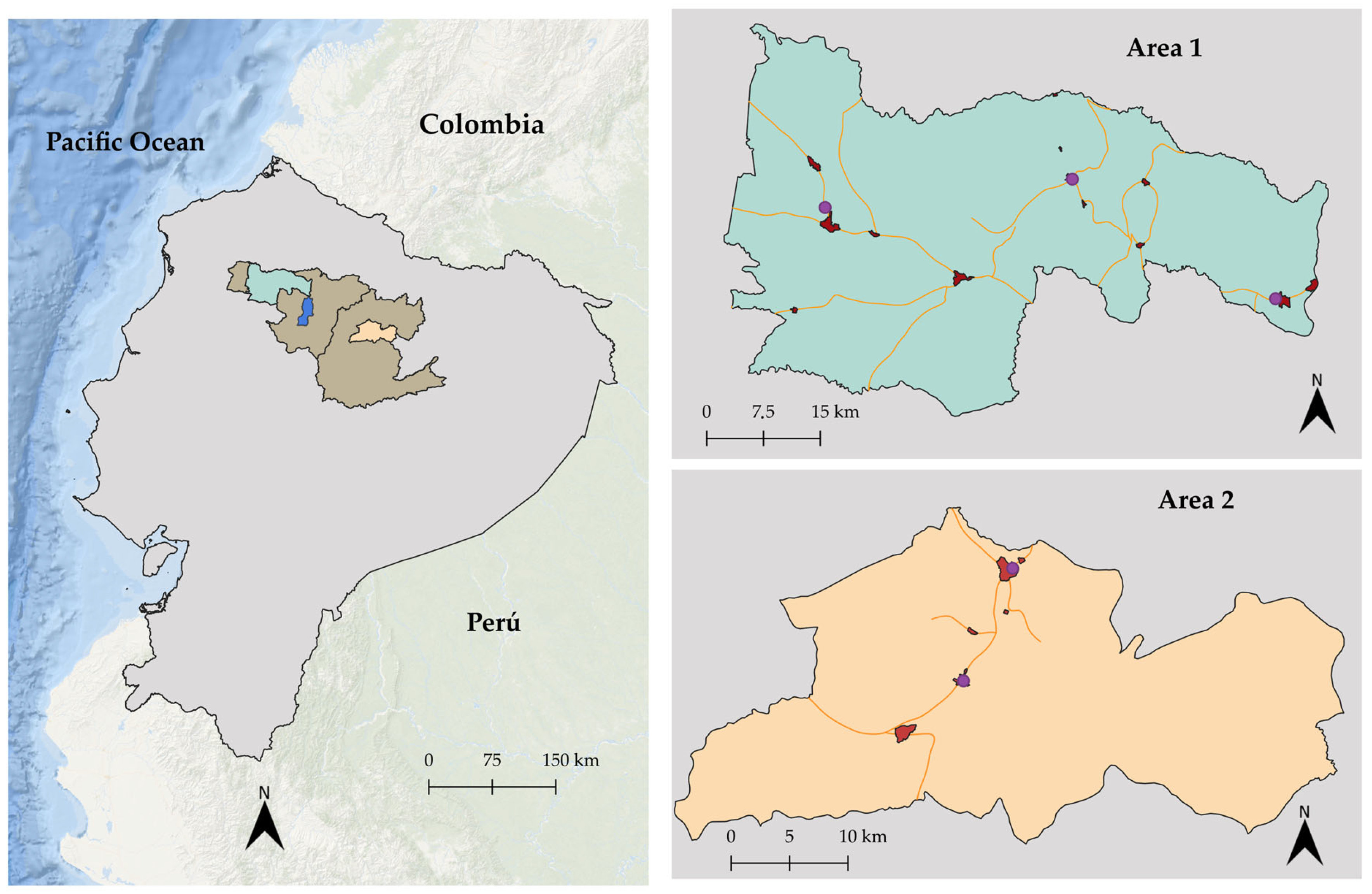

| Product | Households (%) | Less or Equal to 10 Years Old | Higher than 10 Years Old | ||||
|---|---|---|---|---|---|---|---|
| Consumers (%) | DC A | DC B | Consumers (%) | DC A | DC B | ||
| Milk | 97 | 98 | 94.76 | 98.24 | 97 | 95.85 | 102.15 |
| Meat | 91 | 91 | 5.88 | 6.45 | 92 | 30.95 | 35.62 |
| Liver | 30 | 32 | 0.14 | 0.35 | 30 | 0.75 | 2.27 |
| Food of Bovine Origin | Median Residue Concentration (µg/kg) | Mean Residue Concentration (µg/kg) | Consumption Percentile 97.5th (kg/day) | Consumption Means (kg/day) | bw (kg) | EDI (µg/bw/day) | EDI (µg/kg bw/day) | Exposure (µg/kg bw/day) | GECDE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 97.5th | Mean | µg/kg bw/day | %ADI | ||||||||||
| Less or equal to 10 years old | Milk | 5.0000 | 12.4571 | 0.2404 | 0.0948 | 13.4935 | 1.1804 | 0.0875 | 0.0891 | 0.0351 | 0.0891 | 0.8908 | |
| A | Meat | 5.0000 | 5.0000 | 0.0118 | 0.0059 | 13.4935 | 0.0294 | 0.0022 | 0.0044 | 0.0022 | 0.0022 | 0.0218 | |
| Liver | 5.0000 | 16.1667 | 0.0008 | 0.0001 | 13.4935 | 0.0022 | 0.0002 | 0.0003 | 0.0001 | 0.0001 | 0.0005 | ||
| TOTAL | 1.2120 | 0.0899 | 0.0937 | 0.0373 | 0.0913 | 0.9131 | |||||||
| Higher than 10 years old | Milk | 5.0000 | 12.4571 | 0.3668 | 0.0958 | 68.3891 | 1.1940 | 0.0175 | 0.0268 | 0.0070 | 0.0268 | 0.0027 | |
| A | Meat | 5.0000 | 5.0000 | 0.0724 | 0.0309 | 68.3891 | 0.1547 | 0.0023 | 0.0053 | 0.0023 | 0.0023 | 0.0002 | |
| Liver | 5.0000 | 16.1667 | 0.0056 | 0.0008 | 68.3891 | 0.0122 | 0.0002 | 0.0004 | 0.0001 | 0.0001 | 0.0000 | ||
| TOTAL | 1.2609 | 0.0200 | 0.0325 | 0.0093 | 0.0291 | 0.0029 | |||||||
| Less or equal to 10 years old | Milk | 5.0000 | 12.4571 | 0.2697 | 0.0982 | 13.4935 | 1.2238 | 0.0907 | 0.0999 | 0.0364 | 0.0999 | 0.9993 | |
| B | Meat | 5.0000 | 5.0000 | 0.0133 | 0.0065 | 13.4935 | 0.0323 | 0.0024 | 0.0049 | 0.0024 | 0.0024 | 0.0239 | |
| Liver | 5.0000 | 16.1667 | 0.0011 | 0.0004 | 13.4935 | 0.0057 | 0.0004 | 0.0004 | 0.0001 | 0.0001 | 0.0013 | ||
| TOTAL | 1.2618 | 0.0935 | 0.1053 | 0.0389 | 0.1025 | 1.0246 | |||||||
| Higher than 10 years old | Milk | 5.0000 | 12.4571 | 0.3668 | 0.1022 | 68.3891 | 1.2725 | 0.0186 | 0.0268 | 0.0075 | 0.0268 | 0.2682 | |
| B | Meat | 5.0000 | 5.0000 | 0.0733 | 0.0356 | 68.3891 | 0.1781 | 0.0026 | 0.0054 | 0.0026 | 0.0026 | 0.0260 | |
| Liver | 5.0000 | 16.1667 | 0.0072 | 0.0023 | 68.3891 | 0.0367 | 0.0005 | 0.0005 | 0.0002 | 0.0002 | 0.0017 | ||
| TOTAL | 1.4873 | 0.0217 | 0.0327 | 0.0102 | 0.0296 | 0.2959 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paucar-Quishpe, V.; Cepeda-Bastidas, D.; Rodríguez-Hidalgo, R.; Pérez-Otáñez, X.; Perez, C.; Enríquez, S.; Guzman, E.; Ulcuango, F.; Grijalva, J.; Vanwambeke, S.O.; et al. Evaluating the Human Risks of Consumption of Foods of Bovine Origin with Ivermectin Residues in Ecuador. Foods 2024, 13, 3470. https://doi.org/10.3390/foods13213470
Paucar-Quishpe V, Cepeda-Bastidas D, Rodríguez-Hidalgo R, Pérez-Otáñez X, Perez C, Enríquez S, Guzman E, Ulcuango F, Grijalva J, Vanwambeke SO, et al. Evaluating the Human Risks of Consumption of Foods of Bovine Origin with Ivermectin Residues in Ecuador. Foods. 2024; 13(21):3470. https://doi.org/10.3390/foods13213470
Chicago/Turabian StylePaucar-Quishpe, Valeria, Darío Cepeda-Bastidas, Richar Rodríguez-Hidalgo, Ximena Pérez-Otáñez, Cecilia Perez, Sandra Enríquez, Erika Guzman, Fernanda Ulcuango, Jorge Grijalva, Sophie O. Vanwambeke, and et al. 2024. "Evaluating the Human Risks of Consumption of Foods of Bovine Origin with Ivermectin Residues in Ecuador" Foods 13, no. 21: 3470. https://doi.org/10.3390/foods13213470
APA StylePaucar-Quishpe, V., Cepeda-Bastidas, D., Rodríguez-Hidalgo, R., Pérez-Otáñez, X., Perez, C., Enríquez, S., Guzman, E., Ulcuango, F., Grijalva, J., Vanwambeke, S. O., Ron-Garrido, L., & Saegerman, C. (2024). Evaluating the Human Risks of Consumption of Foods of Bovine Origin with Ivermectin Residues in Ecuador. Foods, 13(21), 3470. https://doi.org/10.3390/foods13213470








