Assessment of the Content of Glycoalkaloids in Potato Snacks Made from Colored Potatoes, Resulting from the Action of Organic Acids and Thermal Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials Characteristic
2.2. Basic Analyses of Raw Materials and Ready-to-Eat Products
2.3. Conditions for Producing Experimental Coloured Potato Grits
2.4. Conditions for Obtaining Extruded Pellets and Colorful Snacks
2.5. Conditions for Obtaining Coloured Potato French Fries
2.6. Sample Preparation for the Chromatographic Analysis of α-Solanine and α-Chaconine
2.7. Apparatus and Conditions of the Glycoalkaloids Separation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Raw Material Characteristics
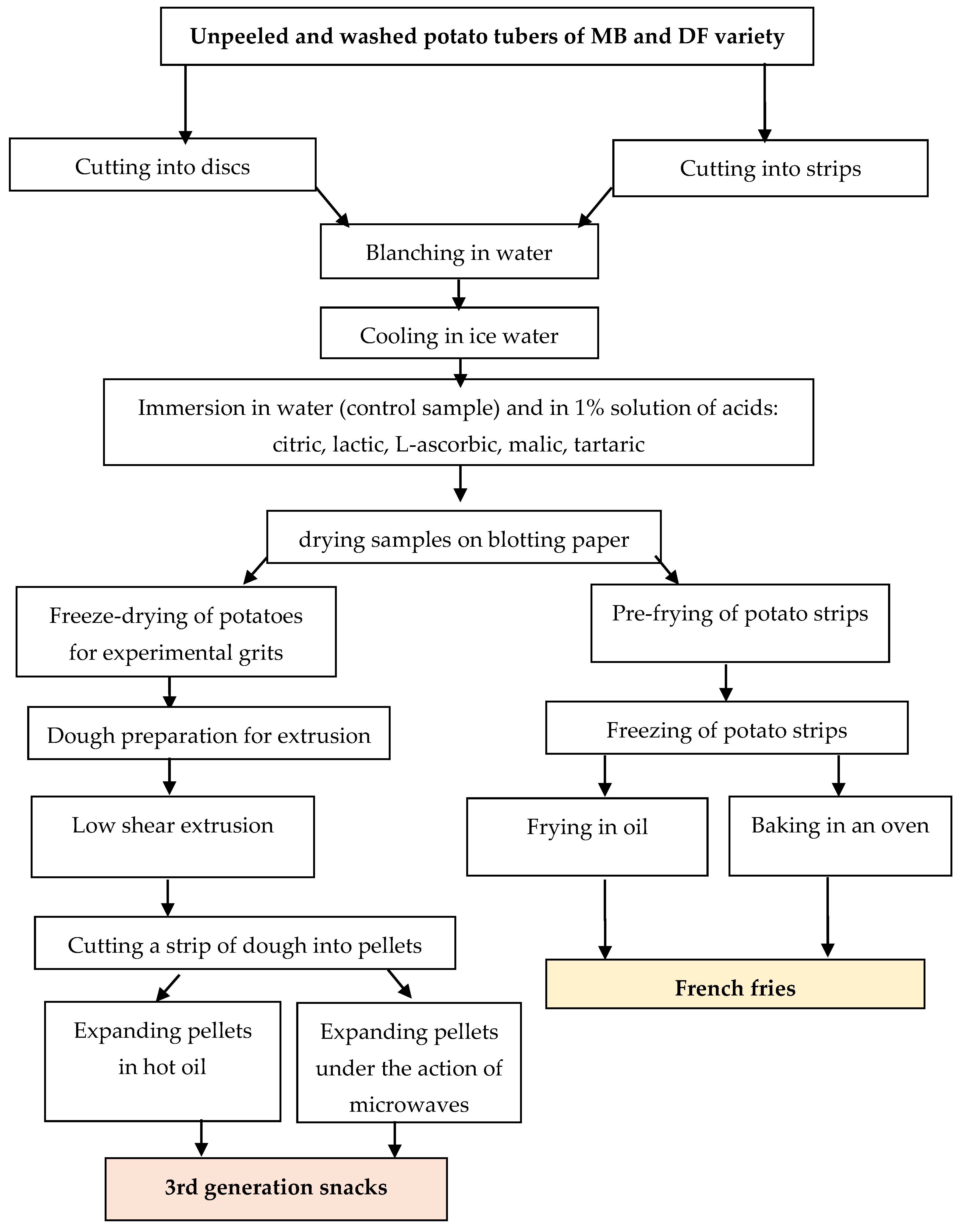
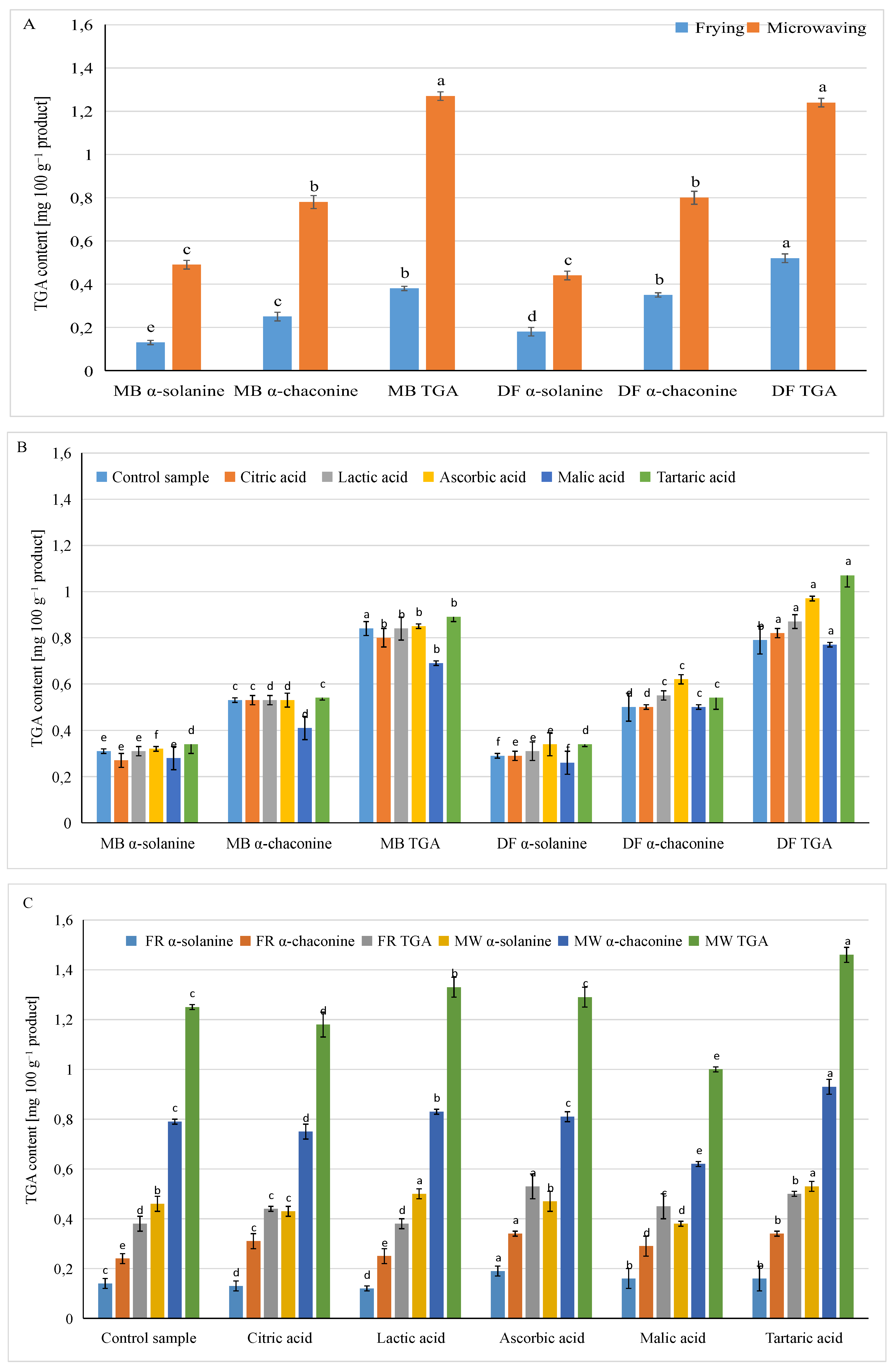
3.2. Influence of Different Factors in the Production of Pellet Snacks on the Content of Glycoalkaloids in Ready Products
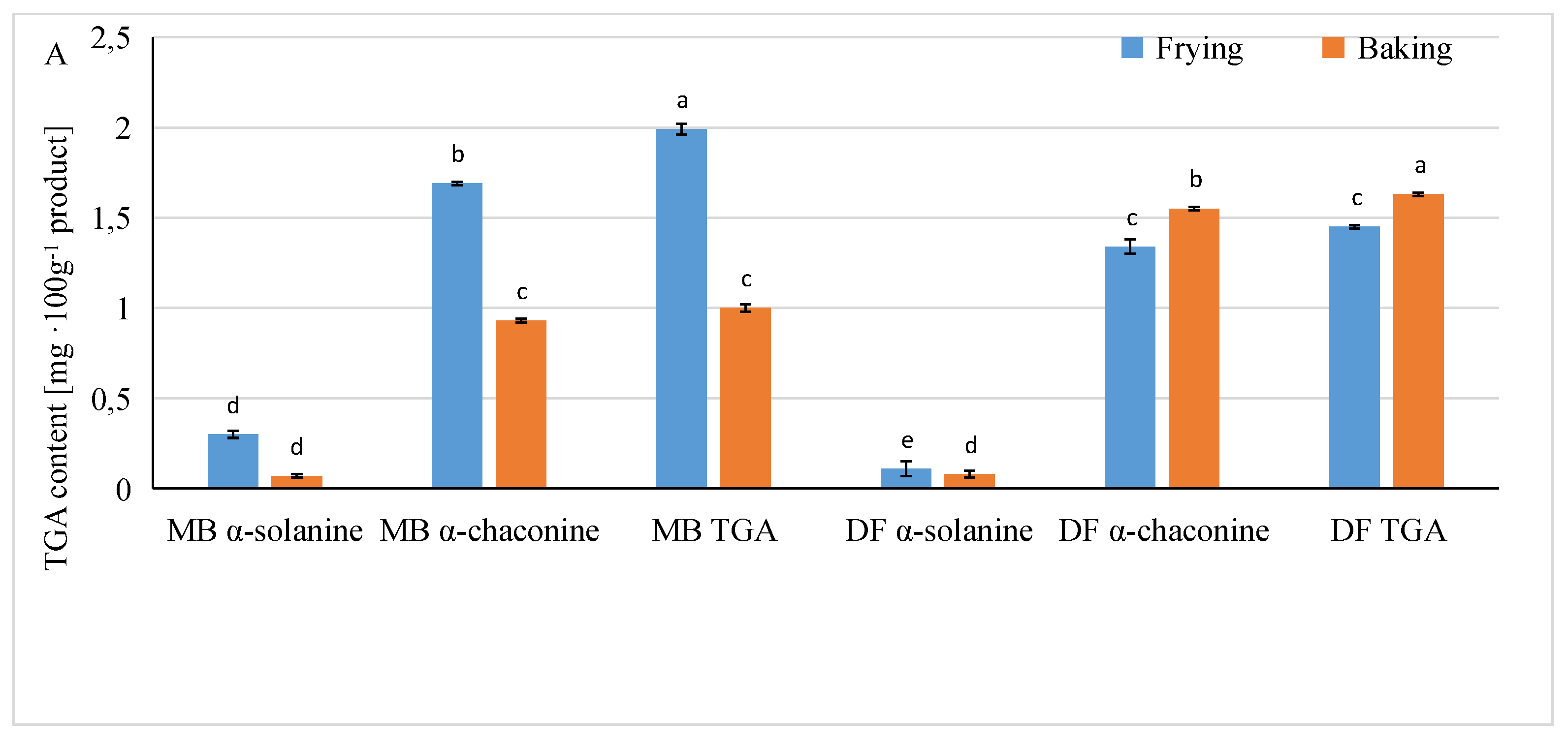
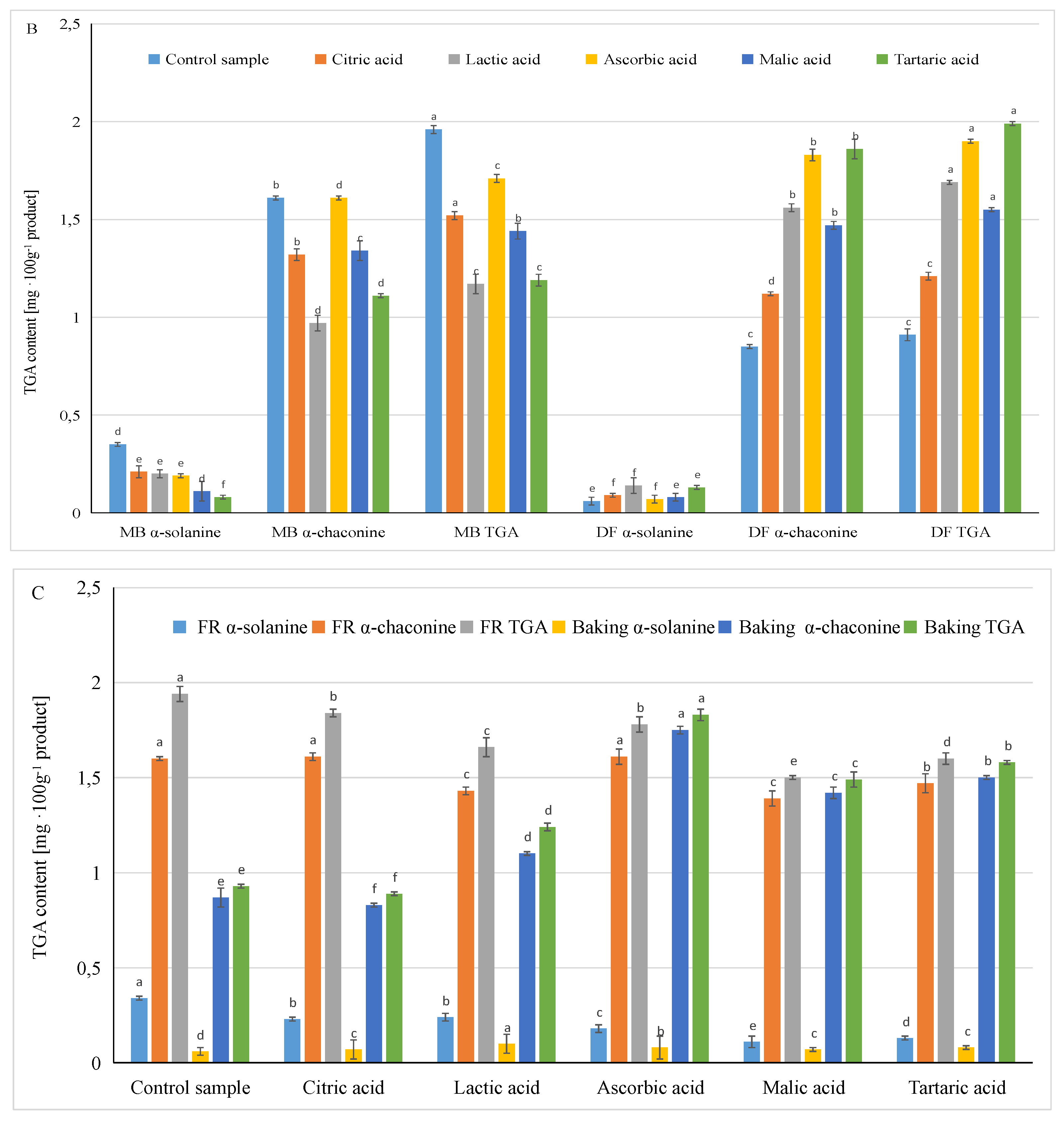
3.3. Influence of Different Factors in the Production of French Fries on the Content of Glycoalkaloids in Ready Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Orsák, M. Coloured potatoes. In Advances in Potato Chemistry and Technology, 2nd ed.; Singh, J., Kaur, L., Eds.; Academic Press: London, UK, 2016; Chapter 9; pp. 249–281. [Google Scholar]
- Ieri, F.; Innocenti, M.; Andrenelli, L.; Vecchio, V.; Mulinacci, N. Rapid HPLC/DAD/MS method to determine phenolic acids, glycoalkaloids and anthocyanins in pigmented potatoes (Solanum tuberosum L.) and correlations with variety and geographical origin. Food Chem. 2011, 125, 750–759. [Google Scholar] [CrossRef]
- Ru, W.; Pang, Y.; Gan, Y.; Liu, Q.; Bao, J. Phenolic Compounds and Antioxidant Activities of Potato Cultivars with White, Yellow, Red and Purple Flesh. Antioxidants 2019, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and Human Health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Berrios, J.D.J.; Powers, J.R.; Tang, J.; Ji, Y. Colored potatoes (Solanum tuberosum L.) dried for antioxidant-rich value-added foods. J. Food Process. Preserv. 2011, 35, 571–580. [Google Scholar] [CrossRef]
- Tian, J.; Chen, J.; Lv, F.; Chen, S.; Chen, J.; Liu, D.; Ye, X. Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes. Food Chem. 2016, 197, 1264–1270. [Google Scholar] [CrossRef]
- Rasheed, H.; Ahmad, D.; Bao, J. Genetic Diversity and Health Properties of Polyphenols in Potato. Antioxidants 2022, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Kano, M.; Takayanagi, T.; Yamacawa, O.; Ishikawa, F. Absorption of acylated antocyanins in rats and humans after ingesting an extract of Ipomoea batatas purple sweet potato tuber. Biosci. Biotechnol. Biochem. 2004, 68, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric. Food Chem. 2006, 15, 8655–8681. [Google Scholar] [CrossRef] [PubMed]
- Jansen, G.; Flamme, W. Coloured potatoes (Solanum tuberosum L.)–anthocyanin content and tuber quality. Genet. Resour. Crop Evol. 2006, 53, 1321–1331. [Google Scholar] [CrossRef]
- Urban, J.; Hamouz, K.; Lachman, J.; Pulkrábek, J.; Pazderů, K. Effect of genotype, flesh colour and environment on the glycoalkaloid content in potato tubers from integrated agriculture. Plant Soil Environ. 2018, 64, 186–191. [Google Scholar] [CrossRef]
- D’Amelia, V.; Sarais, G.; Fais, G.; Dessì, D.; Giannini, V.; Garramone, R.; Carputo, D.; Melito, S. Biochemical Characterization and Effects of Cooking Methods on Main Phytochemicals of Red and Purple Potato Tubers, a Natural Functional Food. Foods 2022, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Deußer, H.; Guignard, C.; Hoffmann, L.; Evers, D. Polyphenol and glycoalkaloid contents in potato cultivars grown in Luxembourg. Food Chem. 2012, 135, 2814–2824. [Google Scholar] [CrossRef] [PubMed]
- Rytel, E.; Tajner-Czopek, A.; Kita, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Hamouz, K. Content of anthocyanins and glycoalkaloids in blue-fleshed potatoes and changes in the content of α-solanine and α-chaconine during manufacture of fried and dried products. Int. J. Food Sci. Technol. 2018, 53, 719–727. [Google Scholar] [CrossRef]
- Friedman, M.; Kozukue, N.; Kim, H.-J.; Choi, S.-H.; Mizuno, M. Glycoalkaloid, phenolic, and flavonoid content and antioxidative activities of conventional nonorganic and organic potato peel powders from commercial gold, red, and Russet potatoes. J. Food Compos. Anal. 2017, 62, 69–75. [Google Scholar] [CrossRef]
- Singh, B.; Dutt, S.; Raigond, P. Potato glycoalkaloids. In Potato: Nutrition and Food Security; Springer: Singapore, 2020; pp. 191–212. ISBN 9789811576621. [Google Scholar]
- Martinez-Garcia, I.; Gaona-Scheytt, C.; Morante-Zarcero, S.; Sierra, I. Development of a Green, Quick, and Efficient Method Based on Ultrasound-Assisted Extraction Followed by HPLC-DAD for the Analysis of Bioactive Glycoalkaloids in Potato Peel Waste. Foods 2024, 13, 651. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Levin, C.E. Glycoalkaloids and Calystegine Alkaloids in Potatoes. In Advances in Potato Chemistry and Technology, 2nd ed.; Singh, J., Kaur, L., Eds.; Academic Press: London, UK, 2016; Chapter 7; pp. 167–194. [Google Scholar]
- Vinci, R.M.; Mestdagh, F.; Van Poucke, C.; Kerkaert, B.; De Muer, N.; Denon, Q.; Van Peteghem, C.; De Meulenaer, B. Implementation of acrylamide mitigation strategies on industrial production of French fires: Challenges and Pitfalls. J. Agric. Food Chem. 2011, 59, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Amaral, R.D.A.; Benedetti, B.C.; Pujolà, M.; Achaerandio, I. Effect of citric acid on browning of fresh-cut potatoes and on texture after frying. Acta Hortic. 2018, 1209, 259–264. [Google Scholar] [CrossRef]
- Oszmiański, J.; Bąkowska, A.; Piacente, S. Thermodynamic characteristics of copigmentation reaction of acylated anthocyanin isolated from blue flowers of Scutellaria baicalensis Georgi with copigments. J. Sci. Food Agric. 2004, 84, 1500–1506. [Google Scholar] [CrossRef]
- Brownmiller, C.; Howard, L.R.; Prior, R.L. Processing and storage on monomeric anthocyanins percent polymeric color, and antioxidant capacity of processed blueberry products. J. Food Sci. 2008, 5, 72–79. [Google Scholar] [CrossRef]
- Thiex, N.J.; Van Erem, T. Determination of water (moisture) and dry matter in animal feed, grain, and forage (plant tissue) by Karl Fischer titration: Collaborative study. J. AOAC Int. 2002, 85, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Houghland, G.V.C. New conversion table for specific gravity, dry matter and starch in potatoes. Am. Pot. J. 1966, 43, 138. [Google Scholar] [CrossRef]
- Lindsay, H.A. A colorimetric estimation of reducing sugars in potatoes with 3,5-dinitrosalicylic acid. Pot. Res. 1973, 16, 176–179. [Google Scholar] [CrossRef]
- Pęksa, A.; Miedzianka, J.; Kita, A.; Tajner-Czopek, A.; Rytel, E. The quality of fried snacks fortified with fiber and protein supplements. Poravinárstvo 2010, 4, 59–64. [Google Scholar] [CrossRef]
- Tajner-Czopek, A.; Kita, A.; Rytel, E. Characteristics of French Fries and Potato Chips in Aspect of Acrylamide Content—Methods of Reducing the Toxic Compound Content in Ready Potato Snacks. Appl. Sci. 2021, 11, 3943. [Google Scholar] [CrossRef]
- Saito, K.; Horie, M.; Hoshino, Y.; Nose, N. High-performance liquid chromatographic determination of glycoalkaloids in potato products. J. Chromatogr. 1990, 508, 141–147. [Google Scholar] [CrossRef]
- Pęksa, A.; Gołubowska, G.; Rytel, E.; Lisińska, G.; Aniołowski, K. Influence of harvest date on glycoalkaloid contents of three potato varieties. Food Chem. 2002, 78, 313–317. [Google Scholar] [CrossRef]
- StatSoft, Inc. Electronic Statistics Textbook; StatSoft: Tulsa, OK, USA, 2013. [Google Scholar]
- Lisińska, G.; Pęksa, A.; Kita, A.; Rytel, E.; Tajner-Czopek, A. The quality of potato for processing and consumption. In Potato III. Food, Global Science Books; Yee, N., Bussel, W.T., Eds.; Unitec: Auckland, New Zeland, 2009; pp. 99–104. [Google Scholar]
- Lachman, J.; Hamouz, K.; Musilová, J.; Hejtmánková, K.; Kotíková, Z.; Pazderů, K.; Domkářová, J.; Pivec, V.; Cimr, J. Effect of peeling and three cooking methods on the content of selected phytochemicals in potato tubers with various colour of flesh. Food Chem. 2013, 138, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Omayio, O.G.; Abong, G.O.; Okoth, M.W. A Review of Occurrence of Glycoalkaloids in Potato and Potato Products. Curr. Res. Nutr. Food Sci. 2016, 4, 195–202. [Google Scholar] [CrossRef]
- Nie, X.; Zhanga, G.; Lvc, S.; Guo, H. Steroidal glycoalkaloids in potato foods as affected by cooking methods. Int. J. Food Prop. 2018, 21, 1875–1887. [Google Scholar] [CrossRef]
- Rytel, E.; Kułakowska, K.; Nemś, A. The effect of chips processing on the content of toxic compounds. It. J. Food Sci. 2012, 24, 376–383. Available online: https://www.proquest.com/docview/1266221529/fulltextPDF/5545A6D922314383PQ/1?accountid=48845&sourcetype=Scholarly%20Journals (accessed on 3 May 2024).
- Kita, A.; Lisińska, G.; Tajner-Czopek, A.; Pęksa, A.; Rytel, E. The properties of Potato Snacks Influenced by the Frying Medium. In Potato IV. Food, Global Science Books; Yee, N., Bussel, W.T., Eds.; Unitec: Auckland, New Zeland, 2009; pp. 93–98. [Google Scholar]
- Liua, J.; Wena, C.; Wang, M.; Wang, S.; Dong, N.; Lei, Z.; Lin, S.; Zhu, B. Enhancing the hardness of potato slices after boiling by combined treatment with lactic acid and calcium chloride: Mechanism and optimization. Food Chem. 2020, 308, 124832. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, J.; Li, M.; Li, C.; Wang, Y.; Shen, M.; Chen, Y.; Nie, S.; Zeng, M.; Che, J.; et al. Effect of acidity regulators on acrylamide and 5-hydroxymethylfurfural formation in French fries: The dual role of pH and acid radical ion. Food Chem. 2022, 371, 131154. [Google Scholar] [CrossRef] [PubMed]
- Negoită, M.; Mihai, A.L.; Hornet, G.A. Influence of Water, NaCl and Citric Acid Soaking Pre-Treatments on Acrylamide Content in French Fries Prepared in Domestic Conditions. Foods 2022, 11, 1204. [Google Scholar] [CrossRef]
- Liu, H.; Roasa, J.; Mats, L.; Zhu, H.; Shao, S. Effect of acid on glycoalkaloids and acrylamide in French fries. Food Addit. Contam. Part A 2020, 37, 938–945. [Google Scholar] [CrossRef]
| Potato Variety | Compound | Raw Unpeeled Potatoes | Dried Unpeeled Potatoes |
|---|---|---|---|
| Dry matter [g·100 g−1] | 22.24 ± 0.23 A | 92.88 ± 0.14 A | |
| Starch [g·100 g−1] | 16.66 ± 0.11 A | 69.58 ± 0.07 A | |
| Reducing sugars [g·100 g−1] | 0.22± 0.08 A | 0.92 ± 0.05 A | |
| α-solanine [mg·100 g−1] | 1.78 ± 0.02 A | 7.42 ± 0.01 A | |
| (MB) | α-chaconine [mg·100 g−1] | 4.33 ± 0.04 A | 18.09 ± 0.02 A |
| TGA [mg·100 g−1] | 6.04 ± 0.11 A | 25.21 ± 0.13 A | |
| α-chaconine/α-solanine | 2.4 | 2.4 | |
| A sum of anthocyanins [mg·100 g−1] | 24.95 ± 0.13 B | 104.2 ± 0.15 B | |
| Dry matter [g·100 g−1] | 21.75 ± 0.19 B | 92.68 ± 0.25 A | |
| Starch [g·100 g−1] | 16.06 ± 0.15 A | 68.43 ± 0.03 B | |
| Reducing sugars [g·100 g−1] | 0.18 ± 0.02 A | 0.77 ± 0.01 A | |
| α-solanine [mg·100 g−1] | 0.61 ± 0.03 B | 2.60 ± 0.02 B | |
| (DF) | α-chaconine [mg·100 g−1] | 1.73 ± 0.01 B | 7.36 ± 0.02 B |
| TGA [mg·100 g−1] | 2.34 ± 0.03 B | 9.96 ± 0.02 B | |
| α-chaconine/α-solanine | 2.8 | 2.8 | |
| A sum of anthocyanins [mg·100 g−1] | 50.38 ± 0.15 A | 214.7 ± 0.11 A |
| Potato Variety | Compound | Experimental Dried Potato | |||||
|---|---|---|---|---|---|---|---|
| Water/Control | Citric Acid | Lactic Acid | Ascorbic Acid | Malic Acid | Tartaric Acid | ||
| Dry matter [g·100 g−1] | 92.21 ± 0.23 B | 93.05 ± 0.17 A | 93.10 ± 0.16 A | 92.75 ± 0.20 A | 93.08 ± 0.14 A | 92.99 ± 0.19 A | |
| α-solanine [mg·100 g−1] | 2.32 ± 0.03 bA | 1.50 ± 0.06 cB | 3.82 ± 0.01 aA | 2.66 ± 0.02 bA | 3.96 ± 0.03 aA | 1.52 ± 0.02 cB | |
| (MB) | α-chaconine [mg·100 g−1] | 7.46 ± 0.02 bA | 4.44 ± 0.02 cdB | 9.12 ± 0.03 aA | 5.25 ± 0.03 cA | 8.20 ± 0.01 abA | 3.82 ± 0.03 dB |
| TGA [mg·100 g−1] | 9.78 ± 0.08 bA | 5.94 ± 0.01 dB | 12.94 ± 0.02 aA | 7.91 ± 0.02 cA | 12.16 ± 0.02 aA | 5.34 ± 0.01 dB | |
| α-chaconine/α-solanine | 3.1 | 2.9 | 2.4 | 2.0 | 2.1 | 2.5 | |
| Dry matter [g·100 g−1] | 93.39 ± 0.18 A | 92.91 ± 0.17 B | 93.54 ± 0.15 A | 92.77 ± 0.19 A | 92.30 ± 0.21 B | 93.01 ± 0.14 A | |
| α-solanine [mg·100 g−1] | 1.54 ± 0.01 abB | 1.58 ± 0.05 abA | 1.29 ± 0.06 cB | 0.92 ± 0.02 cB | 1.70 ± 0.01 aB | 1.66 ± 0.04 aA | |
| (DF) | α-chaconine [mg·100 g−1] | 5.51 ± 0.03 abB | 6.10 ± 0.06 aA | 4.69 ± 0.08 abB | 3.37 ± 0.02 bB | 3.88 ± 0.02 bB | 6.44 ± 0.03 aA |
| TGA [mg·100 g−1] | 7.04 ± 0.02 abB | 7.68 ± 0.04 abA | 5.98 ± 0.07 bcB | 4.29 ± 0.02 cB | 5.58 ± 0.01 bcB | 8.10 ± 0.03 aA | |
| α-chaconine/α-solanine | 3.6 | 3.9 | 3.6 | 3.7 | 2.3 | 3.9 | |
| Factor | α-Solanine | α-Chaconine [mg·100 g−1] | TGA |
|---|---|---|---|
| ANOVA Test | |||
| Potato variety | NS | *** | *** |
| Expanding method | *** | *** | *** |
| Acid type | *** | *** | *** |
| Potato variety x acid type | *** | *** | *** |
| Expanding method x acid type | *** | *** | *** |
| Potato variety x expanding method | *** | *** | *** |
| LSD multiple range test | |||
| Expanding method | |||
| Frying | 0.15 b | 0.30 b | 0.45 b |
| Microwaving | 0.47 a | 0.79 a | 1.26 a |
| Type of acid | |||
| Control | 0.30 cd | 0.52 bc | 0.82 c |
| Citric acid | 0.29 c | 0.53 b | 0.81 cd |
| Lactic acid | 0.32 ab | 0.54 b | 0.86 bc |
| Ascorbic acid | 0.33 ab | 0.58 ab | 0.91 ab |
| Malic acid | 0.27 c | 0.46 c | 0.73 d |
| Tartaric acid | 0.35 a | 0.64 a | 0.99 a |
| Potato variety | |||
| Mulberry Beauty | 0.31 a | 0.51 b | 0.82 b |
| Double Fun | 0.31 a | 0.57 a | 0.88 a |
| Factor | α-Solanine | α-Chaconine [mg·100 g−1] | TGA |
|---|---|---|---|
| ANOVA Test | |||
| Potato variety | *** | *** | *** |
| Preparing method | *** | *** | *** |
| Acid type | * | * | NS |
| Potato variety x preparing method | *** | *** | *** |
| Preparing method x acid type | *** | ** | ** |
| Potato variety x acid type | *** | *** | *** |
| LSD multiple range test | |||
| Preparing method | |||
| Frying | 0.20 a | 1.51 a | 1.71 a |
| Baking | 0.08 b | 1.24 b | 1.32 b |
| Type of acid | |||
| Control | 0.20 a | 1.23 b | 1.43 a |
| Citric acid | 0.14 ab | 1.22 b | 1.36 a |
| Lactic acid | 0.17 ab | 1.26 b | 1.43 a |
| Ascorbic acid | 0.13 ab | 1.67 a | 1.80 a |
| Malic acid | 0.09 b | 1.40 ab | 1.50 a |
| Tartaric acid | 0.10 ab | 1.49 ab | 1.59 a |
| Potato variety | |||
| Mulberry Beauty | 0.18 a | 1.31 b | 1.49 a |
| Double Fun | 0.09 b | 1.45 a | 1.54 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pęksa, A.; Tajner-Czopek, A.; Gryszkin, A.; Miedzianka, J.; Rytel, E.; Wolny, S. Assessment of the Content of Glycoalkaloids in Potato Snacks Made from Colored Potatoes, Resulting from the Action of Organic Acids and Thermal Processing. Foods 2024, 13, 1712. https://doi.org/10.3390/foods13111712
Pęksa A, Tajner-Czopek A, Gryszkin A, Miedzianka J, Rytel E, Wolny S. Assessment of the Content of Glycoalkaloids in Potato Snacks Made from Colored Potatoes, Resulting from the Action of Organic Acids and Thermal Processing. Foods. 2024; 13(11):1712. https://doi.org/10.3390/foods13111712
Chicago/Turabian StylePęksa, Anna, Agnieszka Tajner-Czopek, Artur Gryszkin, Joanna Miedzianka, Elżbieta Rytel, and Szymon Wolny. 2024. "Assessment of the Content of Glycoalkaloids in Potato Snacks Made from Colored Potatoes, Resulting from the Action of Organic Acids and Thermal Processing" Foods 13, no. 11: 1712. https://doi.org/10.3390/foods13111712
APA StylePęksa, A., Tajner-Czopek, A., Gryszkin, A., Miedzianka, J., Rytel, E., & Wolny, S. (2024). Assessment of the Content of Glycoalkaloids in Potato Snacks Made from Colored Potatoes, Resulting from the Action of Organic Acids and Thermal Processing. Foods, 13(11), 1712. https://doi.org/10.3390/foods13111712








