The Effects of Interventions with Glucosinolates and Their Metabolites in Cruciferous Vegetables on Inflammatory Bowel Disease: A Review
Abstract
:1. Introduction
2. Cruciferous Vegetables and Glucosinolates
3. Physiological Functions of GLSs and Metabolites
3.1. Metabolism of GLSs In Vivo
3.2. Alleviating Effects of GLSs and Their Metabolites on Colitis
| Models | Inducement | Intervention | Effects | References |
|---|---|---|---|---|
| C57BL6/J-Ahrb/b and Ahrd/d mice, 8–10 weeks | drinking 3.5% DSS water for 6 days | 15% broccoli diet for 14 days beforehand | broccoli diet significantly attenuated the clinical manifestation of splenomegaly and DAI in Ahrb/b and Ahrd/d mice | [46] |
| C57BL/6 mice, male, 8–10 weeks | drinking 2.5% DSS for one week | 10% raw broccoli (RB), 10% lightly cooked broccoli (CB) | both CB and RB effectively reduced DAI, extended colon length and induced less blood endotoxin and less severe colon lesions | [39] |
| interleukin (IL)-10-knockout mice on C57BL/6 background | inoculation with Helicobacter hepaticus | diet with 10% raw broccoli sprouts | broccoli sprout diet reduced weight stagnation, fecal blood and diarrhea, enhanced gut microbiota richness and reduced the prevalence and abundance of pathobiont bacteria triggering inflammation | [47] |
| C57BL/6J mice, female and male | drinking 2% DSS water for 5 days for SPF mice and 1% DSS for GF mice | 5% steamed broccoli sprout (SBS) diet for 4–6 weeks | SBS decreased DSS-induced colitis via the gut microbiota converting cruciferous vegetables into bioactive metabolites, promoting anti-inflammatory effects | [15] |
| C57BL/6J mice. male and female, 7–8 weeks | aater with 3% DSS for 1 week, followed 1 week of recovery and then 1 week 3% DSS | red cabbage juice (RCJ) | RCJ significantly improved body weight and survival of mice, decreased DAI scores, improved intestinal barrier integrity by enhancing the expression of colonic mucins and tight junction (TJ) proteins and the abundance of SCFA-producing bacteria and increased PPAR-γ activation | [48] |
| Wistar rats, male | drinking 4% DSS water for 6 days, mild colitis | diet with 8750 mg/kg broccoli sprout extract (BSE) | diets with BE reduced the DSS-induced rise in the expression of pro-inflammatory mediators NFκB, MCP-1, COX2 and VCAM-1 | [49] |
| C57BL/6J mice male, 6 weeks old | 25 g/L DSS water | 370 mg/kg·day BSE dissolved in 0.2 mL of skim milk | BSE administration increased body weight, improved antioxidant activities and restored the intestinal barrier through enhancing TJ protein expression | [50] |
| C57BL/6J mice, male, 7 weeks | drinking 3% DSS water for 5 days (acute UC) and cyclic rotations of 2.5% DSS water for 30 d | moringa seed extract (MSE) | MSE decreased DAI scores and colon weight/length ratios, increased colon lengths, reduced colonic inflammation and damage in acute UC, decreased colonic pro-inflammatory expression and downregulated gene expression of pro-inflammatory activity | [51] |
| Sprague Dawley rats, male, 3 months | intrarectal injection with 2,4-dinitrobenzenesulfonic acid (DNBS, 20 mg in 0.25 mL of 50% ethanol) | Eruca sativa defatted seed meal (0.1~1 g/kg p.o) | administration of E. sativa seed (1 g/kg) promoted colon recovery from injury and decreased enteric gliosis | [52] |
| C57BL/6J mice, male, 6 weeks | water consisting of 2.5% (w/v) DSS | gavage with BSE (370 mg/kg·day) or with Bifidobacterium longum CCFM1206 (109 CFU/mL) | combined treatment of B. longum CCFM1206 and BSE ameliorated DSS-induced colitis symptoms, mitigated colonic inflammatory levels and oxidative injury and restored the intestinal barrier | [53] |
| C57BL/6 mice, 8 weeks old | drinking 2.5% DSS for 9 days | diet supplemented with GRP (600 ppm) for 4 weeks | GRP attenuated body weight loss, DAI and colon shortening, maintained the colonic structure, inhibited inflammatory reactions and reduced colonic macrophage infiltration | [40] |
| C57BL/6 mice | drinking 4% DSS water for 5 days, acute colitis | pretreatment with 25 mg/kg·b.w. SFN per os for 7 days | SFN pretreatment significantly minimized body weight loss and DAI, extended colon lengths and relieved colon inflammation | [37] |
| C57BL/6 mice, male, 8 weeks old | drinking 3% DSS water | AITC in corn oil | AITC intervention showed less body weight loss, fewer colitis symptoms and longer colons, lessened the disruption of colonic histological structure and decreased mucosal inflammation | [54] |
| C57BL/6 mice, male, 8 weeks old | drinking 3% DSS water for 7 days | DIM | DIM significantly ameliorated the clinical symptoms and histological features, reduced inflammatory cell infiltration and suppressed the expression of pro-inflammatory cytokines and vascular endothelial growth factors | [55] |
| C57BL/6 mice, female, 6 weeks old | drinking 2.5% DSS water for 7 days | AITC (10 mg/kg/day) for 7 days | AITC could attenuate the severity of colitis through enhancing the intestinal barrier, including both TJ protein and mucin expression | [45] |
| BALB/cJ and C57BL/6 mice, female | 50 μL intrarectal injections of 1 mg of TNBS in 50% ethanol | I3C (40 mg/kg in 0.05% DMSO/corn oil) | I3C repressed colonic inflammation and prevented microbial dysbiosis, increasing a group of butyrate-producing gram-positive bacteria, which was correlated with an increase in IL-22 | [43] |
| C57BL/6 (18~22 g), male | drinking 2.5% DSS water | SFN intragastric administration 20 mg/kg·days for 2 weeks | SFN treatment increased body weight and colon length, decreased the colon damage scores and myeloperoxidase (MPO) activity, reversed DSS-induced gut microbiota dysbiosis and restored the abundance of Butyricicoccus | [29] |
| Sprague Dawley rats | intracolonic single administration of 2 mL of 4% acetic acid | SFN (15 mg/kg) by oral gavage daily for 2 weeks | SFN maintained the length and weight of the colon and improved morphological changes by improving antioxidant ability, elevating mitochondrial biogenesis and suppressing DNA polymerization | [42] |
| C57BL/6 mice, male | drinking 2% DSS water for 7 days | SFN (2.5, 5, 10 and 20 mg/kg body weight) | SFN treatment alleviated the changes in colon length, DAI scores and pathological damages, partially recovered gut microbiota disorder and enhanced the content of volatile fatty acids | [41] |
| C57BL/6JNifdc mice, male, 6–8 weeks old | 2.5% DSS was gavaged for 7 days | SFN (20, 40, 10 mg/kg·days) | SFN effectively attenuated intestinal inflammation through skewing the switching from classically (M1) to alternatively (M2) activated phenotypes both in intestinal and bone marrow-derived macrophages, leading to changes in the inflammatory mediators | [38] |
| human colonic cancer cell lines HT-29 and Caco-2 | treated with IL-1β (1 ng/mL) for 5 h | DIM | DIM mainly recovered the intestinal permeability of differentiated Caco-2 cells through increasing TJ protein expression and significantly enhanced the transepithelial electrical resistance of the cell monolayer | [44] |
| Caenorhabditis elegans, wild-type strain N2 and the mutant strain SS104 | feeding with Pseudomonas aeruginosa PAO1 | DIM | DIM relieved the damaged intestinal permeability and prolonged the lifespan of C. elegans fed P. aeruginosa | [44] |
4. Mechanism of GLSs and Their Metabolites in Alleviating IBD
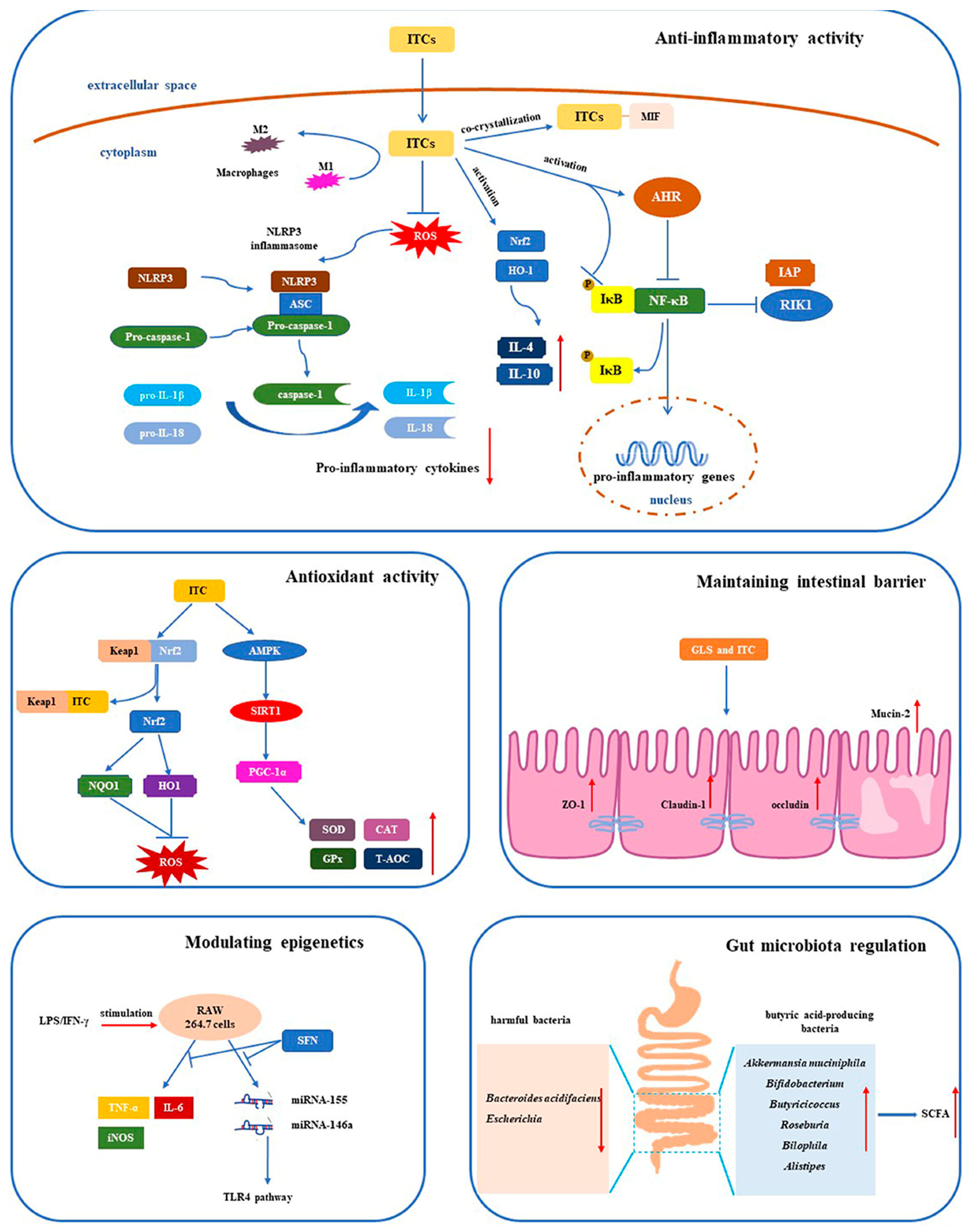
4.1. Regulation of Gut Microbiota
4.1.1. Regulation of Gut Microbiota Composition
4.1.2. Promoting the Production of SCFAs
4.2. Antioxidant Activity
4.3. Anti-Inflammatory Activity
4.3.1. Downregulating Inflammatory Mediators
4.3.2. Inhibiting NF-κB
4.4. Maintaining the Intestinal Barrier
4.5. Other Mechanisms
5. Processing Strategy of GLS-Containing Cruciferous Vegetable
5.1. Pretreatment
5.2. Heat Treatment
| Materials | GLSs Before Treatment | Processing Method | Detection Method | GLSs and Their Metabolites After Treatment | Ref. |
|---|---|---|---|---|---|
| broccoli cauliflower, white cabbages, red cabbages, Chinese cabbages, baby cabbages, white radish roots and red radish roots | Total GLS contents varied among vegetable type and could be ordered as follows: red radish root > broccoli > white cabbage > red cabbage > white radish root > baby cabbage > Chinese cabbage >cauliflower. The dominant GLS also depended on each vegetable type. For example, GRP accounted for 58.77% and 47.33% of the total GLS contents of broccoli and red cabbage, respectively. | blanching (30 s) and cooling at 2–4 °C for 5 min | HPLC quadrupole time of flight (QTOF) | blanching had little influence on the total GLS contents | [124] |
| QF −/+ boiling (8 min) | Total GLS contents of QF groups were higher than other groups for each species. High treatment temperature in VD resulted in a low GLS content. Blanching VFD is suitable for GLS preservation. Boiling led to a further decrease in the GLS content. The order of total GLS contents for each species was QF-B > VFD-B > VD-B > OD-B. There were significant differences in the stability among different GLSs or cruciferous vegetables. | ||||
| OD −/+boiling (8 min) | |||||
| VD −/+ boiling (8 min) | |||||
| VFD −/+ boiling (8 min) | |||||
| red cabbage | 0.21 µmol/g FW of ITCs but 0.62 µmol/g FW ETNs and 0.17 µmol/g FW nitriles formed after homogenization | aqueous heat treatment at 100 °C | UHPLC, GC-MS | 4-pentenenitrile ↓, 3-butenyl isothiocyanate ↑ and 1-cyano-3,4epithiobutane ↓ after short heat treatment (2–3 min), where nitriles accounted for 92% after 120 min of heat treatment | [121] |
| white cabbage | 0.72 µmol/g FW of ITCs, 0.50 µmol/g FW of ETNs and 0.16 µmol/g of nitriles | aqueous heat treatment at 100 °C | UHPLC, GC-MS | 3-butenenitrile ↓, 2-propenyl isothiocyanate ↑ and 1-cyano-2,3-epithiopropane ↓ after 3 min of heating, where nitriles accounted for 99.5% after 120 min of heat treatment | [121] |
| kohlrabi | 0.85 µmol/g FW of nitriles, 0.34 µmol/g FW ITCs and no ETNs | aqueous heat treatment at 100 °C | UHPLC, GC-MS | 4-(methylthio)pentanenitrile ↓ and 4-(methylthio)butyl isothiocyanate ↑ after 3 min of heating, where nitriles accounted for 99% after 120 min of heat treatment | [121] |
| red cabbage | the main GLSs were GRP, PRO, GIB and GBS | heating in boiling water | HPLC-DAD-ToF-MS, GC-MS | GLS degradation products included 3-butenenitrile, 5-(methylsulfinyl) pentanenitrile, indole-3-acetonitrile, 4-pentenenitrile, 3-phenylpropanenitrile and 1-cyano-2,3-epithiopropane. Formation of the corresponding nitriles increased over time, and ITCs did not accumulate in broths during boiling. | [126] |
| kohlrabi | the main GLSs were GER followed by glucoiberverin and smaller amounts of GIB, GRP and several indolic GLSs | heating in boiling water | HPLC-DAD-ToF-MS, GC-MS | Degradation products included 5-(methylthio) pentanenitrile, 4-(methylthio)butanenitrile, 4-(methylsulfinyl)butanenitrile, 3-(methylthio) propyl ITC and 4-(methylthio) butyl ITC. The relative ITC concentration steadily declined, and the corresponding nitriles increased over heating time. | [126] |
| red cabbage | high content of SIN, GIB and GRP as well as GBS and low amounts of GNA, glucoiberverin, GER and gluconasturtiin (GNS) | freshly prepared homogenates incubated for 1 h | UHPLC-DAD-TOF-MS, GC-MS | GLS hydrolysis product differed depending on the structure, mainly including corresponding ETNs, nitrile, amine and ITCs. SIN yielded high amounts of ETNs and amine, followed by ITC. | [122] |
| white cabbage | mainly SIN and GIB as well as GBS and lower amounts of GNA, glucoiberverin, GRP and GNS. | freshly prepared homogenates incubated for 1 h | UHPLC-DAD-TOF-MS, GC-MS | GLS hydrolysis product differed depending on their structure, mainly included corresponding ETN, nitrile, amine and ITCs. GIB and GRP yielded high amounts of nitrile, and ITC yielded and low levels of amine. | [122] |
| broccoli seeds | Aliphatic GLS content was 54.5−218.7 μmol/g fresh weight, accounting for >90% of the total GLS. The major GLSs were GRP and GER in 27 samples and PRO in 7 samples. | enzymatic degradation (ground and incubated at 25 °C for 2 h) | HPLC, GC-FID | ITC, nitrile and ETNs of SIN, GNA, GIB, GER and PRO, such as glucomesonitrile, SFN and butenylsulfuroside cyclonitrile | [129] |
| leaf mustard | content of SIN, GNA, PRO, GBS, 4-methoxyglucobrassicin, neoglucobrassicin and GNS ranges among the varieties | fermentation (20 °C for 4 days) | HPLC, GC-MS | three ITCs, three EPNs and two CNs, including SIN-ITC, GNA-ITC, GNS-ITC, SIN-EPN, GNA-EPN, Pro-EPN, SIN-CN and GNA-CN | [130] |
| broccoli seed extract | GRP | 24 h of anaerobic fermentation with B. longum | HPLC, UHPLC Q Exactive MS | SFN, SFN−L-cysteine and erucin | [53] |
5.3. Drying and Freezing
5.4. Fermentation
5.5. Other Observations
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abraham, C.; Cho, J.H. Inflammatory Bowel Disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kotze, P.G.; Underwood, F.E.; Damião, A.O.M.C.; Ferraz, J.G.P.; Saad-Hossne, R.; Toro, M.; Iade, B.; Bosques-Padilla, F.; Teixeira, F.V.; Juliao-Banos, F.; et al. Progression of Inflammatory Bowel Diseases Throughout Latin America and the Caribbean: A Systematic Review. Clin. Gastroenterol. Hepatol. 2020, 18, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cheon, J.H. Incidence and Prevalence of Inflammatory Bowel Disease across Asia. Yonsei Med. J. 2021, 62, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Singh, S.; Boland, B.S.; Jess, T.; Moore, A.A. Management of inflammatory bowel diseases in older adults. Lancet Gastroenterol. Hepatol. 2023, 8, 368–382. [Google Scholar] [CrossRef]
- Wei, L.-Y.; Zhang, J.-K.; Zheng, L.; Chen, Y. The functional role of sulforaphane in intestinal inflammation: A review. Food Funct. 2022, 13, 514–529. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Du, H.; Psychiatry, B. Inflammatory bowel disease: A potential pathogenic factor of Alzheimer’s disease. Prog. Neuro-Psychopharmacol. 2022, 119, 110610. [Google Scholar] [CrossRef]
- Rogler, G. Gastrointestinal and liver adverse effects of drugs used for treating IBD. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 157–165. [Google Scholar] [CrossRef]
- Fitzpatrick, J.A.; Melton, S.L.; Yao, C.K.; Gibson, P.R.; Halmos, E.P. Dietary management of adults with IBD—The emerging role of dietary therapy. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 652–669. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Li, X.; Zhou, D.; Wang, Y.; Wen, X.; Wang, C.; Liu, X.; Feng, Y.; Li, B.; et al. Functional foods and intestinal homeostasis: The perspective of in vivo evidence. Trends Food Sci. Technol. 2021, 111, 475–482. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Yang, Z.-Y.; Gong, T.-T.; Liu, Y.-S.; Liu, F.-H.; Wen, Z.-Y.; Li, X.-Y.; Gao, C.; Luan, M.; Zhao, Y.-H.; et al. Cruciferous vegetable consumption and multiple health outcomes: An umbrella review of 41 systematic reviews and meta-analyses of 303 observational studies. Food Funct. 2022, 13, 4247–4259. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hebuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN guideline on Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Zhang, T.; Holman, J.; McKinstry, D.; Trindade, B.C.; Eaton, K.A.; Mendoza-Castrejon, J.; Ho, S.; Wells, E.; Yuan, H.; Wen, B.; et al. A steamed broccoli sprout diet preparation that reduces colitis via the gut microbiota. J. Nutr. Biochem. 2023, 112, 109215. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Wu, C.; Rao, Z.; Du, L.; Zhou, Y. Cruciferous vegetable and isothiocyanate intake and multiple health outcomes. Food Chem. 2022, 375, 131816. [Google Scholar] [CrossRef]
- Lv, Q.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. The Cellular and Subcellular Organization of the Glucosinolate-Myrosinase System against Herbivores and Pathogens. Int. J. Mol. Sci. 2022, 23, 1577. [Google Scholar] [CrossRef]
- Suzuki, C.; Ohnishi-Kameyama, M.; Sasaki, K.; Murata, T.; Yoshida, M. Behavior of Glucosinolates in Pickling Cruciferous Vegetables. J. Agric. Food Chem. 2006, 54, 9430–9436. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, S.; Liu, Y.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H.; Wang, Y.; Xu, D. Characterization of glucosinolates in 80 broccoli genotypes and different organs using UHPLC-Triple-TOF-MS method. Food Chem. 2021, 334, 127519. [Google Scholar] [CrossRef]
- Hwang, I.M.; Park, B.; Dang, Y.M.; Kim, S.-Y.; Seo, H.Y. Simultaneous direct determination of 15 glucosinolates in eight Brassica species by UHPLC-Q-Orbitrap-MSN. Food Chem. 2019, 282, 127–133. [Google Scholar] [CrossRef]
- Park, C.H.; Park, S.-Y.; Park, Y.J.; Kim, J.K.; Park, S.U. Metabolite Profiling and Comparative Analysis of Secondary Metabolites in Chinese Cabbage, Radish, and Hybrid xBrassicoraphanus. J. Agric. Food Chem. 2020, 68, 13711–13719. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Tian, Y.-X.; Jiang, M.; Yuan, Q.; Chen, Q.; Zhang, Y.; Luo, Y.; Zhang, F.; Tang, H.-R. Variation in the main health-promoting compounds and antioxidant activity of whole and individual edible parts of baby mustard (Brassica juncea var. gemmifera). RSC Adv. 2018, 8, 33845–33854. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Vyas, D. Myrosinase: Insights on structural, catalytic, regulatory, and environmental interactions. Crit. Rev. Biotechnol. 2019, 39, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Sikorska-Zimny, K.; Beneduce, L. The Metabolism of Glucosinolates by Gut Microbiota. Nutrients 2021, 13, 2750. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Charron, C.S.; Novotny, J.A.; Peng, B.; Yu, L.; Chen, P. Profiling glucosinolate metabolites in human urine and plasma after broccoli consumption using non-targeted and targeted metabolomic analyses. Food Chem. 2020, 309, 125660. [Google Scholar] [CrossRef]
- Martins, T.; Ferreira, T.; Colaço, B.; Medeiros-Fonseca, B.; Pinto, M.d.L.; Barros, A.N.; Venâncio, C.; Rosa, E.; Antunes, L.M.; Oliveira, P.A.; et al. Absorption and Excretion of Glucosinolates and Isothiocyanates after Ingestion of Broccoli (Brassica oleracea L. var italica) Leaf Flour in Mice: A Preliminary Study. Nutraceuticals 2023, 3, 540–555. [Google Scholar] [CrossRef]
- Liou, C.S.; Sirk, S.J.; Diaz, C.A.C.; Klein, A.P.; Fischer, C.R.; Higginbottom, S.K.; Erez, A.; Donia, M.S.; Sonnenburg, J.L.; Sattely, E.S. A Metabolic Pathway for Activation of Dietary Glucosinolates by a Human Gut Symbiont. Cell 2020, 180, 717–728.e719. [Google Scholar] [CrossRef]
- Shakour, Z.T.; Shehab, N.G.; Gomaa, A.S.; Wessjohann, L.A.; Farag, M.A. Metabolic and biotransformation effects on dietary glucosinolates, their bioavailability, catabolism and biological effects in different organisms. Biotechnol. Adv. 2022, 54, 107784. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, L.; Li, C.; Wu, H.; Ran, D.; Zhang, Z. Sulforaphane alter the microbiota and mitigate colitis severity on mice ulcerative colitis induced by DSS. AMB Express 2020, 10, 119. [Google Scholar] [CrossRef]
- Yuanfeng, W.; Chengzhi, L.; Ligen, Z.; Juan, S.; Xinjie, S.; Yao, Z.; Jianwei, M. Approaches for enhancing the stability and formation of sulforaphane. Food Chem. 2021, 345, 128771. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Hoeflinger, J.L.; Neme, B.P.; Jeffery, E.H.; Miller, M.J. Dietary Broccoli Alters Rat Cecal Microbiota to Improve Glucoraphanin Hydrolysis to Bioactive Isothiocyanates. Nutrients 2017, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.-y.; Ke, Y.-s.; Zhao, H.-h.; Wang, L.; Jia, C.; Liu, W.-z.; Fu, Q.-h.; Shi, M.-n.; Cui, J.; Li, S.-c. Role of colonic microbiota in the pathogenesis of ulcerative colitis. BMC Gastroenterol. 2019, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli consumption affects the human gastrointestinal microbiota. J. Nutr. Biochem. 2019, 63, 27–34. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Sousa, A.S.; Reis, C.A.; Pintado, M.M. Phenylethyl Isothiocyanate: A Bioactive Agent for Gastrointestinal Health. Molecules 2022, 27, 794. [Google Scholar] [CrossRef]
- El Badawy, S.A.; Ogaly, H.A.; Abd-Elsalam, R.M.; Azouz, A.A. Benzyl isothiocyanates modulate inflammation, oxidative stress, and apoptosis via Nrf2/HO-1 and NF-κB signaling pathways on indomethacin-induced gastric injury in rats. Food Funct. 2021, 12, 6001–6013. [Google Scholar] [CrossRef]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016. [Google Scholar] [CrossRef]
- Wagner, A.E.; Will, O.; Sturm, C.; Lipinski, S.; Rosenstiel, P.; Rimbach, G. DSS-induced acute colitis in C57BL/6 mice is mitigated by sulforaphane pre-treatment. J. Nutr. Biochem. 2013, 24, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tang, J.; Li, C.; Liu, J.; Liu, H. Sulforaphane attenuates dextran sodium sulphate induced intestinal inflammation via IL-10/STAT3 signaling mediated macrophage phenotype switching. Food Sci. Hum. Wellness 2022, 11, 129–142. [Google Scholar] [CrossRef]
- Wang, Y.; Jeffery, E.H.; Miller, M.J.; Wallig, M.A.; Wu, Y. Lightly cooked broccoli is as effective as raw broccoli in mitigating dextran sulfate sodium-induced colitis in mice. Nutrients 2018, 10, 748. [Google Scholar] [CrossRef]
- Tian, Q.; Xu, Z.; Sun, Q.; Iniguez, A.B.; Du, M.; Zhu, M.J. Broccoli-Derived Glucoraphanin Activates AMPK/PGC1α/NRF2 Pathway and Ameliorates Dextran-Sulphate-Sodium-Induced Colitis in Mice. Antioxidants 2022, 11, 2404. [Google Scholar] [CrossRef]
- He, C.; Gao, M.; Zhang, X.; Lei, P.; Yang, H.; Qing, Y.; Zhang, L. The Protective Effect of Sulforaphane on Dextran Sulfate Sodium-Induced Colitis Depends on Gut Microbial and Nrf2-Related Mechanism. Front. Nutr. 2022, 9, 893344. [Google Scholar] [CrossRef] [PubMed]
- Alattar, A.; Alshaman, R.; Al-Gayyar, M.M.H. Therapeutic effects of sulforaphane in ulcerative colitis: Effect on antioxidant activity, mitochondrial biogenesis and DNA polymerization. Redox Rep. 2022, 27, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Busbee, P.B.; Menzel, L.; Alrafas, H.R.; Dopkins, N.; Becker, W.; Miranda, K.; Tang, C.; Chatterjee, S.; Singh, U.P.; Nagarkatti, M.; et al. Indole-3-carbinol prevents colitis and associated microbial dysbiosis in an IL-22-dependent manner. JCI Insight 2020, 5, 127551. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Le, T.A.N.; Lee, S.Y.; Song, D.G.; Hong, S.C.; Cha, K.H.; Lee, J.W.; Pan, C.H.; Kang, K.e. 3,3′-Diindolylmethane Improves Intestinal Permeability Dysfunction in Cultured Human Intestinal Cells and the Model Animal Caenorhabditis elegans. J. Agric. Food Chem. 2019, 67, 9277–9285. [Google Scholar] [CrossRef]
- Kim, M.W.; Choi, S.; Kim, S.Y.; Yoon, Y.S.; Kang, J.-H.; Oh, S.H. Allyl Isothiocyanate Ameliorates Dextran Sodium Sulfate-Induced Colitis in Mouse by Enhancing Tight Junction and Mucin Expression. Int. J. Mol. Sci. 2018, 19, 2025. [Google Scholar] [CrossRef]
- Hubbard, T.D.; Murray, I.A.; Nichols, R.G.; Cassel, K.; Podolsky, M.; Kuzu, G.; Tian, Y.; Smith, P.; Kennett, M.J.; Patterson, A.D.; et al. Dietary broccoli impacts microbial community structure and attenuates chemically induced colitis in mice in an Ah receptor dependent manner. J. Funct. Foods 2017, 37, 685–698. [Google Scholar] [CrossRef]
- Holcomb, L.; Holman, J.M.; Hurd, M.; Lavoie, B.; Colucci, L.; Hunt, B.; Hunt, T.; Kinney, M.; Pathak, J.; Mawe, G.M.; et al. Early life exposure to broccoli sprouts confers stronger protection against enterocolitis development in an immunological mouse model of inflammatory bowel disease. bioRxiv Prepr. Serv. Biol. 2023, 8, e00688-23. [Google Scholar] [CrossRef]
- Wilson, E.J.; Natesh, N.S.; Ghadermazi, P.; Pothuraju, R.; Prajapati, D.R.; Pandey, S.; Kaifi, J.T.; Dodam, J.R.; Bryan, J.N.; Lorson, C.L.; et al. Red Cabbage Juice-Mediated Gut Microbiota Modulation Improves Intestinal Epithelial Homeostasis and Ameliorates Colitis. Int. J. Mol. Sci. 2024, 25, 539. [Google Scholar] [CrossRef]
- Mueller, K.; Blum, N.M.; Mueller, A.S. Examination of the Anti-Inflammatory, Antioxidant, and Xenobiotic-Inducing Potential of Broccoli Extract and Various Essential Oils during a Mild DSS-Induced Colitis in Rats. ISRN Gastroenterol. 2013, 2013, 710856. [Google Scholar] [CrossRef]
- Wu, J.; Guo, W.; Cui, S.; Tang, X.; Zhang, Q.; Lu, W.; Jin, Y.; Zhao, J.; Mao, B.; Chen, W. Broccoli seed extract rich in polysaccharides and glucoraphanin ameliorates DSS-induced colitis via intestinal barrier protection and gut microbiota modulation in mice. J. Sci. Food Agric. 2023, 103, 1749–1760. [Google Scholar] [CrossRef]
- Kim, Y.; Wu, A.G.; Jaja-Chimedza, A.; Graf, B.L.; Waterman, C.; Verzi, M.P.; Raskin, I. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS ONE 2017, 12, e0184709. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, E.; Micheli, L.; Pagnotta, E.; Matteo, R.; Parisio, C.; Toti, A.; Ferrara, V.; Ciampi, C.; Martelli, A.; Testai, L.; et al. Beneficial Effects of Eruca sativa Defatted Seed Meal on Visceral Pain and Intestinal Damage Resulting from Colitis in Rats. Foods 2022, 11, 580. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cui, S.; Tang, X.; Zhang, Q.; Jin, Y.; Zhao, J.; Mao, B.; Zhang, H. Bifidobacterium longum CCFM1206 Promotes the Biotransformation of Glucoraphanin to Sulforaphane That Contributes to Amelioration of Dextran-Sulfate-Sodium-Induced Colitis in Mice. J. Agric. Food Chem. 2023, 71, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Davaatseren, M.; Hwang, J.-T.; Park, J.H.; Kim, M.-S.; Wang, S.; Sung, M.J. Allyl Isothiocyanate Ameliorates Angiogenesis and Inflammation in Dextran Sulfate Sodium-Induced Acute Colitis. PLoS ONE 2014, 9, e102975. [Google Scholar] [CrossRef]
- Jeon, E.J.; Davaatseren, M.; Hwang, J.T.; Park, J.H.; Hur, H.J.; Lee, A.S.; Sung, M.J. Effect of Oral Administration of 3,3′-Diindolylmethane on Dextran Sodium Sulfate-Induced Acute Colitis in Mice. J. Agric. Food Chem. 2016, 64, 7702–7709. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Vestergaard, M.V.; Allin, K.H.; Eriksen, C.; Zakerska-Banaszak, O.; Arasaradnam, R.P.; Alam, M.T.; Kristiansen, K.; Brix, S.; Jess, T. Gut microbiota signatures in inflammatory bowel disease. United Eur. Gastroent. 2024, 12, 22–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, C.; Huang, S.; Sun, J.; Song, X.; Nishanbaev, S.Z.; Benito, M.J.; Wu, Y. Effects of Polyphenols and Glucosinolates in Broccoli Extract on Human Gut Microorganisms Based on Simulation In Vitro. ACS Omega 2022, 7, 45096–45106. [Google Scholar] [CrossRef]
- He, C.; Huang, L.; Lei, P.; Liu, X.; Li, B.; Shan, Y. Sulforaphane normalizes intestinal flora and enhances gut barrier in mice with BBN-induced bladder cancer. Mol. Nutr. Food Res. 2018, 62, 1800427. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Chen, T.; Shi, L.; Wang, D.; Tang, D. Regulatory role of short-chain fatty acids in inflammatory bowel disease. Cell Commun. Signal. 2022, 20, 1–10. [Google Scholar] [CrossRef]
- Peng, K.; Xia, S.; Xiao, S.; Yu, Q. Short-chain fatty acids affect the development of inflammatory bowel disease through intestinal barrier, immunology, and microbiota: A promising therapy? J. Gastroenterol. Hepatol. 2022, 37, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, K.; Konop, M.; Bielinska, K.; Hutsch, T.; Dziekiewicz, M.; Banaszkiewicz, A.; Ufnal, M. Inflammatory bowel disease is associated with increased gut-to-blood penetration of short-chain fatty acids: A new, non-invasive marker of a functional intestinal lesion. Exp. Physiol. 2019, 104, 1226–1236. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Kaczmarczyk, O.; Dąbek-Drobny, A.; Woźniakiewicz, M.; Paśko, P.; Dobrowolska-Iwanek, J.; Woźniakiewicz, A.; Piątek-Guziewicz, A.; Zagrodzki, P.; Mach, T.; Zwolińska-Wcisło, M. Fecal levels of lactic, succinic and short-chain fatty acids in patients with ulcerative colitis and crohn disease: A pilot study. J. Clin. Med. 2021, 10, 4701. [Google Scholar] [CrossRef] [PubMed]
- Rhimi, S.; Kriaa, A.; Mariaule, V.; Saidi, A.; Drut, A.; Jablaoui, A.; Akermi, N.; Maguin, E.; Hernandez, J.; Rhimi, M. The Nexus of Diet, Gut Microbiota and Inflammatory Bowel Diseases in Dogs. Metabolites 2022, 12, 1176. [Google Scholar] [CrossRef]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, C.; Zhu, J.; Lin, Q.; Yu, M.; Wen, J.; Feng, J.; Hu, C. Sodium butyrate ameliorates oxidative stress-induced intestinal epithelium barrier injury and mitochondrial damage through AMPK-mitophagy pathway. Oxid. Med. Cell. Longev. 2022, 2022, 3745135. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Guan, G.; Lan, S. Implications of Antioxidant Systems in Inflammatory Bowel Disease. BioMed Res. Int. 2018, 2018, 1290179. [Google Scholar] [CrossRef]
- Collins, D.; Hogan, A.M.; Winter, D.C. Microbial and viral pathogens in colorectal cancer. Lancet Oncol. 2011, 12, 504–512. [Google Scholar] [CrossRef]
- Chodur, G.M.; Olson, M.E.; Wade, K.L.; Stephenson, K.K.; Nouman, W.; Garima; Fahey, J.W. Wild and domesticated Moringa oleifera differ in taste, glucosinolate composition, and antioxidant potential, but not myrosinase activity or protein content. Sci. Rep. 2018, 8, 7995. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Wu, Q. Sulforaphane protects intestinal epithelial cells against lipopolysaccharide-induced injury by activating the AMPK/SIRT1/PGC-1ɑ pathway. Bioengineered 2021, 12, 4349–4360. [Google Scholar] [CrossRef] [PubMed]
- Cedrowski, J.; Dąbrowa, K.; Przybylski, P.; Krogul-Sobczak, A.; Litwinienko, G. Antioxidant activity of two edible isothiocyanates: Sulforaphane and erucin is due to their thermal decomposition to sulfenic acids and methylsulfinyl radicals. Food Chem. 2021, 353, 129213. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shao, R.; Wang, N.; Zhou, N.; Du, K.; Shi, J.; Wang, Y.; Zhao, Z.; Ye, X.; Zhang, X. Sulforaphane activates a lysosome-dependent transcriptional program to mitigate oxidative stress. Autophagy 2021, 17, 872–887. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Liu, W.; Li, X.; Chen, W.; Liu, Z.; Wen, J.; Liu, Z. A Protective Role of the NRF2-Keap1 Pathway in Maintaining Intestinal Barrier Function. Oxid. Med. Cell. Longev. 2019, 2019, 1759149. [Google Scholar] [CrossRef] [PubMed]
- Egbujor, M.C.; Petrosino, M.; Zuhra, K.; Saso, L. The role of organosulfur compounds as Nrf2 activators and their antioxidant effects. Antioxidants 2022, 11, 1255. [Google Scholar] [CrossRef]
- Petkovic, M.; Leal, E.C.; Alves, I.; Bose, C.; Palade, P.T.; Singh, P.; Awasthi, S.; Børsheim, E.; Dalgaard, L.T.; Singh, S.P. Dietary supplementation with sulforaphane ameliorates skin aging through activation of the Keap1-Nrf2 pathway. J. Nutr. Biochem. 2021, 98, 108817. [Google Scholar] [CrossRef]
- Clifford, T.; Acton, J.P.; Cocksedge, S.P.; Davies, K.A.B.; Bailey, S.J. The effect of dietary phytochemicals on nuclear factor erythroid 2-related factor 2 (Nrf2) activation: A systematic review of human intervention trials. Mol. Biol. Rep. 2021, 48, 1745–1761. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Kloska, D.; Grochot-Przęczek, A.; Feelisch, M.; Cuadrado, A.; van Goor, H. Personalized redox medicine in inflammatory bowel diseases: An emerging role for HIF-1α and NRF2 as therapeutic targets. Redox Biol. 2023, 60, 102603. [Google Scholar] [CrossRef]
- Cheng, X.-R.; Yu, B.-T.; Song, J.; Ma, J.-H.; Chen, Y.-Y.; Zhang, C.-X.; Tu, P.-H.; Muskat, M.N.; Zhu, Z.-G. The Alleviation of Dextran Sulfate Sodium (DSS)-Induced Colitis Correlate with the log P Values of Food-Derived Electrophilic Compounds. Antioxidants 2022, 11, 2406. [Google Scholar] [CrossRef]
- Melim, C.; Lauro, M.R.; Pires, I.M.; Oliveira, P.J.; Cabral, C. The role of glucosinolates from cruciferous vegetables (Brassicaceae) in gastrointestinal cancers: From prevention to therapeutics. Pharmaceutics 2022, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Suzuki, K. The integrative role of sulforaphane in preventing inflammation, oxidative stress and fatigue: A review of a potential protective phytochemical. Antioxidants 2020, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Holman, J.; Hurd, M.; Moses, P.L.; Mawe, G.M.; Zhang, T.; Ishaq, S.L.; Li, Y. Interplay of broccoli/broccoli sprout bioactives with gut microbiota in reducing inflammation in inflammatory bowel diseases. J. Nutr. Biochem. 2023, 113, 109238. [Google Scholar] [CrossRef] [PubMed]
- Polinska, B.; Matowicka-Karna, J.; Kemona, H. The cytokines in inflammatory bowel disease. Postep. Hig. Med. Dosw. 2009, 63, 389–394. [Google Scholar]
- Miller, C.A. Expression of the human aryl hydrocarbon receptor complex in yeast—Activation of transcription by indole compounds. J. Biol. Chem. 1997, 272, 32824–32829. [Google Scholar] [CrossRef]
- Neavin, D.R.; Liu, D.; Ray, B.; Weinshilboum, R.M. The role of the aryl hydrocarbon receptor (AHR) in immune and inflammatory diseases. Int. J. Mol. Sci. 2018, 19, 3851. [Google Scholar] [CrossRef]
- Peng, C.; Wu, C.; Xu, X.; Pan, L.; Lou, Z.; Zhao, Y.; Jiang, H.; He, Z.; Ruan, B. Indole-3-carbinol ameliorates necroptosis and inflammation of intestinal epithelial cells in mice with ulcerative colitis by activating aryl hydrocarbon receptor. Exp. Cell Res. 2021, 404, 112638. [Google Scholar] [CrossRef]
- Garcia-Ibañez, P.; Núñez-Sánchez, M.A.; Oliva-Bolarín, A.; Martínez-Sánchez, M.A.; Ramos-Molina, B.; Ruiz-Alcaraz, A.J.; Moreno, D.A. Anti-inflammatory potential of digested Brassica sprout extracts in human macrophage-like HL-60 cells. Food Funct. 2023, 14, 112–121. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; He, Q.; Yu, L.; Pham, Q.; Cheung, L.; Zhang, Z.; Kim, Y.S.; Smith, A.D.; Wang, T.T. Dietary indole-3-carbinol alleviated spleen Enlargement, enhanced IgG response in C3H/HeN mice infected with Citrobacter rodentium. Nutrients 2020, 12, 3148. [Google Scholar] [CrossRef]
- Abu-Qatouseh, L. Sulforaphane from broccoli attenuates inflammatory hepcidin by reducing IL-6 secretion in human HepG2 cells. J. Funct. Foods 2020, 75, 104210. [Google Scholar]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Sulforaphane protects cells against lipopolysaccharide-stimulated inflammation in murine macrophages. Antioxidants 2019, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-kappa B Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Mahn, A.; Castillo, A. Potential of sulforaphane as a natural immune system enhancer: A review. Molecules 2021, 26, 752. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Bonay, M.; Vanhee, V.; Vinit, S.; Deramaudt, T.B. Comparative effectiveness of 4 natural and chemical activators of Nrf2 on inflammation, oxidative stress, macrophage polarization, and bactericidal activity in an in vitro macrophage infection model. PLoS ONE 2020, 15, e0234484. [Google Scholar] [CrossRef]
- Bahiraii, S.; Brenner, M.; Yan, F.; Weckwerth, W.; Heiss, E.H. Sulforaphane diminishes moonlighting of pyruvate kinase M2 and interleukin 1β expression in M1 (LPS) macrophages. Front. Immunol. 2022, 13, 935692. [Google Scholar] [CrossRef] [PubMed]
- Trivedi-Parmar, V.; Jorgensen, W.L. Advances and insights for small molecule inhibition of macrophage migration inhibitory factor. J. Med. Chem. 2018, 61, 8104–8119. [Google Scholar] [CrossRef]
- Spencer, E.S.; Dale, E.J.; Gommans, A.L.; Rutledge, M.T.; Vo, C.T.; Nakatani, Y.; Gamble, A.B.; Smith, R.A.; Wilbanks, S.M.; Hampton, M.B. Multiple binding modes of isothiocyanates that inhibit macrophage migration inhibitory factor. Eur. J. Med. Chem. 2015, 93, 501–510. [Google Scholar] [CrossRef]
- Guerrero-Alonso, A.; Antunez-Mojica, M.; Medina-Franco, J.L. Chemoinformatic Analysis of Isothiocyanates: Their Impact in Nature and Medicine. Mol. Inf. 2021, 40, 2100172. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Liu, B.; Zhang, Y.; Pan, X.; Yu, X.-Y.; Shen, Z.; Song, Y.-H. Inflammasomes as therapeutic targets in human diseases. Signal Transduct. Target. Ther. 2021, 6, 247. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Kiser, C.; Gonul, C.P.; Olcum, M.; Genc, S. Inhibitory effects of sulforaphane on NLRP3 inflammasome activation. Mol. Immunol. 2021, 140, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, S.; Sun, S.; Li, Z.; Guo, B. Inflammasome activation has an important role in the development of spontaneous colitis. Mucosal Immunol. 2014, 7, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-J.; Dong, J.-Y.; Qiu, Y.; Zhang, G.-L.; Wei, K.; He, L.-H.; Sun, Y.-N.; Jiang, H.-Z.; Zhang, S.-S.; Guo, X.-R.; et al. Sulforaphane decreases oxidative stress and inhibits NLRP3 inflammasome activation in a mouse model of ulcerative colitis. Biomed. Pharmacother. 2024, 175, 116706. [Google Scholar] [CrossRef] [PubMed]
- Park, W.S.; Lee, J.; Na, G.; Park, S.; Seo, S.-K.; Choi, J.S.; Jung, W.-K.; Choi, I.-W. Benzyl Isothiocyanate Attenuates Inflammasome Activation in Pseudomonas aeruginosa LPS-Stimulated THP-1 Cells and Exerts Regulation through the MAPKs/NF-κB Pathway. Int. J. Mol. Sci. 2022, 23, 1228. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Saleh, H.A.; Ramdan, E.; Elmazar, M.M.; Azzazy, H.M.; Abdelnaser, A. Comparing the protective effects of resveratrol, curcumin and sulforaphane against LPS/IFN-γ-mediated inflammation in doxorubicin-treated macrophages. Sci. Rep. 2021, 11, 545. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef]
- Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Natural indoles, indole-3-carbinol and 3, 3′-diindolymethane, inhibit T cell activation by staphylococcal enterotoxin B through epigenetic regulation involving HDAC expression. Toxicol. Appl. Pharm. 2014, 274, 7–16. [Google Scholar] [CrossRef] [PubMed]
- López-Chillón, M.T.; Carazo-Díaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.A.; Villaño, D. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clin. Nutr. 2019, 38, 745–752. [Google Scholar] [CrossRef]
- Lohning, A.; Kidachi, Y.; Kamiie, K.; Sasaki, K.; Ryoyama, K.; Yamaguchi, H. 6-(methylsulfinyl) hexyl isothiocyanate (6-MITC) from Wasabia japonica alleviates inflammatory bowel disease (IBD) by potential inhibition of glycogen synthase kinase 3 beta (GSK-3β). Eur. J. Med. Chem. 2021, 216, 113250. [Google Scholar] [CrossRef]
- Chang, Y.; Zhai, L.; Peng, J.; Wu, H.; Bian, Z.; Xiao, H. Phytochemicals as regulators of Th17/Treg balance in inflammatory bowel diseases. Biomed. Pharmacother. 2021, 141, 111931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lei, F.; Jiang, T.; Xie, L.; Huang, P.; Li, P.; Huang, Y.; Tang, X.; Gong, J.; Lin, Y. H19/miR-675-5p targeting SFN enhances the invasion and metastasis of nasalpharyngeal cancer cells. Curr. Molec. Pharmacol. 2019, 12, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.L.; Kala, R.; Tollefsbol, T.O. Mechanisms for the inhibition of colon cancer cells by sulforaphane through epigenetic modulation of microRNA-21 and human telomerase reverse transcriptase (hTERT) down-regulation. Curr. Cancer Drug Targets 2018, 18, 97–106. [Google Scholar] [CrossRef]
- Gao, L.; Cheng, D.; Yang, J.; Wu, R.; Li, W.; Kong, A.-N. Sulforaphane epigenetically demethylates the CpG sites of the miR-9-3 promoter and reactivates miR-9-3 expression in human lung cancer A549 cells. J. Nutr. Biochem. 2018, 56, 109–115. [Google Scholar] [CrossRef]
- Nandini, D.; Rao, R.S.; Deepak, B.; Reddy, P.B. Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy. J. Oral Maxillofac. Pathol. JOMFP 2020, 24, 405. [Google Scholar] [CrossRef]
- Li, Z.; Rana, T.M. Therapeutic targeting of microRNAs: Current status and future challenges. Nat. Rev. Drug Discov. 2014, 13, 622–638. [Google Scholar] [CrossRef] [PubMed]
- Krishnachaitanya, S.S.; Liu, M.; Fujise, K.; Li, Q. MicroRNAs in inflammatory bowel disease and its complications. Int. J. Mol. Sci. 2022, 23, 8751. [Google Scholar] [CrossRef]
- Jung, H.; Kim, J.S.; Lee, K.H.; Tizaoui, K.; Terrazzino, S.; Cargnin, S.; Smith, L.; Koyanagi, A.; Jacob, L.; Li, H. Roles of microRNAs in inflammatory bowel disease. Int. J. Biol. Sci. 2021, 17, 2112. [Google Scholar] [CrossRef]
- Dacosta, C.; Bao, Y. The role of MicroRNAs in the chemopreventive activity of sulforaphane from cruciferous vegetables. Nutrients 2017, 9, 902. [Google Scholar] [CrossRef]
- Wu, J.; Cui, S.; Liu, J.; Tang, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. The recent advances of glucosinolates and their metabolites: Metabolism, physiological functions and potential application strategies. Crit. Rev. Food Sci. 2023, 63, 4217–4234. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Kuehn, C.; Nickel, M.; Rohn, S.; Dekker, M. Leaching and degradation kinetics of glucosinolates during boiling of Brassica oleracea vegetables and the formation of their breakdown products. Food Chem. 2018, 263, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Andernach, L.; Witzel, K.; Hanschen, F.S. Glucosinolate-derived amine formation in Brassica oleracea vegetables. Food Chem. 2023, 405, 134907. [Google Scholar] [CrossRef] [PubMed]
- Sikorska-Zimny, K.; Beneduce, L. The glucosinolates and their bioactive derivatives in Brassica: A review on classification, biosynthesis and content in plant tissues, fate during and after processing, effect on the human organism and interaction with the gut microbiota. Crit. Rev. Food Sci. 2021, 61, 2544–2571. [Google Scholar] [CrossRef]
- Luo, S.; An, R.; Zhou, H.; Zhang, Y.; Ling, J.; Hu, H.; Li, P. The glucosinolate profiles of Brassicaceae vegetables responded differently to quick-freezing and drying methods. Food Chem. 2022, 383, 132624. [Google Scholar] [CrossRef]
- Bello, C.; Maldini, M.; Baima, S.; Scaccini, C.; Natella, F. Glucoraphanin and sulforaphane evolution during juice preparation from broccoli sprouts. Food Chem. 2018, 268, 249–256. [Google Scholar] [CrossRef]
- Renz, M.; Dekker, M.; Rohn, S.; Hanschen, F.S. Plant matrix concentration and redox status influence thermal glucosinolate stability and formation of nitriles in selected Brassica vegetable broths. Food Chem. 2023, 404, 134594. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.; Andernach, L.; Kanzler, C.; Hanschen, F.S. Novel transformation products from glucosinolate-derived thioglucose and isothiocyanates formed during cooking. Food Res. Int. 2022, 157, 111237. [Google Scholar] [CrossRef]
- Hoffmann, H.; Ott, C.; Raupbach, J.; Andernach, L.; Renz, M.; Grune, T.; Hanschen, F.S. Assessing Bioavailability and Bioactivity of 4-Hydroxythiazolidine-2-Thiones, Newly Discovered Glucosinolate Degradation Products Formed During Domestic Boiling of Cabbage. Front. Nutr. 2022, 9, 941286. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Zhao, Z.; Sheng, X.; Shen, Y.; Gu, H. Natural Variation of Glucosinolates and Their Breakdown Products in Broccoli (Brassica oleracea var. italica) Seeds. J. Agric. Food Chem. 2019, 67, 12528–12537. [Google Scholar] [CrossRef]
- Di, H.; Ma, J.; Zhang, Y.; Wei, J.; Yang, J.; Ma, J.; Bian, J.; Xu, J.; Huang, Z.; Tang, Y.; et al. Correlations between flavor and glucosinolates and changes in quality-related physiochemical characteristics of Guizhou suancai during the fermentation process. Food Chem. 2023, 405, 134965. [Google Scholar] [CrossRef]
| Classification of GLS | Compound Name | Glucosinolate Content (μmol/g) | |||
|---|---|---|---|---|---|
| Kale [20] Brassica oleracea L. | Broccoli [19] B. oleracea L. var. Italic) | Chinese Cabbage [21] B. rapa var. Glabra Rule) | Mustard [22] B. juncea | ||
| aliphatic GLS | glucoiberin (GIB) | 4.78~5.42 | 0~0.878 | N/A | N/A |
| glucoberteroin | N/A | 0.008~6.273 | 0.35~0.38 | N/A | |
| glucoraphanin (GRP) | N/A | 0.136~14.973 | N/A | N/A | |
| progoitrin (PRO) | 0.08~0.70 | 0~4.537 | 0.71~0.87 | N/A | |
| sinigrin (SIN) | 1.64~1.78 | 0~3.161 | N/A | 13.95~17.67 | |
| glucoalyssin | N/A | 0~2.728 | 1.11~1.19 | 0.45~0.53 | |
| gluconapin (GNA) | N/A | N/A | 0.23~0.27 | 0.12~0.18 | |
| percentage (%) | 84.40~85.08% | 27.78~48% | 18.93~23.20% | 71.56~90.69% | |
| indole GLS | glucobrasscin (GBS) | 0.98~1.04 | 0.103~27.690 | 3.94~4.36 | 0.43~0.59 |
| neoglucobrasscin | 0.07~0.33 | 0.018~45.954 | 3.54~3.56 | N/A | |
| glucobrassicanapin | N/A | N/A | 0.85~0.91 | 0.20~0.28 | |
| 4-hydroxyglucobrassicin | N/A | 0.014~3.289 | 0.08~0.12 | 0.23~0.35 | |
| 4-methoxyglucobrassicin | N/A | 0.014~3.915 | 0.96~1.12 | 0.28~0.4 | |
| percentage (%) | 13.74~14.64% | 43.67~70.99% | 73.90~86.22% | 3.50~6.47% | |
| aromatic GLS | gluconasturtiin (GNS) | N/A | 0.004–0.441 | N/A | 0.22~0.34 |
| sinalbin | N/A | N/A | N/A | N/A | |
| glucotropaeolin | N/A | 0–0.040 | N/A | N/A | |
| percentage (%) | 0 | 0.42~1.33% | 0 | 1.08~2.13% | |
| total (μmol/g) | 7.64~9.36 | 0.30~113.88 | 11.68~12.68 | 15.93~20.29 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Zhang, X.; Li, F.; Lei, X.; Ge, L.; Li, H.; Zhao, N.; Ming, J. The Effects of Interventions with Glucosinolates and Their Metabolites in Cruciferous Vegetables on Inflammatory Bowel Disease: A Review. Foods 2024, 13, 3507. https://doi.org/10.3390/foods13213507
Zhao J, Zhang X, Li F, Lei X, Ge L, Li H, Zhao N, Ming J. The Effects of Interventions with Glucosinolates and Their Metabolites in Cruciferous Vegetables on Inflammatory Bowel Disease: A Review. Foods. 2024; 13(21):3507. https://doi.org/10.3390/foods13213507
Chicago/Turabian StyleZhao, Jichun, Xiaoqin Zhang, Fuhua Li, Xiaojuan Lei, Lihong Ge, Honghai Li, Nan Zhao, and Jian Ming. 2024. "The Effects of Interventions with Glucosinolates and Their Metabolites in Cruciferous Vegetables on Inflammatory Bowel Disease: A Review" Foods 13, no. 21: 3507. https://doi.org/10.3390/foods13213507
APA StyleZhao, J., Zhang, X., Li, F., Lei, X., Ge, L., Li, H., Zhao, N., & Ming, J. (2024). The Effects of Interventions with Glucosinolates and Their Metabolites in Cruciferous Vegetables on Inflammatory Bowel Disease: A Review. Foods, 13(21), 3507. https://doi.org/10.3390/foods13213507






