Screening of the Nutritional Properties, Bioactive Components, and Antioxidant Properties in Legumes
Abstract
1. Introduction
2. Materials and Methods
2.1. Legumes Sources
2.2. Chemical Composition Analysis
2.3. Amino Acid Composition Analysis
2.4. Determination of In Vitro Protein Digestibility
2.5. Color Analysis
2.6. Preparation of Methanolic Extracts for Bioactive Compounds and Antioxidant Capacity
2.7. Determination of Total Polyphenolic Content (TPC)
2.8. Determination of Total Flavonoid Content (TFC)
2.9. Determination of Antioxidant Capacity Through DPPH Method
2.10. Determination of Antioxidant Capacity Through ABTS Method
2.11. Determination of Antioxidant Capacity Through FRAP Method
2.12. Determination of Antioxidant Capacity Through CUPRAC Method
2.13. Photochemiluminescence (PCL) Assay
2.14. Development of the Relative Antioxidant Capacity Index (RACI)
2.15. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Legume Samples
3.2. Amino Acid Composition
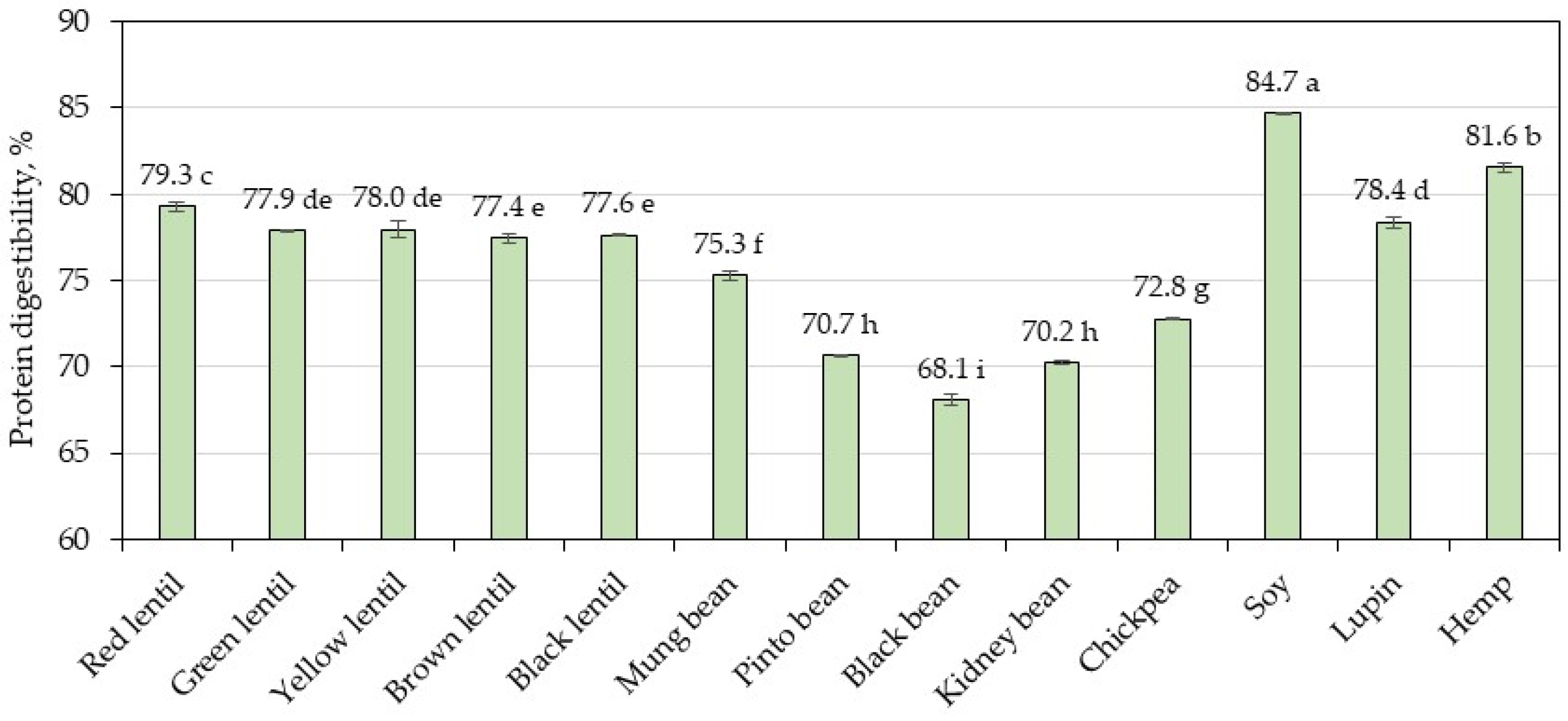
3.3. Protein Digestibility
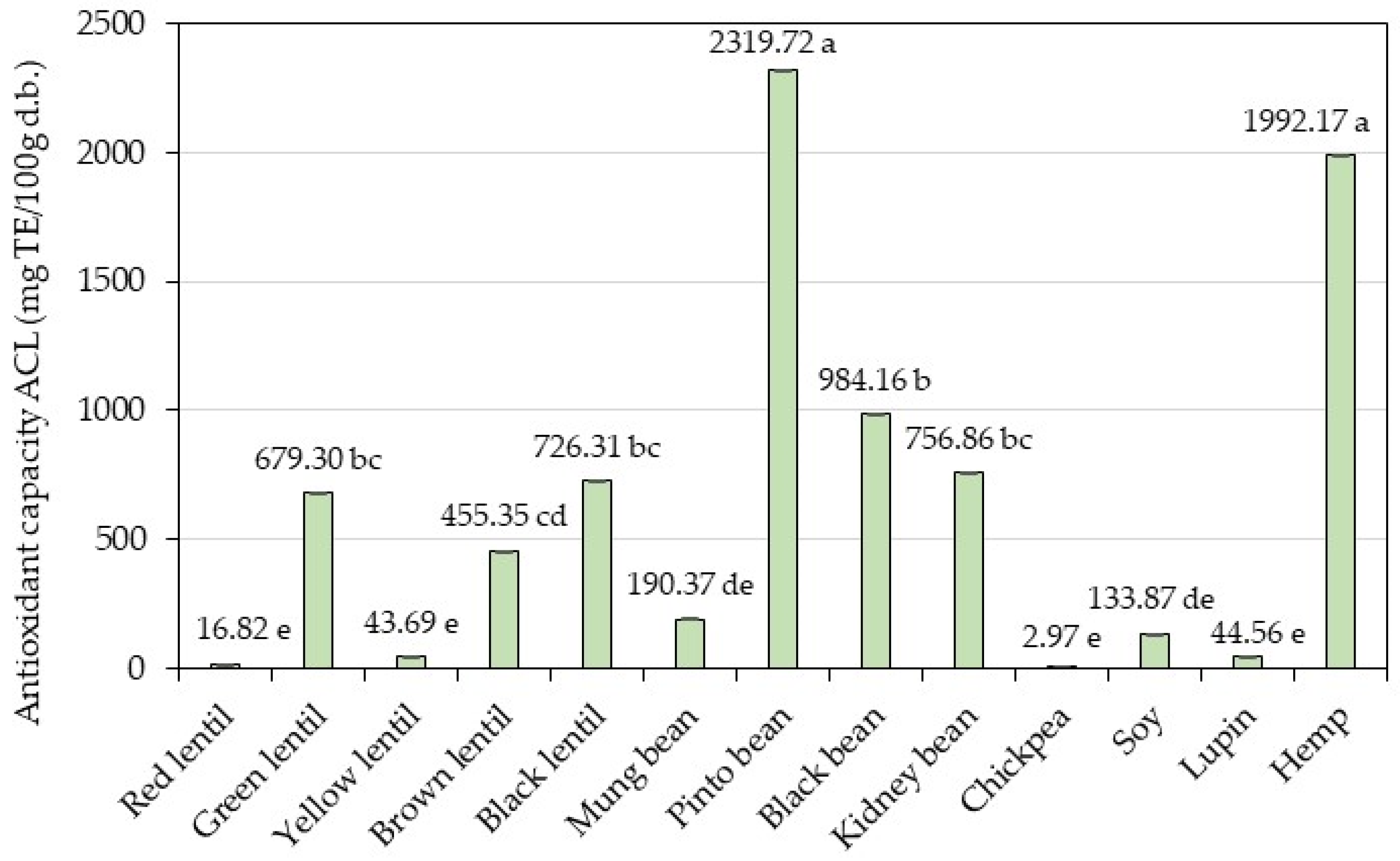
3.4. Bioactive Compounds Content
3.5. Antioxidant Capacity by Different Assays
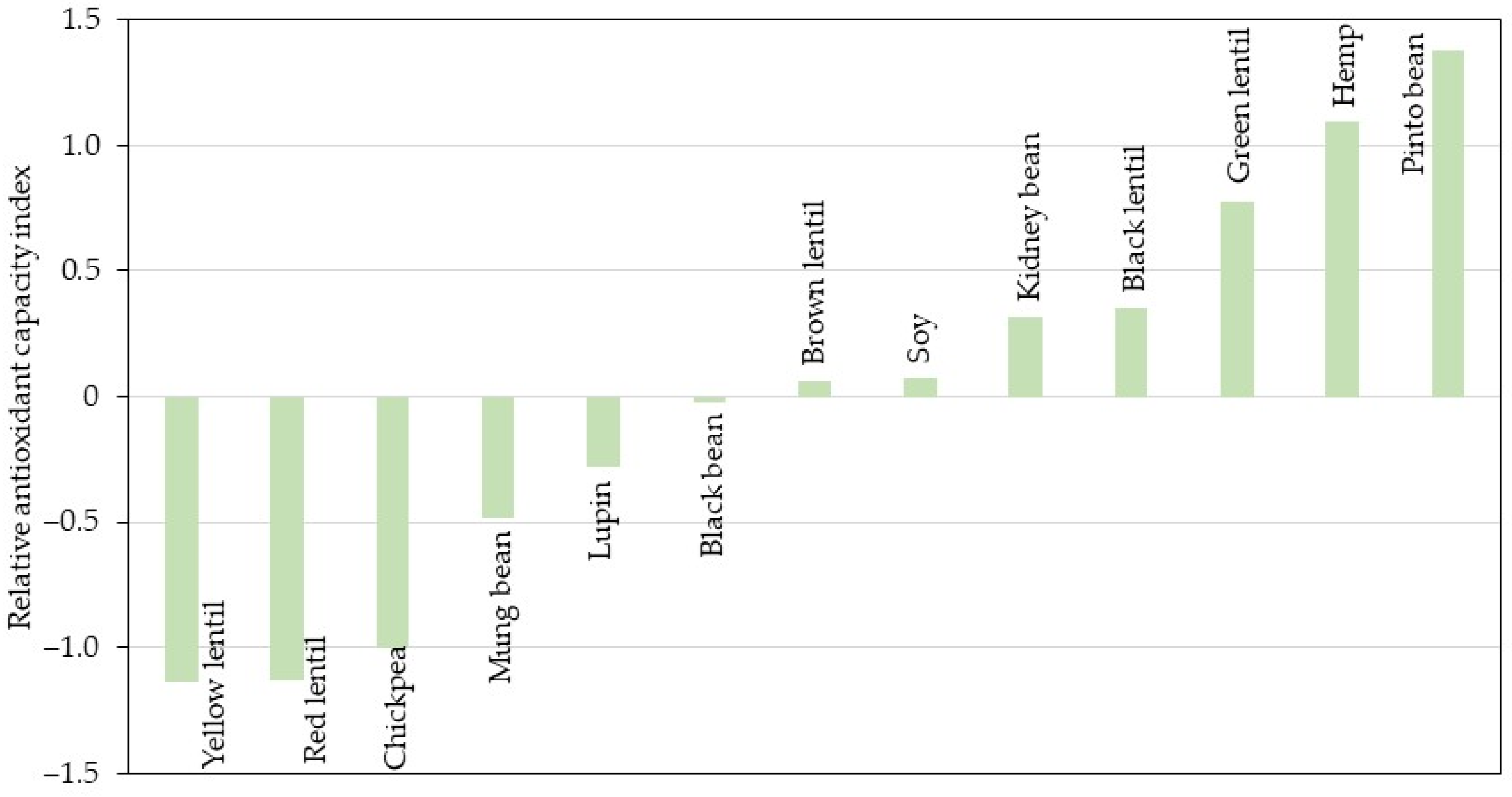
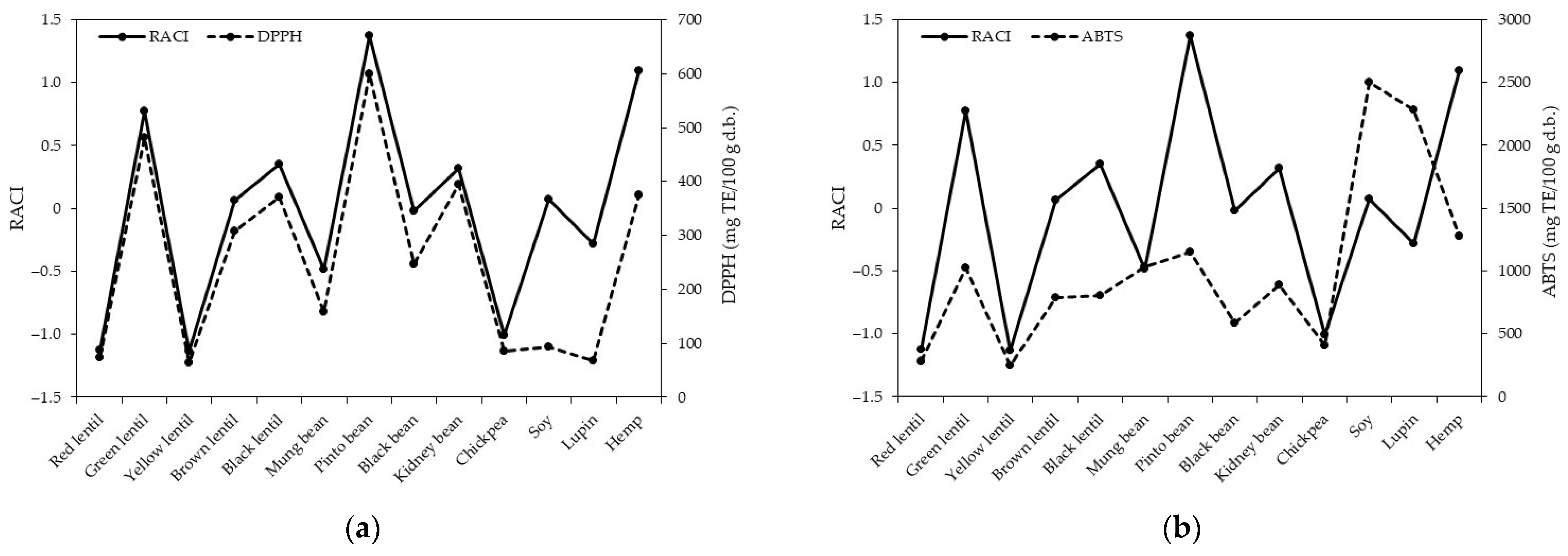
3.6. Calculation of the Relative Antioxidant Capacity Index
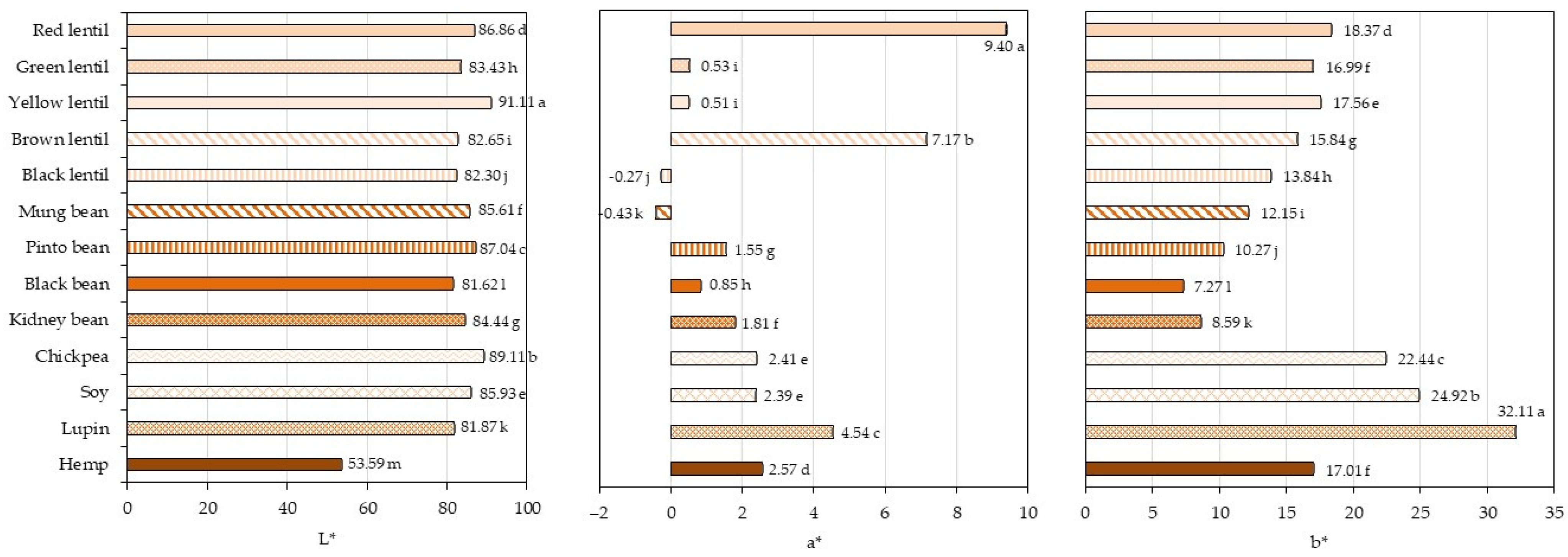
3.7. Determination of Color Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects 2022: Summary of Results; UN DESA/POP/2022/TR/NO. 3; United Nations: New York, NY, USA, 2022; ISBN 978-92-1-148373-4. [Google Scholar]
- Yanni, A.E.; Iakovidi, S.; Vasilikopoulou, E.; Karathanos, V.T. Legumes: A Vehicle for Transition to Sustainability. Nutrients 2024, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Ramsing, R.; Rahman, N.; Kraemer, K.; Bloem, M.W. Legumes as a sustainable source of protein in human diets. Glob. Food Sec. 2021, 28, 100520. [Google Scholar] [CrossRef]
- Carbas, B.; Machado, N.; Pathania, S.; Brites, C.; Rosa, E.A.; Barros, A.I. Potential of legumes: Nutritional value, bioactive properties, innovative food products, and application of eco-friendly tools for their assessment. Food Rev. Int. 2021, 39, 160–188. [Google Scholar] [CrossRef]
- Martín-Cabrejas, M.A. Legumes: An Overview. In Legumes: Nutritional Quality, Processing and Potential Health Benefits; Martín-Cabrejas, M.A., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 1–18. ISBN 978-1-78801-572-1. [Google Scholar]
- Chughtai, M.F.J.; Khaliq, A.; Zahoor, T.; Ahsan, S.; Liaqat, A.; Nadeem, M.; Mehmood, T.; Tahir, A.B.; Saeed, K.; Junaid-ur-Rehman, S. Meat Replacers and Meal Plans Based on Plant Protein Isolates for Human Consumption. In Plant Protein Foods; Manickavasagan, A., Lim, L.T., Ali, A., Eds.; Springer: Cham, Switzerland, 2022; pp. 439–465. ISBN 978-3-030-91205-5. [Google Scholar]
- Salim, R.; Nehvi, I.B.; Mir, R.A.; Tyagi, A.; Ali, S.; Bhat, O.M. A review on anti-nutritional factors: Unraveling the natural gateways to human health. Front. Nutr. 2023, 10, 1215873. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Tomas, M.; Ozkan, G.; Ozdal, T.; Capanoglu, E. In vitro digestibility of plant proteins: Strategies for improvement and health implications. Curr. Opin. Food Sci. 2024, 57, 101148. [Google Scholar] [CrossRef]
- Kumar, Y.; Basu, S.; Goswami, D.; Devi, M.; Shanker Shivhare, U.; Kumar Vishwakarma, R. Anti-nutritional compounds in pulses: Implications and alleviation methods. Legum. Sci. 2022, 4, e111. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Schmidt, H.d.O.; Oliveira, V.R.d. Overview of the Incorporation of Legumes into New Food Options: An Approach on Versatility, Nutritional, Technological, and Sensory Quality. Foods 2023, 12, 2586. [Google Scholar] [CrossRef]
- Pasqualone, A.; Costantini, M.; Coldea, T.E.; Summo, C. Use of Legumes in Extrusion Cooking: A Review. Foods 2020, 9, 958. [Google Scholar] [CrossRef]
- Dutta, A.; Trivedi, A.; Nath, C.P.; Gupta, D.S.; Hazra, K. A comprehensive review on grain legumes as climate-smart crops: Challenges and prospects. Environ. Chall. 2022, 7, 100479. [Google Scholar] [CrossRef]
- Liu, K. Chemistry and Nutritional Value of Soybean Components. In Soybeans; Liu, K., Ed.; Springer: Boston, MA, USA, 1997; pp. 25–113. ISBN 978-1-4613-5711-7. [Google Scholar]
- Joehnke, M.S.; Jeske, S.; Ispiryan, L.; Zannini, E.; Arendt, E.K.; Bez, J.; Sørensen, J.C.; Petersen, I.L. Nutritional and anti-nutritional properties of lentil (Lens culinaris) protein isolates prepared by pilot-scale processing. Food Chem. X 2021, 9, 100112. [Google Scholar] [CrossRef] [PubMed]
- Kaale, L.D.; Siddiq, M.; Hooper, S. Lentil (Lens culinaris Medik) as nutrient-rich and versatile food legume: A review. Legum. Sci. 2023, 5, e169. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-Rich Dry Common Beans (Phaseolus vulgaris L.) and Their Health Benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Murray, R.; Zelman, K.M. The Nutritional Value and Health Benefits of Chickpeas and Hummus. Nutrients 2016, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef]
- Mazumder, K.; Biswas, B.; Kerr, P.G.; Blanchard, C.; Nabila, A.; Golder, M.; Gulzarul Aziz, M.; Farahnaky, A. Comparative assessment of nutritional, thermal, rheological and functional properties of nine Australian lupin cultivars. Sci. Rep. 2021, 11, 21515. [Google Scholar] [CrossRef]
- Fechner, A.; Schweiggert, U.; Hasenkopf, K.; Jahreis, G. Lupine Kernel Fiber: Metabolic Effects in Human Intervention Studies and Use as a Supplement in Wheat Bread. In Flour and Breads and their Fortification in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2011; pp. 463–473. ISBN 978-0-12-380886-8. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 21st ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Long, W. Automated Amino Acid Analysis Using an Agilent Poroshell HPH-C18 Column; Agilent Technologies: Santa Clara, CA, USA, 2017. [Google Scholar]
- Food and Agriculture Organization (FAO). Dietary protein quality evaluation in human nutrition: Report of an FAO expert consultation. FAO Food Nutr. Pap. 2013, 92, 1–66. [Google Scholar]
- Corgneau, M.; Gaiani, C.; Petit, J.; Nikolova, Y.; Banon, S.; Ritié-Pertusa, L.; Thanh Lam Le, D.; Scher, J. Nutritional quality evaluation of commercial protein supplements. Int. J. Food Sci. Technol. 2019, 54, 2586–2594. [Google Scholar] [CrossRef]
- Li, Z.; Hong, T.; Shen, G.; Gu, Y.; Guo, Y.; Han, J. Amino Acid Profiles and Nutritional Evaluation of Fresh Sweet–Waxy Corn from Three Different Regions of China. Nutrients 2022, 14, 3887. [Google Scholar] [CrossRef]
- Hsu, H.W.; Vavak, D.L.; Satterlee, L.D.; Miller, G.A. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- Horszwald, A.; Andlauer, W. Characterisation of bioactive compounds in berry juices by traditional photometric and modern microplate methods. J. Berry Res. 2011, 1, 189–199. [Google Scholar] [CrossRef]
- Celik, S.E.; Ozyürek, M.; Güçlü, K.; Apak, R. Determination of antioxidants by a novel on-line HPLC-cupric reducing antioxidant capacity (CUPRAC) assay with post-column detection. Anal. Chim. Acta 2010, 674, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Popov, I.N.; Lewin, G. Photochemiluminescent detection of antiradical activity; IV: Testing of lipid-soluble antioxidant. J. Biochem. Bioph. Meth. 1996, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Tanumihardjo, S.A. An integral approach to evaluate food antioxidant capacity. J. Food Sci. 2007, 72, 159–165. [Google Scholar] [CrossRef]
- Piergiovanni, A.R. Nutritional Characteristics of Black Lentil from Soleto: A Single-Flower Vetch Landrace of Apulia Region (Southern Italy). Foods 2021, 10, 2863. [Google Scholar] [CrossRef]
- Sinković, L.; Pipan, B.; Šibul, F.; Nemeš, I.; Tepić Horecki, A.; Meglić, V. Nutrients, Phytic Acid and Bioactive Compounds in Marketable Pulses. Plants 2023, 12, 170. [Google Scholar] [CrossRef]
- Grela, E.R.; Kiczorowska, B.; Samolińska, W.; Matras, J.; Kiczorowski, P.; Rybiński, W.; Hanczakowska, E. Chemical composition of leguminous seeds: Part I—Content of basic nutrients, amino acids, phytochemical compounds, and antioxidant activity. Eur. Food Res. Technol. 2017, 243, 1385–1395. [Google Scholar] [CrossRef]
- Wang, N.; Daun, J.K. Effects of variety and crude protein content on nutrients and anti-nutrients in lentils (Lens culinaris). Food Chem. 2006, 95, 493–502. [Google Scholar] [CrossRef]
- Gunathunga, C.; Senanayake, S.; Jayasinghe, M.A.; Brennan, C.S.; Truong, T.; Marapana, U.; Chandrapala, J. Germination effects on nutritional quality: A comprehensive review of selected cereals and pulses changes. J. Food Compost. Anal. 2024, 128, 106024. [Google Scholar] [CrossRef]
- Viktorinová, K.; Petřeková, K.; Šimek, J.; Hartman, I.; Hertel, V. Nutrition and Sensory Evaluation of Germinated Legumes. Kvasný Průmysl 2020, 63, 270–276. [Google Scholar] [CrossRef]
- Garrido-Galand, S.; Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. The potential of fermentation on nutritional and technological improvement of cereal and legume flours: A review. Food Res. Int. 2021, 145, 110398. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Schop, M.; de Boer, I.J.M.; Huppertz, T. Protein Quality in Perspective: A Review of Protein Quality Metrics and Their Applications. Nutrients 2022, 14, 947. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Huub Waterval, W.A.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Wang, T.; Luo, Y. A review on plant-based proteins from soybean: Health benefits and soy product development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Espinosa-Páez, E.; Alanis-Guzmán, M.G.; Hernández-Luna, C.E.; Baez-González, J.G.; Amaya-Guerra, C.A.; Andrés-Grau, A.M. Increasing antioxidant activity and protein digestibility in phaseolus vulgaris and avena sativa by fermentation with the Pleurotusostreatus fungus. Molecules 2017, 22, 2275. [Google Scholar] [CrossRef]
- Khattab, R.Y.; Arntfield, S.D.; Nyachoti, C.M. Nutritional quality of legume seeds as affected by some physical treatments. Part 1. Protein quality evaluation. LWT 2009, 42, 1107–1112. [Google Scholar] [CrossRef]
- Ohanenye, I.C.; Tsopmo, A.; Ejike, C.E.C.C.; Udenigwe, C.C. Germination as a bioprocess for enhancing the quality and nutritional prospects of legume proteins. Trends Food Sci Technol. 2020, 101, 213–222. [Google Scholar] [CrossRef]
- Ohanenye, I.C.; Ekezie, F.C.; Sarteshnizi, R.A.; Boachie, R.T.; Emenike, C.U.; Sun, X.; Nwachukwu, I.D.; Udenigwe, C.C. Legume Seed Protein Digestibility as Influenced by Traditional and Emerging Physical Processing Technologies. Foods 2022, 11, 2299. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages, and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Xia, M.; Li, M.; de Souza, T.S.P.; Barrow, C.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS2 Characterization of Phenolic Compounds in Different Lentil (Lens culinaris M.) Sam-ples and Their Antioxidant Capacity. Front. Biosci. 2023, 28, 44. [Google Scholar] [CrossRef]
- Durazzo, A.; Turfani, V.; Azzini, E.; Maiani, G.; Carcea, M. Phenols, lignans and antioxidant properties of legume and sweet chestnut flours. Food Chem. 2013, 140, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Baik, B.-K. Antioxidant activity and phenolic content of lentils (Lens culinaris), chickpeas (Cicer arietinum L.), peas (Pisum sativum L.) and soybeans (Glycine max), and their quantitative changes during processing. Int. J. Food Sci. Technol. 2008, 43, 1971–1978. [Google Scholar] [CrossRef]
- Shi, Z.; Yao, Y.; Zhu, Y.; Ren, G. Nutritional composition and antioxidant activity of twenty mung bean cultivars in China. Crop J. 2016, 4, 398–406. [Google Scholar] [CrossRef]
- Fonseca Hernández, D.; Mojica, L.; Berhow, M.A.; Brownstein, K.; Lugo Cervantes, E.; Gonzalez de Mejia, E. Black and pinto beans (Phaseolus vulgaris L.) unique mexican varieties exhibit antioxidant and anti-inflammatory potential. Food Res. Int. 2023, 169, 112816. [Google Scholar] [CrossRef] [PubMed]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Cui, S.W.; Li, Y.; Xu, S.; Wu, Y.; Wang, J.; Bai, Z.; et al. Nutrients, phytochemicals and antioxidant activities of 26 kidney bean cultivars. Food Chem. Toxicol. 2017, 108, 467–477. [Google Scholar] [CrossRef]
- Giusti, F.; Caprioli, G.; Ricciutelli, M.; Vittori, S.; Sagratini, G. Determination of fourteen polyphenols in pulses by high performance liquid chromatography-diode array detection (HPLC-DAD) and correlation study with antioxidant activity and colour. Food Chem. 2017, 221, 689–697. [Google Scholar] [CrossRef]
- Rocchetti, G.; Lucini, L.; Rodriguez, J.M.L.; Barba, F.J.; Giuberti, G. Gluten-free flours from cereals, pseudocereals and legumes: Phenolic fingerprints and in vitro antioxidant properties. Food Chem. 2019, 271, 157–164. [Google Scholar] [CrossRef]
- Chen, P.X.; Tang, Y.; Marcone, M.F.; Pauls, P.K.; Zhang, B.; Liu, R.; Tsao, R. Characterization of free, conjugated and bound phenolics and lipophilic antioxidants in regular- and non-darkening cranberry beans (Phaseolus vulgaris L.). Food Chem. 2015, 185, 298–308. [Google Scholar] [CrossRef]
- Mastura, H.Y.; Hasnah, H.; Dang, T.N. Total phenolic content and antioxidant capacity of beans: Organic vs inorganic. Int. Food Res. J. 2017, 24, 510–517. [Google Scholar]
- Carbas, B.; Machado, N.; Oppolzer, D.; Ferreira, L.; Queiroz, M.; Brites, C.; As Rosa, E.; Barros, A.I. Nutrients, Antinutrients, Phenolic Composition, and Antioxidant Activity of Common Bean Cultivars and their Potential for Food Applications. Antioxidants 2020, 9, 186. [Google Scholar] [CrossRef]
- Mekky, R.H.; del Mar Contreras, M.; El-Gindi, M.R.; Abdel-Monem, A.R.; Ab-del-Sattar, E.; Segura-Carretero, A. Profiling of phenolic and other compounds from Egyptian cultivars of chickpea (Cicer arietinum L.) and antioxidant activity: A comparative study. RSC Adv. 2015, 5, 17751–17767. [Google Scholar] [CrossRef]
- Siger, A.; Czubinski, J.; Kachlicki, P.; Dwiecki, K.; Lampart-Szczapa, E.; Nogala-Kalucka, M. Antioxidant activity and phenolic content in three lupin species. J. Food Compos. Anal. 2012, 25, 190–197. [Google Scholar] [CrossRef]
- Vonapartis, E.; Marie-Pier, A.; Seguin, P.; Mustafa, A.F.; Charron, J.B. Seed composition of ten industrial hemp cultivars approved for production in Canada. J. Food Compos. Anal. 2015, 39, 8–12. [Google Scholar] [CrossRef]
- Farinon, B.; Costantini, L.; Molinari, R.; Di Matteo, G.; Garzoli, S.; Ferri, S.; Ceccantoni, B.; Mannina, L.; Merendino, N. Effect of malting on nutritional and antioxidant properties of the seeds of two industrial hemp (Cannabis sativa L.) cultivars. Food Chem. 2022, 370, 131348. [Google Scholar] [CrossRef]
- Taaifi, Y.; Benmoumen, A.; Belhaj, K.; Aazza, S.; Abid, M.; Azeroual, E.; Elamrani, A.; Mansouri, F.; Serghini Caid, H. Seed composition of non-industrial hemp (Cannabis sativa L.) varieties from four regions in northern Morocco. Int. J. Food Sci. Technol. 2021, 56, 5931–5947. [Google Scholar] [CrossRef]
- Kalefetoğlu Macar, T.K.; Macar, O.; Mart, D.İ. Variability in Some Biochemical and Nutritional Characteristics in Desi and Turkish Kabuli Chickpea (Cicer arietinum L.) Types. Celal Bayar Univ. J. Sci. 2017, 13, 677–680. [Google Scholar] [CrossRef]
- Josipović, A.; Sudar, R.; Sudarić, A.; Jurković, V.; Matoša Kočar, M.; Markulj Kulundžić, A. Total phenolic and total flavonoid content variability of soybean genotypes in eastern Croatia. Croat. J. Food Sci. Technol. 2016, 8, 60–65. [Google Scholar] [CrossRef]
- Rashid, A.; Ali, V.; Khajuria, M.; Sheenam, F.; Jamwal, S.; Lone, J.F.; Gairola, S.; Vyas, D. Antioxidant analysis in seeds of four different accessions of Cannabis sativa L. from Jammu. Res. Rev. Biotechnol. Biosci. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Faugno, S.; Piccolella, S.; Sannino, M.; Principio, L.; Crescente, G.; Baldi, G.M.; Fiorentino, N.; Pacifico, S. Can agronomic practices and cold-pressing extraction parameters affect phenols and polyphenols content in hempseed oils? Ind. Crops Prod. 2019, 130, 511–519. [Google Scholar] [CrossRef]
- Eseberri, I.; Trepiana, J.; Léniz, A.; Gómez-García, I.; Carr-Ugarte, H.; González, M.; Portillo, M.P. Variability in the Beneficial Effects of Phenolic Compounds: A Review. Nutrients 2022, 14, 1925. [Google Scholar] [CrossRef]
- Giusti, F.; Capuano, E.; Sagratini, G.; Pellegrini, N. A comprehensive investigation of the behaviour of phenolic compounds in legumes during domestic cooking and in vitro digestion. Food Chem. 2019, 285, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Vollmannova, A.; Lidikova, J.; Musilova, J.; Snirc, M.; Bojnanska, T.; Urminska, D.; Tirdilova, I.; Zetochova, E. White Lupin as a Promising Source of Antioxidant Phenolics for Functional Food Production. J. Food Qual. 2021, 512236. [Google Scholar] [CrossRef]
- Quintero-Soto, M.F.; Saracho-Peña, A.G.; Chavez-Ontiveros, J.; Garzon-Tiznado, J.A.; Pineda-Hidalgo, K.V.; Delgado-Vargas, F.; Lopez-Valenzuela, J.A. Phenolic profiles and their contribution to the antioxidant activity of selected chickpea genotypes from Mexico and ICRISAT collections. Plant Foods Hum. Nutr. 2018, 73, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Gandhi, H.; Toor, B.S.; Kaur, A.; Kaut, J. Effect of processing treatments on physicochemical, functional and thermal characteristics of lentils (Lens culinaris). J. Food Meas. Charact. 2022, 16, 4603–4614. [Google Scholar] [CrossRef]
- Xu, B.J.; Yuan, S.H.; Chang, S.K.C. Comparative analyses of phenolic composition, antioxidant capacity, and color of cool season legumes and other selected food legumes. J. Food Sci. 2007, 72, S167–S177. [Google Scholar] [CrossRef]

| Samples | Protein | Fat | Ash | Starch | Fiber |
|---|---|---|---|---|---|
| Red lentil | 28.22 ± 0.49 f | 1.16 ± 0.06 g | 2.64 ± 0.05 h | 53.57 ± 0.15 a | 10.01 ± 0.05 k |
| Green lentil | 28.95 ± 0.18 e | 0.77 ± 0.10 ij | 3.11 ± 0.03 f | 28.76 ± 0.04 h | 33.44 ± 0.20 a |
| Yellow lentil | 28.19 ± 0.36 f | 1.27 ± 0.01 fg | 2.45 ± 0.02 i | 37.66 ± 0.11 f | 25.19 ± 0.05 d |
| Brown lentil | 28.37 ± 0.12 ef | 0.66 ± 0.02 j | 2.51 ± 0.05 i | 50.20 ± 0.30 b | 12.71 ± 0.31 j |
| Black lentil | 33.23 ± 0.59 d | 0.80 ± 0.02 hi | 2.81 ± 0.05 g | 39.79 ± 0.04 e | 18.64 ± 0.16 g |
| Mung bean | 22.36 ± 0.25 i | 0.92 ± 0.02 h | 3.94 ± 0.05 d | 50.24 ± 0.22 b | 17.77 ± 0.12 h |
| Pinto bean | 23.42 ± 0.02 h | 1.41 ± 0.01 e | 4.21 ± 0.01 c | 39.52 ± 0.17 e | 26.33 ± 0.09 c |
| Black bean | 24.79 ± 0.30 g | 1.21 ± 0.01 fg | 4.18 ± 0.01 c | 47.65 ± 0.24 c | 16.68 ± 0.08 i |
| Kidney bean | 28.34 ± 0.14 f | 1.32 ± 0.01 ef | 3.64 ± 0.03 e | 36.38 ± 0.12 g | 24.76 ± 0.11 d |
| Chickpea | 24.80 ± 0.12 g | 5.70 ± 0.01 d | 2.52 ± 0.01 i | 41.90 ± 0.14 d | 19.44 ± 0.17 f |
| Soy | 43.86 ± 0.30 b | 24.36 ± 0.10 a | 5.25 ± 0.02 b | 15.19 ± 0.08 j | 9.70 ± 0.01 k |
| Lupin | 42.24 ± 0.02 c | 7.45 ± 0.13 c | 3.10 ± 0.02 f | 16.57 ± 0.11 i | 28.59 ± 0.11 b |
| Hemp | 47.25 ± 0.17 a | 10.42 ± 0.02 b | 8.50 ± 0.12 a | 7.28 ± 0.04 k | 23.46 ± 0.23 e |
| Samples | His | Ile | Leu | Lys | Met | Cys | Thr | Val | |
| Red lentil | 2.93 ± 0.08 a | 4.81 ± 0.16 a | 8.43 ± 0.08 a | 7.68 ± 0.10 a | 0.87 ± 0.06 f | 0.78 ± 0.04 de | 4.54 ± 0.06 a | 5.06 ± 0.08 a | |
| Green lentil | 2.37 ± 0.04 f | 3.92 ± 0.12 d | 6.74 ± 0.31 g | 6.33 ± 0.08 de | 0.83 ± 0.02 f | 0.75 ± 0.02 def | 3.55 ± 0.06 g | 3.97 ± 0.10 i | |
| Yellow lentil | 2.58 ± 0.02 cd | 4.42 ± 0.07 bc | 7.52 ± 0.21 cde | 6.87 ± 0.12 b | 0.94 ± 0.06 ef | 0.75 ± 0.06 def | 3.87 ± 0.10 f | 4.56 ± 0.06 cde | |
| Brown lentil | 2.43 ± 0.02 ef | 4.01 ± 0.06 d | 6.85 ± 0.02 fg | 6.14 ± 0.04 ef | 0.82 ± 0.04 f | 0.75 ± 0.02 def | 3.44 ± 0.02 g | 4.10 ± 0.04 hi | |
| Black lentil | 2.60 ± 0.08 cd | 4.48 ± 0.10 ab | 7.90 ± 0.09 bc | 6.74 ± 0.20 bc | 0.84 ± 0.05 f | 0.65 ± 0.08 ef | 4.08 ± 0.05 de | 4.74 ± 0.11 bc | |
| Mung bean | 2.53 ± 0.05 de | 4.13 ± 0.10 cd | 7.43 ± 0.10 de | 6.56 ± 0.12 cd | 1.32 ± 0.03 c | 1.02 ± 0.03 b | 3.39 ± 0.08 g | 4.63 ± 0.08 bcd | |
| Pinto bean | 2.61 ± 0.03 bcd | 4.49 ± 0.07 ab | 7.85 ± 0.14 bcd | 6.61 ± 0.07 bc | 1.07 ± 0.03 e | 0.88 ± 0.03 cd | 4.45 ± 0.07 ab | 4.77 ± 0.03 b | |
| Black bean | 2.40 ± 0.03 ef | 4.15 ± 0.21 cd | 7.23 ± 0.09 ef | 5.97 ± 0.03 f | 1.40 ± 0.07 c | 0.97 ± 0.03 bc | 4.12 ± 0.04 cd | 4.32 ± 0.03 fg | |
| Kidney bean | 2.52 ± 0.02 de | 4.00 ± 0.10 d | 7.31 ± 0.10 e | 6.14 ± 0.10 ef | 1.31 ± 0.06 c | 1.07 ± 0.08 b | 3.98 ± 0.07 def | 4.20 ± 0.10 gh | |
| Chickpea | 2.48 ± 0.03 def | 4.13 ± 0.17 cd | 7.21 ± 0.13 ef | 5.42 ± 0.07 g | 1.60 ± 0.09 b | 1.49 ± 0.03 a | 3.85 ± 0.07 f | 4.40 ± 0.03 ef | |
| Soy | 2.70 ± 0.04 bc | 4.80 ± 0.04 a | 8.39 ± 0.10 a | 6.18 ± 0.04 ef | 1.11 ± 0.04 de | 1.39 ± 0.02 a | 4.33 ± 0.05 b | 4.50 ± 0.07 def | |
| Lupin | 3.02 ± 0.03 a | 4.59 ± 0.05 ab | 8.10 ± 0.04 ab | 4.88 ± 0.09 h | 1.26 ± 0.08 cd | 0.63 ± 0.01 f | 4.27 ± 0.04 bc | 4.17 ± 0.04 ghi | |
| Hemp | 2.74 ± 0.07 b | 4.12 ± 0.06 cd | 7.12 ± 0.17 efg | 4.03 ± 0.01 i | 2.05 ± 0.07 a | 1.45 ± 0.07 a | 3.93 ± 0.02 ef | 4.66 ± 0.03 bcd | |
| FAO * | 1.6 | 3.0 | 6.1 | 4.8 | 2.3 | 2.5 | 4.0 | ||
| Samples | Phe | Tyr | Glu | Asp | Arg | Ala | Gly | Pro | Ser |
| Red lentil | 5.79 ± 0.06 a | 2.98 ± 0.06 b | 21.25 ± 0.06 c | 13.91 ± 0.10 a | 9.43 ± 0.18 d | 4.77 ± 0.08 a | 4.46 ± 0.10 bc | 4.98 ± 0.39 bcd | 5.89 ± 0.10 a |
| Green lentil | 4.68 ± 0.04 de | 2.42 ± 0.02 d | 15.92 ± 0.02 j | 10.43 ± 0.02 i | 7.27 ± 0.15 g | 3.82 ± 0.06 d | 3.57 ± 0.02 ghi | 3.25 ± 0.07 f | 4.68 ± 0.04 g |
| Yellow lentil | 5.19 ± 0.15 b | 2.61 ± 0.02 c | 18.11 ± 0.06 g | 11.47 ± 0.04 g | 8.94 ± 0.06 e | 4.20 ± 0.06 bc | 3.90 ± 0.10 ef | 4.26 ± 0.17 de | 5.18 ± 0.06 e |
| Brown lentil | 4.66 ± 0.07 de | 2.38 ± 0.02 de | 16.88 ± 0.08 i | 11.03 ± 0.14 h | 8.34 ± 0.10 f | 3.81 ± 0.07 d | 3.61 ± 0.04 ghi | 3.86 ± 0.11 ef | 4.75 ± 0.06 fg |
| Black lentil | 5.29 ± 0.09 b | 2.73 ± 0.08 c | 19.50 ± 0.09 e | 12.97 ± 0.17 c | 10.48 ± 0.15 b | 4.32 ± 0.05 b | 4.09 ± 0.05 de | 4.41 ± 0.39 cde | 5.47 ± 0.07 cd |
| Mung bean | 5.72 ± 0.08 a | 2.26 ± 0.03 e | 18.49 ± 0.05 f | 11.71 ± 0.03 f | 6.24 ± 0.05 h | 4.20 ± 0.03 bc | 3.52 ± 0.05 hi | 4.81 ± 0.36 bcd | 4.87 ± 0.03 f |
| Pinto bean | 5.65 ± 0.09 a | 2.62 ± 0.03 c | 17.56 ± 0.03 h | 13.08 ± 0.05 c | 5.92 ± 0.15 h | 4.05 ± 0.05 c | 3.66 ± 0.07 gh | 4.84 ± 0.35 bcd | 5.86 ± 0.03 a |
| Black bean | 5.17 ± 0.03 bc | 2.60 ± 0.04 c | 15.25 ± 0.05 k | 12.02 ± 0.03 e | 6.03 ± 0.03 h | 3.79 ± 0.03 d | 3.52 ± 0.11 hi | 4.55 ± 0.21 cde | 5.39 ± 0.03 d |
| Kidney bean | 5.33 ± 0.12 b | 2.44 ± 0.06 d | 16.77 ± 0.02 i | 12.11 ± 0.02 e | 6.24 ± 0.07 h | 3.69 ± 0.02 d | 3.44 ± 0.06 i | 3.90 ± 0.24 ef | 5.59 ± 0.06 bc |
| Chickpea | 5.83 ± 0.11 a | 2.36 ± 0.04 de | 18.22 ± 0.03 g | 12.38 ± 0.07 d | 9.97 ± 0.14 c | 4.29 ± 0.07 b | 3.77 ± 0.07 fg | 4.76 ± 0.45 bcd | 5.22 ± 0.04 e |
| Soy | 5.67 ± 0.07 a | 3.59 ± 0.04 a | 22.56 ± 0.02 b | 13.45 ± 0.04 b | 8.54 ± 0.05 f | 4.67 ± 0.12 a | 4.28 ± 0.04 cd | 6.42 ± 0.14 a | 5.74 ± 0.04 ab |
| Lupin | 4.62 ± 0.03 e | 3.60 ± 0.06 a | 27.61 ± 0.06 a | 12.41 ± 0.06 d | 12.70 ± 0.11 a | 4.12 ± 0.03 c | 4.76 ± 0.03 a | 5.53 ± 0.21 b | 5.74 ± 0.01 ab |
| Hemp | 4.91 ± 0.12 cd | 3.07 ± 0.09 b | 20.66 ± 0.03 d | 11.97 ± 0.06 e | 12.61 ± 0.10 a | 4.75 ± 0.12 a | 4.64 ± 0.09 ab | 5.24 ± 0.2 3 bc | 5.52 ± 0.06 cd |
| FAO * | 4.1 | ||||||||
| Samples | AAS * | PDCAAS | EAAI ** | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| His | Ile | Leu | Lys | Cys + Met | Phe + Tyr | Thr | Val | |||
| Red lentil | 1.83 | 1.60 | 1.38 | 1.60 | 0.72 | 2.14 | 1.82 | 1.26 | 0.57 | 84.39 |
| Green lentil | 1.48 | 1.31 | 1.11 | 1.32 | 0.68 | 1.73 | 1.42 | 0.99 | 0.53 | 69.28 |
| Yellow lentil | 1.61 | 1.47 | 1.23 | 1.43 | 0.74 | 1.90 | 1.55 | 1.14 | 0.57 | 76.47 |
| Brown lentil | 1.52 | 1.34 | 1.12 | 1.28 | 0.68 | 1.72 | 1.38 | 1.03 | 0.53 | 69.48 |
| Black lentil | 1.63 | 1.49 | 1.30 | 1.40 | 0.65 | 1.96 | 1.63 | 1.18 | 0.50 | 76.86 |
| Mung bean | 1.58 | 1.38 | 1.22 | 1.37 | 1.02 | 1.95 | 1.35 | 1.16 | 0.77 | 77.26 |
| Pinto bean | 1.63 | 1.50 | 1.29 | 1.38 | 0.85 | 2.02 | 1.78 | 1.19 | 0.60 | 80.54 |
| Black bean | 1.50 | 1.38 | 1.18 | 1.24 | 1.03 | 1.89 | 1.65 | 1.08 | 0.70 | 76.73 |
| Kidney bean | 1.58 | 1.33 | 1.20 | 1.28 | 1.03 | 1.89 | 1.59 | 1.05 | 0.73 | 76.62 |
| Chickpea | 1.55 | 1.38 | 1.18 | 1.13 | 1.34 | 2.00 | 1.54 | 1.10 | 0.80 | 78.62 |
| Soy | 1.69 | 1.60 | 1.38 | 1.29 | 1.09 | 2.26 | 1.73 | 1.13 | 0.92 | 84.39 |
| Lupin | 1.89 | 1.53 | 1.33 | 1.02 | 0.82 | 2.00 | 1.71 | 1.04 | 0.64 | 77.40 |
| Hemp | 1.71 | 1.37 | 1.17 | 0.84 | 1.52 | 1.94 | 1.57 | 1.17 | 0.68 | 78.25 |
| Samples | TPC, mg GAE/100 g d.b | TFC, mg QE/100 g d.b. |
|---|---|---|
| Red lentil | 85.89 ± 1.31 h | 10.80 ± 0.21 g |
| Green lentil | 327.31 ± 1.82 b | 23.67 ± 0.22 d |
| Yellow lentil | 69.00 ± 0.71 i | 5.44 ± 0.01 h |
| Brown lentil | 247.56 ± 1.97 e | 19.67 ± 0.11 e |
| Black lentil | 248.38 ± 2.19 e | 28.57 ± 0.22 b |
| Mung bean | 260.62 ± 2.36 d | 45.47 ± 0.22 a |
| Pinto bean | 425.19 ± 2.04 a | 0.24 ± 0.11 j |
| Black bean | 209.53 ± 2.04 g | 5.93 ± 0.22 h |
| Kidney bean | 302.05 ± 2.00 c | 17.93 ± 0.20 f |
| Chickpea | 91.64 ± 2.15 h | 0.16 ± 0.01 j |
| Soy | 242.06 ± 0.98 e | 2.43 ± 0.23 i |
| Lupin | 230.29 ± 0.60 f | 26.22 ± 0.36 c |
| Hemp | 432.56 ± 4.58 a | 18.13 ± 1.01 f |
| Samples | DPPH | ABTS | FRAP | CUPRAC |
|---|---|---|---|---|
| mg TE/100 g d.b. | ||||
| Red lentil | 73.76 ± 2.30 j | 285.59 ± 0.46 k | 44.53 ± 1.74 j | 298.43 ± 3.70 j |
| Green lentil | 480.46 ± 0.62 b | 1024.27 ± 0.56 e | 351.62 ± 5.73 a | 1060.27 ± 2.99 c |
| Yellow lentil | 63.48 ± 2.32 k | 245.97 ± 0.91 j | 43.64 ± 1.36 j | 314.41 ± 7.45 j |
| Brown lentil | 307.38 ± 1.83 e | 789.89 ± 0.97 h | 228.79 ± 4.44 f | 787.23 ± 4.84 e |
| Black lentil | 371.94 ± 0.36 d | 807.10 ± 1.51 g | 285.04 ± 4.98 d | 842.87 ± 4.70 d |
| Mung bean | 158.73 ± 2.12 g | 1036.50 ± 0.56 e | 117.49 ± 2.28 h | 524.62 ± 2.98 h |
| Pinto bean | 600.00 ± 0.72 a | 1149.57 ± 0.95 d | 332.20 ± 4.21 b | 1093.65 ± 3.90 b |
| Black bean | 246.13 ± 0.26 f | 587.82 ± 0.25 i | 215.99 ± 3.08 f | 684.19 ± 4.17 f |
| Kidney bean | 395.63 ± 1.15 c | 887.82 ± 0.28 f | 241.27 ± 3.80 e | 834.64 ± 5.98 d |
| Chickpea | 84.62 ± 2.45 i | 408.09 ± 0.58 j | 46.53 ± 1.07 j | 408.69 ± 7.88 i |
| Soy | 93.42 ± 1.56 h | 2499.54 ± 0.78 a | 181.83 ± 1.90 g | 678.86 ± 4.87 f |
| Lupin | 68.19 ± 0.6 8 j,k | 2276.06 ± 1.48 b | 79.70 ± 1.12 i | 596.60 ± 3.28 g |
| Hemp | 375.08 ± 1.43 d | 1273.99 ± 1.33 c | 300.53 ± 4.88 c | 1208.31 ± 6.02 a |
| Method | DPPH | ABTS | FRAP | CUPRAC | ACL |
|---|---|---|---|---|---|
| DPPH | 1 | −0.045 | 0.9276 | 0.8783 | 0.8221 |
| ABTS | - | 1 | 0.1656 | 0.3005 | 0.0354 |
| FRAP | - | - | 1 | 0.9457 | 0.7643 |
| CUPRAC | - | - | - | 1 | 0.8305 |
| ACL | - | - | - | - | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Multescu, M.; Culetu, A.; Susman, I.E. Screening of the Nutritional Properties, Bioactive Components, and Antioxidant Properties in Legumes. Foods 2024, 13, 3528. https://doi.org/10.3390/foods13223528
Multescu M, Culetu A, Susman IE. Screening of the Nutritional Properties, Bioactive Components, and Antioxidant Properties in Legumes. Foods. 2024; 13(22):3528. https://doi.org/10.3390/foods13223528
Chicago/Turabian StyleMultescu, Mihaela, Alina Culetu, and Iulia Elena Susman. 2024. "Screening of the Nutritional Properties, Bioactive Components, and Antioxidant Properties in Legumes" Foods 13, no. 22: 3528. https://doi.org/10.3390/foods13223528
APA StyleMultescu, M., Culetu, A., & Susman, I. E. (2024). Screening of the Nutritional Properties, Bioactive Components, and Antioxidant Properties in Legumes. Foods, 13(22), 3528. https://doi.org/10.3390/foods13223528






