Nitrogen Storage in Rice: Analysis of Physical Quality by Respiration, Weight, and Storage According to Nitrogen Ratio
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
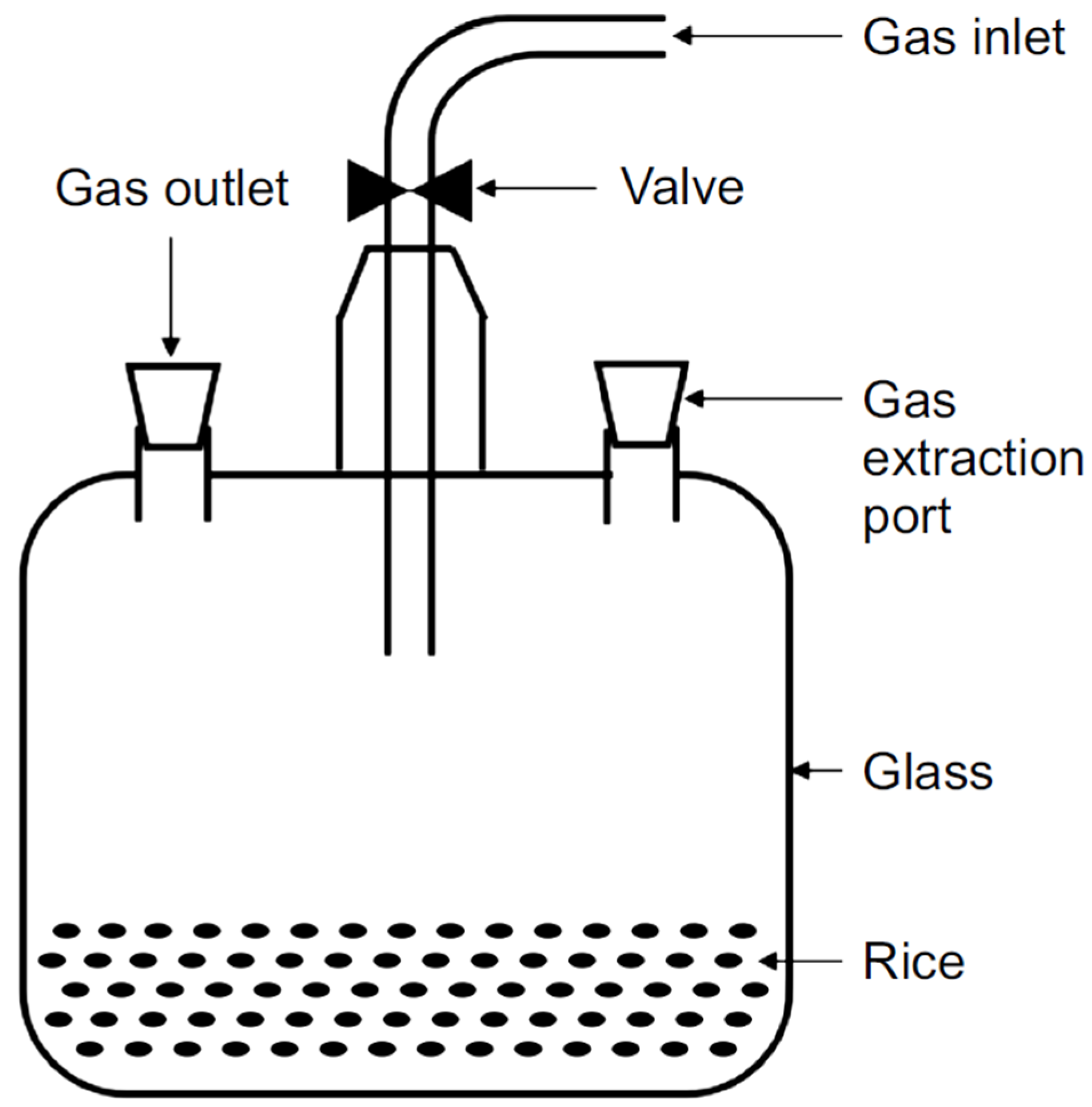
2.2. Respiration Experiment
2.3. Respiration Model and Dry Mass Loss
2.4. Quality During Storage
2.5. Statistical Analysis
3. Results and Discussion
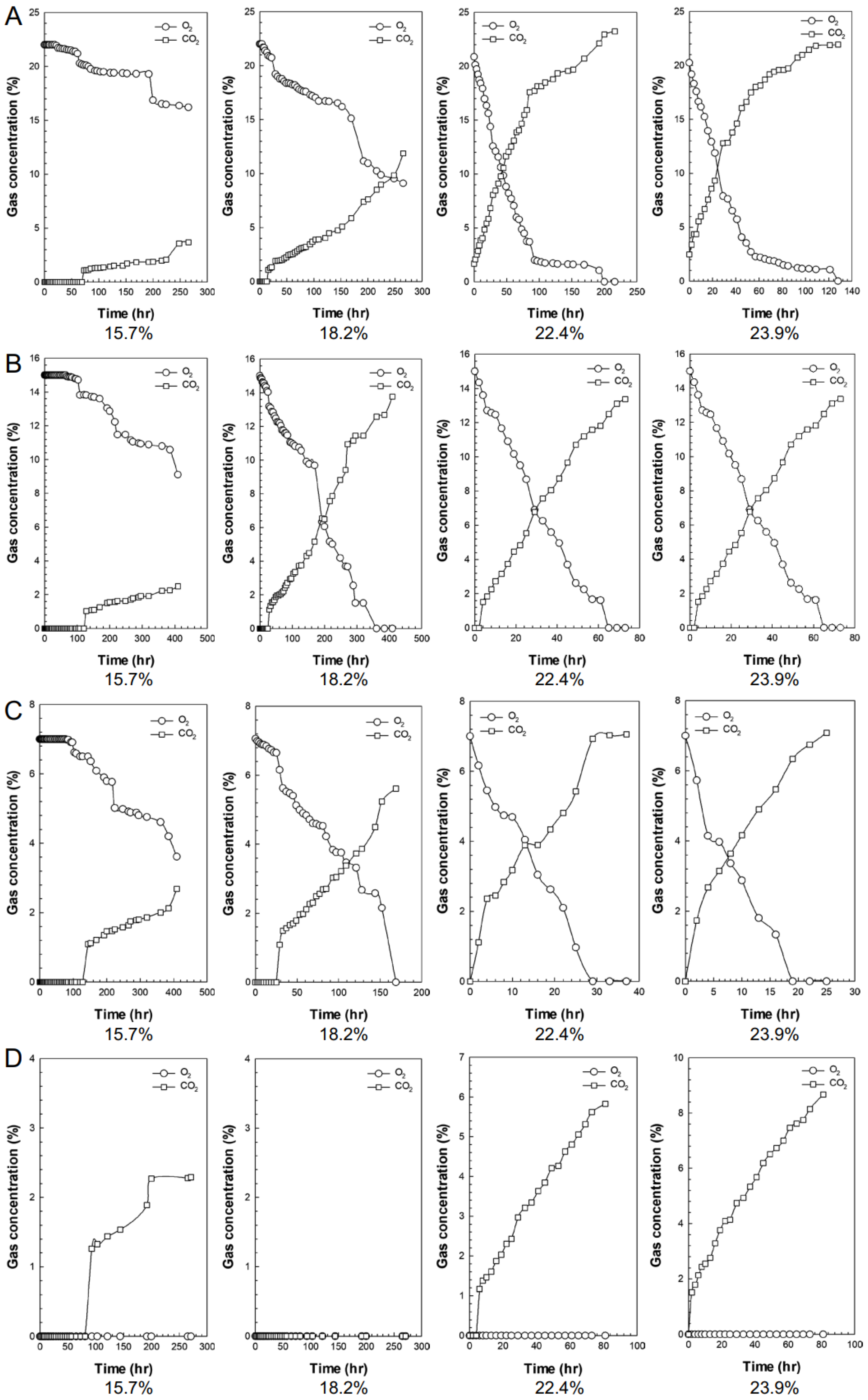
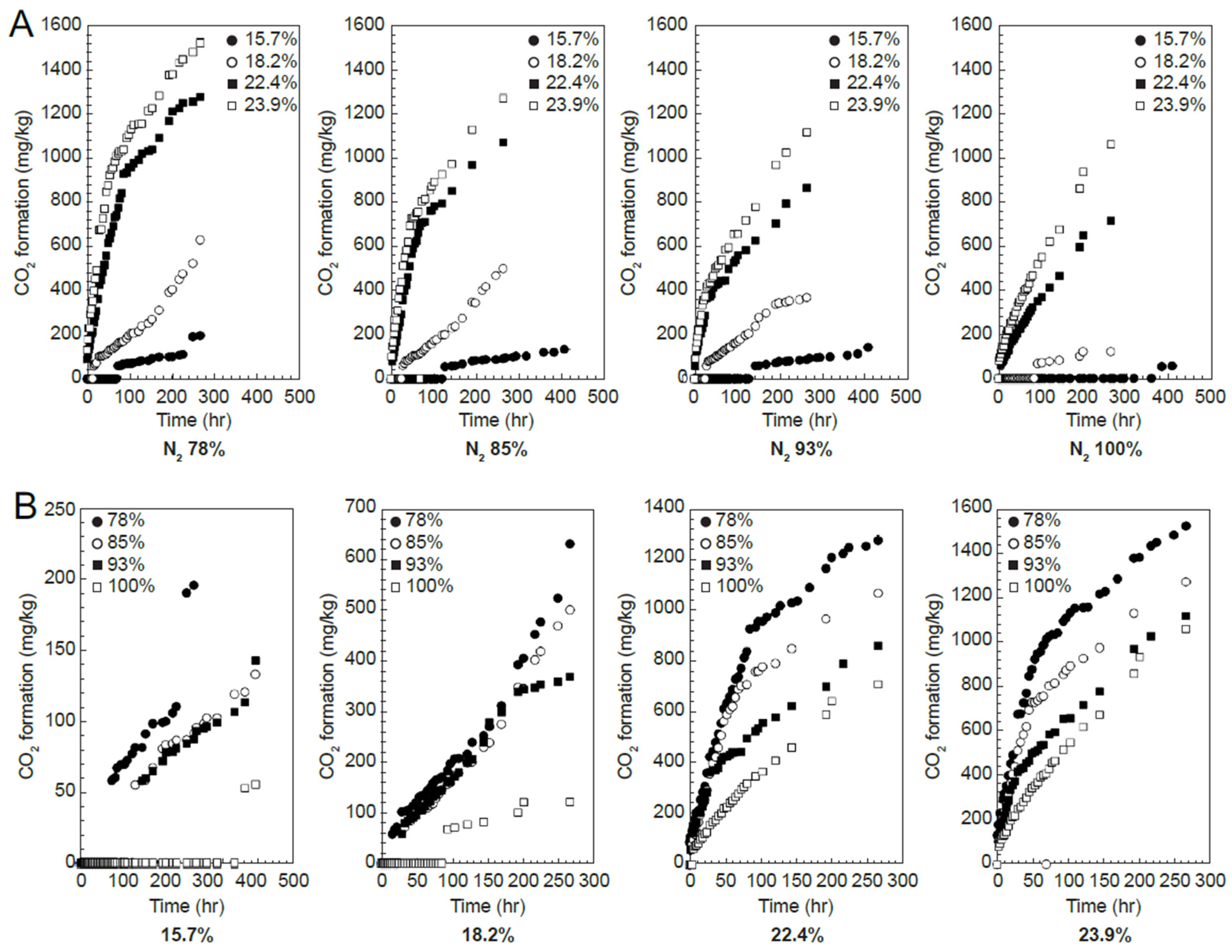
3.1. Gas Composition and Change in Respiration Volume
3.2. Respiration Model and DML
3.3. Storage Characteristics
3.3.1. Moisture Content
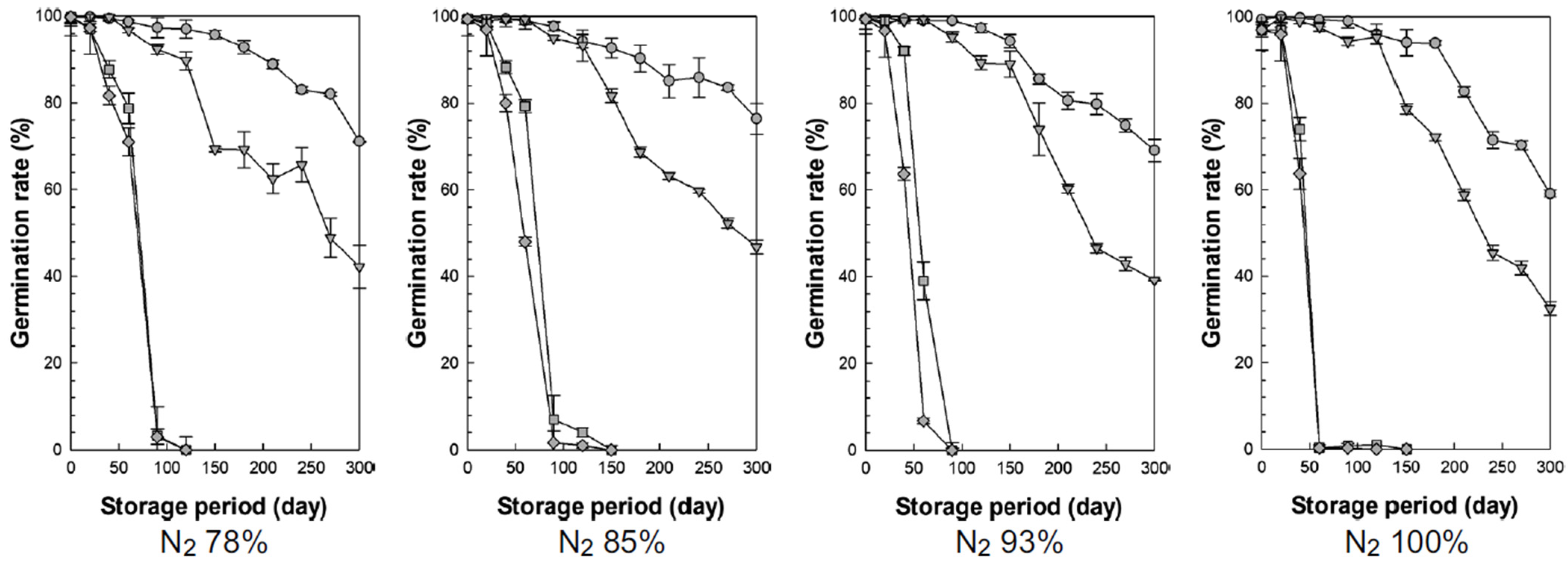
3.3.2. Germination Rate
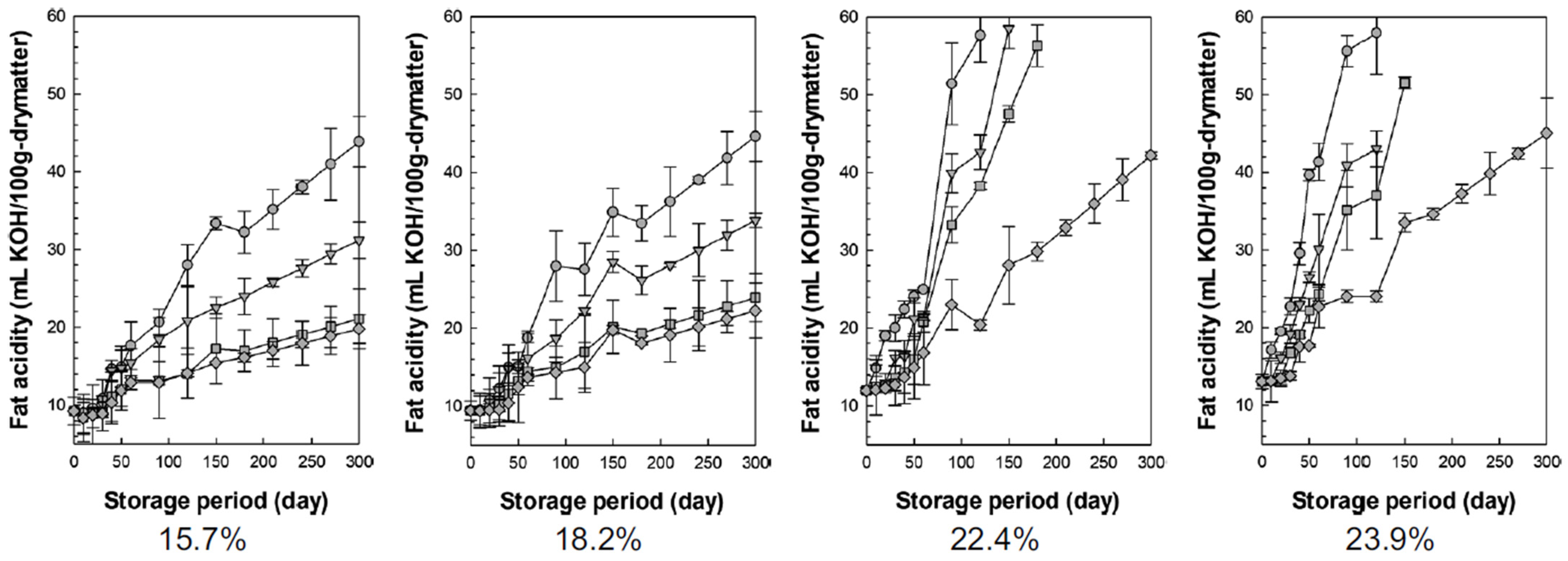
3.3.3. Fat Acidity
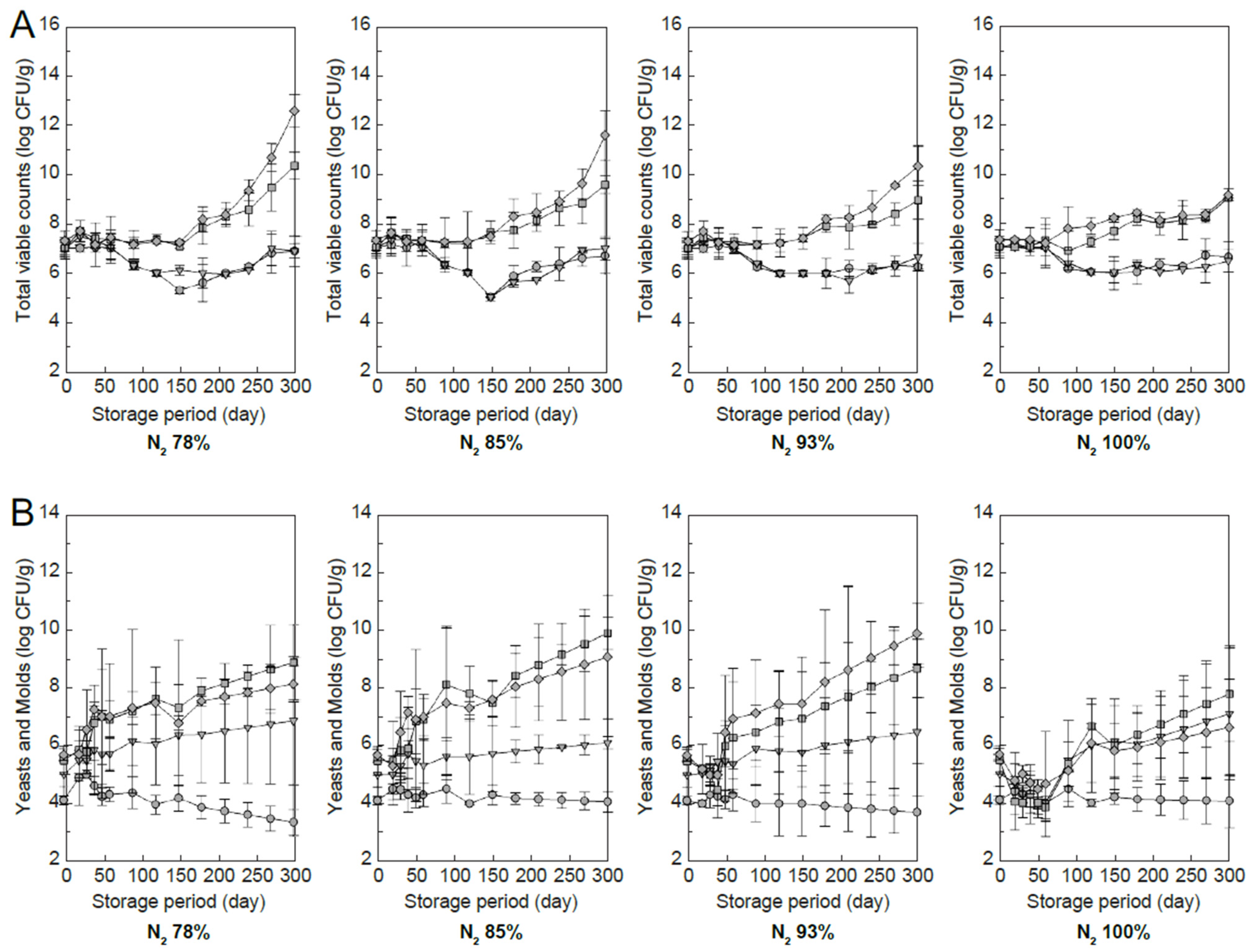
3.3.4. Total Bacterial and Mold Counts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, X.; Fang, C.; Zhang, W.; Lu, L.; Guo, Z.; Li, S.; Chen, M. Change in volatiles, soluble sugars and fatty acids of glutinous rice, japonica rice and indica rice during storage. LWT 2023, 174, 114416. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhou, R.; Ma, M. Kinetic studies on soluble sugar profile in rice during storage: Derivation using the Laplace transform. Innov. Food Sci. Emerg. Technol. 2022, 76, 102915. [Google Scholar] [CrossRef]
- Choi, S.; Seo, H.S.; Lee, K.R.; Lee, S.; Lee, J.; Lee, J. Effect of milling and long-term storage on volatiles of black rice (Oryza sativa L.) determined by headspace solid-phase microextraction with gas chromatography-mass spectrometry. Food Chem. 2019, 276, 572–582. [Google Scholar] [CrossRef]
- Afzal, I.; Bakhtavar, M.A.; Ishfaq, M.; Sagheer, M.; Baributsa, D. Maintaining dryness during storage contributes to higher maize seed quality. J. Stored Prod. Res. 2017, 72, 49–53. [Google Scholar] [CrossRef]
- Bartosik, R.; Urcola, H.; Cardoso, L.; Maciel, G.; Busato, P. Silo-bag system for storage of grains, seeds and by-products: A review and research agenda. J. Stored Prod. Res. 2023, 100, 102061. [Google Scholar] [CrossRef]
- Ziegler, V.; Paraginski, R.T.; Ferreira, C.D. Grain storage systems and effects of moisture, temperature and time on grain quality—A review. J. Stored Prod. Res. 2021, 91, 101770. [Google Scholar] [CrossRef]
- Kuyu, C.G.; Tola, Y.B.; Mohammed, A.; Mengesh, A.; Mpagalile, J.J. Evaluation of different grain storage technologies against storage insect pests over an extended storage time. J. Stored Prod. Res. 2022, 96, 101945. [Google Scholar] [CrossRef]
- Timm, N.d.S.; Coradi, P.C.; Cañizares, L.; Jappe, S.N.; Ferreira, C.D.; Lutz, É. Effects of the storage temperature and time of corn from the center and extremities of corncob on quality parameters. J. Cereal Sci. 2023, 110, 103645. [Google Scholar] [CrossRef]
- Ubhi, G.S.; Sadaka, S. Temporal valuation of corn respiration rates using pressure sensors. J. Stored Prod. Res. 2015, 61, 39–47. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Lu, Q. Exploration of mechanisms for internal deterioration of wheat seeds in postharvest storage and nitrogen atmosphere control for properties protection. Crop Sci. 2018, 58, 823–836. [Google Scholar] [CrossRef]
- Salazar, N.; Caladcad, J.A.; Villeta, R. Predictive modelling on the effects of the critical parameters in grain storage systems: A case study in the Philippines. J. Stored Prod. Res. 2024, 107, 102341. [Google Scholar] [CrossRef]
- Barreto, A.A.; Abalone, R.; Gastón, A.; Bartosik, R. Analysis of storage conditions of a wheat silo-bag for different weather conditions by computer simulation. Biosyst. Eng. 2013, 116, 497–508. [Google Scholar] [CrossRef]
- Huang, H.; Danao, M.C.; Rausch, K.D.; Singh, V. Diffusion and production of carbon dioxide in bulk corn at various temperatures and moisture contents. J. Stored Prod. Res. 2013, 55, 21–26. [Google Scholar] [CrossRef]
- Müller, A.; Nunes, M.T.; Maldaner, V.; Coradi, P.C.; de Moraes, R.S.; Martens, S.; Leal, A.F.; Pereira, V.F.; Marin, C.K. Rice drying, storage and processing: Effects of post-harvest operations on grain quality. Rice Sci. 2022, 29, 16–30. [Google Scholar] [CrossRef]
- Thompson, T.L. Temporary storage of high-moisture shelled corn using continuous aeration. Trans. ASAE 1972, 15, 0333–0337. [Google Scholar] [CrossRef]
- Da Silva, W.S.V.; Vanier, N.L.; Ziegler, V.; de Oliveira, M.; Guerra Dias, A.R.G.; Elias, M.C. Effects of using Eolic exhausters as a complement to conventional aeration on the quality of rice stored in metal silos. J. Stored Prod. Res. 2014, 59, 76–81. [Google Scholar] [CrossRef]
- Xiao, Y.; Xie, L.; Li, Y.; Li, C.; Yu, Y.; Hu, J.; Li, G. Impact of low temperature on the chemical profile of sweet corn kernels during post-harvest storage. Food Chem. 2024, 431, 137079. [Google Scholar] [CrossRef]
- Sun, S.; Li, B.; Yang, T.; Luo, F.; Zhao, J.; Cao, J.; Lin, Q. Preservation mechanism of high concentration carbon dioxide controlled atmosphere for paddy rice storage based on quality analyses and molecular modeling tools. J. Cereal Sci. 2019, 85, 279–285. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Gong, Z.; Liu, X.; Lv, H.; Zhao, Y. Changes in Quality Characteristics and Metabolite Composition of Low-Temperature and Nitrogen-Modified Atmosphere in Indica Rice during Storage. Foods 2024, 13, 2968. [Google Scholar] [CrossRef]
- Shejbal, J. Preservation of cereal grains in nitrogen atmospheres. Resour. Recovery Conserv. 1979, 4, 13–29. [Google Scholar] [CrossRef]
- Hashem, M.Y.; Ahmed, A.A.; Ahmed, S.S.; Khalil, S.S.; Mahmoud, Y.A. Comparative susceptibility of Corcyra cephalonica (Lepidoptera: Pyralidae) eggs to carbon dioxide and nitrogen at different temperatures. J. Stored Prod. Res. 2016, 69, 99–105. [Google Scholar] [CrossRef]
- Navarro, S. The use of modified and controlled atmospheres for the disinfestation of stored products. J. Pest Sci. 2012, 85, 301–322. [Google Scholar] [CrossRef]
- Neethirajan, S.; Freund, M.S.; Jayas, D.S.; Shafai, C.; Thomson, D.J.; White, N.D.G. Development of carbon dioxide (CO2) sensor for grain quality monitoring. Biosyst. Eng. 2010, 106, 395–404. [Google Scholar] [CrossRef]
- Taher, H.I.; Urcola, H.A.; Cendoya, M.G.; Bartosik, R.E. Predicting soybean losses using carbon dioxide monitoring during storage in silo bags. J. Stored Prod. Res. 2019, 82, 1–8. [Google Scholar] [CrossRef]
- Haritos, V.S.; Damcevski, K.A.; Dojchinov, G. Improved efficacy of ethyl formate against stored grain insects by combination with carbon dioxide in a ‘dynamic’application. Pest Manag. Sci. Former. Pestic. Sci. 2006, 62, 325–333. [Google Scholar] [CrossRef]
- Grigg, B.C.; Siebenmorgen, T.J. Impacts of kernel thickness and associated physical properties on milling yields of long-grain rice. Appl. Eng. Agric. 2015, 31, 505–511. [Google Scholar] [CrossRef][Green Version]
- Maciel, G.; de la Torre, D.A.; Cardoso, L.M.; Cendoya, M.G.; Wagner, J.R.; Bartosik, R.E. Determination of safe storage moisture content of soybean expeller by means of sorption isotherms and product respiration. J. Stored Prod. Res. 2020, 86, 101567. [Google Scholar] [CrossRef]
- Marcos Valle, F.J.; Gastón, A.; Abalone, R.M.; de la Torre, D.A.; Castellari, C.C.; Bartosik, R.E. Study and modelling the respiration of corn seeds (Zea mays L.) during hermetic storage. Biosyst. Eng. 2021, 208, 45–57. [Google Scholar] [CrossRef]
- Ho, P.L.; Tran, D.T.; Hertog, M.L.A.T.M.; Nicolaï, B.M. Modelling respiration rate of dragon fruit as a function of gas composition and temperature. Sci. Hortic. 2020, 263, 109138. [Google Scholar] [CrossRef]
- Nishiyama, M.; Kleijn, S.; Aquilanti, V.; Kasai, T. Temperature dependence of respiration rates of leaves, 18O-experiments and super-Arrhenius kinetics. Chem. Phys. Lett. 2009, 482, 325–329. [Google Scholar] [CrossRef]
- Benítez, S.; Chiumenti, M.; Sepulcre, F.; Achaerandio, I.; Pujolá, M. Modeling the effect of storage temperature on the respiration rate and texture of fresh cut pineapple. J. Food. Eng. 2012, 113, 527–533. [Google Scholar] [CrossRef]
- Mundim, K.C.; Baraldi, S.; Machado, H.G.; Vieira, F.M.C. Temperature coefficient (Q10) and its applications in biological systems: Beyond the Arrhenius theory. Ecol. Model. 2020, 431, 109127. [Google Scholar] [CrossRef]
- Waghmare, R.B.; Mahajan, P.V.; Annapure, U.S. Modelling the effect of time and temperature on respiration rate of selected fresh-cut produce. Postharvest Biol. Technol. 2013, 80, 25–30. [Google Scholar] [CrossRef]
- Raudienė, E.; Rušinskas, D.; Balčiūnas, G.; Juodeikienė, G.; Gailius, D. Carbon dioxide respiration rates in wheat at various temperatures and moisture contents. Mapan 2017, 32, 51–58. [Google Scholar] [CrossRef]
- AACC. AACC Method 02-01.02 Fat Acidity-General Method. In AACC Approved Methods Analysis; Cereals & Grains Association: Eagan, MN, USA, 1999. [Google Scholar]
- Jacob, A.; Sudagar, I.P.; Pandiselvam, R.; Rajkumar, P.; Rajavel, M. Effect of packaging materials and storage temperature on the physicochemical and microbial properties of ultrasonicated mature coconut water during storage. Food Control 2023, 149, 109693. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, T.; Geng, S.; Liang, F.; Wang, T. Effect of accumulated temperature on flavour and microbial diversity of japonica rice during storage. J. Stored Prod. Res. 2021, 92, 101779. [Google Scholar] [CrossRef]
- Taher, H.; San Martino, S.; Abadía, M.B.; Bartosik, R.E. Respiration of barley seeds (Hordeum vulgare L.) under different storage conditions. J. Stored Prod. Res. 2023, 104, 102178. [Google Scholar] [CrossRef]
- Kamara, M.M.; El-Aty, S.M.A.; Elgamal, H.W.; Soleiman, M.R.; Mousa, M.K.; Ueno, T. Effect of storage temperature on storage efficacy, germination and physical characters of some paddy rice cultivars during different storage periods. J. Fac. Agric. Kyushu Univ. 2019, 64, 61–69. [Google Scholar] [CrossRef]
- Dowell, F.E.; Dowell, C.N. Reducing grain storage losses in developing countries. Qual. Assur. Saf. Crop. Foods 2017, 9, 93–100. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.; Holmes, B.; Muck, R. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [PubMed]
- Chidananda, K.P.; Chelladurai, V.; Jayas, D.S.; Alagusundaram, K.; White, N.D.G.; Fields, P.G. Respiration of pulses stored under different storage conditions. J. Stored Prod. Res. 2014, 59, 42–47. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, Q.W.; Kim, H.; Kim, H.J. Changes in the chemical, physical, and sensory properties of rice according to its germination rate. Food Chem. 2022, 388, 133060. [Google Scholar] [CrossRef]
- Ghosh, M.; Gorain, J.; Pal, A.K.; Saha, B.; Chakraborti, P.; Avinash, B.; Pyngrope, D.M.; Banerjee, S.; Sarkar, S. Study of seed morphology and influence of ageing and storage conditions on germination and seedling vigour of non-Basmati aromatic rice. J. Stored Prod. Res. 2021, 93, 101863. [Google Scholar] [CrossRef]
- Nithya, U.; Chelladurai, V.; Jayas, D.S.; White, N.D.G. Safe storage guidelines for durum wheat. J. Stored Prod. Res. 2011, 47, 328–333. [Google Scholar] [CrossRef]
- Karunakaran, C.; Muir, W.E.; Jayas, D.S.; White, N.D.G.; Abramson, D. Safe storage time of high moisture wheat. J. Stored Prod. Res. 2001, 37, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Lv, Y.Y.; Wang, Y.L.; Jia, F.; Wang, J.S.; Hu, Y.S. Physiochemical changes in wheat of different hardnesses during storage. J. Stored Prod. Res. 2017, 72, 161–165. [Google Scholar] [CrossRef]
- Tahir, A.; Afzal, I.; Khalid, E.; Razzaq, M.; Arif, M.A.R. Rice seed longevity in the context of seed moisture contents and hypoxic conditions in the storage environment. Seed Sci. Res. 2023, 33, 39–49. [Google Scholar] [CrossRef]
- Rani, P.R.; Chelladurai, V.; Jayas, D.S.; White, N.D.G.; Kavitha-Abirami, C.V. Storage studies on pinto beans under different moisture contents and temperature regimes. J. Stored Prod. Res. 2013, 52, 78–85. [Google Scholar] [CrossRef]
- Malegori, C.; Buratti, S.; Benedetti, S.; Oliveri, P.; Ratti, S.; Cappa, C.; Lucisano, M. A modified mid-level data fusion approach on electronic nose and FT-NIR data for evaluating the effect of different storage conditions on rice germ shelf life. Talanta 2020, 206, 120208. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Chen, F.; Yong, F. Lipid oxidation of brown rice stored at different temperatures. Int. J. Food Sci. Technol. 2017, 52, 188–195. [Google Scholar] [CrossRef]
- Qiu, Z.; Wu, F.; Hu, H.; Guo, J.; Wu, C.; Wang, P.; Ling, J.; Cui, Y.; Ye, J.; Fang, G.; et al. Deciphering the Microbiological Mechanisms Underlying the Impact of Different Storage Conditions on Rice Grain Quality. Foods 2024, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Salman, H.; Copeland, L. Effect of storage on fat acidity and pasting characteristics of wheat flour. Cereal Chem. 2007, 84, 600–606. [Google Scholar] [CrossRef]
- Qu, C.; Li, W.; Yang, Q.; Xia, Y.; Lu, P.; Hu, M. Metabolic mechanism of nitrogen modified atmosphere storage on delaying quality deterioration of rice grains. Food Chem. X 2022, 16, 100519. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.; Kumar, S.; Kotwaliwale, N.; Singh, K.K. Critical factors responsible for fungi growth in stored food grains and non-chemical approaches for their control. Ind. Crops Prod. 2017, 108, 162–182. [Google Scholar] [CrossRef]
- Moses, J.A.; Jayas, D.S.; Alagusundaram, K. Climate change and its implications on stored food grains. Agric. Res. 2015, 4, 21–30. [Google Scholar] [CrossRef]



| a | b | c | d | e | f |
|---|---|---|---|---|---|
| 2.8337 | −0.2895 | 0.00738 | −1440.4 | 74.0155 | −1.0245 |
| RMSE | SSE | r2 | |
|---|---|---|---|
| 78 | 0.097 | 0.041 | 0.995 |
| 85 | 0.154 | 0.025 | 0.996 |
| 93 | 0.166 | 0.121 | 0.975 |
| 100 | 0.134 | 0.076 | 0.982 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, D.G.; Han, J.W.; Ahn, J.H.; Kim, H. Nitrogen Storage in Rice: Analysis of Physical Quality by Respiration, Weight, and Storage According to Nitrogen Ratio. Foods 2024, 13, 3530. https://doi.org/10.3390/foods13223530
Shin DG, Han JW, Ahn JH, Kim H. Nitrogen Storage in Rice: Analysis of Physical Quality by Respiration, Weight, and Storage According to Nitrogen Ratio. Foods. 2024; 13(22):3530. https://doi.org/10.3390/foods13223530
Chicago/Turabian StyleShin, Dong Gwan, Jae Woong Han, Jae Hwan Ahn, and Hoon Kim. 2024. "Nitrogen Storage in Rice: Analysis of Physical Quality by Respiration, Weight, and Storage According to Nitrogen Ratio" Foods 13, no. 22: 3530. https://doi.org/10.3390/foods13223530
APA StyleShin, D. G., Han, J. W., Ahn, J. H., & Kim, H. (2024). Nitrogen Storage in Rice: Analysis of Physical Quality by Respiration, Weight, and Storage According to Nitrogen Ratio. Foods, 13(22), 3530. https://doi.org/10.3390/foods13223530





