Probiotication of Plum Pulp and Conditions Effects Freeze-Drying in Cell Viability, Functional Properties and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plum Pulp Processing
2.2. Inoculation of BAL in Plum Pulp
2.3. Characterization of Fermented Plum Pulp
2.3.1. pH and Total Titratable Acidity
2.3.2. Water Content and Water Activity
2.3.3. Total Phenolic Compounds (TPC)
2.3.4. Total Tannins (TT)
2.3.5. Total Carotenoids (TC)
2.3.6. Total Flavonoids (TF) and Anthocyanins (TA)
2.3.7. Cell Viability
2.4. FD Process of Probiotic Plum Pulp
2.4.1. Process Yield
2.4.2. Survival Rate
2.5. In Vitro Antioxidant Activity
2.5.1. Free Radical Scavenging by DPPH (1,1-Diphenyl-2-picrylhydrazyl)
2.5.2. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS)
2.5.3. Antioxidant Activity by Iron Reducing Power (FRAP)
2.6. Viability During Storage
2.7. Statistical Analysis
3. Results
3.1. Physicochemical, Bioactive, and Cellular Viability Characterization of Probiotic Plum Pulp
3.2. Optimization of the FD Process of Probiotic Plum Pulp
3.3. Water Content and Water Activity of Probiotic Plum Pulp Powder
3.4. Bioactive Compounds of Probiotic Plum Pulp Powder
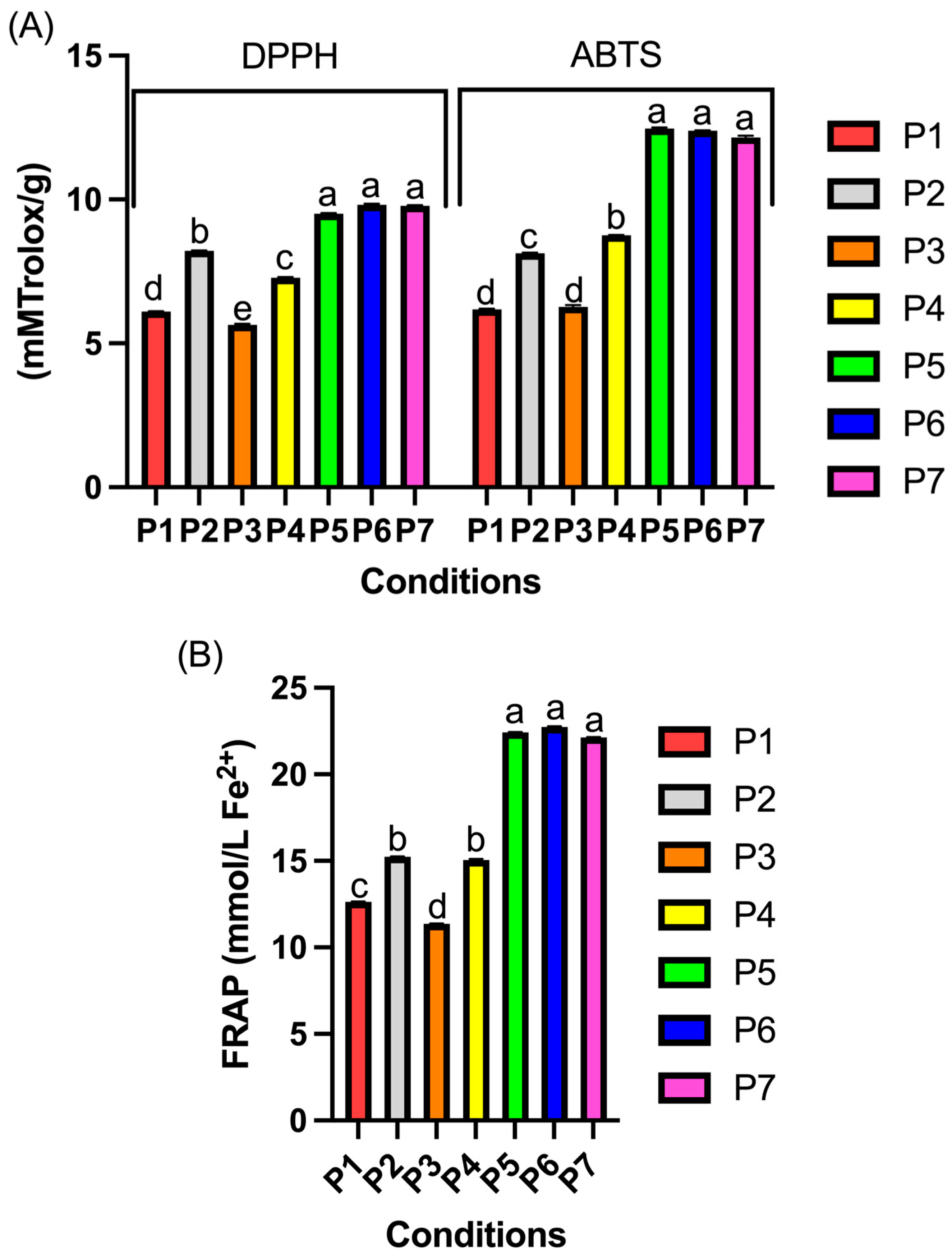
3.5. Antioxidant Activity of Probiotic Plum Pulp Powder
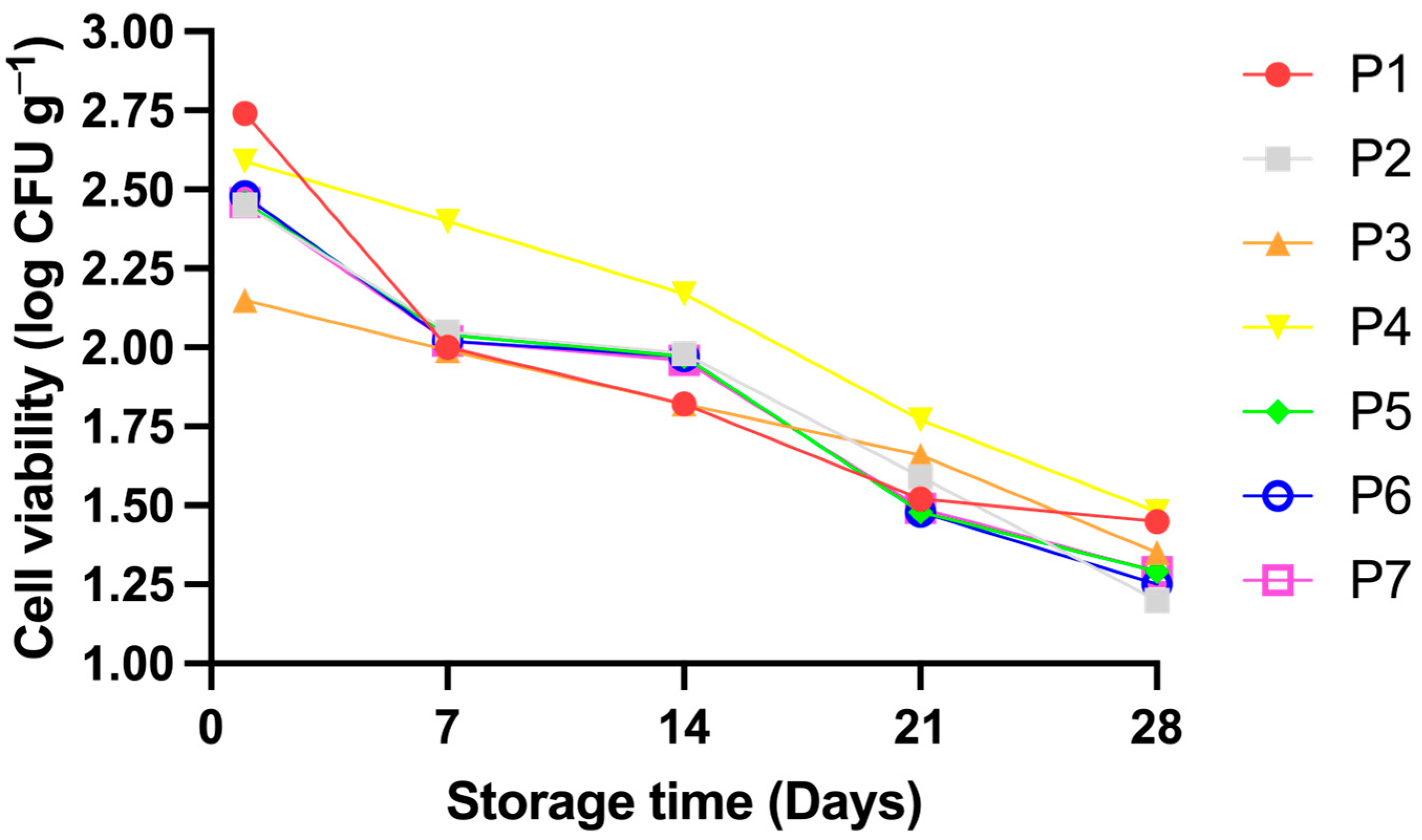
3.6. Probiotic Viability During Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markets and Markets. Dairy Alternatives Market by Source (Soy, Almond, Coconut, Rice, Oats, Hemp), Application (Milk, Cheese, Yogurt, Ice Creams, Creamers), Distribution Channel (Supermarkets, Health Stores, Pharmacies), Formulation and Region—Forecast to 2027. Available online: https://www.marketsandmarkets.com/Market-Reports/dairy-alternative-plant-milk-beverages-market-677.html (accessed on 2 July 2023).
- Min, M.; Bunt, C.R.; Mason, S.L.; Hussain, M.A. Non-dairy probiotic food products: An emerging group of functional foods. Crit. Rev. Food Sci. Nutr. 2018, 59, 2626–2641. [Google Scholar] [CrossRef] [PubMed]
- Meena, L.; Buvaneswaran, M.; Byresh, T.S.; Sunil, S.K.; Rawson, A.; Venkatachalapatia, N. Effect of Ultrasound Treatment on White Finger Millet-Based Probiotic Beverage. Meas. Food 2023, 11, 100. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Immune boosting functional foods and their mechanisms: A critical evaluation of probiotics and prebiotics. Biomed. Pharmacother. 2020, 130, 110625. [Google Scholar] [CrossRef] [PubMed]
- Seyedzade, H.S.; Khorshidian, N.; Mohammadi, M. An Insight into the Potential Application of Symbiotic Edible Films and Coatings in Food Products. Front. Nutr. 2022, 9, 875368. [Google Scholar] [CrossRef]
- Hyrslova, I.; Krausova, G.; Smolova, J.; Barbora, S.; Tomáš, B.; Malinska, H.; Hüttl, M.; Antonín, K.; Ladislav, C.; Doskocil, I. Functional Properties of Chlorella vulgaris, Colostrum, and Bifidobacteria, and Their Potential for Application in Functional Foods. Appl. Sci. 2021, 11, 5264. [Google Scholar] [CrossRef]
- Santos, N.C.; Almeida, R.L.J.; Monteiro, S.S.; Silva, V.M.A.; Lima, T.L.B.; Saraiva, M.M.T.; Rocha, A.P.T. Ultrasound and Microwaves Reduce Stress in Probiotics during Avocado Drying: Impact on Mass Transfer and Cell Viability. Food Biosci. 2024, 61, 104655. [Google Scholar] [CrossRef]
- Albuquerque, A.P.; Rodrigues, T.J.A.; Beserra, Y.A.S.; Vieira, A.F.; Almeida, R.L.J.; Santos, N.C.; Rocha, A.P.T. Viability of the Probiotic Bacterium (Bifidobacterium animalis ssp. lactis) in Umbu-Caja Pulp. J. Food Meas. Charact. 2023, 18, 812–822. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef]
- Holkem, A.T.; Favaro-Trindade, C.S.; Lacroix, M. Study of Anticancer Properties of Proanthocyanidin-Rich Cinnamon Extract in Combination with Bifidobacterium animalis Subsp. lactis BLC1 and Resistance of These Free and Co-Encapsulated Materials under In Vitro Simulated Gastrointestinal Conditions. Food Res. Int. 2020, 134, 109274. [Google Scholar] [CrossRef]
- Cai, Y.; Quan, H.; Liu, Y.; Han, X.; Lu, Y.; Lan, X.; Guo, X. The Impact of Thermal Pretreatment on Phytochemical Profiles and Bioactivity of Freeze-Dried Lily Bulbs (Lilium lancifolium). Food Chem. X 2024, 22, 101284. [Google Scholar] [CrossRef]
- Albuquerque, A.P.; Rodrigues, T.J.A.; da Silva, L.R.; Santos, N.C.; Rocha, A.P.T.; Gomes, J.P. Impact of Drying Technique on Umbu-Cajá Pulp with the Addition of Probiotic Culture: Optimization, Cell Viability, Physicochemical, and Functional Properties. Food Bioeng. 2024, 3, 12083. [Google Scholar] [CrossRef]
- Ge, S.; Han, J.; Sun, Q.; Zhou, Q.; Ye, Z.; Li, P.; Gu, Q. Research Progress on Improving the Freeze-Drying Resistance of Probiotics: A Review. Trends Food Sci. Technol. 2024, 147, 104425. [Google Scholar] [CrossRef]
- Fonseca, M.T.; Vital, A.C.; Silva, M.B.; Monteiro, S.S.; Nascimento, A.; Trindade, A.P.; Lisboa, H.M.; Pasquali, M.B. Improving the Stability of Spray-Dried Probiotic Acerola Juice: A Study on Hydrocolloids’ Efficacy and Process Variables. Food Bioprod. Process. 2024, 147, 209–218. [Google Scholar] [CrossRef]
- ANVISA. Informe Técnico n° 33, de 25 de Outubro de 2007. Hidróxido de Sódio—INS 524; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2007.
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- AOAC. Official Methods of Analysis, 20th ed.; AOAC: Washington, DC, USA, 2012; p. 3100. [Google Scholar]
- Waterhouse, A. Folin-ciocalteau micro method for total phenol in wine. Am. J. Enol. Vitic. 2006, 48, 357–363. [Google Scholar]
- Goldstein, J.L.; Swain, T. Changes in Tannins in Ripening Fruits. Phytochemistry 1963, 2, 371–383. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Francis, F.J.; Markakis, P.C. Food Colorants: Anthocyanins. Crit. Rev. Food Sci. Nutr. 1989, 28, 273–314. [Google Scholar] [CrossRef]
- ISO 20128:2006; Milk Products—Enumeration of Presumptive Lactobacillus Acidophilus on a Selective Medium—Colony-Count Technique at 37 °C. International Organization for Standardization (ISO): Geneva, Switzerland, 2006.
- Dias, C.O.; Opuski de Almeida, J.D.S.; Pinto, S.S.; Santana, F.C.O.; Verruck, S.; Müller, C.M.O.; Prudêncio, E.S.; Amboni, R.D.M.C. Development and Physico-Chemical Characterization of Microencapsulated Bifidobacteria in Passion Fruit Juice: A Functional Non-Dairy Product for Probiotic Delivery. Food Biosci. 2018, 24, 26–36. [Google Scholar] [CrossRef]
- Rufino, M.d.S.M.; Alves, R.E.; Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and Antioxidant Capacities of 18 Non-Traditional Tropical Fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Andrade, V.T.; Castro, R.J.S. Fermented Grain-Based Beverages as Probiotic Vehicles and Their Potential Antioxidant and Antidiabetic Properties. Biocatal. Agric. Biotechnol. 2023, 53, 102873. [Google Scholar] [CrossRef]
- Silva, L.C.; Kunigk, C.J. Efeito do pH na Sobrevivência de Bifidobacterium animalis em Matriz de Fruta. Braz. J. Food Res. 2019, 10, 118–130. [Google Scholar] [CrossRef]
- Oliveira, D.; Vidal, L.; Ares, G.; Walter, E.H.; Rosenthal, A.; Deliza, R. Sensory, Microbiological and Physicochemical Screening of Probiotic Cultures for the Development of Non-Fermented Probiotic Milk. LWT-Food Sci. Technol. 2017, 79, 234–241. [Google Scholar] [CrossRef]
- Santos, N.C.; Almeida, R.L.J.; da Silva, G.M.; Monteiro, S.S.; de Alcântara Ribeiro, V.H.; de França Silva, A.P.; de Almeida Mota, M.M. Influence of high hydrostatic pressure (HHP) pretreatment on plum (Prunus salicina) drying: Drying approach, physical, and morpho-structural properties of the powder and total phenolic compounds. J. Food Process. Preserv. 2022, 46, e16968. [Google Scholar] [CrossRef]
- Maia, M.S.; Domingos, M.M.; de São José, J.F.B. Viability of Probiotic Microorganisms and the Effect of Their Addition to Fruit and Vegetable Juices. Microorganisms 2023, 11, 1335. [Google Scholar] [CrossRef]
- Cassani, L.; Gerbino, E.; del Rosario Moreira, M.; Gómez-Zavaglia, A. Influence of Non-Thermal Processing and Storage Conditions on the Release of Health-Related Compounds after In Vitro Gastrointestinal Digestion of Fiber-Enriched Strawberry Juices. J. Funct. Foods 2018, 40, 128–136. [Google Scholar] [CrossRef]
- Soares, M.B.; Martinez, R.C.; Pereira, E.P.; Balthazar, C.F.; Cruz, A.G.; Ranadheera, C.S.; Sant’Ana, A.S. The Resistance of Bacillus, Bifidobacterium, and Lactobacillus Strains with Claimed Probiotic Properties in Different Food Matrices Exposed to Simulated Gastrointestinal Tract Conditions. Food Res. Int. 2019, 125, 108542. [Google Scholar] [CrossRef]
- Monteiro, S.; Beserra, Y.A.S.; Oliveira, H.M.L.; Pasquali, M.A.B. Production of Probiotic Passion Fruit (Passiflora edulis Sims f. flavicarpa Deg.) Drink Using Lactobacillus reuteri and Microencapsulation via Spray Drying. Foods 2020, 9, 335. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Bujna, E.; Fekete, N.; Tran, A.T.; Rezessy-Szabo, J.M.; Prasad, R.; Nguyen, Q.D. Probiotic Beverage from Pineapple Juice Fermented with Lactobacillus and Bifidobacterium Strains. Front. Nutr. 2019, 6, 54. [Google Scholar] [CrossRef]
- Bujna, E.; Farkas, N.A.; Tran, A.M.; Dam, M.S.; Nguyen, Q.D. Lactic Acid Fermentation of Apricot Juice by Mono- and Mixed Cultures of Probiotic Lactobacillus and Bifidobacterium Strains. Food Sci. Biotechnol. 2018, 27, 547–554. [Google Scholar] [CrossRef]
- Meenu, M.; Kaur, S.; Kaur, M.; Mradula, M.; Khandare, K.; Xu, B.; Pati, P.K. The golden era of fruit juices-based probiotic beverages: Recent advancements and future possibilities. Process Biochem. 2024, 142, 113–135. [Google Scholar] [CrossRef]
- DePaz, R.; Pansare, S.; Patel, S. Freeze-Drying Above the Glass Transition Temperature in Amorphous Protein Formulations While Maintaining Product Quality and Improving Process Efficiency. J. Pharm. Sci. 2016, 105, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Jawan, R.; Abbasiliasi, S.; Tan, J.S.; Kapri, M.R.; Mustafa, S.; Halim, M.; Ariff, A.B. Influence of Type and Concentration of Lyoprotectants, Storage Temperature, and Storage Duration on Cell Viability and Antibacterial Activity of Freeze-Dried Lactic Acid Bacterium, Lactococcus lactis Gh1. Dry Technol. 2022, 40, 1774–1790. [Google Scholar] [CrossRef]
- Rodrigues, T.J.A.; Albuquerque, A.P.; Da Silva, L.R.; Silva, H.A.; Pasquali, M.A.B.; Araujo, G.T.; Rocha, A.P.T. Production of Probiotic Cajá Fruit (Spondias mombin) Powder Using Bifidobacterium animalis ssp. lactis B94 via Spouted Bed. Food Sci. Technol. 2022, 42, e27821. [Google Scholar] [CrossRef]
- Semyonov, D.; Ramon, O.; Kaplun, Z.; Levin-Brener, L.; Gurevich, N.; Shimoni, E. Microencapsulation of Lactobacillus paracasei by Spray Freeze Drying. Food Res. Int. 2010, 43, 193–202. [Google Scholar] [CrossRef]
- Wang, G.-Q.; Pu, J.; Yu, X.-Q.; Xia, Y.-J.; Ai, L.-Z. Influence of freezing temperature before freeze-drying on the viability of various Lactobacillus plantarum strains. J. Dairy Sci. 2020, 103, 3066–3075. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C. Optimization of Spray Drying Conditions for Developing Nondairy Based Probiotic Sohiong Fruit Powder. Int. J. Fruit Sci. 2021, 21, 193–204. [Google Scholar] [CrossRef]
- Nami, Y.; Lornezhad, G.; Kiani, A.; Abdullah, N.; Haghshenas, B. Alginate-Persian Gum-Prebiotics Microencapsulation Impacts on the Survival Rate of Lactococcus lactis ABRIINW-N19 in Orange Juice. LWT 2020, 124, 109190. [Google Scholar] [CrossRef]
- Azmi, L.; Shukla, I.; Rao, C.V.; Jawaid, T.; Kamal, M.; Alkhamees, O.A.; Alsanad, S.M. Optimization of Ultrasonic Extraction of Major Phenolic Components from Flowers of Ghav-Patta (Argyreia Speciosa Linn.) Via Response Surface Model. Pharm. Chem. J. 2021, 55, 591–599. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Dellarosa, N.; Tylewicz, U.; Laghi, L.; Romani, S.; Dalla Rosa, M.; Witrowa-Rajchert, D. The Influence of Carrier Material on Some Physical and Structural Properties of Carrot Juice Microcapsules. Food Chem. 2017, 236, 134–141. [Google Scholar] [CrossRef]
- Rishabh, D.; Athira, A.; Preetha, R.; Nagamaniammai, G. Freeze Dried Probiotic Carrot Juice Powder for Better Storage Stability of Probiotic. J. Food Sci. Technol. 2021, 60, 916–924. [Google Scholar] [CrossRef]
- Aryaee, H.; Ariaii, P.; Zare, D.; Mirdamadi, S.; Naghizadeh Raeisi, S. Evaluation of the Physicochemical Characteristics of a Blend Fruit Juice Powder Mixed with Lactiplantibacillus plantarum: A Comparison of Spray Drying and Freeze Drying. J. Food Process. Preserv. 2023, 47, 5597647. [Google Scholar] [CrossRef]
- Ma, Y.; Yi, J.; Jin, X.; Li, X.; Feng, S.; Bi, J. Freeze-Drying of Fruits and Vegetables in Food Industry: Effects on Phytochemicals and Bioactive Properties Attributes—A Comprehensive Review. Food Rev. Int. 2022, 39, 6611–6629. [Google Scholar] [CrossRef]
- Abbas, M.S.; Afzaal, M.; Saeed, F.; Asghar, A.; Jianfeng, L.; Ahmad, A.; Shah, M.A. Probiotic Viability as Affected by Encapsulation Materials: Recent Updates and Perspectives. Int. J. Food Prop. 2023, 26, 1324–1350. [Google Scholar] [CrossRef]
- Sultana, M.; Chan, E.S.; Janarthanan, P.; Choo, W.S. Functional Orange Juice with Lactobacillus casei and Tocotrienol-Enriched Flaxseed Oil Co-Encapsulation: Physicochemical Properties, Probiotic Viability, Oxidative Stability, and Sensorial Acceptability. LWT 2023, 188, 115388. [Google Scholar] [CrossRef]
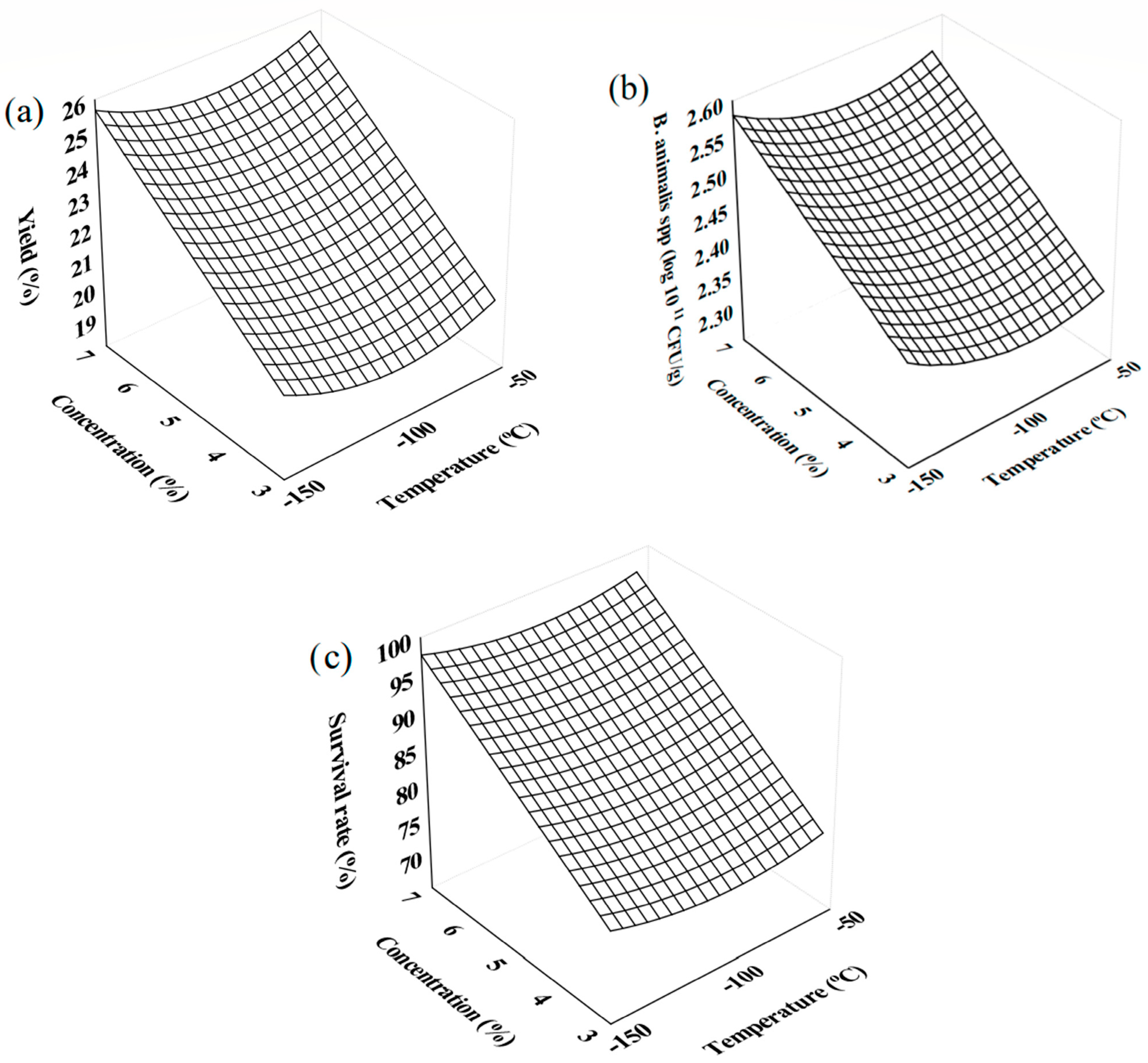
| Experiments | T (°C) | Concentration (%) | Yield (%) | Cell Viability (11 log CFU/g) | Survival Rate (%) |
|---|---|---|---|---|---|
| P1 | −150 (−1) | 3 (−1) | 19.51 | 2.36 | 73.01 |
| P2 | −150 (−1) | 7 (+1) | 25.67 | 2.58 | 97.66 |
| P3 | −50 (+1) | 3 (−1) | 19.36 | 2.32 | 72.10 |
| P4 | −50 (+1) | 7 (+1) | 25.65 | 2.56 | 97.49 |
| P5 | −100 (0) | 5 (0) | 22.18 | 2.44 | 85.15 |
| P6 | −100 (0) | 5 (0) | 22.25 | 2.46 | 85.32 |
| P7 | −100 (0) | 5 (0) | 22.20 | 2.41 | 85.33 |
| ANOVA | |||||
| R2 | 99.98 | 97.28 | 99.99 | ||
| Adj R2 | 99.98 | 93.18 | 99.99 | ||
| p-value | <0.0008 | <0.0117 | <0.00114 | ||
| Fc/Ft | 5.56 | 1.42 | 28.50 | ||
| Parameters | Fresh Pulp | Fermented Pulp |
|---|---|---|
| pH | 3.36 ± 0.05 b | 5.87 ± 0.03 a |
| Total titratable acidity (g of citric acid/100 g) | 1.32 ± 0.09 a | 0.73 ± 0.04 b |
| Water content (%) | 87.55 ± 0.40 a | 87.18 ± 0.29 a |
| Water activity, aw | 0.98 ± 0.00 a | 0.98 ± 0.00 a |
| TPC (mg GAE/100 g) | 1775.33 ± 2.67 b | 2259.11 ± 1.80 a |
| TT (mg TAE/100 g) | 724.65 ± 1.73 b | 1145.53 ± 2.07 a |
| TF (mg/100 g) | 39.67 ± 0.55 b | 44.85 ± 0.26 a |
| TA (mg/100 g) | 3.64 ± 0.15 b | 9.26 ± 0.23 a |
| TC (µg/100 g) | 48.94 ± 0.34 a | 72.97 ± 0.19 a |
| Cell viability (CFU/mL) | N/D | 2.98 × 1011 |
| Experiments | Water Content (%) | Water Activity | TPC (mg GAE/100 g) | TT (mg TAE/100 g) | TF (mg/100 g) | TA (mg/100 g) | TC (µg/100 g) |
|---|---|---|---|---|---|---|---|
| P1 | 3.46 ± 0.03 b | 0.220 ± 0.01 a | 1002.05 ± 0.03 g | 5218.28 ± 0.03 e | 92.04 ± 0.03 c | 24.61 ± 0.03 f | 72.98 ± 0.22 c |
| P2 | 3.49 ± 0.05 b | 0.224 ± 0.00 a | 8958.56 ± 0.01 d | 5079.46 ± 0.03 f | 83.87 ± 0.01 e | 16.26 ± 0.02 g | 42.20 ± 0.12 e |
| P3 | 3.76 ± 0.01 a | 0.228 ± 0.02 a | 1095.11 ± 0.01 f | 5378.04 ± 0.01 d | 95.46 ± 0.02 b | 27.12 ± 0.04 e | 72.97 ± 0.11 c |
| P4 | 3.51 ± 0.04 b | 0.224 ± 0.01 a | 8355.12 ± 0.04 e | 5020.36 ± 0.06 g | 91.17 ± 0.01 d | 29.44 ± 0.01 d | 44.45 ± 0.14 d |
| P5 | 3.55 ± 0.03 b | 0.227 ± 0.01 a | 9615.77 ± 0.05 b | 6764.24 ± 0.04 a | 120.81 ± 0.20 a | 34.70 ± 0.01 a | 96.71 ± 0.05 a |
| P6 | 3.43 ± 0.02 b | 0.221 ± 0.01 a | 9588.8 ± 0.02 a | 6703.34 ± 0.06 c | 120.28 ± 0.35 a | 35.47 ± 0.03 c | 95.84 ± 0.23 b |
| P7 | 3.54 ± 0.01 b | 0.225 ± 0.02 a | 9506.73 ± 0.03 c | 6750.02 ± 0.02 b | 120.98 ± 0.05 a | 35.54 ± 0.02 b | 95.49 ± 0.31 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregório, M.; Araújo, M.; Albuquerque, A.; Rodrigues, T.; Santos, N.C.; Fonseca, M.T.; Costa, M.E.d.; Tomé, A.; Gomes, J.; Gouveia, D.; et al. Probiotication of Plum Pulp and Conditions Effects Freeze-Drying in Cell Viability, Functional Properties and Antioxidant Activity. Foods 2024, 13, 3551. https://doi.org/10.3390/foods13223551
Gregório M, Araújo M, Albuquerque A, Rodrigues T, Santos NC, Fonseca MT, Costa MEd, Tomé A, Gomes J, Gouveia D, et al. Probiotication of Plum Pulp and Conditions Effects Freeze-Drying in Cell Viability, Functional Properties and Antioxidant Activity. Foods. 2024; 13(22):3551. https://doi.org/10.3390/foods13223551
Chicago/Turabian StyleGregório, Mailson, Morgana Araújo, Aline Albuquerque, Thais Rodrigues, Newton C. Santos, Maria Tereza Fonseca, Maria Eduarda da Costa, Anna Tomé, Josivanda Gomes, Deyzi Gouveia, and et al. 2024. "Probiotication of Plum Pulp and Conditions Effects Freeze-Drying in Cell Viability, Functional Properties and Antioxidant Activity" Foods 13, no. 22: 3551. https://doi.org/10.3390/foods13223551
APA StyleGregório, M., Araújo, M., Albuquerque, A., Rodrigues, T., Santos, N. C., Fonseca, M. T., Costa, M. E. d., Tomé, A., Gomes, J., Gouveia, D., Lisboa, H. M., & Rocha, A. P. (2024). Probiotication of Plum Pulp and Conditions Effects Freeze-Drying in Cell Viability, Functional Properties and Antioxidant Activity. Foods, 13(22), 3551. https://doi.org/10.3390/foods13223551









