Application of a Multi-Gas Detector for Monitoring Gas Composition in Minced Beef During Storage
Abstract
1. Introduction
2. Materials and Methods
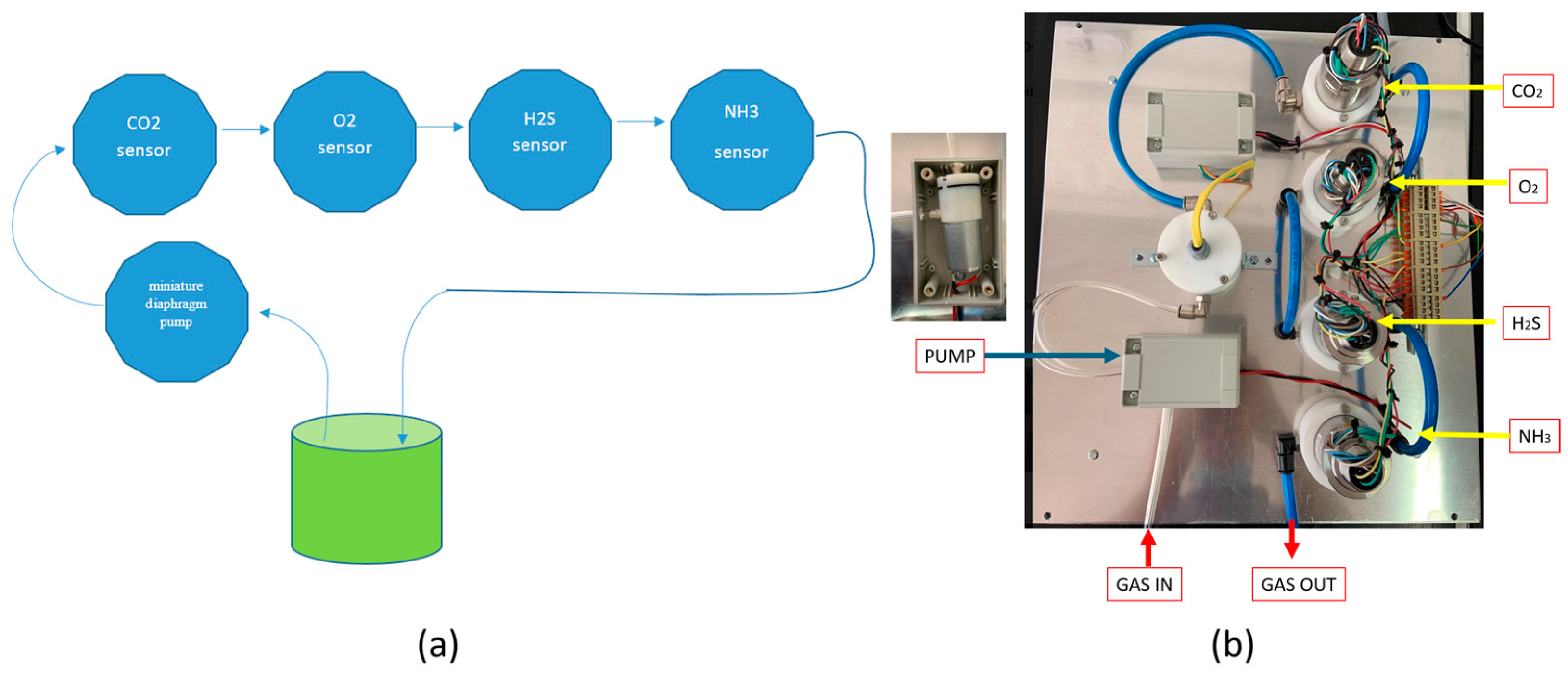
2.1. Construction of the Device
2.2. The Meat Preparation
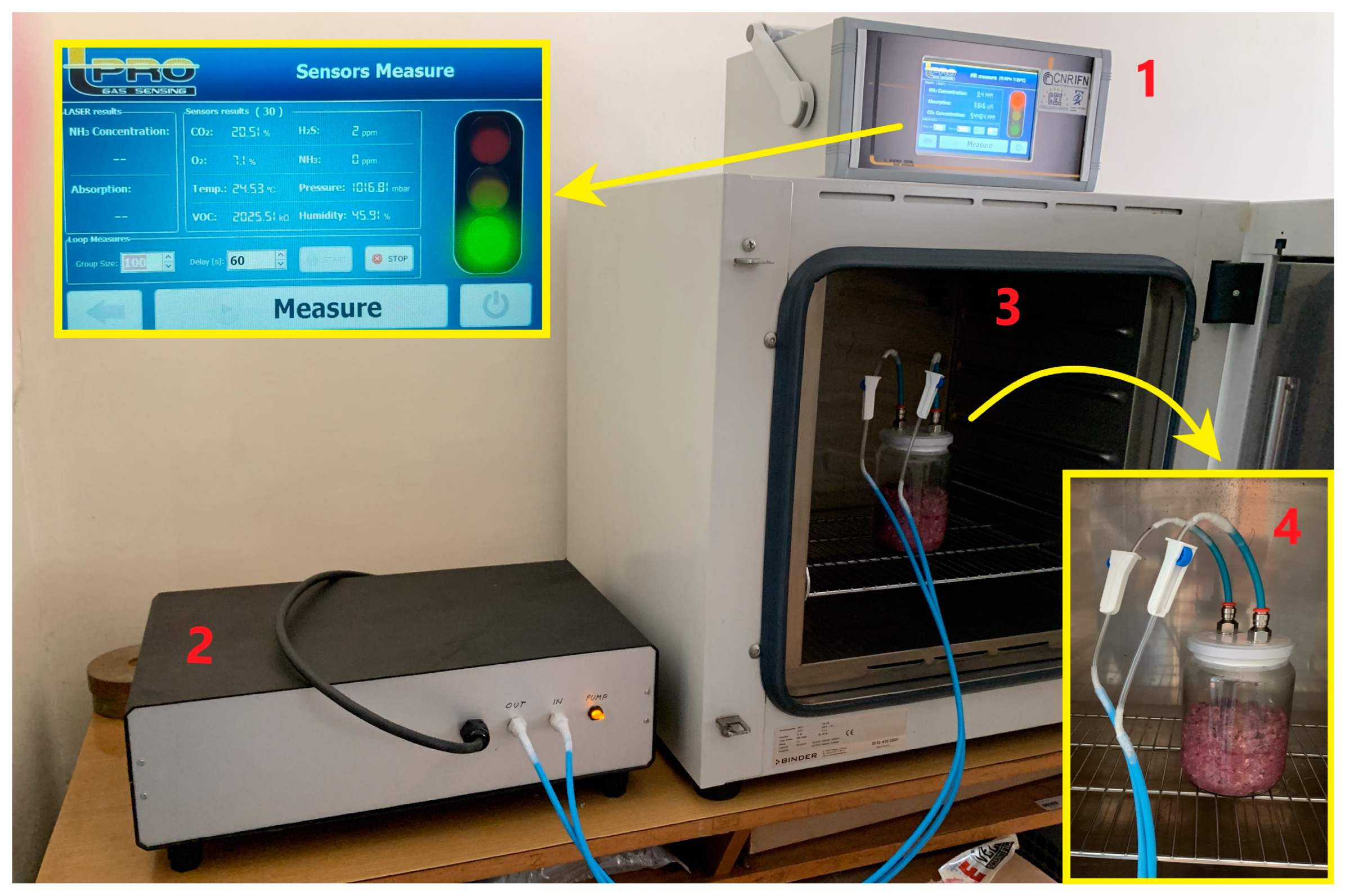
2.3. Gas Measurement
2.4. Microbial Analysis
2.5. Statistical Analysis
3. Results and Discussion
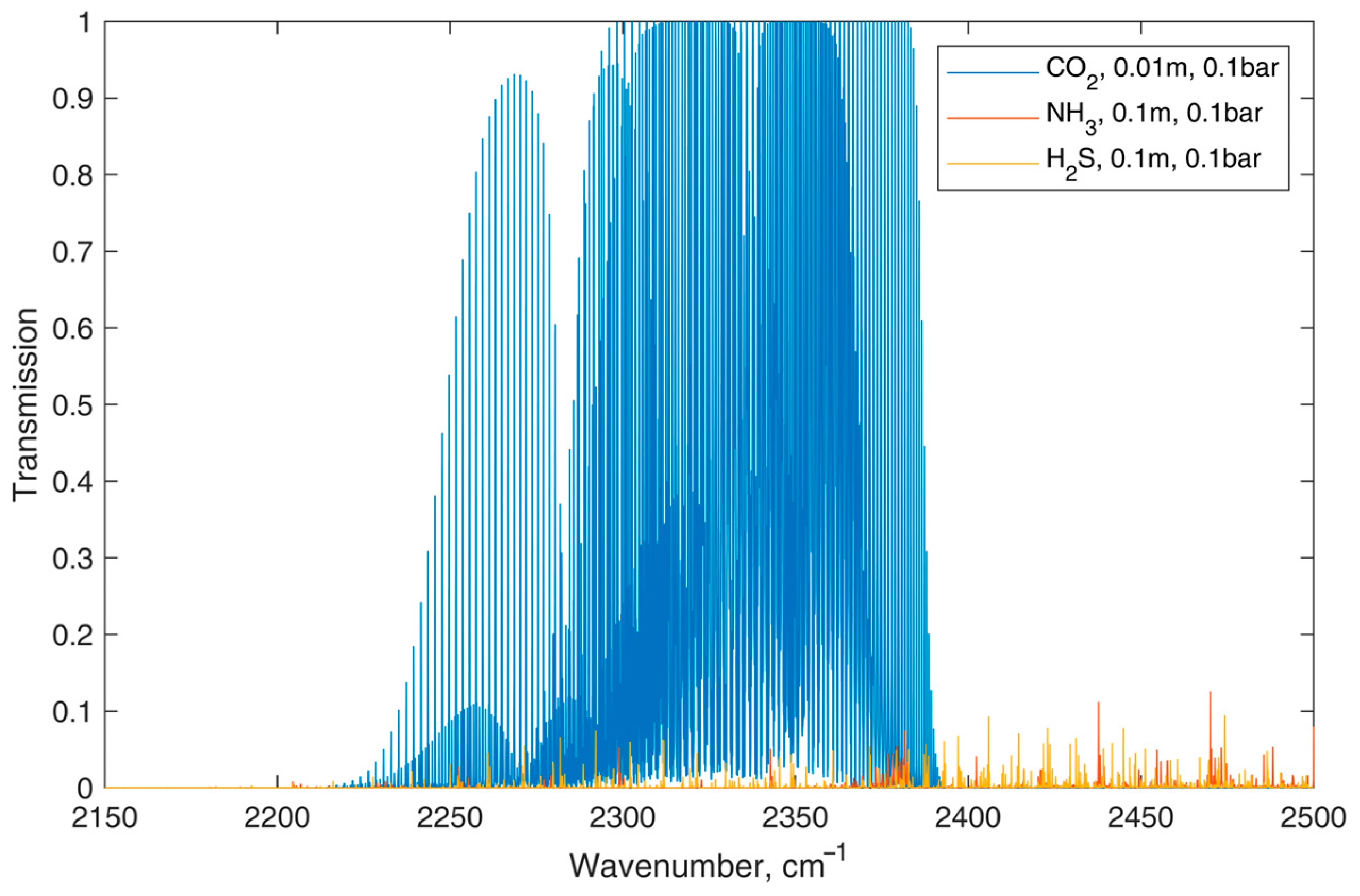
3.1. The Device Construction and Characteristics
- Test 1: Low H2S Concentration (2 ppm)
- Test 2: High H2S Concentration (70 ppm)
- Test 3: Mixed H2S and CO2 Concentrations (50 ppm H2S, 25% CO2)
- Test 4: High NH3 Concentration (240 ppm)
Summary of Cross-Correlation Results
3.2. Gas Content and Microbial Composition During the Meat Storage
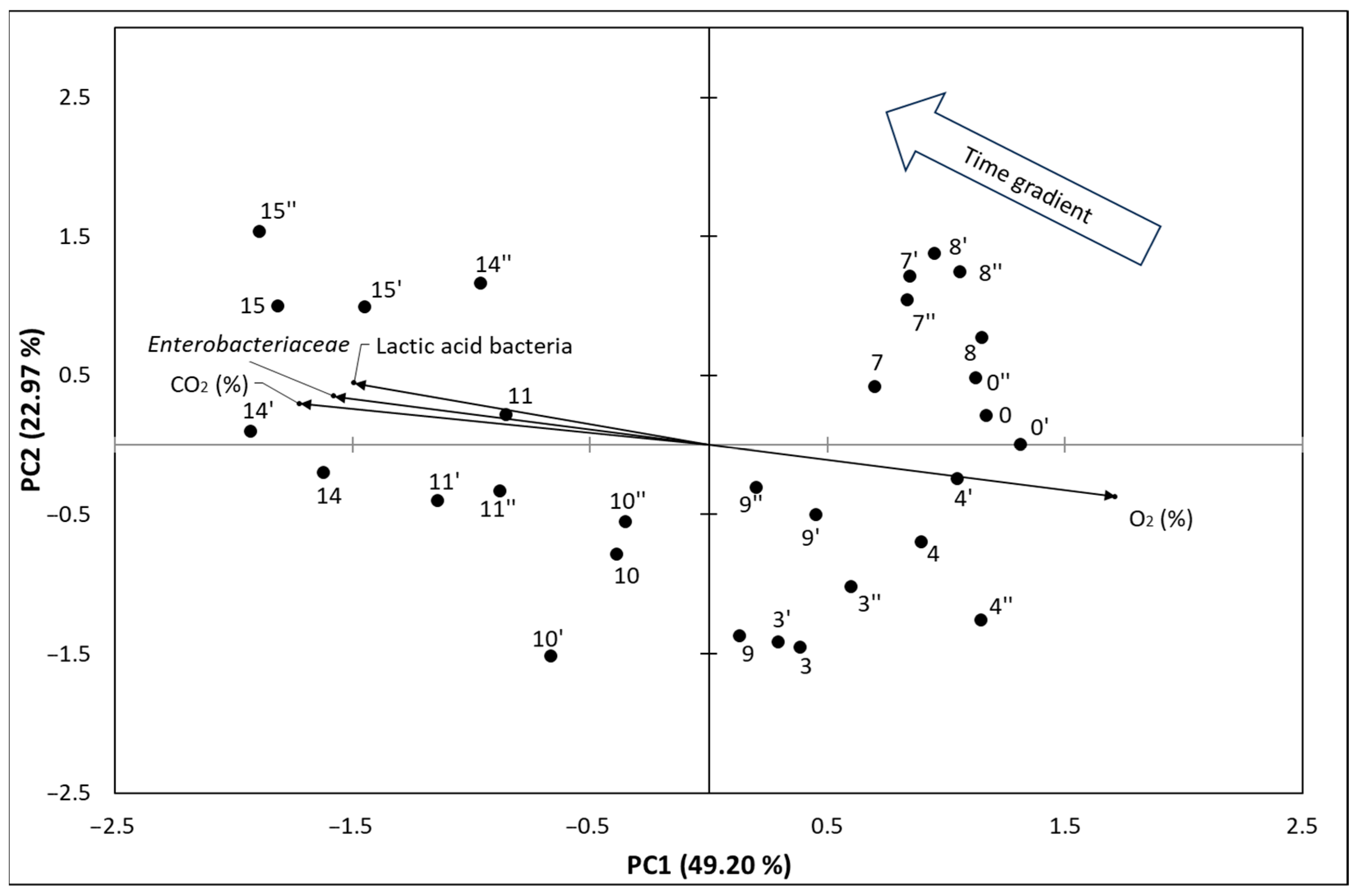
3.3. Principal Component Analysis of the Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.J.E. Spoilage microbiota associated to the storage of raw meat in different conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Huang, X.; Mahunu, G.K. Exploratory review on the safety of edible raw fish per the hazard factors and their detection methods. Trends Food Sci. Technol. 2017, 59, 37–48. [Google Scholar] [CrossRef]
- Dave, D.; Ghaly, A.E. Meat spoilage mechanisms and preservation techniques: A critical review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Ray, B. Fundamental Food Microbiology, 3rd ed.; CRC Press: London, UK, 2005; pp. 81–92. [Google Scholar]
- Danilović, B.; Savić, D.; Cocola, L.; Fedel, M.; Poletto, L. Determination of CO2 content in the headspace of spoiled yogurt packages. J. Food Qual. 2018, 1, 8121606. [Google Scholar] [CrossRef]
- Dold, J.; Kehr, C.; Hollmann, C.; Langowski, H.-C. Non-Destructive Measuring Systems for the Evaluation of High Oxygen Stored Poultry: Development of Headspace Gas Composition, Sensory and Microbiological Spoilage. Foods 2022, 11, 592. [Google Scholar] [CrossRef]
- Lovestead, T.M.; Bruno, T.J. Detection of poultry spoilage markers from headspace analysis with cryoadsorption on a short alumina PLOT column. Food Chem. 2010, 121, 1274–1282. [Google Scholar] [CrossRef]
- Kim, H.K.; An, D.S.; Yam, K.L.; Lee, D.S. Package Headspace Composition Changes of Chill-Stored Perishable Foods in Relation to Microbial Spoilage. Packag. Technol. Sci. 2011, 24, 343–352. [Google Scholar] [CrossRef]
- Ghasemi-Varnamkhasti, M.; Mohtasebi, S.S.; Siadat, M.; Balasubramanian, S. Meat quality assessment by electronic nose (machine olfaction technology). Sensors 2009, 9, 6058–6083. [Google Scholar] [CrossRef]
- Kartika, V.S.; Rivai, M.; Purwanto, D. Spoiled meat classification using semiconductor gas sensors, image processing and neural network. In Proceedings of the 2018 International Conference on Information and Communications Technology (ICOIACT), Yogyakarta, Indonesia, 6–7 March 2018; pp. 418–423. [Google Scholar] [CrossRef]
- Chang, Z.; Sun, Y.; Zhang, Y.; Gao, Y.; Weng, X.; Chen, D.; David, L.; Xie, J. Bionic Optimization Design of Electronic Nose Chamber for Oil and Gas Detection. J. Bionic Eng. 2018, 15, 533–544. [Google Scholar] [CrossRef]
- Chen, D.H.; Ye, S.B.; Weng, X.H.; Tong, J.; Chang, Z.Y. Artificial olfactory system technology on chicken freshness detection. Appl. Mech. Mater. 2014, 461, 801–808. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, Y.; Wang, C.; Shi, H.; Wang, S.; Yong, C.; Gong, Y.; Zhang, W.; Zou, X. Non-Destructive Detection of Chicken Freshness Based on Electronic Nose Technology and Transfer Learning. Agriculture 2023, 13, 496. [Google Scholar] [CrossRef]
- Pellissery, A.J.; Vinayamohan, P.G.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Spoilage bacteria and meat quality. In Meat Quality Analysis; Academic Press: London, UK, 2020; pp. 307–334. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, M.; Adhikari, B. Advances of electronic nose and its application in fresh foods: A review. Crit. Rev. Food Sci. Nutr. 2017, 58, 2700–2710. [Google Scholar] [CrossRef]
- Bosch, S.; de Menezes, R.X.; Pees, S.; Wintjens, D.J.; Seinen, M.; Bouma, G.; Kuyvenhoven, J.; Stokkers, P.C.F.; de Meij, T.G.J.; de Boer, N.K.H. Electronic Nose Sensor Drift Affects Diagnostic Reliability and Accuracy of Disease-Specific Algorithms. Sensors 2022, 22, 9246. [Google Scholar] [CrossRef]
- Falasconi, M.; Concina, I.; Gobbi, E.; Sberveglieri, V.; Pulvirenti, A.; Sberveglieri, G. Electronic Nose for Microbiological Quality Control of Food Products. Int. J. Electrochem. 2012, 2012, 715763. [Google Scholar] [CrossRef]
- Fuśnik, Ł.; Szafraniak, B.; Paleczek, A.; Grochala, D.; Rydosz, A. A Review of Gas Measurement Set-Ups. Sensors 2022, 22, 2557. [Google Scholar] [CrossRef]
- Lewander, M.; Guan, Z.G.; Persson, L.; Olsson, A.; Svanberg, S. Food monitoring based on diode laser gas spectroscopy. Appl. Phys. B 2008, 93, 619–625. [Google Scholar] [CrossRef]
- Lewander, M.; Lundin, P.; Svensson, T.; Svanberg, S.; Olsson, A. Non-intrusive measurements of headspace gas composition in liquid food packages made of translucent materials. Packag. Technol. Sci. 2011, 24, 271–280. [Google Scholar] [CrossRef]
- Mulier, M.; Zeninari, V.; Joly, L.; Decarpenterie, T.; Parvitte, B.; Jeandet, P.; Liger-Belair, G. Development of a compact CO2 sensor based on near-infrared laser technology for enological applications. Appl. Phys. B 2009, 94, 725–733. [Google Scholar] [CrossRef]
- Lundin, P.; Cocola, L.; Lewander, M.; Olsson, A.; Svanberg, S. Non-intrusive headspace gas measurements by laser spectroscopy–Performance validation by a reference sensor. J. Food Eng. 2012, 111, 612–617. [Google Scholar] [CrossRef]
- Cocola, L.; Allermann, H.; Fedel, M.; Sønderby, S.; Tondello, G.; Bardenstein, A.; Poletto, L. Validation of an in-line non-destructive headspace oxygen sensor. Food Packag. Shelf Life 2016, 9, 38–44. [Google Scholar] [CrossRef]
- Cocola, L.; Fedel, M.; Tondello, G.; Poletto, L. A Modular Approach of Different Geometries for Non-invasive Oxygen Measurement inside Moving Food Packages. Packag. Technol. Sci. 2017, 30, 159–170. [Google Scholar] [CrossRef]
- Veličković, A.; Danilović, B.; Cocola, L.; Fedel, M.; Poleto, L.; De Marchi, M.; Franzoi, M.; Savić, D. Measurement of ammonia realised by spontaneous microbiota in beef during storage. In Microbial Diversity as a Source of Novelty: Function, Adaptation and Exploitation; University of Turin: Catania, Italy, 2019; pp. 431–432. [Google Scholar]
- ISO 1443:1973; Meat and Meat Products—Determination of Total Fat Content. International Organisation for Standardization: Geneva, Switzerland, 1973.
- ISO 937:1978; Meat and Meat Products—Determination of Nitrogen Content. International Organisation for Standardization: Geneva, Switzerland, 1978.
- ISO 1442:1997; Meat and Meat Products—Determination of Moisture Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 1997.
- Kim, H.J.; Lee, Y.J.; Eun, J.B. Changes in the microbiological characteristics of Korean native cattle (Hanwoo) beef exposed to ultraviolet (UV) irradiation prior to refrigeration. Korean J. Food Sci. Anim. Resour. 2014, 34, 815. [Google Scholar] [CrossRef]
- Teng, S.; Gan, J.; Chen, Y.; Yang, L.; Ye, K. The Application of Ultraviolet Treatment to Prolong the Shelf Life of Chilled Beef. Foods 2023, 12, 2410. [Google Scholar] [CrossRef]
- Ruparelia, J.; Rabari, A.; Joshi, N.; Jha, C.K. Isolation methods for evaluation of extremophilic microbial diversity from Antarctica region. In Microbial Diversity in Hotspots; Academic Press: London, UK, 2022; pp. 267–289. [Google Scholar] [CrossRef]
- Stachowiak, D.; Jaworski, P.; Krzaczek, P.; Maj, G.; Nikodem, M. Laser-based monitoring of CH4, CO2, NH3, and H2S in animal farming—System characterization and initial demonstration. Sensors 2018, 18, 529. [Google Scholar] [CrossRef]
- Bugbee, B.; Blonquist, M. Absolute and Relative Gas Concentration: Understanding Oxygen in Air. 2006, Volume 27, pp. 1–9. Available online: https://www.apogeeinstruments.com/content/o2s_correcting.pdf (accessed on 6 November 2024).
- Marx, Í.M.G. Emerging Trends of Electrochemical Sensors in Food Analysis. Electrochem 2023, 4, 42–46. [Google Scholar] [CrossRef]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Modern Food Microbiology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Cerveny, J.; Meyer, J.D.; Hall, P.A. Microbiological spoilage of meat and poultry products. In Compendium of the Microbiological Spoilage of Foods and Beverages; Springer: Berlin/Heidelberg, Germany, 2009; pp. 69–86. [Google Scholar] [CrossRef]
- Comi, G.; Iacumin, L. Identification and process origin of bacteria responsible for cavities and volatile off-flavour compounds in artisan cooked ham. Int. J. Food Sci. Technol. 2012, 47, 114–121. [Google Scholar] [CrossRef]
- Pennacchia, C.; Ercolini, D.; Villani, F. Spoilage-related microbiota associated with chilled beef stored in air or vacuum pack. Food Microbiol. 2011, 28, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Húngaro, H.M.; Caturla, M.Y.; Horita, C.N.; Furtado, M.M.; Sant’Ana, A.S. Blown pack spoilage in vacuum-packaged meat: A review on clostridia as causative agents, sources, detection methods, contributing factors and mitigation strategies. Trends Food Sci. Technol. 2016, 52, 123–138. [Google Scholar] [CrossRef]
- Matindoust, S.; Farzi, G.; Nejad, M.B.; Shahrokhabadi, M.H. Polymer-based gas sensors to detect meat spoilage: A review. React. Funct. Polym. 2021, 165, 104962. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, Y.; Luo, F.; Lin, C.; Wang, J.; Qiu, B.; Lin, Z. Multicolor hydrogen sulfide sensor for meat freshness assessment based on Cu2+-modified boron nitride nanosheets-supported subnanometer gold nanoparticles. Food Chem. 2022, 381, 132278. [Google Scholar] [CrossRef] [PubMed]
- Jos, H.J.; in’t Veld, J.H.H. Microbial and biochemical spoilage of foods: An overview. Int. J. Food Microbiol. 1996, 33, 1–18. [Google Scholar] [CrossRef]
- Anklam, E.H.; Belitz, D.; Grosch, W.; Schieberle, P. Food Chem. In Analytical and Bioanalytical Chemistry, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 10–11. [Google Scholar] [CrossRef]
- García-Cano, I.; Rocha-Mendoza, D.; Ortega-Anaya, J.; Wang, K.; Kosmerl, E.; Jiménez-Flores, R. Lactic acid bacteria isolated from dairy products as potential producers of lipolytic, proteolytic and antibacterial proteins. Appl. Microbiol. Biotechnol. 2019, 103, 5243–5257. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Pobiega, K.; Piwowarek, K.; Kot, A.M. Characteristics of the Proteolytic Enzymes Produced by Lactic Acid Bacteria. Molecules 2021, 26, 1858. [Google Scholar] [CrossRef]
- Tofteskov, J.; Hansen, J.S.; Bailey, N.P. Modelling the autoxidation of myoglobin in fresh meat under modified atmosphere packing conditions. J. Food Eng. 2017, 214, 129–136. [Google Scholar] [CrossRef]
- Mortazavi, S.M.H.; Kaur, M.; Farahnaky, A.; Torley, P.J.; Osborn, A.M. Microbial and Quality Attributes of Beef Steaks under High-CO2 Packaging: Emitter Pads versus Gas Flushing. Foods 2024, 13, 2913. [Google Scholar] [CrossRef]
- Mortazavi, S.M.H.; Kaur, M.; Javanmardi, F.; Khosravi, A.; Shahbazi, Y. The Pathogenic and Spoilage Bacteria Associated with Red Meat and Application of Different Approaches of High CO2 Packaging to Extend Product Shelf-Life. Crit. Rev. Food Sci. Nutr. 2021, 62, 2340–2361. [Google Scholar] [CrossRef]
- Esmer, O.K.; Irkin, R.; Degirmencioglu, N.; Degirmencioglu, A. The effects of modified atmosphere gas composition on microbiological criteria, color and oxidation values of minced beef meat. Meat Sci. 2011, 88, 221–226. [Google Scholar] [CrossRef]
- Rouger, A.; Moriceau, N.; Prévost, H.; Remenant, B.; Zagorec, M. Diversity of bacterial communities in French chicken cuts stored under modified atmosphere packaging. Food Microbiol. 2018, 70, 7–16. [Google Scholar] [CrossRef]
- Kolbeck, S.; Reetz, L.; Hilgarth, M.; Vogel, R.F. Quantitative oxygen consumption and respiratory activity of meat spoiling bacteria upon high oxygen modified atmosphere. Front. Microbiol. 2019, 10, 2398. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Paramithiotis, S.; Kagkli, D.M.; Nychas, G.J.E. Lactic acid bacteria population dynamics during minced beef storage under aerobic or modified atmosphere packaging conditions. Food Microbiol. 2010, 27, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, J.; Zhu, L.; Luo, X.; Mao, Y.; Hopkins, D.L.; Zhang, Y.; Dong, P. Effect of modified atmosphere packaging on shelf life and bacterial community of roast duck meat. Food Res. Int. 2020, 137, 109645. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Guðjónsdóttir, M.; Prieto, M.A.; Garcia-Oliveira, P.; Simal-Gandara, J.; Marini, F.; Di Donato, F.; D’Archivio, A.A.; Biancolillo, A. Application of Novel Techniques for Monitoring Quality Changes in Meat and Fish Products during Traditional Processing Processes: Reconciling Novelty and Tradition. Processes 2020, 8, 988. [Google Scholar] [CrossRef]
- Ammor, M.S.; Argyri, A.; Nychas, G.J.E. Rapid monitoring of the spoilage of minced beef stored under conventionally and active packaging conditions using Fourier transform infrared spectroscopy in tandem with chemometrics. Meat Sci. 2009, 81, 507–514. [Google Scholar] [CrossRef]
- Janda, J.M.; Lopez, D.L. The family Enterobacteriaceae. In Practical Handbook of Microbiology, 4th ed.; CRC Press: London, UK, 2021; pp. 353–362. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Nychas, G.J.E.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef]
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef]
 ; enterobacteria–
; enterobacteria– ) in minced beef meat (230 g) during incubation at 4 °C. The error bars represent standard errors in experimental data. Values followed by different letters (from “a” to “g”) indicate statistically significant differences at p < 0.05 (Tukey’s HSD test).
) in minced beef meat (230 g) during incubation at 4 °C. The error bars represent standard errors in experimental data. Values followed by different letters (from “a” to “g”) indicate statistically significant differences at p < 0.05 (Tukey’s HSD test).
 ; enterobacteria–
; enterobacteria– ) in minced beef meat (230 g) during incubation at 4 °C. The error bars represent standard errors in experimental data. Values followed by different letters (from “a” to “g”) indicate statistically significant differences at p < 0.05 (Tukey’s HSD test).
) in minced beef meat (230 g) during incubation at 4 °C. The error bars represent standard errors in experimental data. Values followed by different letters (from “a” to “g”) indicate statistically significant differences at p < 0.05 (Tukey’s HSD test).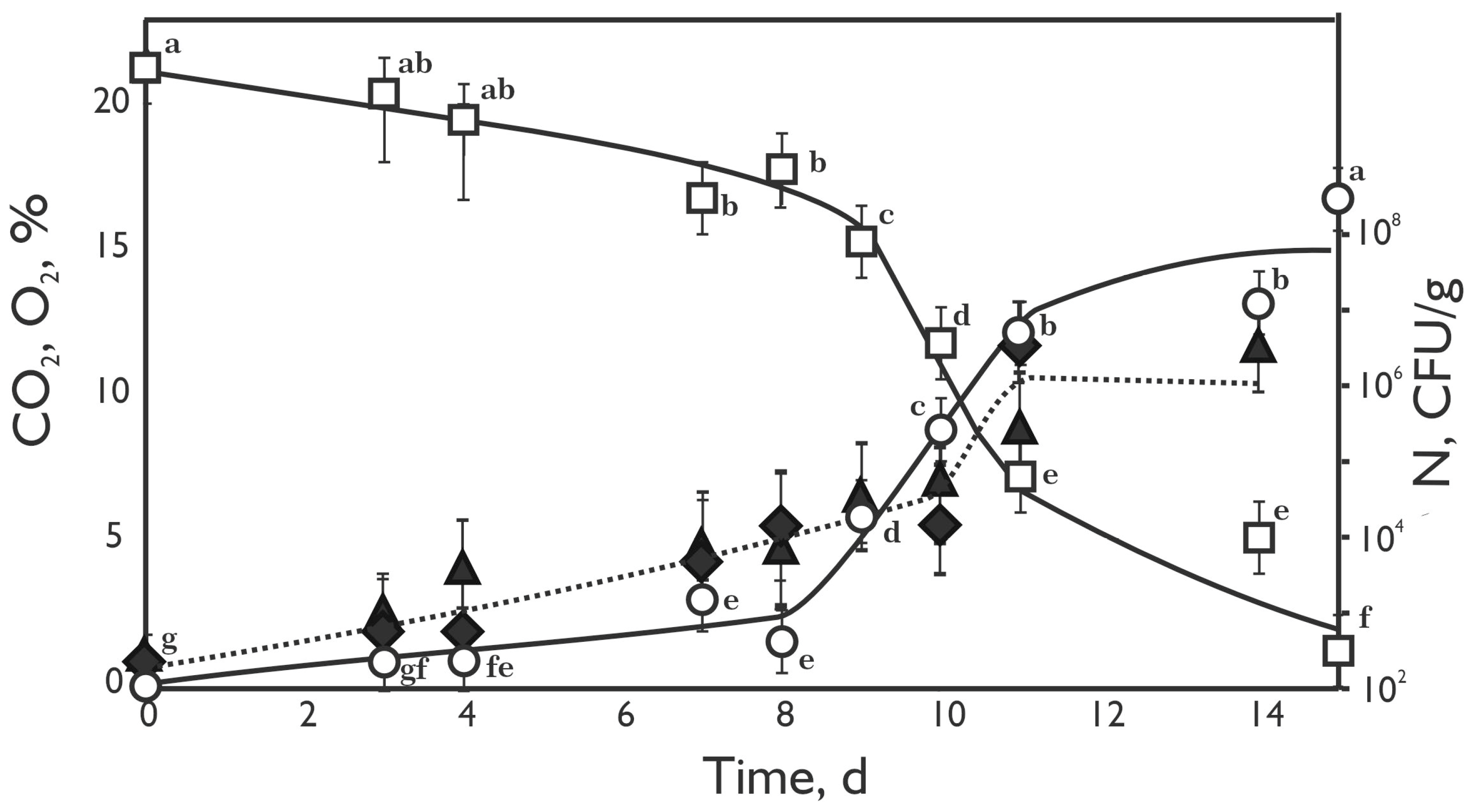
| Detection Gas | ||||
|---|---|---|---|---|
| Characteristic | Carbon Dioxide | Ammonia | Hydrogen Sulphide | Oxygen |
| Sensor type | pyroelectric | Pellistors | Pellistors | electrochemical |
| Detection range | 0–20 vol% | 0–100 ppm | 0–100 ppm | 0–25 vol% |
| Repeatability % of F.S range | ±2% | ±10% | ±2% | ±2% |
| Resolution | 0.1% | 1 ppm | 0.1 ppm | 0.2 vol% |
| Typical Response Time, s | <30 | <60 | <30 | <10 |
| Hydrogen Sulphide, ppm | Ammonia, ppm | |||
|---|---|---|---|---|
| Gas | Concentration | Concentration Equivalent | Concentration | Concentration Equivalent |
| Hydrogen Sulphide | 50 | 50 | 70 | 70 |
| Carbon dioxide | 4000 | 0 | 4000 | 0 |
| Ammonia | 70 | 0 | 70 | 1 |
| Oxygen | 20,900 | 0 | 20,900 | 0 |
| Variables | CO2 | O2 | Lactic Acid Bacteria | Enterobacteriaceae |
|---|---|---|---|---|
| CO2 | 1.00 | −0.99 ** | 0.82 ** | 0.89 ** |
| O2 | <0.001 | 1.00 | −0.85 ** | −0.91 ** |
| Lactic acid bacteria | 0.004 | 0.002 | 1.00 | 0.87 ** |
| Enterobacteriaceae | <0.001 | <0.001 | 0.001 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veličković, A.; Cocola, L.; Fedel, M.; Danilović, B.; De Marchi, M.; Poletto, L.; Savić, D. Application of a Multi-Gas Detector for Monitoring Gas Composition in Minced Beef During Storage. Foods 2024, 13, 3553. https://doi.org/10.3390/foods13223553
Veličković A, Cocola L, Fedel M, Danilović B, De Marchi M, Poletto L, Savić D. Application of a Multi-Gas Detector for Monitoring Gas Composition in Minced Beef During Storage. Foods. 2024; 13(22):3553. https://doi.org/10.3390/foods13223553
Chicago/Turabian StyleVeličković, Aleksandar, Lorenzo Cocola, Massimo Fedel, Bojana Danilović, Massimo De Marchi, Luca Poletto, and Dragiša Savić. 2024. "Application of a Multi-Gas Detector for Monitoring Gas Composition in Minced Beef During Storage" Foods 13, no. 22: 3553. https://doi.org/10.3390/foods13223553
APA StyleVeličković, A., Cocola, L., Fedel, M., Danilović, B., De Marchi, M., Poletto, L., & Savić, D. (2024). Application of a Multi-Gas Detector for Monitoring Gas Composition in Minced Beef During Storage. Foods, 13(22), 3553. https://doi.org/10.3390/foods13223553







