Abstract
The incidence of multiple-organ cancers has recently increased due to simultaneous exposure to various environmental carcinogens. Houttuynia cordata Thunb. (H. cordata) is recognized for its many health benefits, including its anti-cancer properties. The fermentation of its leaves has been shown to significantly enhance the bioflavonoid content and its bioactivities. This study aimed to evaluate the effectiveness of fermented H.cordata leaf (FHCL) extracts against combined carcinogens and investigate the underlying mechanisms. The crude ethanolic extract of FHCL was partitioned to obtain hexane- (HEX), dichloromethane- (DCM), ethyl acetate- (ETAC), butanol- (nBA), and residue fractions. The crude ethanolic extract (200–250 μg/mL) and the DCM fraction (50 μg/mL) significantly reduced NO production in RAW264.7 macrophages. In addition, the crude extract and the DCM and ETAC fractions showed anti-genotoxicity against aflatoxin B1 and 2-amino-3,4-dimethylimidazo [4,5-f]quinoline (MeIQ) in Salmonella typhimurium assays (S9+). Despite demonstrating genotoxicity in the Salmonella mutation assay (with and without S9 activation), oral administration of the crude extract at 500 mg/kg of body weight (bw) for 40 days in rats did not induce micronucleated hepatocytes, indicating that the extract is non-genotoxic in vivo. Moreover, the crude extract significantly decreased Phase I but increased Phase II xenobiotic-metabolizing enzyme activities in the rats. Next, the anti-cancer effects of FHCL were evaluated in a dual-organ carcinogenesis model of the colon and liver in rats induced by 1,2-dimethylhydrazine (DMH) and diethylnitrosamine (DEN), respectively. The crude extract significantly reduced not only the number and size of glutathione S-transferase placental form positive foci in the liver (at doses of 100 and 500 mg/kg bw) but also the number of aberrant crypt foci in rat colons (at 500 mg/kg bw). Furthermore, FHCL significantly reduced the expression of proliferating cell nuclear antigen (PCNA) in the colon (at 100 and 500 mg/kg bw) and liver (at 500 mg/kg bw) of the treated rats. In conclusion, FHCL exhibits significant preventive properties against colon and liver cancers in this dual-organ carcinogenesis model. Its mechanisms of action may involve anti-inflammatory effects, the prevention of genotoxicity, the modulation of xenobiotic-metabolizing enzymes, and the inhibition of cancer cell proliferation. These findings support the use of FHCL as a natural supplement for preventing cancer.
1. Introduction
Multiple primary cancers are defined as the synchronous or metachronous occurrence of two or more primary malignant tumors in the same individual, with an increasing incidence reaching up to 17%, making it a significant concern in oncology [1,2]. The colon and liver are major sites for multiple primary cancers of the digestive system, with incidences of 48.9% and 15.2%, respectively [3]. The occurrence of a first primary colorectal cancer is frequently associated with the development of a second primary cancer, often in the colon and liver. Similarly, primary liver cancer usually leads to secondary primary cancers in organs such as the lung, head and neck, and colorectal regions [4,5]. Environmental and lifestyle factors contribute to more than 90% of cancer incidences [1,6,7], with exposure to carcinogens being a significant risk for the development of multiple cancers [8,9]. Carcinogens like heterocyclic amines, dimethylhydrazine, nitrosamines, and aflatoxin B1 (AFB1) are known to promote colorectal and liver cancers [10,11,12,13]. These carcinogens are metabolized by Phase I xenobiotic enzymes into reactive metabolites that likely cause mutations and initiate oxidative and inflammatory responses, promoting tumor development by enhancing genotoxicity and cell proliferation, and inhibiting apoptosis [14,15,16,17]. Modulating these metabolic enzymes and cellular processes is a promising strategy for cancer prevention, especially for primary cancers induced by environmental carcinogens. However, applying these strategies to prevent multiple cancers is challenging. Natural products often show diminished efficacy in multi-organ cancer models compared to single-carcinogen treatments [18,19,20,21,22]. This highlights the complexity of understanding their full effects in more realistic models of cancer prevention.
Houttuynia cordata Thunb. (H. cordata), a medicinal plant widely used in Asia and Southeast Asia [23], emerges as a promising candidate for cancer prevention. Traditionally, it has been used to treat respiratory, digestive, and skin conditions. Previous studies have demonstrated that the extracts of H. cordata can suppress Phase I enzyme expression in high-fat diet rats, prevent genetic mutations induced by mutagens in Salmonella bacteria, and reduce inflammatory cytokine in lipopolysaccharide (LPS)-induced RAW 264.7 cells [24,25,26]. Moreover, extracts of H. cordata have shown anti-cancer activity against colon (HT29) and liver (HepG2) cancer cell lines and have been found to suppress human hepatocellular carcinoma growth in nude mice models [27,28,29]. It is noteworthy that this plant contains bioactive flavonoids, including rutin, hyperoside, quercetin, and quercitrin [30,31]. These flavonoids have been reported to exert various biological activities, including antioxidant, anti-inflammatory, and anti-cancer effects [32,33].
Fermentation is a traditional technique used in traditional medicine to enhance herbal efficacy and reduce the adverse effects of herbal medicines [34,35]. Notably, the fermentation of H. cordata leaf with Bacillus strains has been shown to enhance the content of key bioactive flavonoids, including rutin, quercetin, and quercitrin, which increase its anti-cancer properties [36]. The bacterial fermented H. cordata leaf has shown significant anti-cancer effects by inducing apoptosis and inhibiting cell proliferation in colon and liver cancer cell lines, as well as by reducing inflammation [37,38,39]. Overall, fermented H. cordata leaf (FHCL) shows promising potential as effective cancer chemopreventive agents.
Despite these favorable findings, there is still a significant gap in understanding the chemopreventive effects of FHCL in animal models, particularly in models of multiple primary cancers induced by environmental carcinogens. Therefore, this study aims to investigate the effects of the ethanolic extract of FHCL in a dual-carcinogenesis model involving 1,2-dimethylhydrazine (DMH)-induced colon and diethylnitrosamine (DEN)-induced liver carcinogenesis. Moreover, this study explores the possible mechanisms of cancer preventive effects, including the modulation of xenobiotic enzymes, anti-inflammatory activity, and anti-genotoxic properties.
2. Materials and Methods
2.1. Chemicals and Reagents
Lipopolysaccharides (LPS) from Escherichia coli O111, Griess reagent, and 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) reagent were obtained from Sigma-Aldrich (St. Louis, MO, USA). Resorufin, ethoxyresorufin, uridine 5′- diphosphate -glucuronic acid (UDP-GA), para-nitrophenol (p-NP), and 3,3′-diaminobenzidine (DAB) were also sourced from Sigma-Aldrich. Standard mutagens, including aflatoxin B1 (AFB1), sodium azide (NaN3), and diethylnitrosamine (DEN), were purchased from the same supplier. Additional mutagens, such as 2-aminoanthracene (2-AA), 2-(2-furyl)-3-(5-nitro-2-furyl)-acrylamide (AF-2), and 2-amino-3,4-dimethylimidazo[4,5-f]quinoline (MeIQ), along with the substrate methoxyresorufin, were obtained from Wako Pure Chemicals (Osaka, Japan). Dimethylhydrazine (DMH) was sourced from the Tokyo Chemical Industry (Tokyo, Japan). Collagenase type IV and 4′,6-diamidino-2-phenylindole (DAPI) were acquired from Invitrogen Corp. (Carlsbad, CA, USA). Additionally, 1-chloro-2,4-dinitrobenzene (CDNB) and bovine serum albumin (BSA) were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Dulbecco’s Modified Eagle Medium (DMEM), penicillin-streptomycin, and fetal bovine serum (FBS) were obtained from Gibco/Invitrogen Crop. (Grand Island, NY, USA). The avidin–biotin–horseradish peroxidase complex (ABC) kit and Vectastain Elite ABC kit (Universal) were obtained from Vector Laboratories Inc. (Burlingame, CA, USA), while the Apoptag detection kit was purchased from Merck Millipore (Darmstadt, Germany). All other chemicals used in this study were of analytical or HPLC grade.
2.2. Preparation of Fermented Houttuynia cordata Leaf (FHCL) Extract and Its Fractions
Houttuynia cordata Thunb. (Queen Sirikit Botanic Garden Herbarium: QBG. No. 131046) was cultivated and harvested in Chai Badan District, Lopburi, Thailand. The harvested plants underwent fermentation and quality control procedures according to the guidelines and regulations managed by Prolac (Thailand) Co., Ltd. (Lamphun, Thailand). In brief, approximately 400 kg of H. cordata leaf was harvested and fermented with 70 kg of sugarcane powder, 150 L of in-house bacteria culture, and 800 L of water [40]. Prolac Co., Ltd. generously provided the fermented Houttuynia cordata leaf (FHCL) powder, which was extracted twice with 70% ethanol over two days and then freeze-dried to obtain the crude extract. This extract was dissolved in deionized (DI) water for in vivo studies.
The crude extract was then partitioned sequentially using hexane, dichloromethane, ethyl acetate, and butanol in a 1:1 ratio, yielding hexane (HEX), dichloromethane (DCM), ethyl acetate (ETAC), butanol (nBA), and residue fractions. All fractions were evaporated, freeze-dried, and dissolved in dimethyl sulfoxide (DMSO) for in vitro studies.
2.3. The Determination of Phytochemicals in the Crude Extract and Its Fractions
The contents of total phenolics, total flavonoids, and total hydrolysable tannins of crude extract and its fractions were analyzed spectrophotometrically using the Folin–Ciocalteu colorimetric method, aluminum chloride colorimetric method, and potassium iodate method, respectively [41,42]. The HPLC technique was employed to analyze specific phenolics and flavonoids in all fractions using reverse-phase HPLC with an Agilent ZORBAX Eclipse Plus C18 column (4.6 × 250 mm, 5 μM) (Agilent Technologies, Santa Clara, CA, USA) as described in a previous study [43]. The concentration of each component in the sample was quantified using a standard curve for each standard compound, including gallic acid, protocatechuic acid, 4-hydroxybenzoic acid, chlorogenic acid, vanillic acid, syringic acid, p-coumaric acid, ferulic acid, ellagic acid, trans-cinnamic acid, catechin, epicatechin, rutin, quercetin, luteolin, apigenin, quercitrin, myricetin, naringenin, kaempferol, and isorhamnetin.
2.4. Cell Culture and Culture Condition
RAW264.7 macrophage cells (ATCC TIB-71, Manassas, VA, USA) were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. The cells were maintained at 37 °C in a humidified atmosphere with 5% CO2.
2.5. A Cytotoxicity Assessment of the Crude Extract and Its Fractions on RAW 264.7 Macrophages
The cytotoxicity of the crude extract and its fractions on RAW 264.7 macrophages was assessed using an MTT assay [44]. RAW 264.7 cells were seeded at a density of 2 × 105 cells/mL and treated with test samples at concentrations of up to 500 μg/mL for 24 h. Following the treatment, the cells were incubated with the MTT reagent at a final concentration of 0.5 mg/mL for two hours to assess cell viability. The formazan crystal was then dissolved with dimethyl sulfoxide (DMSO). The absorbance of the solubilized formazan was then measured at 540 nm and 630 nm. The results were reported as IC20, and a concentration below IC20 was considered non-cytotoxic. Concentrations of the fractions that did not exceed their IC20 values were used for further investigation of their potential effects on LPS-induced NO production in RAW 264.7 macrophages.
2.6. The Determination of the Anti-Inflammatory Activity of the Crude Extract and Its Fractions
The anti-inflammatory activity of the crude extract and its fractions was determined using a nitric oxide (NO) scavenging assay [44]. RAW 264.7 cells were seeded at a density of 2 × 105 cells/mL and treated with various concentrations of the fractions. After two hours of treatment, the cells were exposed to 1 μg/mL of LPS for 24 h, and then the supernatant was collected to evaluate the NO concentration using Griess reagent. The NO concentration was measured at 550 nm and quantified using a sodium nitrite standard curve. The results were expressed as the relative NO production to the LPS-induced NO alone.
2.7. An Evaluation of the In Vitro Genotoxicity and Anti-Genotoxicity of the Crude Extract and Its Fractions Using an Ames Test
The genotoxicity and anti-genotoxicity tests of the crude extract and its fractions were performed using Salmonella typhimurium strains TA 98 and TA100 in both the presence (+S9) and absence (−S9) of metabolic activation as described previously [45]. The bacterial tester strains were kindly provided by Dr. Kei-Ichi Sugiyama, National Institute of Health, Tokyo, Japan.
For the genotoxicity assay, various concentrations of each fraction were mixed with the bacteria in the presence (+S9) or absence (−S9) of the S9 mixture. This mixture was incubated at 37 °C for 20 min in a shaking water bath. Subsequently, top agar containing L-histidine and D-biotin was added and poured onto minimal agar plates, which were then incubated at 37 °C for 48 h. The mutagens 2AA and AF-2 were used as positive controls in the presence and absence of metabolic activation, respectively. The number of revertant colonies was counted and expressed as a mutagenic index (MI), which is the ratio of the number of revertant colonies to the control. A sample was identified as mutagenic if the MI exceeded two.
For the anti-genotoxicity test, non-cytotoxic doses of each fraction were mixed with the bacteria, mutagen, and either the +S9 or −S9 buffer. The mixture was incubated at 37 °C for 20 min and then poured onto minimal agar plates, followed by further incubation at 37 °C for 48 h. AFB1 and MeIQ were used as indirect mutagens for TA98 and TA100, respectively, under metabolic activation conditions. AF-2 and NaN3 were used as direct mutagens for TA98 and TA100, respectively, under conditions without metabolic activation. Anti-genotoxic activity was expressed as the average number of His+ revertant colonies per plate and as a percentage of inhibition.
2.8. Animals
All Wistar rats used in this study were obtained from Nomura Siam International Co., Ltd. in Bangkok, Thailand. They were housed in the Animal House at the Faculty of Medicine, Chiang Mai University, under standard environmental conditions throughout the experiments. The oral dosage was determined from the results of an acute oral toxicity test, which was conducted following OECD guideline No. 425 [46]. The experimental protocols for rats were approved by the Animal Ethics Committee of the Faculty of Medicine, Chiang Mai University (Protocol No. 07/2563, approved on 17 April 2020, and Protocol No. 38/2563, approved on 26 July 2020). All procedures were performed in compliance with relevant guidelines and regulations, adhering to the recommendations outlined in the ARRIVE guidelines.
2.9. In Vivo Genotoxicity Assessment Using Micronucleus Test
The genotoxicity of crude FHCL was assessed in vivo by the micronucleus test, following a modified method previously described in [47]. Four-week-old male Wistar rats were randomly divided into two groups, with five rats per group. Group 1 received deionized water and served as the negative control, while Group 2 was fed crude extract by oral gavage at a concentration of 500 mg/kg of body weight (bw) for 40 days. On day 41, all rats underwent partial hepatectomy to accelerate the proliferation of mutated cells. On day 43, the rats were anesthetized, and hepatocytes were isolated using a two-step collagenase perfusion method as described previously [48]. The isolated hepatocytes were stained with DAPI and counted under a fluorescent microscope (Olympus AX70, Tokyo, Japan). Micronucleated cells were scored according to the criteria described by Cliet et al. [49].
2.10. Measurement of Xenobiotic-Metabolizing Enzyme Activities in the Livers of Rats Receiving Crude FHCL
The remaining rat liver samples from Section 2.9 were homogenized to prepare cytosolic and microsomal lysates by following a previously described method [50]. The protein concentrations of these lysates were measured using the Lowry method in the comparison of a standard curve of bovine serum albumin [51].
The activities of cytochrome P450 (CYP), 1A1, and 1A2 were assessed using ethoxyresorufin-O-deethylation (EROD) and methoxyresorufin-O-demethylation (MROD) assays, respectively. Each substrate was mixed with isolated liver microsomes, NADPH, and magnesium chloride. The production of resorufin, the end-product of both assays, was measured by Agilent BioTek Synergy H4 Hybrid Microplate Reader (Winooski, VT, USA) at excitation and emission wavelengths of 520 nm and 590 nm, respectively. The enzyme activities were calculated from the linear equation of a resorufin standard curve and expressed as units per mg of protein [52].
The activity of CYP2E1 was measured by quantifying p-nitrocatechol formation from p-nitrophenol (p-NP). The liver microsomes were pre-incubated with p-NP, NADPH, magnesium chloride, D-glucose-6 phosphate, and glucose-6 phosphate dehydrogenase. The reaction was then stopped with 10% TCA and centrifuged at 10,000 g for 5 min. The supernatant was neutralized with NaOH, and the end-product, 4-nitrocatechol, was monitored by Agilent BioTek Synergy H4 Hybrid Microplate Reader (Winooski, VT, USA) at 535 nm. The CYP2E1 activity in each sample was calculated from the linear equation generated by a 4-nitrocatechol standard curve and expressed as units per mg of protein [50].
The activity of CYP3A2 was measured using the erythromycin N-demethylation (ENDM) assay. CYP3A2 activity was determined using an Agilent BioTek Synergy H4 Hybrid Microplate Reader (Winooski, VT, USA), based on a formaldehyde standard curve at a wavelength of 405 nm, and expressed as units per mg of protein [52].
Glutathione S-transferase (GST) activity was assessed using 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate. Liver cytosol was added to a reaction mixture containing phosphate buffer and glutathione. After 90 s, the absorbance of the CDNB-GSH conjugate was measured by Agilent BioTek Synergy H4 Hybrid Microplate Reader (Winooski, VT, USA) at 340 nm. The activity was calculated using a molar extinction coefficient of 9.6 M−1 cm−1 and expressed as units per mg of protein [52].
UDP-glucuronosyl transfersase (UGT) activity was analyzed by measuring the conjugation of p-NP. Liver microsomes were pre-incubated with a reaction mixture consisting of Tris, magnesium chloride, p-NP, and UDP-glucuronic acid (UDP-GA). The reaction was stopped with 10% TCA, followed by centrifugation at 10,000 rpm for 5 min. The supernatant was mixed with NaOH, and the conjugated p-NP level was quantified by Agilent BioTek Synergy H4 Hybrid Microplate Reader (Winooski, VT, USA) at 405 nm and calculated using a molar extinction coefficient of 9.6 M−1 cm−1 and expressed as units per mg of protein [52].
2.11. Anti-Carcinogenicity Assessment of Crude FHCL Using a Dual-Organ Model in Rats
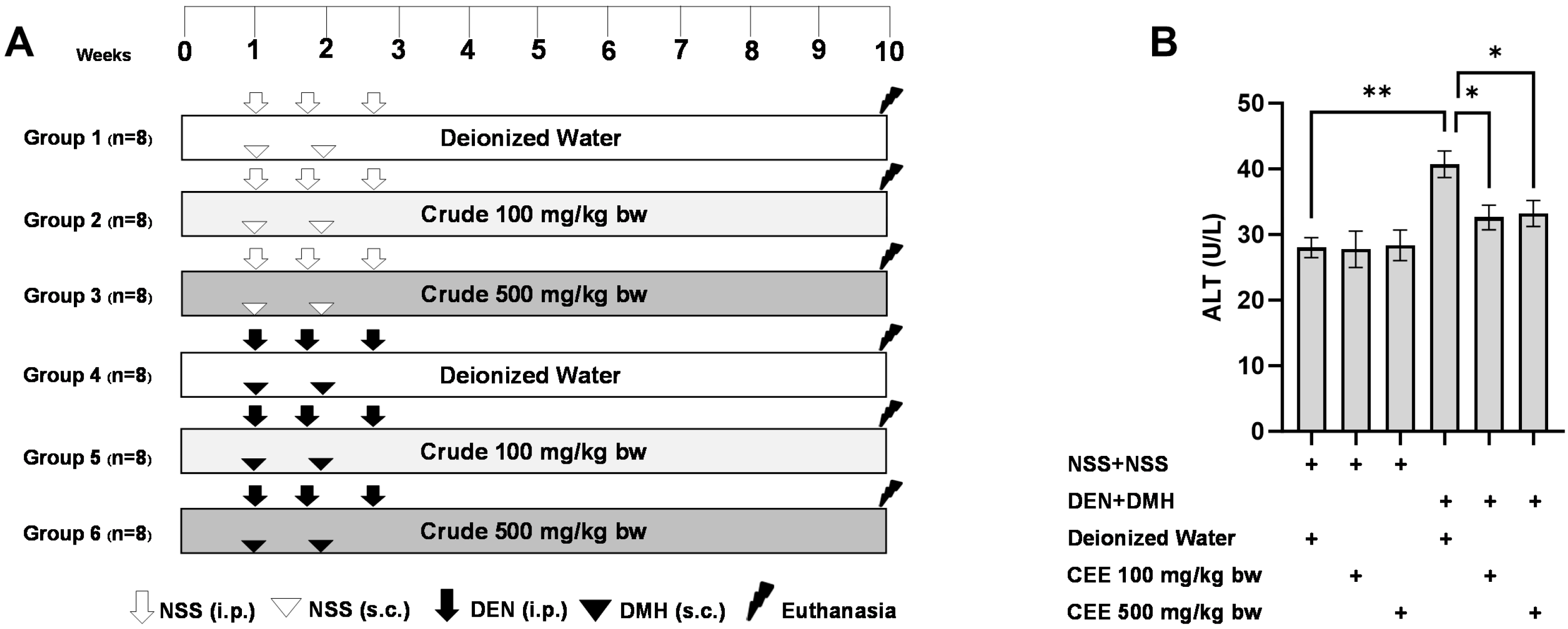
The anticarcinogenic effects of crude FHCL ethanolic extract were examined using a dual-organ carcinogenicity model following a methodology described previously [18,53]. Four-week-old male rats were randomly assigned to six groups, each comprising eight rats. To induce colorectal carcinogenesis, Groups 4 to 6 were injected subcutaneously (s.c.) with 40 mg/kg bw of DMH on days 7 and 14. These groups also intraperitoneally (i.p.) received 100 mg/kg bw of DEN on days 7, 11, and 18 to induce hepatocarcinogenesis. Conversely, Groups 1 to 3 were administered injections of 0.9% normal saline (NSS) either subcutaneously or intraperitoneally instead of DMH and DEN, respectively.
For the treatment, Groups 1 and 4, serving as vehicle controls, were administered deionized water via gavage five days a week. Groups 2 and 5 received 100 mg/kg bw of the crude extract via gavage five days a week, whereas Groups 3 and 6 received 500 mg/kg bw of the crude extract via gavage. At the end of the ten-week study, the rats were humanely euthanized. Blood samples were collected for serum alanine aminotransferase (ALT) level measurement, which was conducted by the Small Animal Hospital at Chiang Mai University, Thailand. Additionally, liver and colon tissues were collected to evaluate preneoplastic lesions and to investigate the expression of cell proliferation- and death-associated markers.
2.12. Determination of Preneoplastic Lesions in Liver and Colon Tissues
Hepatic preneoplastic lesions were identified by detecting glutathione S-transferase placental form (GST-P)-positive foci using an immunohistochemical staining technique [54]. Initially, liver sections were deparaffinized and immersed in a solution containing 3% hydrogen peroxide (H2O2) and skimmed milk to block endogenous peroxidase activity and reduce non-specific binding, respectively. Following this, the slides were incubated with a rabbit polyclonal anti-rat GST-P antibody (MBL, Nagoya, Japan). An avidin–biotin complex (ABC) kit and the substrate diaminobenzidine (DAB) were then used to develop brown coloration, followed by counterstaining with hematoxylin. The numbers and sizes of GST-P-positive foci were quantified using the Leica Application Suite (LAS) Interactive Measurement program (Leica Microsystems, Wetzlar, Germany).
Colonic preneoplastic lesions were identified by staining aberrant crypt foci (ACF) with methylene blue [54]. Formalin-fixed colons were immersed in a 2% methylene blue solution for staining. The total number of aberrant crypts per rat and the number of aberrant crypts per focus were then evaluated using a light microscope (Nikon Corporation, Tokyo, Japan).
2.13. Determination of Cell Proliferation Marker Using Immunohistochemical Staining
Immunohistochemical staining for proliferating cell nuclear antigen (PCNA), a cell proliferation marker, was conducted following a previously described method [18]. Liver and colon sections were first deparaffinized and then treated with 10 mM citrate buffer (pH 6.0) using autoclaving for antigen retrieval. The sections were then incubated with monoclonal mouse anti-rat PCNA antibodies (BioLegend, San Diego, CA, USA), followed by treatment with biotinylated secondary antibodies. An Elite avidin–biotin complex kit was used in combination with DAB for visualization, with hematoxylin serving as a counterstain. The quantification of PCNA-positive cells was performed using a light microscope (Leica Microsystems, Wetzlar, Germany).
2.14. Determination of Apoptotic Cells Using Terminal Deoxynucleotidyltransferase (TdT)–dUTP Nick End Labeling (TUNEL) Assay
Apoptotic cells in the liver and colon were identified using an ApopTag Peroxidase in situ kit as previously described by Thumvijit et al., 2014 [55]. The procedures were carried out according to the kit instructions, with color development achieved through the application of DAB. The number of apoptotic cells was then counted using a light microscope (Leica Microsystems, Wetzlar, Germany).
2.15. Statistics Analysis
Data are presented as means ± standard deviation (SD). Statistical comparisons between groups in the micronucleus test were performed using the independent Student’s t-test. For other analyses, a one-way analysis of variance (ANOVA) was conducted, followed by a Bonferroni post hoc analysis using GraphPad Prism 9.0 software (GraphPad Software, Boston, MA, USA). Differences were considered significant when p ≤ 0.05. The significance level for each result is indicated in the corresponding graphs and tables.
3. Results
3.1. Phytochemical Components in FHCL and Its Fractions
The ethanolic extract of FHCL was sequentially partitioned, yielding hexane (HEX), dichloromethane (DCM), ethyl acetate (ETAC), butanol (nBA), and residue fractions, with the percentage yields detailed in Table 1. The residue had the highest yield, followed by nBA, HEX, ETAC, and DCM, respectively. A spectrophotometric analysis showed that the crude FHCL extract was rich in total phenolics, flavonoids, and hydrolysable tannins, with 21.09, 16.58, and 4.05 mg/g of dried powder, respectively. Among the fractions, ETAC had the highest phenolic (25.33 mg/g of crude) and flavonoid (23.25 mg/g of crude) contents, while both nBA (11.43 mg/g of crude) and ETAC (11.33 mg/g of crude) had the highest levels of total hydrolysable tannins. These results suggest that flavonoids were the predominant polyphenols in the crude extract and its fractions. An HPLC analysis further revealed that the crude extract contained flavonoids such as rutin (0.644 mg/g of dried powder), quercetin (0.088 mg/g of dried powder), and quercitrin (0.050 mg/g of dried powder), with quercitrin being the most abundant. In contrast, phenolics including protocatechuic acid, chlorogenic acid, vanillic acid, and p-coumaric acid were present in lower amounts and were below detectable levels using HPLC. Among the fractions, protocatechuic acid (1.741 mg/g of crude), chlorogenic acid (2.897 mg/g of crude), and rutin (4.934 mg/g of crude) were noted for their high concentrations in the ETAC fraction. Yet, the DCM fraction was rich in vanillic acid (0.048 mg/g of crude), p-coumaric acid (0.508 mg/g of crude), and quercetin (2.153 mg/g of crude) (Table 1).

Table 1.
Percentage yield and analysis of phytochemical constituents of crude ethanolic extract of FHCL and its fractions determined by spectrophotometry and HPLC.
3.2. Anti-Inflammatory Activities of FHCL Extract and Its Fractions on Lipopolysaccharide (LPS)-Stimulated RAW 264.7 Macrophage Cell Lines
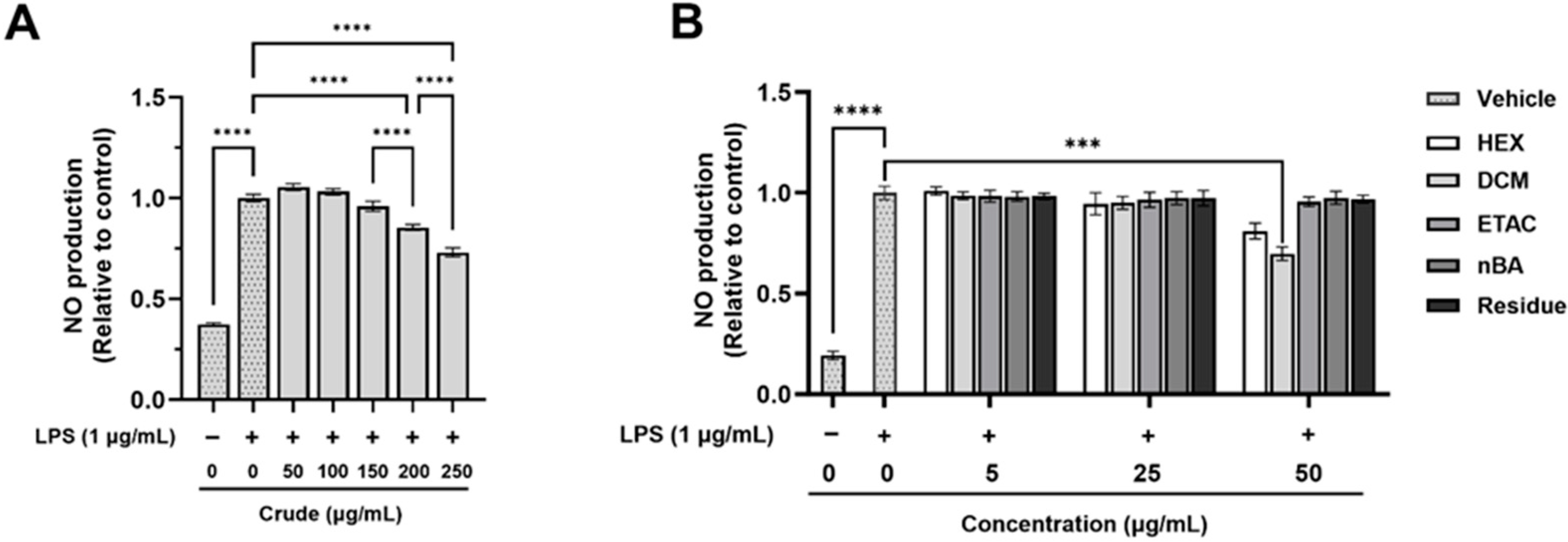
The anti-inflammatory effects of the crude extract and its fractions were evaluated using non-toxic doses (Table S1) on LPS-induced nitric oxide (NO) production in RAW 264.7 macrophages. The stimulation of RAW 264.7 macrophage cells with LPS led to a significant increase in NO production compared to non-LPS-treated cells (Figure 1). However, pre-treatment with the crude extract at 200 and 250 µg/mL significantly reduced LPS-induced NO production by 14.55% and 26.89%, respectively (Figure 1A). The DCM fraction at a concentration of 50 µg/mL also significantly inhibited LPS-induced NO production by 30.20% compared to cells treated with LPS alone; however, the other fractions exhibited no significant changes (Figure 1B). Interestingly, calculations based on the % yield of the crude extract indicate that a 250 µg/mL dose of the crude extract contains approximately 10 µg/mL of the DCM fraction, which is below the effective dose for DCM. The results suggest that the crude extract is more effective than the individual fractions, as it can reduce NO production at much lower concentrations. These findings highlight the strong efficacy of the crude FHCL extract in preventing inflammation by significantly lowering NO production from inflammatory cells. Additionally, both the crude extract and the DCM fraction contain bioactive compounds that contribute to their anti-inflammatory effects.
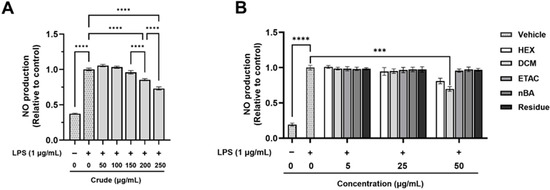
Figure 1.
The effect of FHCL crude ethanolic extract (A) and its fractions (B) on nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages was assessed using Griess reagents. The results are presented as the mean ± standard deviation from three independent experiments. Significance levels are indicated as *** p ≤ 0.001 and **** p ≤ 0.0001. HEX: hexane fraction; DCM: dichloromethane fraction; ETAC: ethyl acetate fraction; nBA: butanol fraction.
3.3. In Vitro Genotoxic Properties of FHCL Crude Extract and Its Fractions
The genotoxic properties of the crude FHCL extract and its fractions were evaluated using the Ames test (Salmonella mutation assay) in bacteria strains TA98 and TA100 with and without metabolic activation (S9 mix). As shown in Table 2, the results reveal that the crude extract caused a two-fold increase in the number of revertant colonies (MI ≥ 2) at concentrations of 1 and 5 µg/plate in both −S9 and +S9 conditions for TA98, indicating that genotoxicity occurs independently of metabolic activation. In TA100, genotoxicity (MI ≥ 2) was observed only at 5 µg/plate of the crude extract under the +S9 condition, while no genotoxicity (MI < 2) was detected under the −S9 condition. This suggests that genotoxic effects are more pronounced in TA98, particularly under the +S9 condition. Interestingly, the DCM and ETAC fractions exhibited MI ≥ 2 at the lowest concentration (0.2 µg/plate) in TA98 under both − and +S9 conditions, with increasing doses resulting in higher MI values. In TA100 under the +S9 condition, ETAC also exhibited MI ≥ 2 at 0.2 µg/plate, whereas DCM displayed this effect starting at 1 µg/plate. However, the genotoxic effect diminished at lower concentrations (0.2 µg/plate for DCM, and 0.2 and 0.1 µg/plate for ETAC) in TA100 under the −S9 condition. Additionally, the HEX and nBA fractions demonstrated MI ≥ 2 only at the highest concentration (5 µg/plate) in TA98. In contrast, the residue fraction showed no genotoxic properties in TA98 or TA100 under both − and +S9 conditions. These findings suggest that all fractions, except the residue, can cause genotoxicity in Salmonella bacteria regardless of the requirement for metabolic activation, with more pronounced effects at higher doses. Next, these genotoxic effects were further validated by performing subsequent animal experiments.

Table 2.
Genotoxicity of FHCL crude ethanolic extract and its fractions using Salmonella mutation assay.
3.4. Anti-Genotoxicity Properties of FHCL Crude Extract and Its Fraction in Salmonella typhimurium
The anti-genotoxic properties of the FHCL crude extract and its fractions were evaluated using Salmonella typhimurium against standard mutagens (Table 3). In the presence of metabolic activation (+S9), significant reductions in revertant colonies of both TA98 and TA100 exposed to aflatoxin B1 (AFB1) and 2-amino-3,4-dimethylimidazo[4,5-f]quinoline (MeIQ) were observed in the groups treated with the crude extract and many fractions, including HEX, DCM, and ETAC, at concentrations ≥ 0.2 mg/plate, indicating anti-genotoxic effects against indirect mutagens. However, their effectiveness likely declined at higher concentrations. The nBA fraction also showed anti-genotoxicity against AFB1 and MeIQ-induced mutagenesis at concentrations ≥ 1 mg/plate, while the residue fraction had no effect. Notably, the DCM fraction exhibited significant reductions in revertant colonies in both strains of bacteria at a lower concentration (0.04 mg/plate) compared to other fractions, demonstrating the highest anti-genotoxic potential among those tested. However, 0.04 mg/plate of the DCM fraction is equivalent to 1 mg/plate of the crude extract based on the % yield calculation. The crude extract at 1 mg/plate achieved 88.1% inhibition, while the DCM fraction at 0.04 mg/plate showed 66.8% inhibition, highlighting the stronger anti-genotoxic effect of the crude extract over its fractions. In contrast, in the absence of metabolic activation (−S9), none of the FHCL fractions demonstrated anti-genotoxic effects against AF-2 and NaN3. These findings suggest that the crude and DCM fractions of FHCL contain anti-mutagenic compounds that are capable of blocking gene mutations induced by indirect mutagens. In addition, these inhibitory effects of the extracts may be attributed to their modulation of xenobiotic-metabolizing enzymes.

Table 3.
Antimutagenicity against direct- and indirect-acting mutagens of crude ethanolic extract of FHCL and its fractions using salmonella mutation assay.
3.5. In Vivo Genotoxicity Assessment of FHCL Crude Extract
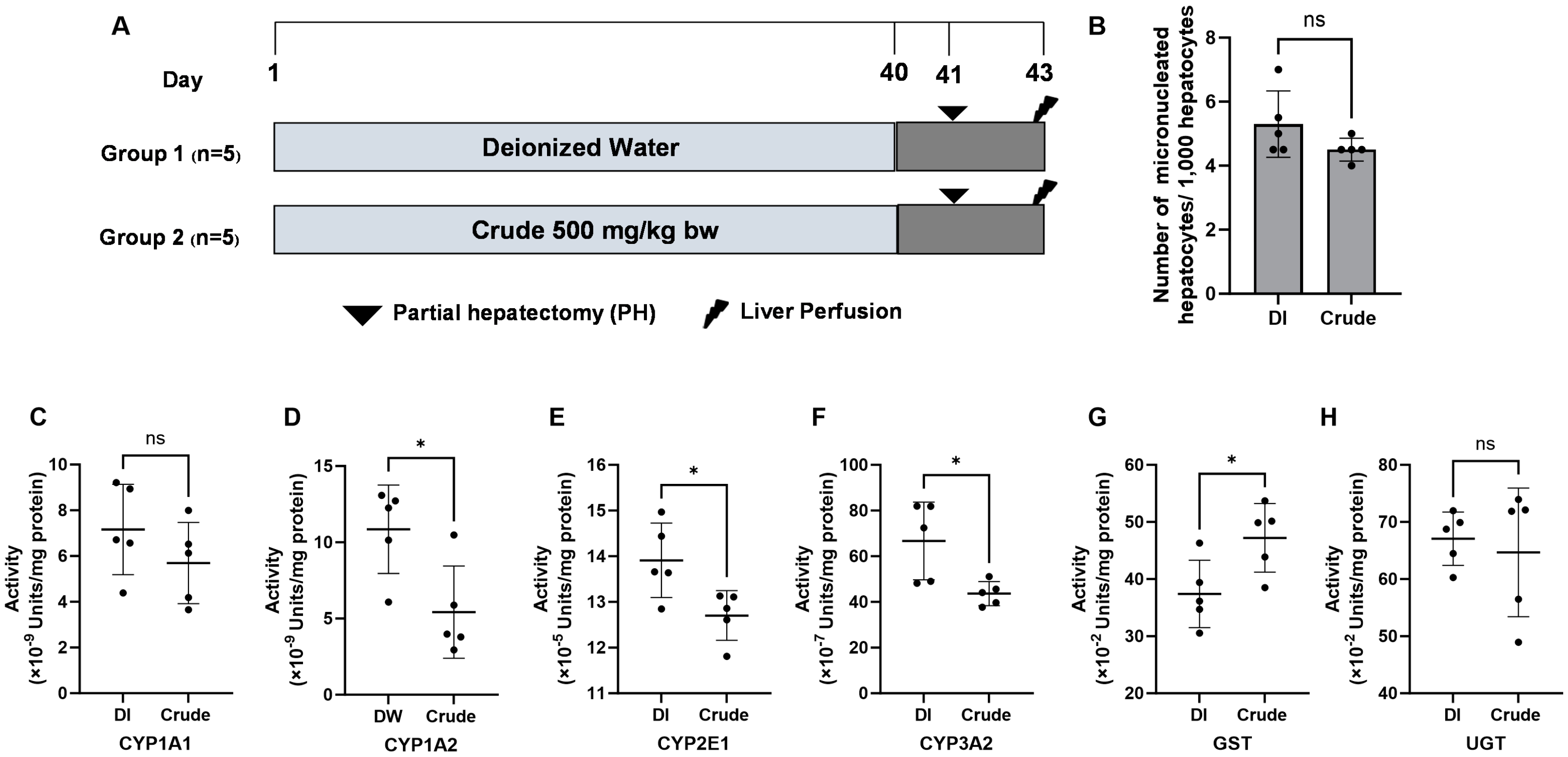
While the FHCL crude extract exhibited the genotoxic potential in bacteria, this study further assessed toxicity using the micronucleus test in an animal model (Figure 2A). The acute toxicity test revealed that the LD50 of the crude extract exceeded 5000 mg/kg bw. Based on OECD guideline No. 425, we chose a dose of 500 mg/kg bw (a 10-fold reduced dose) for subsequent experiments. The micronucleus test indicated no significant differences in the number of micronucleated hepatocytes in rats administered 500 mg/kg bw of crude extract compared to the non-treated control groups (Figure 2B), suggesting that the extract is non-genotoxic in vivo.
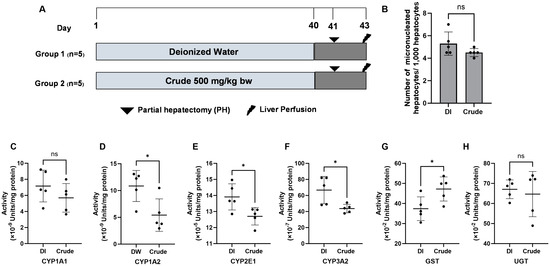
Figure 2.
The genotoxicity protocol. (A) The effect of crude FHCL ethanolic extract on micronucleated hepatocytes (B) and the activities of xenobiotic-metabolizing enzymes were evaluated in rat liver. Deionized (DI) water was used as the negative control. The enzymes studied included Phase I enzymes CYP1A1 (C), CYP1A2 (D), CYP2E1 (E), and CYP3A2 (F) and Phase II enzymes glutathione S-transferases (GST) (G) and UDP-glucuronosyltransferase (UGT) (H). Specific assays were performed for each enzyme. The results are presented as the mean ± standard deviation. Statistical significance was indicated by * (p ≤ 0.05) when compared to the vehicle control group. “ns” denotes non-significant differences (p > 0.05).
3.6. Effect of FHCL Crude Extract on Xenobiotic-Metabolizing Enzymes Activities in Rat Livers
Xenobiotic-metabolizing enzymes are essential for detoxifying and eliminating harmful foreign compounds, known as xenobiotics; however, some metabolites of these xenobiotics can inadvertently lead to genotoxicity. Next, we investigated whether the FHCL crude extract alters the activities of Phase I and Phase II xenobiotic-metabolizing enzymes in rat liver. The crude extract at 500 mg/kg bw significantly reduced the activities of Phase I enzymes, including CYP1A2, CYP2E1, and CYP3A2, while decreased activity was also noted for CYP1A1 (Figure 2C–F). In contrast, the crude extract significantly increased the activity of the Phase II enzyme glutathione S-transferases (GST), but not UDP-glucuronosyltransferase (UGT) (Figure 2G,H). The results highlight the potential of the extract to reduce chemical-induced genotoxicity by modulating xenobiotic-metabolizing enzyme activities in the liver.
3.7. The Anti-Carcinogenic Potential of FHCL Crude Extract in a Dual-Organ Carcinogenesis Model in Rats
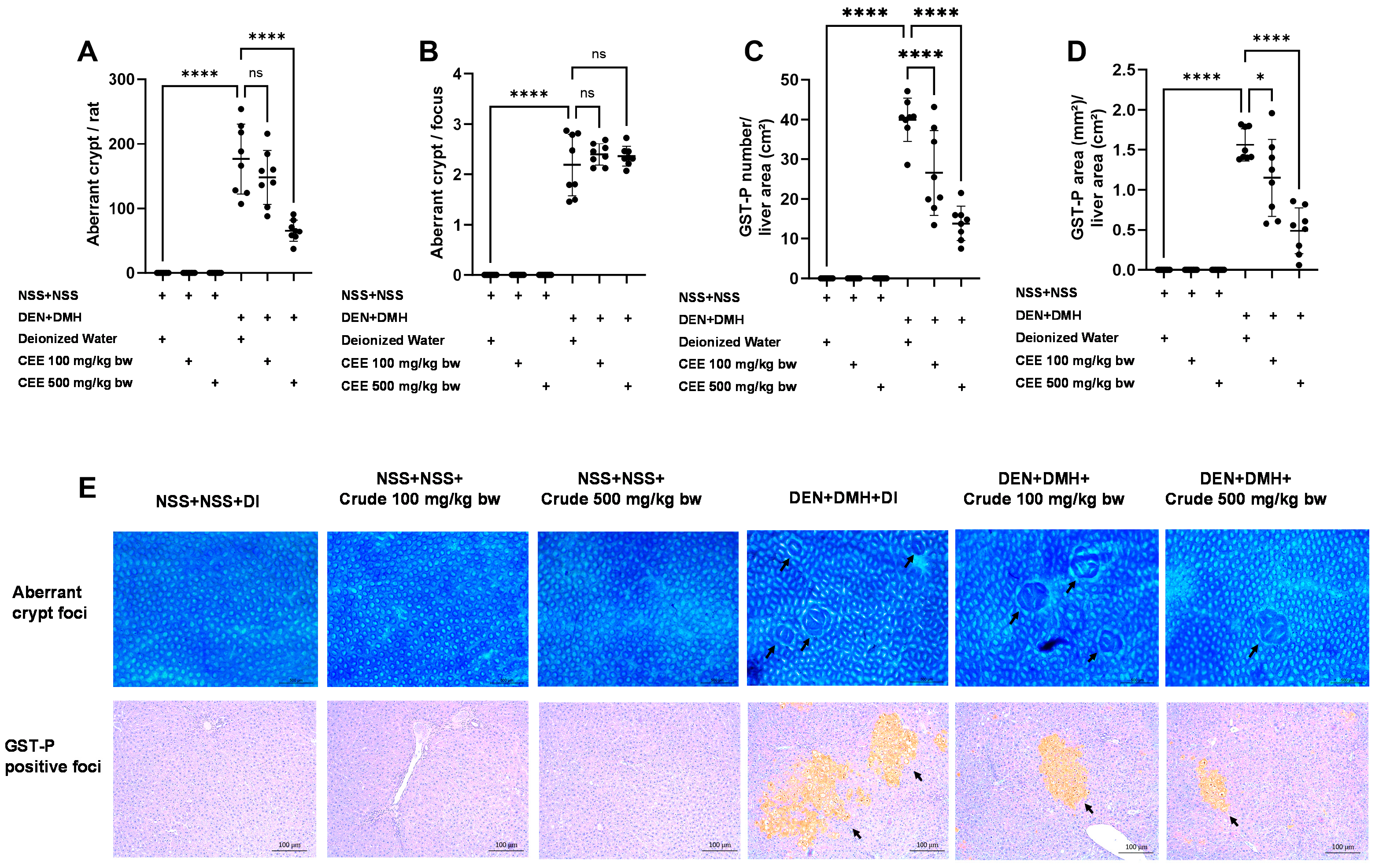
Since the crude extract exhibited the most effective anti-carcinogenic effects in vitro, its ability to reduce preneoplastic lesions in rat colons and livers was evaluated using a DMH- and DEN-induced carcinogenesis model (Figure 3A). Exposure to these carcinogens resulted in marked increases in serum alanine aminotransferase (ALT) levels and number of preneoplastic lesions, including aberrant crypt foci (ACF) in the colon and glutathione S-transferase placental form (GST-P) in the liver, compared to the group receiving normal saline (NSS) alone (Figure 3 and Figure 4). Administration of the crude extract at doses of 100 and 500 mg/kg bw in the rats exposed to carcinogens significantly lowered the serum ALT levels compared to the untreated group exposed to the same carcinogens (Figure 3B), indicating a protective effect against carcinogen-induced liver damage. Furthermore, treatment at 500 mg/kg bw significantly reduced the number of colonic aberrant crypts by over 60%, without affecting their size, compared to the control group treated with carcinogens (Figure 4A,B). Interestingly, crude extract at concentrations of 100 and 500 mg/kg bw significantly decreased either the number or the area of GST-P-positive foci by more than half in the liver samples compared to the carcinogen-treated control group (Figure 4C,D). These results suggest that FHCL is effective in controlling the initiation of lesion growth in the colon and liver following carcinogen exposure. However, its effectiveness in preventing the progression of lesions seems to be restricted to the liver during the early stages of carcinogenesis. Importantly, the rats treated with the crude extract at doses of up to 500 mg/kg bw did not cause any spontaneous preneoplastic formation in the colons and livers, indicating that the extract is non-carcinogenic to these organs.
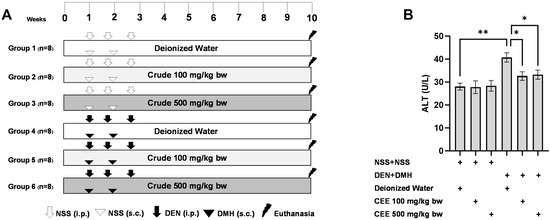
Figure 3.
The study protocol for the dual-organ carcinogenesis model, including the timeline for administering carcinogens (DEN and DMH) and crude FHCL extract in rats (A). The serum alanine aminotransferase (ALT) levels in rats (B). Normal saline (NSS) was used as the control for the carcinogen-treated group, while deionized (DI) water served as the control for the crude extract treatment. The data are presented as the mean ± standard deviation (n = 8). Statistical significance is indicated by * (p ≤ 0.05) and ** (p ≤ 0.01).
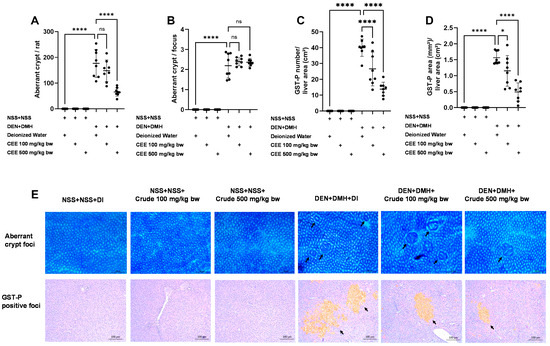
Figure 4.
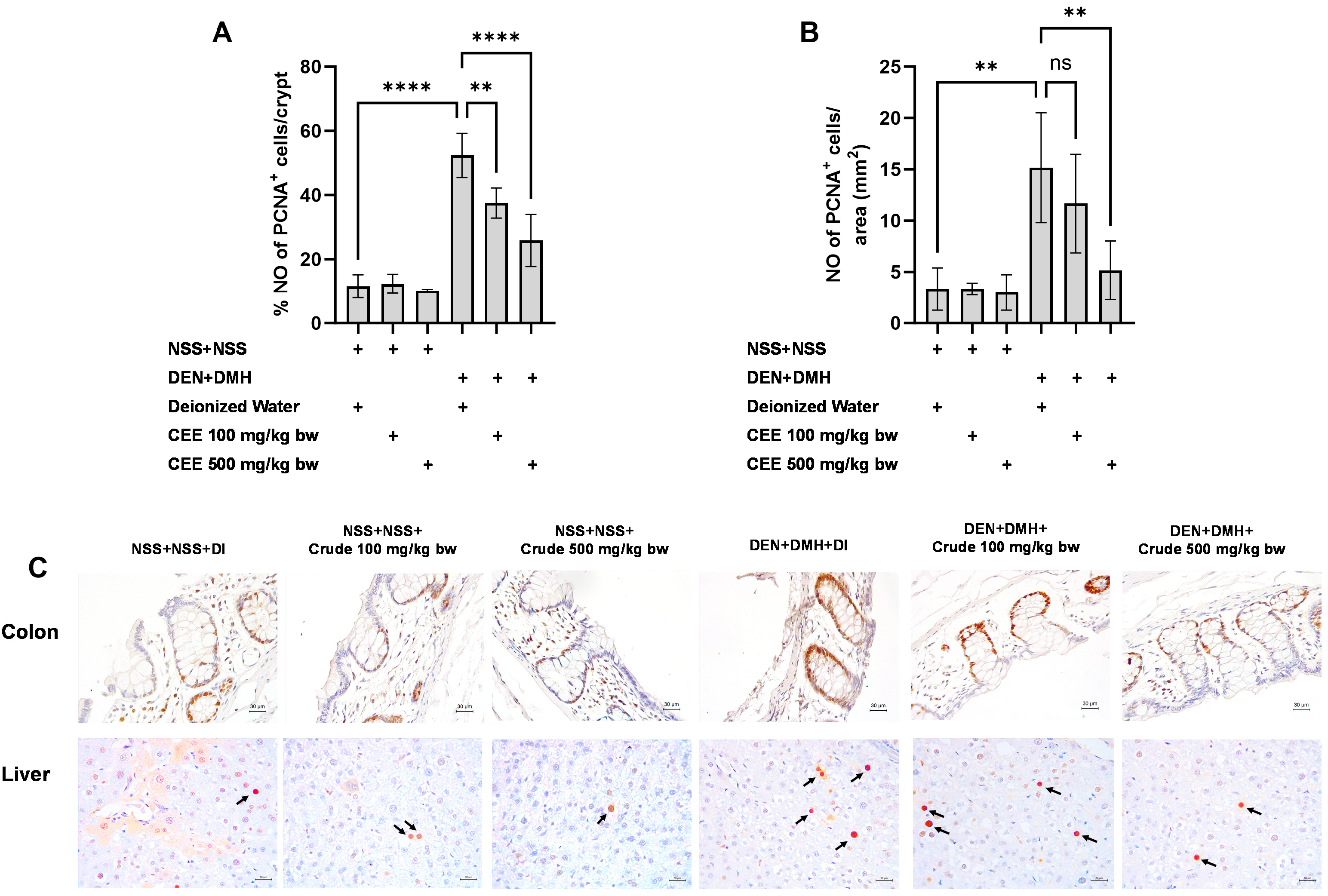
The effects of a ten-week administration period of crude FHCL extract on preneoplastic lesions induced by dual carcinogens in rat colons and livers. The analysis of preneoplastic lesions in the colon includes the number (A) and area (B) of aberrant crypt foci (ACF), identified with 0.2% methylene blue staining. For the liver, preneoplastic lesions are evaluated by the number (C) and size (D) of glutathione S-transferase placental form (GST-P)-positive foci, detected through immunohistochemistry. The figure shows representative images of preneoplastic lesions (ACF and GST-P-positive foci indicated by arrows) in the colons (40× magnification) and livers (100× magnification) (E). The data are presented as the mean ± standard deviation (n = 8). Statistical significance is indicated by * (p ≤ 0.05) and **** (p ≤ 0.0001). “ns” indicates non-significant differences (p > 0.05).
3.8. Effect of Crude FHCL Extract on Cell Proliferation and Cell Apoptosis
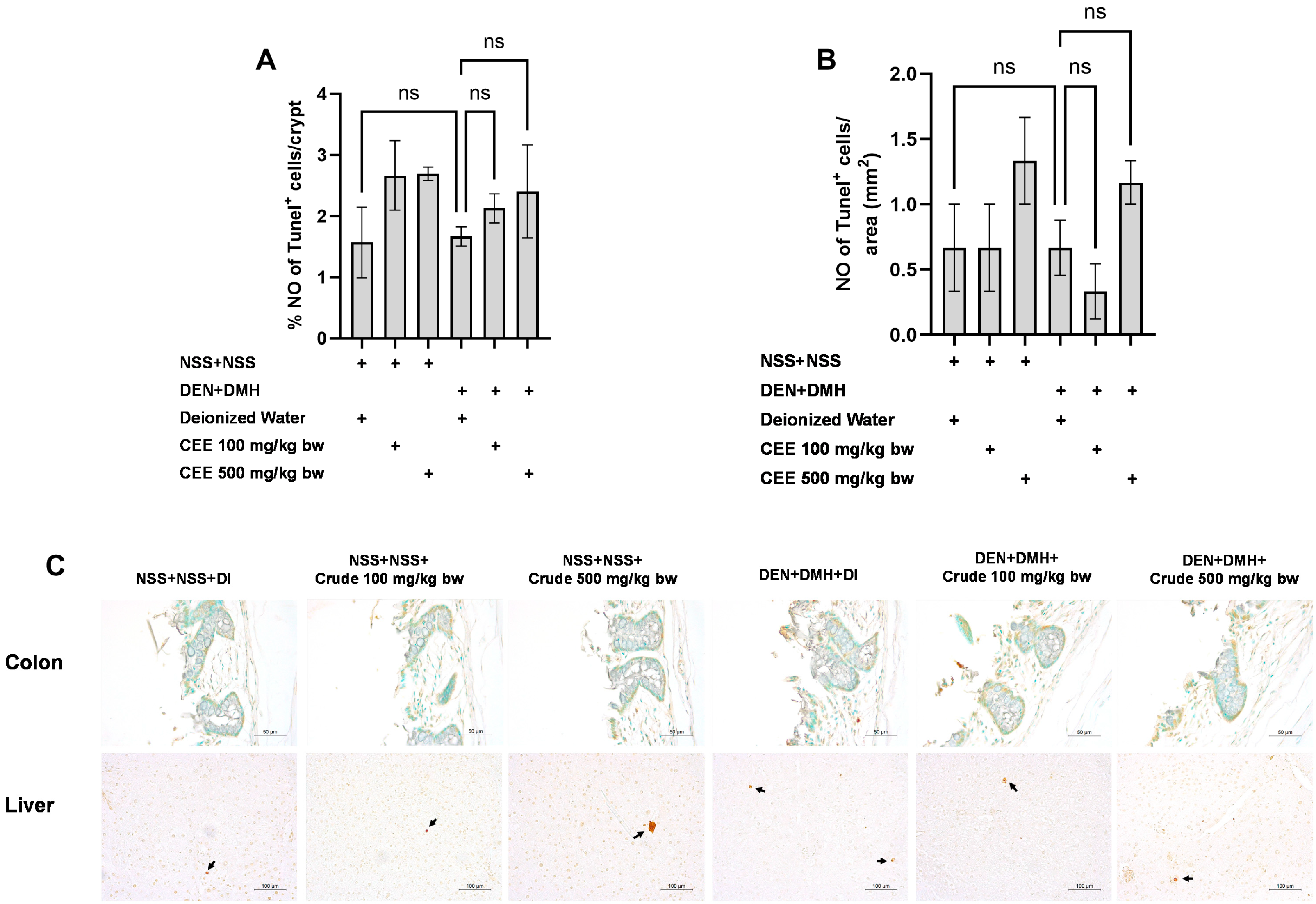
We further explored the mechanisms of the extract’s effects on cellular proliferation and apoptosis in the colon and liver. In rats exposed to DMH and DEN, the number of proliferating cell nuclear antigen (PCNA)-positive cells significantly increased by three-fold in both the colon and liver samples compared to the group that received NSS alone, indicating that carcinogen exposure enhances cellular proliferation. The administration of the crude extract at doses of 100 and 500 mg/kg bw significantly reduced the number of PCNA-positive cells in the colons of rats treated with the carcinogens (Figure 5A). In the liver, a marked reduction in PCNA-positive cells was observed when the carcinogen-treated rats received FHCL extract at a dose of 500 mg/kg bw (Figure 5B). However, treatment with the crude extract did not cause significant changes in the number of TUNEL-positive cells, an indicator of apoptosis, in either the colons or livers of rats treated with carcinogens (Figure 6). These findings suggest that FHCL crude extract primarily inhibits the formation and progression of preneoplastic lesions in the early stages of carcinogenesis by affecting cell proliferation rather than apoptosis. Furthermore, treatment with the crude extract alone did not significantly alter cell proliferation or apoptosis in either the colon or liver tissues of normal rats (Figure 5 and Figure 6), indicating that it does not induce spontaneous changes in these two processes.
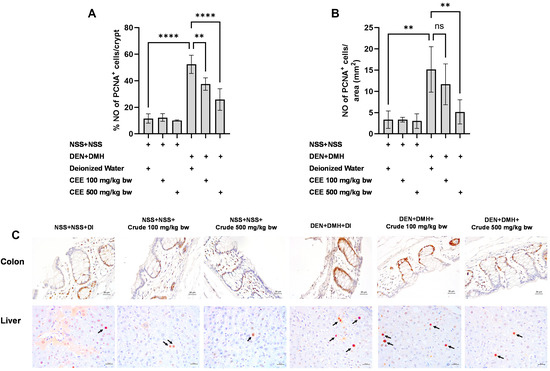
Figure 5.
Effect of crude FHCL extract on early-stage carcinogenesis in rats: modulation of cell proliferation. Immunohistochemical staining was used to quantify proliferating cell nuclear antigen (PCNA)-positive cells in colon (A) and liver (B). Figure displays representative immunohistochemical images of PCNA-positive cells in liver sections (400× magnification) and colon sections (400× magnification) (C). In colon, PCNA-positive cells exhibit brownish staining, while PCNA-positive hepatic cells are indicated by arrows. Data are presented as mean ± standard deviation. Statistical significance is denoted by ** (p ≤ 0.01) and **** (p ≤ 0.0001). “ns” indicates non-significant differences (p > 0.05).
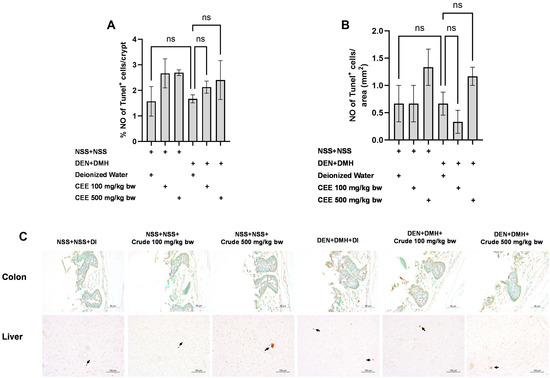
Figure 6.
Effect of crude FHCL extract on early-stage carcinogenesis in rats: modulation of cell apoptosis. Immunohistochemical staining was used to quantify TUNEL-positive cells in colon (A) and liver (B). Figure displays representative immunohistochemical images of TUNEL-positive cells in colon sections (400× magnification) and liver sections (200× magnification) (C). TUNEL-positive cells in colon exhibit brownish staining, while TUNEL-positive hepatic cells are indicated by arrows. Data are presented as mean ± standard deviation. “ns” indicates non-significant differences (p > 0.05).
4. Discussion
This research study offers valuable new insights into the potential of fermented Houttuynia cordata leaf (FHCL) to prevent multiple primary cancers induced by combined carcinogens in animal models. Using a dual carcinogen-induced rat model, the study shows that crude ethanolic extract markedly reduces preneoplastic lesions in the liver and colon during early carcinogenesis. This effect is mainly due to the modulation of key cellular processes, particularly the inhibition of cell proliferation. Additionally, this study highlights the potential of FHCL in reducing inflammation and inhibiting genotoxic effects by modulating detoxification pathways in Phases I and II.
The genotoxic effects on oncogenes and tumor suppressor genes play a crucial role in the development of carcinogenesis [56]. Flavonoid compounds in FHCL, such as rutin, quercetin, and quercitrin, have been shown to induce genotoxicity in Salmonella bacteria models TA98 and TA100 both with and without metabolic activation, with a more pronounced effect being observed in TA98 under +S9 conditions [57,58]. Crude FHCL, as well as its HEX, DCM, ETAC, and nBA fractions, which contain these flavonoids, also exhibited mutagenicity, particularly in TA98 under +S9 conditions. Quercetin and its derivatives interact with DNA through hydrogen bonding and other non-covalent interactions, such as groove binding and intercalation [59,60]. Rutin and quercitrin, due to their glycosylation, show lower genotoxicity compared to quercetin, as their glycosyl groups generate steric hindrance that impedes DNA interaction [61]. These interactions can disrupt the DNA structure, causing replication errors, point mutations, frameshift mutations, or other genetic alterations, especially in rapidly dividing bacterial cells [62,63]. The genotoxic effects were more pronounced in the DCM and ETAC fractions, which have higher concentrations of quercetin, compared to the crude extract. This highlights the advantage of using the crude extract over fractions, as the crude extract may neutralize some of the genotoxic effects of the flavonoids.
Despite these genotoxic effects in bacterial models, the crude FHCL extract and its fractions exhibited significant anti-genotoxic in the same assays. The crude FHCL extract and its fractions, except the residue, could prevent the genotoxic effects of indirect mutagens which induce a frameshift in TA 98 and point mutations in TA 100. This suggests that while these flavonoids may induce genotoxicity, they also have the potential to stabilize DNA and protect it from oxidative damage by reducing DNA accessibility to ROS [64,65,66]. However, the protective effect is concentration-dependent, and higher concentrations of the FHCL and its fractions may reduce their anti-genotoxicity effect, with prolonged exposure potentially causing extensive DNA damage [67]. In contrast to bacterial models, animal studies have shown that quercetin and its derivatives do not exhibit genotoxicity, likely due to their rapid metabolism and lower absorption [68,69]. This highlights the limitations of bacterial models, which lack the complete pharmacokinetic pathways for metabolizing xenobiotic compounds and have less effective DNA repair systems compared to animal models [70,71]. Furthermore, FHCL, containing these flavonoids, has demonstrated no genotoxicity in this study and has shown no acute or sub-chronic toxicity in animal studies or healthy human volunteers, thereby supporting its safety [40,72].
Environmental carcinogens typically require metabolic activation by xenobiotic-metabolizing enzymes to manifest their genotoxic effects. For example, DEN and DMH are primarily metabolized by the Phase I enzyme CYP2E1 [53], while AFB1 is processed by CYP1A2 and CYP3A [73]. Similarly, MeIQ is metabolized by CYP1A1 and CYP1A2 [74]. Exposure to these carcinogens has been reported to increase the expression of Phase I enzymes such as CYP2E1 and CYP1A, mediated by key regulatory proteins and receptors including pregnane X receptor (PXR), chimeric antigen receptor (CAR), aryl hydrocarbon receptor (AhR), and cytochrome P450 reductase (CPR) [53,75]. Concurrently, these carcinogens often reduce the activity of Phase II enzymes like UGT and GST, which are essential for detoxification [53,76]. In this study, FHCL was found to specifically affect indirect mutagens, which require metabolic activation by xenobiotic-metabolizing enzymes. The crude extract and DCM fraction of FHCL, known for their significant anti-genotoxic properties in in vitro models, contain key compounds such as vanillic acid, p-coumaric acid, quercetin, and quercitrin. Vanillic acid notably reduced the expression levels of Phase I enzymes, including CYP1A2 and CYP2E1, and enhanced the expression of detoxification system genes such as glutathione S-transferase alpha 5 (GSTA-5) and nuclear factor erythroid 2-related factor 2 (Nrf2) [77,78]. Quercitrin also inhibited CYP450 activities [79]. Likewise, p-coumaric acid decreased CYP1A1 enzyme activity, while quercetin modulated various Phase I and II enzymes, including CYP1A2, CYP3A4, and UGT, through mRNA expression and enzyme activities [80,81]. These findings are supported by the rat model results, which show decreased activities of Phase I enzymes CYP1A2, CYP2E1, and CYP3A2 and increased Phase II enzyme GST following a 40-day treatment with crude FHCL. Although CYP1A1 and UGT did not show significant differences, a trend toward modulation was observed. However, the potential impact of FHCL on the biotransformation system raises concerns about its interaction with other drugs metabolized by Phase I and II enzymes. Further research is required to fully understand these interactions and evaluate the broader implications of FHCL when used in conjunction with other pharmaceuticals.
In a combined-carcinogen treatment model, FHCL was found to reduce the formation of preneoplastic lesions in both the liver and colon. However, its impact on the progression of these lesions was significantly pronounced only in the liver, potentially due to the high concentration of xenobiotic-metabolizing enzymes in this organ [82]. Controlling inflammatory cells has been shown to inhibit the development of preneoplastic cancer cells [83]. FHCL and its fractions, particularly the crude and DCM fractions, demonstrated the potential to reduce NO production from macrophages, which are key inflammatory cells within tumor microenvironments [84]. Compounds like vanillic acid, p-coumaric acid, quercetin, and quercitrin, which are predominantly found in the crude and DCM fractions, have also been reported to reduce inflammation by inhibiting NO production from inflammatory cells [85,86,87,88]. This inhibition can disrupt pathways that promote cell proliferation and inhibit apoptosis within the tumor microenvironment [89]. These findings align with the observation that crude FHCL, which strongly reduced NO production, also significantly decreased cell proliferation in rat colon and liver tissues. Although this study did not find a significant effect of FHCL on cell apoptosis in preneoplastic lesions in either the liver or colon, previous studies have suggested that FHCL has the potential to induce apoptosis [37]. The lack of observable apoptotic activity in this study might be attributed to the use of a combined carcinogen treatment model, which tends to induce more severe effects compared to models involving single carcinogens [18].
Combining various compounds has been shown to enhance the activity of pharmaceuticals and mitigate the side effects of individual compounds [90]. These findings align with the observation that the crude FHCL extract demonstrates greater effectiveness in reducing inflammation and genotoxicity in vitro compared to individual fractions despite containing lower concentrations of phytochemicals. This superior performance is due to the synergistic interactions among the diverse phytochemical components in the crude extract, which supports its use in animal models. Not surprisingly, the crude extract significantly modulates xenobiotic-metabolizing enzymes and markedly reduces preneoplastic lesions and cell proliferation in the liver and colon within a robust animal carcinogenesis model. Furthermore, the crude extract neutralizes the potential toxicity associated with these compounds, as evidenced by the absence of genotoxicity or acute toxicity in animal studies. These findings further reinforce the safety and efficacy of the crude extract as a cancer-preventive agent.
5. Conclusions
This study highlights the significant chemopreventive potential of fermented Houttuynia cordata leaf (FHCL) against primary cancers of the liver and colon induced by combined carcinogens. The findings suggest that FHCL exerts its effects through multiple mechanisms, including controlling cell proliferation, inhibiting inflammation, preventing genotoxicity, and modulating xenobiotic-metabolizing enzymes. Importantly, FHCL has been shown to be safe in animal studies. These results emphasize the potential for FHCL to be developed as a preventive agent in cancer management. Further research is necessary to investigate the pharmacological interactions of FHCL with other compounds, especially its effects on xenobiotic-metabolizing enzymes, in order to fully understand its safety and efficacy in combination therapies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/foods13223645/s1, Table S1: IC20 of crude ethanolic extract of FHCL and its fractions in Raw 264.7 cell lines.
Author Contributions
C.S.: Conceptualization, Formal Analysis, Investigation, Methodology, Validation, Visualization, and Writing—Original Draft. S.T.: Methodology, Formal Analysis, and Writing—Review and Editing. P.P.: Conceptualization, Formal Analysis, Methodology, Project Administration, Supervision, Writing—Review and Editing, and Funding Acquisition. A.W.: Conceptualization, Formal Analysis, Methodology, Project Administration, Supervision, and Writing—Review and Editing. R.W.: Conceptualization, Funding Acquisition, Project Administration, and Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Research and Researcher for Industries (RRi) as issued by the Thailand Research Fund, grant number PHD62I0025, and the Faculty of Medicine, Chiang Mai University, grant numbers 158-2563.
Institutional Review Board Statement
This study was conducted following the Declaration of Helsinki and approved by the Animal Ethics Committee of the Faculty of Medicine, Chiang Mai University (Protocol No. 07/2563, approved on 17 April 2020, and Protocol No. 38/2563, approved on 26 July 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank Prolac Co., Ltd., Thailand, for kindly providing us with the sample materials, and Dr. Kei-Ichi Sugiyama of the National Institute of Health, Tokyo, Japan, for generously providing Salmonella typhimurium strains TA98 and TA100 for this study. Additionally, we extend our gratitude to Ruth Leatherman, Faculty of Medicine, Chiang Mai University, for proofreading the English of this article. This research work was partially supported by Chiang Mai University.
Conflicts of Interest
Prolac (Thailand) Co., Ltd., along with the Thailand Research Fund (TRF), co-funded the Research and Researcher for Industry (RRi) project. However, none of the authors have any affiliation with this company. The TRF provided major financial support through a Ph.D. scholarship (PHD62I0025), while the company contributed THB 125,000 in cash and provided the fermented leaf samples.
References
- Vogt, A.; Schmid, S.; Heinimann, K.; Frick, H.; Herrmann, C.; Cerny, T.; Omlin, A. Multiple primary tumours: Challenges and approaches, a review. ESMO Open 2017, 2, e000172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; He, L.; Zhou, C.; Cheng, X.; Li, Q.; Tang, Y.; Li, F.; Huang, T.; Tu, S. A pancancer analysis of the clinical and genomic characteristics of multiple primary cancers. Sci. Rep. 2024, 14, 2367. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Chu, C.H.; Chang, W.H.; Hsu, T.C.; Lin, S.C.; Liu, C.C.; Yang, A.M.; Shih, S.C. Clinical analysis of multiple primary malignancies in the digestive system: A hospital-based study. World J. Gastroenterol. 2005, 11, 4215–4219. [Google Scholar] [CrossRef] [PubMed]
- Tanjak, P.; Suktitipat, B.; Vorasan, N.; Juengwiwattanakitti, P.; Thiengtrong, B.; Songjang, C.; Therasakvichya, S.; Laiteerapong, S.; Chinswangwatanakul, V. Risks and cancer associations of metachronous and synchronous multiple primary cancers: A 25-year retrospective study. BMC Cancer 2021, 21, 1045. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, M.; Ju, X.; Lin, A.; Zhou, C.; Shen, J.; Liu, Z.; Tang, B.; Cheng, Q.; Wang, Y.; et al. Correlation between second and first primary cancer: Systematic review and meta-analysis of 9 million cancer patients. Br. J. Surg. 2023, 111, znad377. [Google Scholar] [CrossRef]
- Wynder, E.L.; Mushinski, M.H.; Spivak, J.C. Tobacco and alcohol consumption in relation to the development of multiple primary cancers. Cancer 1977, 40, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Travis, L.B.; Rabkin, C.S.; Brown, L.M.; Allan, J.M.; Alter, B.P.; Ambrosone, C.B.; Begg, C.B.; Caporaso, N.; Chanock, S.; DeMichele, A. Cancer survivorship—Genetic susceptibility and second primary cancers: Research strategies and recommendations. J. Natl. Cancer Inst. 2006, 98, 15–25. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef]
- Copur, M.S.; Manapuram, S. Multiple primary tumors over a lifetime. Oncology 2019, 33, 629384. [Google Scholar]
- Tricker, A.; Preussmann, R. Carcinogenic N-nitrosamines in the diet: Occurrence, formation, mechanisms and carcinogenic potential. Mutat. Res./Genet. Toxicol. 1991, 259, 277–289. [Google Scholar] [CrossRef]
- Cao, W.; Yu, P.; Yang, K.; Cao, D. Aflatoxin B1: Metabolism, toxicology, and its involvement in oxidative stress and cancer development. Toxicol. Mech. Methods 2022, 32, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Sinha, R.; Millikan, R.C.; Martin, C.F.; Newman, B.; Gammon, M.D.; Ammerman, A.S.; Sandler, R.S. Heterocyclic Amines, Meat Intake, and Association with Colon Cancer in a Population-based Study. Am. J. Epidemiol. 2003, 157, 434–445. [Google Scholar] [CrossRef]
- Newaz, S.; Fang, W.F.; Strobel, H.W. Metabolism of the carcinogen 1, 2-dimethylhydrazine by isolated human colon microsomes and human colon tumor cells in culture. Cancer 1983, 52, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Wanibuchi, H.; Morimura, K.; Gonzalez, F.J.; Fukushima, S. Role of CYP2E1 in diethylnitrosamine-induced hepatocarcinogenesis in vivo. Cancer Res. 2007, 67, 11141–11146. [Google Scholar] [CrossRef]
- Rosenberg, D.W.; Giardina, C.; Tanaka, T. Mouse models for the study of colon carcinogenesis. Carcinogenesis 2009, 30, 183–196. [Google Scholar] [CrossRef]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef]
- Singai, C.; Pitchakarn, P.; Taya, S.; Phannasorn, W.; Wongpoomchai, R.; Wongnoppavich, A. Chemopreventive Potential of Phyllanthus emblica Fruit Extract against Colon and Liver Cancer Using a Dual-Organ Rat Carcinogenesis Model. Pharmaceuticals 2024, 17, 818. [Google Scholar] [CrossRef]
- Ito, N.; Hasegawa, R.; Imaida, K.; Hirose, M.; Shirai, T. Medium-term liver and multi-organ carcinogenesis bioassays for carcinogens and chemopreventive agents. Exp. Toxicol. Pathol. 1996, 48, 113–119. [Google Scholar] [CrossRef]
- Shivapurkar, N.; Tang, Z.C.; Frost, A.; Alabaster, O. A rapid dual organ rat carcinogenesis bioassay for evaluating the chemoprevention of breast and colon cancer. Cancer Lett. 1996, 100, 169–179. [Google Scholar] [CrossRef]
- Hirose, M.; Hoshiya, T.; Akagi, K.; Takahashi, S.; Hara, Y.; Ito, N. Effects of green tea catechins in a rat multi-organ carcinogenesis model. Carcinogenesis 1993, 14, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Ushida, Y.; Sekine, K.; Kuhara, T.; Takasuka, N.; Iigo, M.; Maeda, M.; Tsuda, H. Possible chemopreventive effects of bovine lactoferrin on esophagus and lung carcinogenesis in the rat. Jpn. J. Cancer Res. 1999, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Laldinsangi, C. The therapeutic potential of Houttuynia cordata: A current review. Heliyon 2022, 8, e10386. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Koppula, S. Houttuynia cordata alleviates high-fat diet-induced non-alcoholic fatty liver in experimental rats. Pharm. Biol. 2015, 53, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; LIU, T.-F.; Chen, C.-M.; Chao, P.-Y.; Chang, T.-J. A study of the antioxidative and antimutagenic effects of Houttuynia cordata Thunb. using an oxidized frying oil-fed model. J. Nutr. Sci. Vitaminol. 2003, 49, 327–333. [Google Scholar] [CrossRef]
- Shin, S.; Joo, S.S.; Jeon, J.H.; Park, D.; Jang, M.-J.; Kim, T.-O.; Kim, H.-K.; Hwang, B.Y.; Kim, K.-Y.; Kim, Y.-B. Anti-inflammatory effects of a Houttuynia cordata supercritical extract. J. Vet. Sci. 2010, 11, 273–275. [Google Scholar] [CrossRef]
- Kang, H.; Koppula, S. Houttuynia cordata attenuates lipid accumulation via activation of AMP-activated protein kinase signaling pathway in HepG2 cells. Am. J. Chin. Med. 2014, 42, 651–664. [Google Scholar] [CrossRef]
- Kim, J.M.; Hwang, I.-H.; Jang, I.-S.; Kim, M.; Bang, I.S.; Park, S.J.; Chung, Y.-J.; Joo, J.-C.; Lee, M.-G. Houttuynia cordata Thunb promotes activation of HIF-1A–FOXO3 and MEF2A pathways to induce apoptosis in human HepG2 hepatocellular carcinoma cells. Integr. Cancer Ther. 2017, 16, 360–372. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Yang, J.-S.; Lin, C.-F.; Shyu, W.-C.; Tsuzuki, M.; Lu, C.-C.; Chen, Y.-F.; Lai, K.-C. Houttuynia cordata Thunb extract induces apoptosis through mitochondrial-dependent pathway in HT-29 human colon adenocarcinoma cells. Oncol. Rep. 2009, 22, 1051–1056. [Google Scholar]
- Yang, L.; Jiang, J.-G. Bioactive components and functional properties of Hottuynia cordata and its applications. Pharm. Biol. 2009, 47, 1154–1161. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, X.; Hu, Q.; Xiao, X.; Jiang, J.; Ma, X.; Wu, M. Houttuynia cordata Thunb: An ethnopharmacological review. Front. Pharmacol. 2021, 12, 714694. [Google Scholar] [CrossRef] [PubMed]
- Naselli, F.; Bellavia, D.; Costa, V.; De Luca, A.; Raimondi, L.; Giavaresi, G.; Caradonna, F. Osteoarthritis in the Elderly Population: Preclinical Evidence of Nutrigenomic Activities of Flavonoids. Nutrients 2023, 16, 112. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Mariappan, S. Traditionally fermented biomedicines, arishtas and asavas from Ayurveda. Indian J. Trad. Know. 2008, 7, 548–556. [Google Scholar]
- Hussain, A.; Bose, S.; Wang, J.-H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 2016, 81, 1–16. [Google Scholar] [CrossRef]
- Kwon, R.H.; Ha, B.J. Increased flavonoid compounds from fermented Houttuynia cordata using isolated six of Bacillus from traditionally fermented Houttuynia cordata. Toxicol. Res. 2012, 28, 117–122. [Google Scholar] [CrossRef]
- Senawong, T.; Khaopha, S.; Misuna, S.; Komaikul, J.; Senawong, G.; Wongphakham, P.; Yunchalard, S. Phenolic acid composition and anticancer activity against human cancer cell lines of the commercially available fermentation products of Houttuynia cordata. Sci. Asia 2014, 40, 420–427. [Google Scholar] [CrossRef]
- Woranam, K.; Senawong, G.; Utaiwat, S.; Yunchalard, S.; Sattayasai, J.; Senawong, T. Anti-inflammatory activity of the dietary supplement Houttuynia cordata fermentation product in RAW264. 7 cells and Wistar rats. PLoS ONE 2020, 15, e0230645. [Google Scholar] [CrossRef]
- Kumnerdkhonkaen, P.; Saenglee, S.; Asgar, M.A.; Senawong, G.; Khongsukwiwat, K.; Senawong, T. Antiproliferative activities and phenolic acid content of water and ethanolic extracts of the powdered formula of Houttuynia cordata Thunb. fermented broth and Phyllanthus emblica Linn. fruit. BMC Complement. Altern. Med. 2018, 18, 130. [Google Scholar] [CrossRef]
- Woranam, K.; Mootsikapun, P.; Senawong, G.; Prompipak, J.; Promdee, L.; Pintaraks, K.; Ketterman, A.J.; Senawong, T. Safety and immunomodulatory activity of Houttuynia cordata fermentation product in healthy volunteers and its effect on antiretroviral-drug level in rats. Food Agric. Immunol. 2022, 33, 80–96. [Google Scholar] [CrossRef]
- Inboot, W.; Taya, S.; Chailungka, A.; Meepowpan, P.; Wongpoomchai, R. Genotoxicity and antigenotoxicity of the methanol extract of Cleistocalyx nervosum var. paniala seed using a Salmonella mutation assay and rat liver micronucleus tests. Mol. Cell. Toxicol. 2012, 8, 19–24. [Google Scholar] [CrossRef]
- Hartzfeld, P.W.; Forkner, R.; Hunter, M.D.; Hagerman, A.E. Determination of Hydrolyzable Tannins (Gallotannins and Ellagitannins) after Reaction with Potassium Iodate. J. Agric. Food Chem. 2002, 50, 1785–1790. [Google Scholar] [CrossRef]
- Insuan, O.; Chariyakornkul, A.; Rungrote, Y.; Wongpoomchai, R. Antimutagenic and Antioxidant Activities of Thai Rice Brans. J. Cancer Prev. 2017, 22, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chariyakornkul, A.; Phannasorn, W.; Mahatheeranont, S.; Wongpoomchai, R. Phytochemical Profile and Chemopreventive Properties of Cooked Glutinous Purple Rice Extracts Using Cell-Based Assays and Rat Model. Foods 2022, 11, 2333. [Google Scholar] [CrossRef]
- Punvittayagul, C.; Sringarm, K.; Chaiyasut, C.; Wongpoomchai, R. Mutagenicity and antimutagenicity of hydrophilic and lipophilic extracts of Thai northern purple rice. Asian Pac. J. Cancer Prev. 2014, 15, 9517–9522. [Google Scholar] [CrossRef]
- Toxicity–Up, Acute Oral. In OECD Guideline for Testing of Chemicals; Organisation for Economic Co-Operation and Development: Paris, France, 2001; pp. 1–14.
- Chariyakornkul, A.; Punvittayagul, C.; Taya, S.; Wongpoomchai, R. Inhibitory effect of purple rice husk extract on AFB1-induced micronucleus formation in rat liver through modulation of xenobiotic metabolizing enzymes. BMC Complement. Altern. Med. 2019, 19, 237. [Google Scholar] [CrossRef]
- Charoensin, S.; Punvittayagul, C.; Pompimon, W.; Mevatee, U.; Wongpoomchai, R. Toxicological and clastogenic evaluation of pinocembrin and pinostrobin isolated from Boesenbergia pandurata in Wistar rats. Thai J. Toxicol. 2020, 25, 29. [Google Scholar]
- Cliet, I.; Fournier, E.; Melcion, C.; Cordier, A. In vivo micronucleus test using mouse hepatocytes. Mutat. Res./Environ. Mutagen. Relat. Subj. 1989, 216, 321–326. [Google Scholar] [CrossRef]
- Phannasorn, W.; Chariyakornkul, A.; Sookwong, P.; Wongpoomchai, R. Comparative Studies on the Hepatoprotective Effect of White and Coloured Rice Bran Oil against Acetaminophen-Induced Oxidative Stress in Mice through Antioxidant- and Xenobiotic-Metabolizing Systems. Oxidative Med. Cell. Longev. 2021, 2021, 5510230. [Google Scholar] [CrossRef]
- Waterborg, J.H. The Lowry Method for Protein Quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 7–10. [Google Scholar]
- Suwannakul, N.; Punvittayagul, C.; Jarukamjorn, K.; Wongpoomchai, R. Purple rice bran extract attenuates the aflatoxin B1-induced initiation stage of hepatocarcinogenesis by alteration of xenobiotic metabolizing enzymes. Asian Pac. J. Cancer Prev. 2015, 16, 3371–3376. [Google Scholar] [CrossRef]
- Punvittayagul, C.; Chariyakornkul, A.; Chewonarin, T.; Jarukamjorn, K.; Wongpoomchai, R. Augmentation of diethylnitrosamine–induced early stages of rat hepatocarcinogenesis by 1, 2-dimethylhydrazine. Drug Chem. Toxicol. 2019, 42, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Punvittayagul, C.; Chariyakornkul, A.; Sankam, P.; Wongpoomchai, R. Inhibitory Effect of Thai Purple Rice Husk Extract on Chemically Induced Carcinogenesis in Rats. Molecules 2021, 26, 360. [Google Scholar] [CrossRef] [PubMed]
- Thumvijit, T.; Taya, S.; Punvittayagul, C.; Peerapornpisal, Y.; Wongpoomchai, R. Cancer chemopreventive effect of Spirogyra neglecta (Hassall) Kützing on diethylnitrosamine-induced hepatocarcinogenesis in rats. Asian Pac. J. Cancer Prev. 2014, 15, 1611–1616. [Google Scholar] [CrossRef]
- DeMarini, D.M. The Role of Genotoxicity in Carcinogenesis; IARC Scientific Publications: Lyon, France, 2021. [Google Scholar]
- Resende, F.A.; Vilegas, W.; Dos Santos, L.C.; Varanda, E.A. Mutagenicity of flavonoids assayed by bacterial reverse mutation (Ames) test. Molecules 2012, 17, 5255–5268. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Serrano, J.; Vettorazzi, A.; Muruzabal, D.; López de Cerain, A.; Azqueta, A. In vitro genotoxicity assessment of functional ingredients: DHA, rutin and α-tocopherol. Food Chem. Toxicol. 2021, 153, 112237. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Tarantilis, P.A.; Polissiou, M.G.; Diamantoglou, S.; Tajmir-Riahi, H.A. DNA Interaction with Naturally Occurring Antioxidant Flavonoids Quercetin, Kaempferol, and Delphinidin. J. Biomol. Struct. Dyn. 2005, 22, 719–724. [Google Scholar] [CrossRef]
- Sha, Y.; Chen, X.; Niu, B.; Chen, Q. The Interaction Mode of Groove Binding Between Quercetin and Calf Thymus DNA Based on Spectrometry and Simulation. Chem. Biodivers. 2017, 14, e1700133. [Google Scholar] [CrossRef]
- Janjua, N.K.; Siddiqa, A.; Yaqub, A.; Sabahat, S.; Qureshi, R.; Haque, S.u. Spectrophotometric analysis of flavonoid–DNA binding interactions at physiological conditions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 74, 1135–1137. [Google Scholar] [CrossRef]
- Czeczot, H.; Tudek, B.; Kusztelak, J.; Szymczyk, T.; Dobrowolska, B.; Glinkowska, G.; Malinowski, J.; Strzelecka, H. Isolation and studies of the mutagenic activity in the Ames test of flavonoids naturally occurring in medical herbs. Mutat. Res./Genet. Toxicol. 1990, 240, 209–216. [Google Scholar] [CrossRef]
- Das, A.; Majumder, D.; Saha, C. Correlation of binding efficacies of DNA to flavonoids and their induced cellular damage. J. Photochem. Photobiol. B Biol. 2017, 170, 256–262. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Gao, L. Mechanism of antioxidant properties of quercetin and quercetin-DNA complex. J. Mol. Model. 2020, 26, 133. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, C.D.; Tarantilis, P.A.; Polissiou, M.G.; Diamantoglou, S.; Tajmir-Riahi, H.A. An Overview of DNA and RNA Bindings to Antioxidant Flavonoids. Cell Biochem. Biophys. 2007, 49, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Geetha, T.; Malhotra, V.; Chopra, K.; Kaur, I.P. Antimutagenic and antioxidant/prooxidant activity of quercetin. Indian J. Exp. Biol. 2005, 43, 61–67. [Google Scholar]
- Alvi, N.; Rizvi, R.; Hadi, S. Interaction of quercetin with DNA. Biosci. Rep. 1986, 6, 861–868. [Google Scholar] [CrossRef]
- Utesch, D.; Feige, K.; Dasenbrock, J.; Broschard, T.H.; Harwood, M.; Danielewska-Nikiel, B.; Lines, T.C. Evaluation of the potential in vivo genotoxicity of quercetin. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2008, 654, 38–44. [Google Scholar] [CrossRef]
- Okamoto, T. Safety of quercetin for clinical application (Review). Int. J. Mol. Med. 2005, 16, 275–278. [Google Scholar] [CrossRef]
- Vijay, U.; Gupta, S.; Mathur, P.; Suravajhala, P.; Bhatnagar, P. Microbial Mutagenicity Assay: Ames Test. Bio-protocol 2018, 8, e2763. [Google Scholar] [CrossRef]
- Jenssen, D.; Ramel, C. The micronucleus test as part of a short-term mutagenicity test program for the prediction of carcinogenicity evaluated by 143 agents tested. Mutat. Res./Rev. Genet. Toxicol. 1980, 75, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasut, C.; Sivamaruthi, B.S.; Duangjitcharoen, Y.; Kesika, P.; Sirilun, S.; Chaiyasut, K.; Peerajan, S. Assessment of subchronic toxicity of fermented Houttuynia cordata Thunb. using rodent model system. Asian J. Pharm. Clin. Res. 2018, 11, 307–311. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Mori, Y.; Koide, A.; Kobayashi, Y.; Furukawa, F.; Hirose, M.; Nishikawa, A. Effects of cigarette smoke and a heterocyclic amine, MeIQx on cytochrome P-450, mutagenic activation of various carcinogens and glucuronidation in rat liver. Mutagenesis 2003, 18, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ayed-Boussema, I.; Pascussi, J.-M.; Maurel, P.; Bacha, H.; Hassen, W. Effect of Aflatoxin B1 on Nuclear Receptors PXR, CAR, and AhR and Their Target Cytochromes P450 mRNA Expression in Primary Cultures of Human Hepatocytes. Int. J. Toxicol. 2012, 31, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhao, L.; Zhang, N.-Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.-S.; Sun, L.-H. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res./Rev. Mutat. Res. 2018, 778, 79–89. [Google Scholar] [CrossRef]
- Punvittayagul, C.; Chariyakornkul, A.; Jarukamjorn, K.; Wongpoomchai, R. Protective Role of Vanillic Acid against Diethylnitrosamine- and 1,2-Dimethylhydrazine-Induced Hepatocarcinogenesis in Rats. Molecules 2021, 26, 2718. [Google Scholar] [CrossRef]
- Altay, A.; Kartal, D.İ.; Sadi, G.; Güray, T.; Yaprak, A.E. Modulation of mRNA expression and activities of xenobiotic metabolizing enzymes, CYP1A1, CYP1A2, CYP2E1, GPx and GSTP1 by the Salicornia freitagii extract in HT-29 human colon cancer cells. Arch. Biol. Sci. 2017, 69, 439–448. [Google Scholar] [CrossRef]
- Pang, C.Y.; Mak, J.W.; Ismail, R.; Ong, C.E. In vitro modulatory effects of flavonoids on human cytochrome P450 2C8 (CYP2C8). Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Zhu, S.t.; Harris, P.J. Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells. Mol. Nutr. Food Res. 2005, 49, 585–593. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, X.; Morris, M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol. Vitr. 2006, 20, 187–210. [Google Scholar] [CrossRef]
- Pavek, P.; Dvorak, Z. Xenobiotic-induced transcriptional regulation of xenobiotic metabolizing enzymes of the cytochrome P450 superfamily in human extrahepatic tissues. Curr. Drug Metab. 2008, 9, 129–143. [Google Scholar] [CrossRef]
- Kiraly, O.; Gong, G.; Olipitz, W.; Muthupalani, S.; Engelward, B.P. Inflammation-induced cell proliferation potentiates DNA damage-induced mutations in vivo. PLoS Genet. 2015, 11, e1004901. [Google Scholar] [CrossRef]
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T.P. The role of macrophages in cancer development and therapy. Cancers 2021, 13, 1946. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-C.; Kim, S.-J.; Kim, D.-S.; Jeon, Y.-D.; Park, S.J.; Lee, H.S.; Um, J.-Y.; Hong, S.-H. Vanillic acid inhibits inflammatory mediators by suppressing NF-κB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.; Reis, M.B.; Coelho, G.D.; Gastaldello, G.H.; Peti, A.P.F.; Rodrigues, D.M.; Bastos, J.K.; Campo, V.L.; Sorgi, C.A.; Faccioli, L.H. Baccharin and p-coumaric acid from green propolis mitigate inflammation by modulating the production of cytokines and eicosanoids. J. Ethnopharmacol. 2021, 278, 114255. [Google Scholar] [CrossRef]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef]
- Camuesco, D.; Comalada, M.; Rodríguez-Cabezas, M.E.; Nieto, A.; Lorente, M.D.; Concha, A.; Zarzuelo, A.; Gálvez, J. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br. J. Pharmacol. 2004, 143, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.J.; Sullivan, F.J.; Giles, F.J.; Glynn, S.A. The yin and yang of nitric oxide in cancer progression. Carcinogenesis 2013, 34, 503–512. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10, S4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).