Effect of Modification by β-Amylase and α-Glucosidase on the Structural and Physicochemical Properties of Maize Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Maize Starch Modified by Dual Enzyme
2.2.2. Determination of Amylose and Amylopectin Ratio
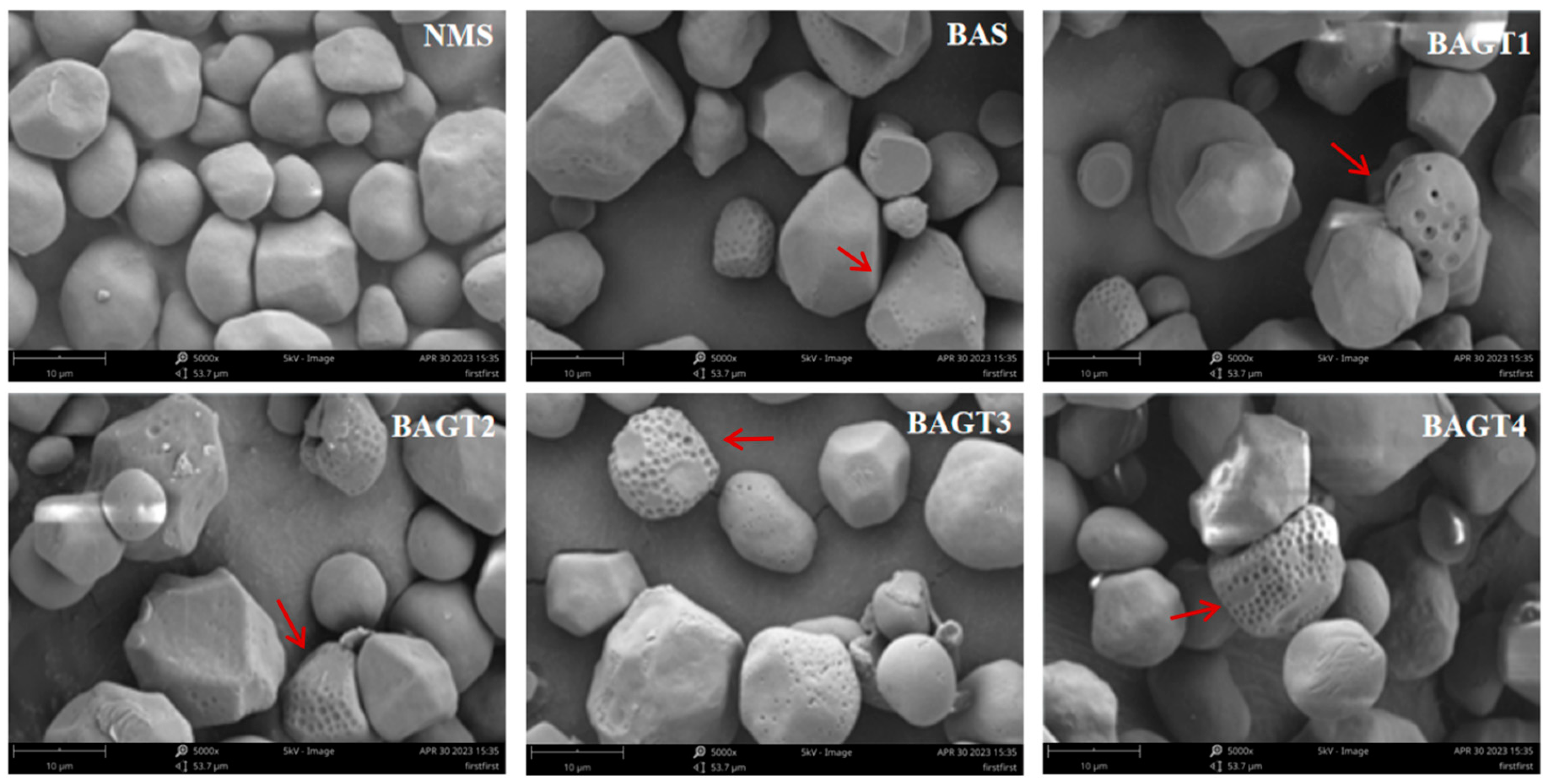
2.2.3. Determination of Starch Granule Morphology
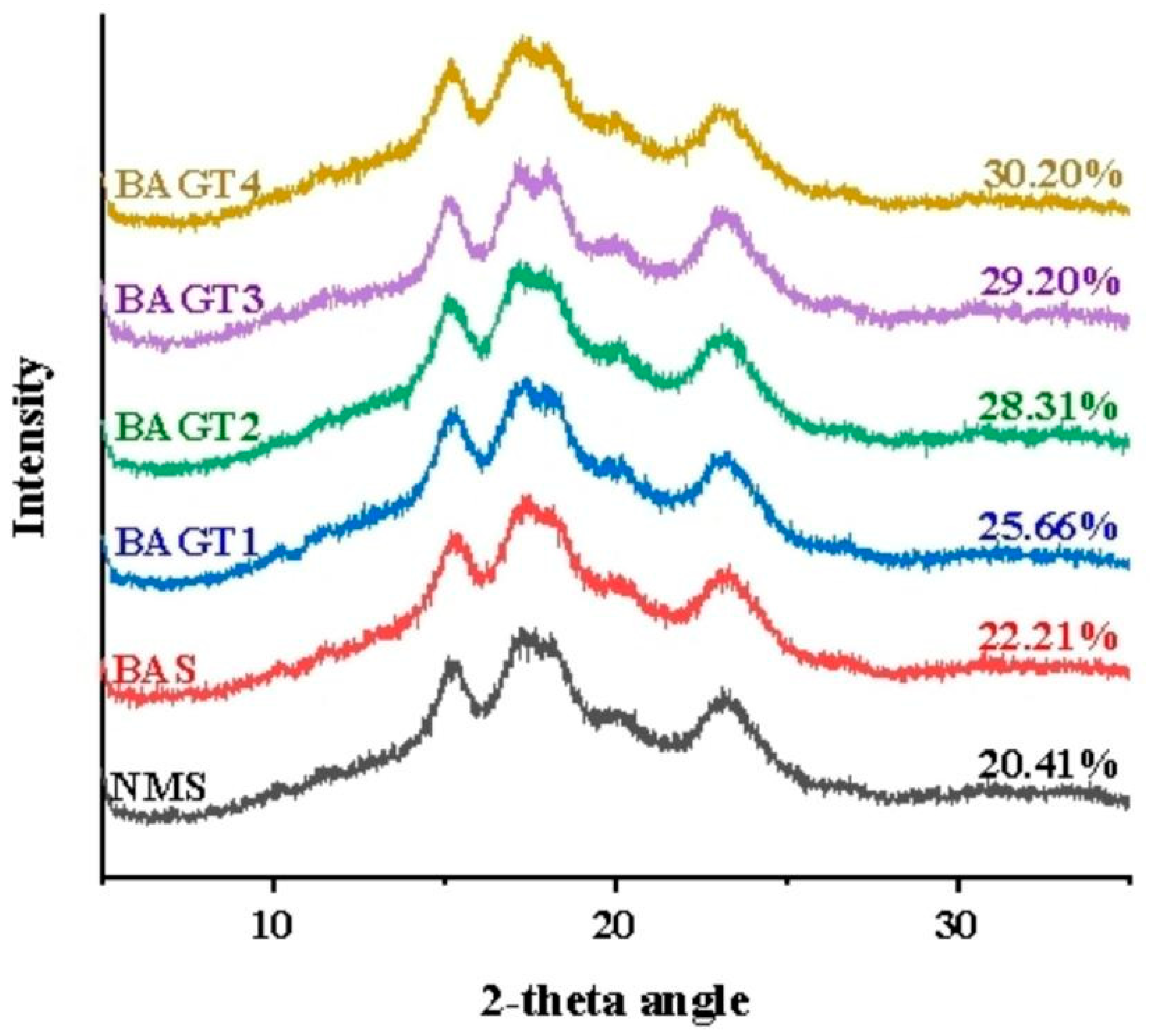
2.2.4. Determination of Starch Crystalline Properties
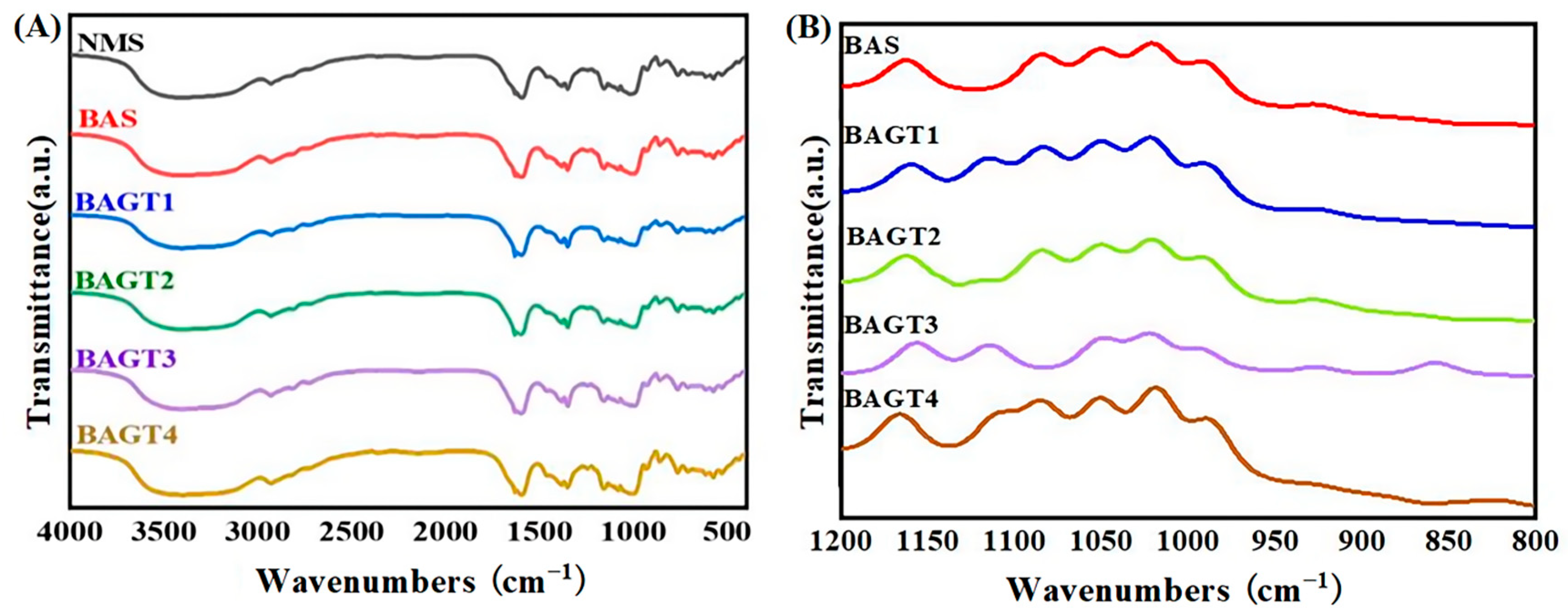
2.2.5. Determination of Fourier Transforms Infrared (FTIR) Spectroscopy
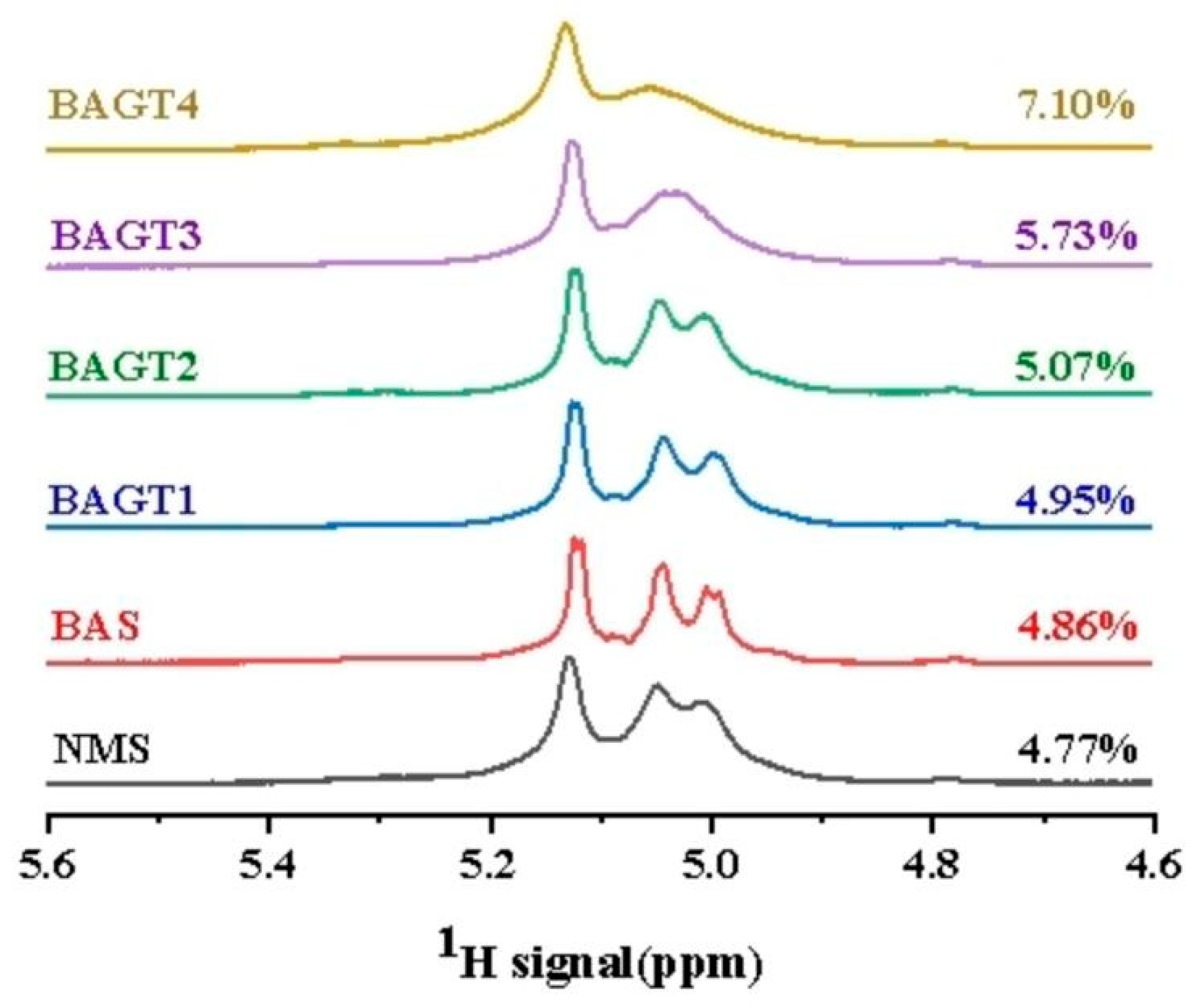
2.2.6. Determination of Starch Branching Degree
2.2.7. Determination of Starch Chain-Length Distribution
2.2.8. Determination of Starch Thermal Properties
2.2.9. Determination of Starch Pasting Properties by RVA
2.2.10. Determination of Starch Light Transmittance
2.2.11. Determination of Starch Solubility and Swelling Power
2.2.12. Statistical Analysis
3. Results and Discussion
3.1. Effect of Dual Enzyme Modification on the Ratio of Amylose/Amylopectin
3.2. Effect of Dual Enzyme Modification on Maize Grain Morphology
3.3. Effect of Dual Enzyme Modification on the Crystal Structure of Maize Starch
3.4. Effect of Enzymatic Modifications on the Short-Range Ordered Structure of Maize Starch
3.5. Effect of Dual Enzyme Modification on the Degree of Branching of Maize Starch
3.6. Effect of Dual Enzyme Modification on the Chain-Length Distribution of Maize Starch
3.7. Effect of Dual Enzyme Modification on the Thermal Properties of Maize Starch
3.8. Effect of Dual Enzyme Modification on the Pasting Property Parameters of Maize Starch
3.9. Effect of Dual Enzyme Modification on the Light Transmittance of Maize Starch
3.10. Effect of Dual Enzyme Modification on Solubility and Swelling Capacity of Maize Starch
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, B.; Ariffin, F.; Bhat, R.; Karim, A.A. Progress in starch modification in the last decade. Food Hydrocoll. 2012, 26, 398–404. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, J.; Wang, Z.; Qin, W.; Wu, Z. Effects of glutathione on structural and digestibility properties of high-hydrostatic-pressure-gelatinized maize starch with different amylose/amylopectin ratios. LWT 2023, 188, 115436. [Google Scholar] [CrossRef]
- Yang, Z.; Swedlund, P.; Hemar, Y.; Mo, G.; Wei, Y.; Li, Z.; Wu, Z. Effect of high hydrostatic pressure on the supramolecular structure of corn starch with different amylose contents. Int. J. Biol. Macromol. 2016, 85, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kong, X.; Ai, Y. Modification of granular waxy, normal and high-amylose maize starches by maltogenic α-amylase to improve functionality. Carbohydr. Polym. 2022, 290, 119503. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Hu, Y.; Li, C.; Yu, Y.; Wang, Y.; Gu, Z.; Hao, Z.; Xiao, Y.; Liu, Y.; Liu, K.; et al. Exploring the formation mechanism of resistant starch (RS3) prepared from high amylose maize starch by hydrothermal-alkali combined with ultrasonic treatment. Int. J. Biol. Macromol. 2024, 258, 128938. [Google Scholar] [CrossRef]
- Cahyana, Y.; Wijaya, E.; Halimah, T.S.; Marta, H.; Suryadi, E.; Kurniati, D. The effect of different thermal modifications on slowly digestible starch and physicochemical properties of green banana flour (Musa acuminata colla). Food Chem. 2019, 274, 274–280. [Google Scholar] [CrossRef]
- Milani, J.M.; Golkar, A. Chapter 8—Starch modification by novel technologies and their functionality. In Food Structure and Functionality; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 157–179. [Google Scholar]
- Cahyana, Y.; Putri, Y.S.E.; Solihah, D.S.; Lutfi, F.S.; Alqurashi, R.M.; Marta, H. Pickering Emulsions as Vehicles for Bioactive Compounds from Essential Oils. Molecules 2022, 27, 7872. [Google Scholar] [CrossRef]
- Handarini, K.; Hamdani, J.S.; Cahyana, Y.; Setiasih, I.S. Gaseous Ozonation at Low Concentration Modifies Functional, Pasting, and Thermal Properties of Arrowroot Starch (Maranta arundinaceae). Starch-Starke 2020, 72, 1900106. [Google Scholar] [CrossRef]
- Zhang, J.; Ran, C.; Jiang, X.; Dou, J. Impact of octenyl succinic anhydride (OSA) esterification on microstructure and physicochemical properties of sorghum starch. LWT 2021, 152, 112320. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.-j.; Sun, P.-l.; Zhang, F.-m.; Linhardt, R.J.; Zhang, A.-q. Chemically modified polysaccharides: Synthesis, characterization, structure activity relationships of action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef]
- Cahyana, Y.; Annisa, N.D.N.; Khoerunnisa, T.K.; Sulastri, S.; Marta, H.; Rialita, T.; Yuliana, T.; Aït-Kaddour, A.; Şumnu, G. Banana starch modified by heat moisture treatment and annealing: Study on digestion kinetics and enzyme affinity. Int. J. Biol. Macromol. 2024, 258, 128771. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Li, R.; Chen, J.; Chen, L.; Xie, F. Favored CH-π interaction between enzymatically modified high amylose starch and resveratrol improves digestion resistance. Food Hydrocoll. 2024, 154, 110137. [Google Scholar] [CrossRef]
- Shah, A.; Masoodi, F.A.; Gani, A.; Ashwar, B. Dual enzyme modified oat starch: Structural characterisation, rheological properties, and digestibility in simulated GI tract. Int. J. Biol. Macromol. 2018, 106, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, C.; Gu, Z.; Cheng, L.; Hong, Y.; Li, Z. Digestion properties of corn starch modified by α-D-glucan branching enzyme and cyclodextrin glycosyltransferase. Food Hydrocoll. 2019, 89, 534–541. [Google Scholar] [CrossRef]
- Karakelle, B.; Kian-Pour, N.; Toker, O.S.; Palabiyik, I. Effect of process conditions and amylose/amylopectin ratio on the pasting behavior of maize starch: A modeling approach. J. Cereal Sci. 2020, 94, 102998. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, Y.; Bai, Y.; Svensson, B. Distinct effects of different α-amylases on cross-linked tapioca starch and gel-improving mechanism. Food Hydrocoll. 2022, 128, 107580. [Google Scholar] [CrossRef]
- Ji, H.; Li, X.; Bai, Y.; Shen, Y.; Jin, Z. Synergetic modification of waxy maize starch by dual-enzyme to lower the in vitro digestibility through modulating molecular structure and malto-oligosaccharide content. Int. J. Biol. Macromol. 2021, 180, 187–193. [Google Scholar] [CrossRef]
- Gui, Y.; Zou, F.; Li, J.; Tang, J.; Guo, L.; Cui, B. Corn starch modification during endogenous malt amylases: The impact of synergistic hydrolysis time of α-amylase and β-amylase and limit dextrinase. Int. J. Biol. Macromol. 2021, 190, 819–826. [Google Scholar] [CrossRef]
- Evans, D.E.; Dambergs, R.; Ratkowsky, D.; Li, C.; Harasymow, S.; Roumeliotis, S.; Eglinton, J.K. Refining the Prediction of Potential Malt Fermentability by Including an Assessment of Limit Dextrinase Thermostability and Additional Measures of Malt Modification, Using Two Different Methods for Multivariate Model Development. J. Inst. Brew. 2010, 116, 86–96. [Google Scholar] [CrossRef]
- Park, K.-H.; Park, J.-H.; Lee, S.; Yoo, S.-H.; Kim, J.-W. Enzymatic Modification of Starch for Food Industry. In Carbohydrate-Active Enzymes; Park, K.-H., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 157–183. [Google Scholar]
- Cui, Y.; Wang, W.; Gao, W.; Shi, M.; Liu, S.; Liu, C.; Zheng, M.; Liu, M.; Liu, H.; Liu, J. Effect of static magnetic field pretreatment on the structure and oil absorption properties of normal maize starch. Food Hydrocoll. 2024, 149, 109589. [Google Scholar] [CrossRef]
- Yashiro, K.; Sato, S.; Kondo, Y.; Nakamura, Y.; Yano, H.; Koda, T.; Nishioka, A.; Takashima, Y.; Matsuba, G. Relationship between the nanometer-scale structures of amylopectin molecules and temperature dependence of internal structures of starch granules in endosperm of starch branching enzyme 2b (be2b) allelic mutant lines from japonica rice. Results Chem. 2024, 9, 101660. [Google Scholar] [CrossRef]
- Gu, F.; Gong, B.; Gilbert, R.G.; Yu, W.; Li, E.; Li, C. Relations between changes in starch molecular fine structure and in thermal properties during rice grain storage. Food Chem. 2019, 295, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Jia, M.; Niu, J.; Zhang, Z.; Xing, B.; Liang, Y.; Li, H.; Zhang, Y.; Ren, G.; Qin, P.; et al. Amylopectin chain length distributions and amylose content are determinants of viscoelasticity and digestibility differences in mung bean starch and proso millet starch. Int. J. Biol. Macromol. 2024, 267, 131488. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Chen, S.; Li, C.; Gu, Z.; Cheng, L.; Hong, Y.; Li, Z. A two-stage modification method using 1,4-α-glucan branching enzyme lowers the in vitro digestibility of corn starch. Food Chem. 2020, 305, 125441. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Keeratiburana, T.; Kain Kirkensgaard, J.J.; Khakimov, B.; Blennow, A.; Hansen, A.R. Generation of short-chained granular corn starch by maltogenic α-amylase and transglucosidase treatment. Carbohydr. Polym. 2021, 251, 117056. [Google Scholar] [CrossRef]
- Chung, J.H.; Han, J.A.; Yoo, B.; Seib, P.A.; Lim, S.T. Effects of molecular size and chain profile of waxy cereal amylopectins on paste rheology during retrogradation. Carbohydr. Polym. 2008, 71, 365–371. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Lin, J.-H. Effects of molecular size and structure of amylopectin on the retrogradation thermal properties of waxy rice and waxy cornstarches. Food Hydrocoll. 2007, 21, 645–653. [Google Scholar] [CrossRef]
- Morikawa, K.; Nishinari, K. Effects of concentration dependence of retrogradation behaviour of dispersions for native and chemically modified potato starch. Food Hydrocoll. 2000, 14, 395–401. [Google Scholar] [CrossRef]
- Chen, L.; Ren, F.; Zhang, Z.; Tong, Q.; Rashed, M.M.A. Effect of pullulan on the short-term and long-term retrogradation of rice starch. Carbohydr. Polym. 2015, 115, 415–421. [Google Scholar] [CrossRef]
- Lin, J.-H.; Lii, C.-y.; Chang, Y.-H. Change of granular and molecular structures of waxy maize and potato starches after treated in alcohols with or without hydrochloric acid. Carbohydr. Polym. 2005, 59, 507–515. [Google Scholar] [CrossRef]
- Di, Y.; Na, R.; Xia, H.; Wang, Y.; Li, F. Irradiation effects on characteristics and ethanol fermentation of maize starch. Int. J. Biol. Macromol. 2023, 246, 125602. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; McClements, D.J.; Yang, T.; Ma, Y.; Ren, F.; Tian, Y.; Jin, Z. Effect of annealing and heat-moisture pretreatments on the oil absorption of normal maize starch during frying. Food Chem. 2021, 353, 129468. [Google Scholar] [CrossRef] [PubMed]
- Takata, H.; Takaha, T.; Okada, S.; Hizukuri, S.; Takagi, M.; Imanaka, T. Structure of the cyclic glucan produced from amylopectin by Bacillus stearothermophilus branching enzyme. Carbohydr. Res. 1996, 295, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jiang, X.; Chen, Y.; Yaqoob, S.; Xiu, L.; Liu, H.; Zheng, M.; Cai, D.; Liu, J. Germination-induced modifications of starch structure, flour-processing characteristics, and in vitro digestive properties in maize. Food Chem. X 2024, 22, 101430. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Herburger, K.; Kirkensgaard, J.J.K.; Khakimov, B.; Hansen, A.R.; Blennow, A. Sequential maltogenic α-amylase and branching enzyme treatment to modify granular corn starch. Food Hydrocoll. 2021, 120, 106904. [Google Scholar] [CrossRef]
- Xia, C.; Zhong, L.; Wang, J.; Zhang, L.; Chen, X.; Ji, H.; Ma, S.; Dong, W.; Ye, X.; Huang, Y.; et al. Structural and digestion properties of potato starch modified using an efficient starch branching enzyme AqGBE. Int. J. Biol. Macromol. 2021, 184, 551–557. [Google Scholar] [CrossRef]
- Rahaman, A.; Kumari, A.; Zeng, X.-A.; Adil Farooq, M.; Siddique, R.; Khalifa, I.; Siddeeg, A.; Ali, M.; Faisal Manzoor, M. Ultrasound based modification and structural-functional analysis of corn and cassava starch. Ultrason. Sonochemistry 2021, 80, 105795. [Google Scholar] [CrossRef]
- Li, X.; Miao, M.; Jiang, H.; Xue, J.; Jiang, B.; Zhang, T.; Gao, Y.; Jia, Y. Partial branching enzyme treatment increases the low glycaemic property and α-1,6 branching ratio of maize starch. Food Chem. 2014, 164, 502–509. [Google Scholar] [CrossRef]
- Cheetham, N.W.H.; Tao, L. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Jo, A.R.; Kim, H.R.; Choi, S.J.; Lee, J.S.; Chung, M.N.; Han, S.K.; Park, C.-S.; Moon, T.W. Preparation of slowly digestible sweet potato Daeyumi starch by dual enzyme modification. Carbohydr. Polym. 2016, 143, 164–171. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, G.; Gao, Q. Preparation and properties of granular cold-water-soluble porous starch. Int. J. Biol. Macromol. 2020, 144, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Hajihashemi, Z.; Nasirpour, A.; Scher, J.; Desobry, S. Interactions among lactose, β-lactoglobulin and starch in co-lyophilized mixtures as determined by Fourier Transform Infrared Spectroscopy. J. Food Sci. Technol. 2014, 51, 3376–3382. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Leng, X.; Zhang, G. The loosening effect of tea polyphenol on the structure of octenyl succinic anhydride modified waxy maize starch. Food Hydrocoll. 2020, 99, 105367. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, Y.-C.; Herrera, A.; Prakash, O. Study of octenyl succinic anhydride-modified waxy maize starch by nuclear magnetic resonance spectroscopy. Carbohydr. Polym. 2011, 83, 407–413. [Google Scholar] [CrossRef]
- Guo, L. Sweet potato starch modified by branching enzyme, β-amylase and transglucosidase. Food Hydrocoll. 2018, 83, 182–189. [Google Scholar] [CrossRef]
- Guo, W.; Yang, L.; Shi, X.; Cong, X.; Cheng, S.; Li, L.; Cheng, H. Effects of color protection and enzymatic hydrolysis on the microstructure, digestibility, solubility and swelling degree of chestnut flour. Food Chem. X 2024, 23, 101770. [Google Scholar] [CrossRef]
- Hanashiro, I.; Abe, J.-i.; Hizukuri, S. A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohydr. Res. 1996, 283, 151–159. [Google Scholar] [CrossRef]
- Hanashiro, I.; Tagawa, M.; Shibahara, S.; Iwata, K.; Takeda, Y. Examination of molar-based distribution of A, B and C chains of amylopectin by fluorescent labeling with 2-aminopyridine. Carbohydr. Res. 2002, 337, 1211–1215. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, L.; Qu, J.; Blennow, A.; Hansen, A.R.; Wu, Y.; Guo, D.; Liu, X. Amylose content and specific fine structures affect lamellar structure and digestibility of maize starches. Food Hydrocoll. 2020, 108, 105994. [Google Scholar] [CrossRef]
- Shin, H.J.; Choi, S.J.; Park, C.S.; Moon, T.W. Preparation of starches with low glycaemic response using amylosucrase and their physicochemical properties. Carbohydr. Polym. 2010, 82, 489–497. [Google Scholar] [CrossRef]
- Kim, E.-J.; Ryu, S.-I.; Bae, H.-A.; Huong, N.T.; Lee, S.-B. Biochemical characterisation of a glycogen branching enzyme from Streptococcus mutans: Enzymatic modification of starch. Food Chem. 2008, 110, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, Y.; Li, C.; Gu, Z.; Cheng, L.; Hong, Y.; Li, Z. Pasting and thermal properties of waxy corn starch modified by 1,4-α-glucan branching enzyme. Int. J. Biol. Macromol. 2017, 97, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Chen, Z.; Ma, R.; Wen, Y.; Li, H.; Wang, J.; Sun, B. Effect of alkyl chain length and amylose/amylopectin ratio on the structure and digestibility of starch-alkylresorcinols inclusion complexes. Food Hydrocoll. 2022, 133, 107900. [Google Scholar] [CrossRef]
- Obadi, M.; Qi, Y.; Xu, B. High-amylose maize starch: Structure, properties, modifications and industrial applications. Carbohydr. Polym. 2023, 299, 120185. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Cai, C.; Gilbert, R.G.; Li, E.; Wang, J.; Wei, C. Relationships between amylopectin molecular structures and functional properties of different-sized fractions of normal and high-amylose maize starches. Food Hydrocoll. 2016, 52, 359–368. [Google Scholar] [CrossRef]
- Li, Y.; Hu, A.; Zheng, J.; Wang, X. Comparative studies on structure and physiochemical changes of millet starch under microwave and ultrasound at the same power. Int. J. Biol. Macromol. 2019, 141, 76–84. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, W.; Pan, Y.; Ali, B.; Xu, D.; Xu, X. Physicochemical, crystalline characterization and digestibility of wheat starch under superheated steam treatment. Food Hydrocoll. 2021, 118, 106720. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, Y.; Ji, H.; Dong, J.; Li, X.; Liu, J.; Jin, Z. Insights into rice starch degradation by maltogenic α–amylase: Effect of starch structure on its rheological properties. Food Hydrocoll. 2022, 124, 107289. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Li, Y.; Jin, D.; Jiang, Y.; Fang, Y.; Lin, Q.; Ding, Y. Effects of OSA-starch-fatty acid interactions on the structural, digestibility and release characteristics of high amylose corn starch. Food Chem. 2024, 454, 139742. [Google Scholar] [CrossRef]
- Wu, K.; Li, C.; Li, Z.; Gu, Z.; Ban, X.; Hong, Y.; Cheng, L.; Kong, H. Enzymatic modification lowers syneresis in corn starch gels during freeze–thaw cycles through 1,4-α-glucan branching enzyme. Int. J. Biol. Macromol. 2024, 269, 132183. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, D.; Guo, D.; Tong, X.; Zhang, Y.; Wang, L. Physicochemical and digestive properties of A- and B-type granules isolated from wheat starch as affected by microwave-ultrasound and toughening treatment. Int. J. Biol. Macromol. 2021, 183, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Hu, X.; Luo, S.; McClements, D.J.; Liang, L.; Liu, C. Effect of endogenous proteins and lipids on starch digestibility in rice flour. Food Res. Int. 2018, 106, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wen, H.; Luo, Y.; Yang, J.; Xiao, W.; Ji, X.; Xie, J. Effects of α-amylase and glucoamylase on the characterization and function of maize porous starches. Food Hydrocoll. 2021, 116, 106661. [Google Scholar] [CrossRef]
| Sample | Amylose % (w/w) | Amylopectin% (w/w) | Amylose/Amylopectin |
|---|---|---|---|
| NMS | 28.77 ± 0.86 a | 70.79 ± 0.10 f | 1: 2.42 ± 0.10 e |
| BAS | 26.16 ± 0.37 b | 73.27 ± 0.13 e | 1: 2.74 ± 0.05 d |
| BAGT1 | 25.16 ± 0.69 c | 74.48 ± 0.03 d | 1: 2.92 ± 0.11 cd |
| BAGT2 | 23.88 ± 0.21 c | 75.35 ± 0.02 c | 1: 3.05 ± 0.04 c |
| BAGT3 | 22.28 ± 0.67 d | 77.17 ± 0.00 b | 1: 3.38 ± 0.28 b |
| BAGT4 | 18.60 ± 0.99 e | 81.71 ± 0.05 a | 1: 4.47 ± 0.28 a |
| Samples | Relative Crystallinity (%) | R1047 cm−1/1022 cm−1 |
|---|---|---|
| NMS | 20.41 | 0.75 ± 0.02 d |
| BAS | 22.21 | 0.77 ± 0.00 d |
| BAGT1 | 25.66 | 0.78 ± 0.00 d |
| BAGT2 | 28.31 | 0.87 ± 0.03 c |
| BAGT3 | 29.20 | 0.93 ± 0.01 b |
| BAGT4 | 30.20 | 1.19 ± 0.05 a |
| Sample | Chain-Length Distribution Ratio (%) | |||
|---|---|---|---|---|
| A (DP3–12) | B1 (DP13–24) | B2 (DP25–36) | B3 (DP ≥ 37) | |
| NMS | 14.97 | 24.92 | 12.26 | 47.86 |
| BAS | 15.15 | 25.81 | 11.74 | 47.30 |
| BAGT1 | 16.16 | 25.73 | 11.18 | 46.93 |
| BAGT2 | 16.19 | 26.51 | 11.27 | 46.03 |
| BAGT3 | 16.21 | 26.66 | 11.16 | 45.79 |
| BAGT4 | 17.87 | 27.44 | 10.48 | 44.21 |
| Sample | To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) |
|---|---|---|---|---|
| NMS | 48.26 ± 15.43 b | 70.09 ± 0.17 a | 95.07 ± 14.98 a | 13.68 ± 4.93 a |
| BAS | 55.30 ± 8.03 ab | 70.56 ± 0.00 d | 87.68 ± 13.50 ab | 11.35 ± 0.79 a |
| BAGT1 | 67.15 ± 0.51 a | 71.08 ± 0.00 a | 77.27 ± 0.31 b | 10.94 ± 0.41 a |
| BAGT2 | 64.00 ± 1.03 a | 70.54 ± 0.02 d | 79.39 ± 2.17 ab | 10.66 ± 1.20 a |
| BAGT3 | 66.63 ± 0.21 a | 70.79 ± 0.00 c | 78.52 ± 0.39 ab | 10.21 ± 0.33 a |
| BAGT4 | 66.53 ± 0.31 a | 70.87 ± 0.00 b | 76.14 ± 0.37 b | 9.13 ± 0.30 a |
| Sample | PV (cp) | TV (cp) | BD (cp) | FV (cp) | SB (cp) |
|---|---|---|---|---|---|
| NMS | 2720.00 ± 20.66 d | 1662.67 ± 14.50 c | 1058.67 ± 34.93 c | 2460.00 ± 20.31 d | 798.00 ± 13.98 d |
| BAS | 3975.67 ± 47.37 a | 2170.00 ± 28.09 a | 1805.00 ± 20.71 b | 3783.50 ± 38.59 a | 1613.67 ± 20.50 a |
| BAGT1 | 3861.67 ± 40.61 b | 1807.50 ± 19.20 b | 2054.50 ± 14.12 a | 3371.50 ± 40.35 b | 1564.00 ± 38.09 a |
| BAGT2 | 3841.00 ± 18.89 b | 1845.00 ± 12.83 b | 1996.50 ± 27.07 a | 3294.00 ± 34.24 b | 1449.00 ± 24.14 b |
| BAGT3 | 3674.33 ± 45.96 c | 1743.50 ± 18.28 b | 1930.00 ± 52.32 a | 3175.33 ± 36.97 c | 1432.50 ± 16.97 b |
| BAGT4 | 3578.00 ± 46.67 c | 1558.50 ± 19.90 c | 2020.00 ± 33.23 a | 2020.00 ± 23.93 e | 1281.00 ± 15.97 c |
| Sample | Transmittance (%) | Solubility (%) | Swelling Power (%) |
|---|---|---|---|
| NMS | 1.40 ± 0.43 d | 20.15 ± 0.15 a | 33.97 ± 0.25 f |
| BAS | 1.42 ± 0.02 d | 17.03 ± 0.17 b | 39.47 ± 0.12 e |
| BAGT1 | 1.61 ± 0.14 c | 15.72 ± 0.23 c | 41.56 ± 0.33 d |
| BAGT2 | 1.93 ± 0.08 b | 15.64 ± 0.07 c | 42.76 ± 0.18 c |
| BAGT3 | 2.04 ± 0.03 a | 14.20 ± 0.13 d | 44.40 ± 0.13 b |
| BAGT4 | 2.16 ± 0.05 a | 13.76 ± 0.07 e | 45.79 ± 0.07 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Xu, J.; Cui, Y.; Ben, D.; Wu, C.; Zhang, J.; Sun, M.; Liu, S.; Zhu, T.; Liu, J.; et al. Effect of Modification by β-Amylase and α-Glucosidase on the Structural and Physicochemical Properties of Maize Starch. Foods 2024, 13, 3763. https://doi.org/10.3390/foods13233763
Jia X, Xu J, Cui Y, Ben D, Wu C, Zhang J, Sun M, Liu S, Zhu T, Liu J, et al. Effect of Modification by β-Amylase and α-Glucosidase on the Structural and Physicochemical Properties of Maize Starch. Foods. 2024; 13(23):3763. https://doi.org/10.3390/foods13233763
Chicago/Turabian StyleJia, Xinge, Jingwen Xu, Yan Cui, Dazhi Ben, Chuyu Wu, Jing Zhang, Mingru Sun, Shuo Liu, Tianhao Zhu, Jingsheng Liu, and et al. 2024. "Effect of Modification by β-Amylase and α-Glucosidase on the Structural and Physicochemical Properties of Maize Starch" Foods 13, no. 23: 3763. https://doi.org/10.3390/foods13233763
APA StyleJia, X., Xu, J., Cui, Y., Ben, D., Wu, C., Zhang, J., Sun, M., Liu, S., Zhu, T., Liu, J., Lin, K., & Zheng, M. (2024). Effect of Modification by β-Amylase and α-Glucosidase on the Structural and Physicochemical Properties of Maize Starch. Foods, 13(23), 3763. https://doi.org/10.3390/foods13233763





