In Vitro Evaluation of Probiotic Activities and Anti-Obesity Effects of Enterococcus faecalis EF-1 in Mice Fed a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Strains and Growth Media
2.3. Screening of E. faecalis EF-1 for Potential Probiotic Properties
2.3.1. Simulated Gastrointestinal Tolerance
2.3.2. Cholesterol-Reducing Rate
2.3.3. Detection of BSH Activity
2.3.4. Detection of Antibacterial Activity
2.3.5. The Inhibition Activity of α-Glucosidase
2.3.6. Fatty Acid Absorption Assay
2.3.7. Measurement of Antibiotic Resistance Phenotypes
2.3.8. Hemolytic Activity
2.4. Animals Experiment Design
2.5. Sample Collection
2.6. Biochemical Assay of Serum and Liver Tissues
2.7. Histological Evaluation
2.7.1. Oil Red O Staining of Liver Tissue
2.7.2. Hematoxylin and Eosin Staining of Liver and Adipose Tissue
2.8. 16S rRNA Sequencing and Processing of Gut Microbiota
2.9. Determination of SCFA Production in HFD-Induced Obese Mice
2.10. Statistical Analysis
3. Results
3.1. Potential Probiotic Properties
3.1.1. Tolerance of E. faecalis EF-1 to Gastric and Intestinal Juices
3.1.2. Cholesterol-Reducing Capacity of E. faecalis EF-1
3.1.3. BSH Activity
3.1.4. Fatty Acid Absorption by E. faecalis EF-1
3.1.5. The Inhibition Effect of α-Glucosidase
3.1.6. Measurement of Antibacterial Activity of E. faecalis EF-1
3.1.7. Antibiotic Resistance
3.1.8. Hemolytic Activity of E. faecalis EF-1
3.2. E. faecalis EF-1 Alleviated HFD-Induced Obesity in Mice
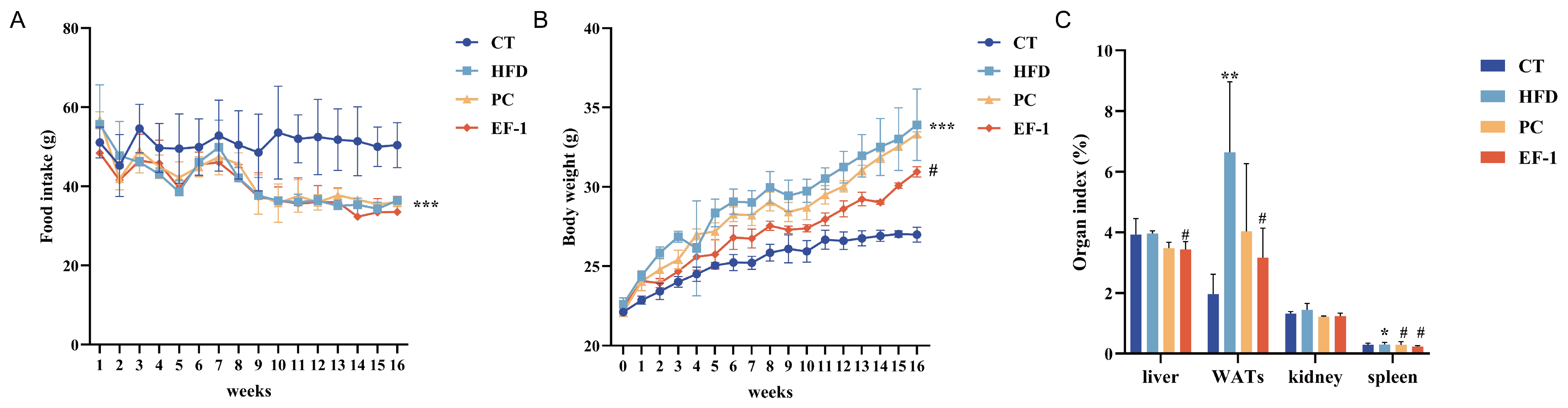
3.2.1. E. faecalis EF-1 Reduced Body Weight in HFD-Induced Obese Mice
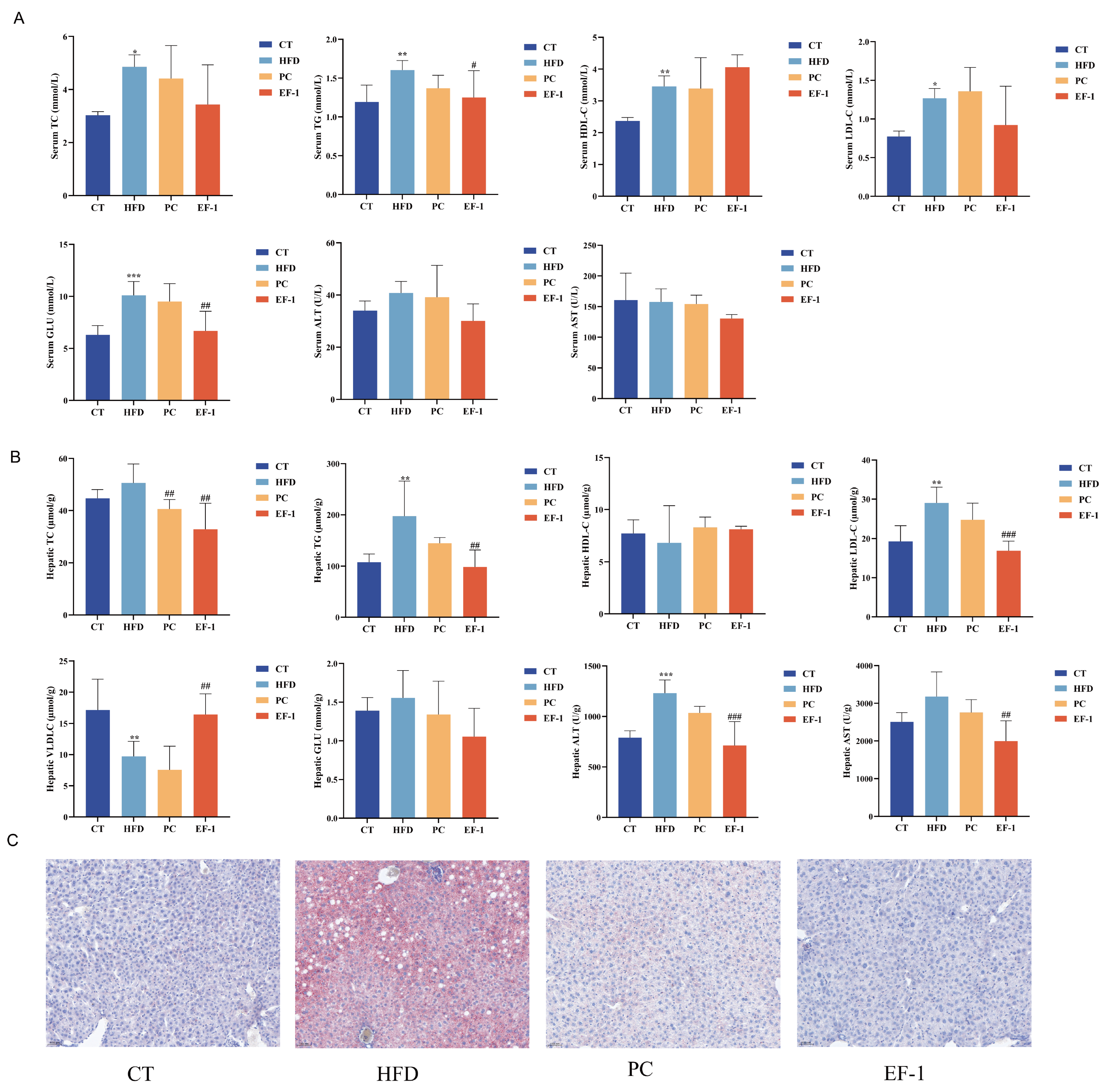
3.2.2. E. faecalis EF-1 Prevented Lipid Accumulation and Reduced Liver and WAT Damage in Obese Mice
3.2.3. E. faecalis EF-1 Prevents the Liver and WAT Damage
3.2.4. E. faecalis EF-1 Regulated the Structure and Composition of the Gut Microbiota in Obese Mice
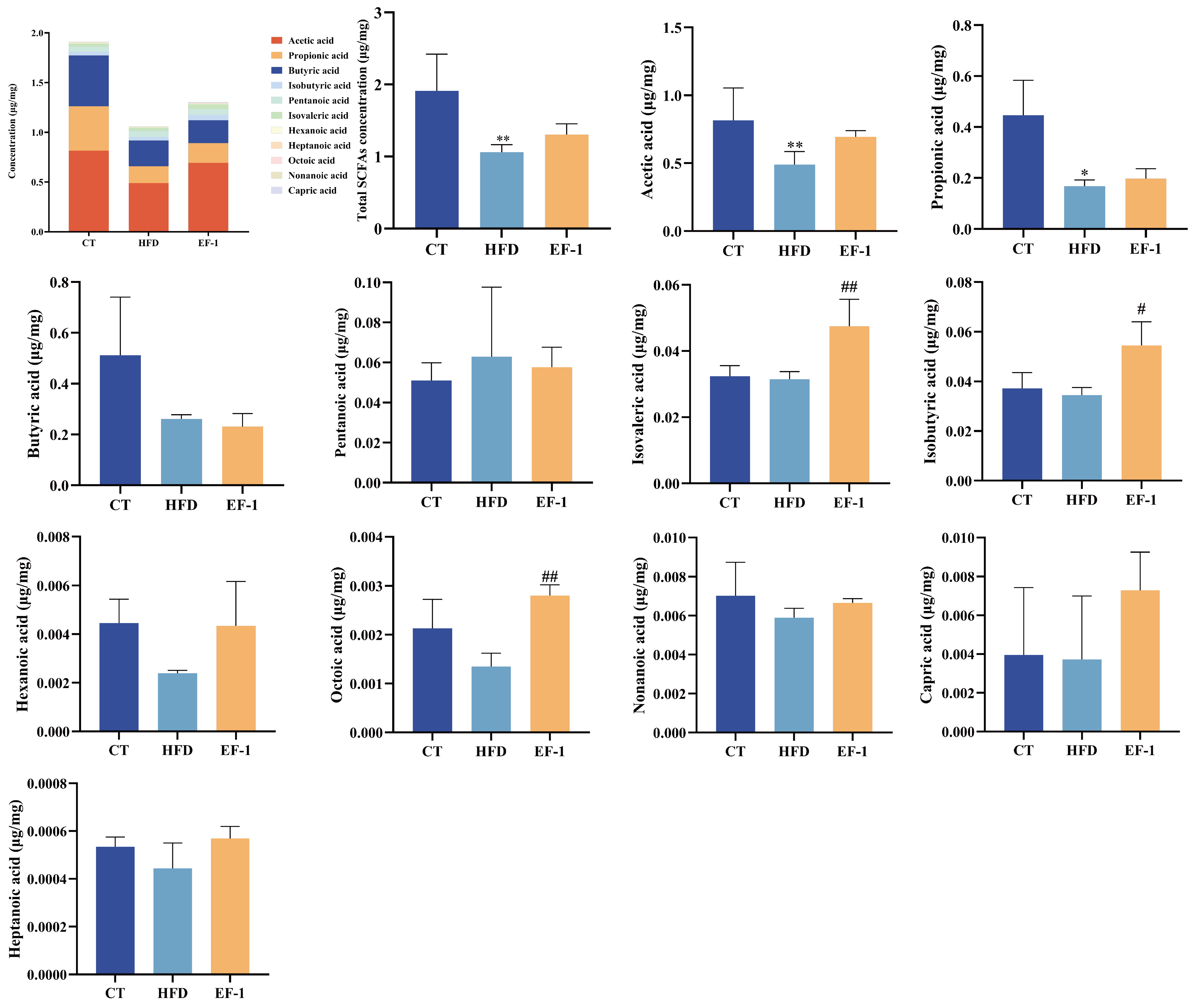
3.2.5. E. faecalis EF-1 Enhanced SCFA Production in HFD-Induced Obese Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Cheong, L.Y.; Xu, A. Intercellular and inter-organ crosstalk in browning of white adipose tissue: Molecular mechanism and therapeutic complications. J. Mol. Cell Biol. 2021, 13, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–s185. Available online: https://www.ajmc.com/view/obesity-definition-comorbidities-causes-burden (accessed on 2 June 2016).
- Wyatt, S.B.; Winters, K.P.; Dubbert, P.M. Overweight and obesity: Prevalence, consequences, and causes of a growing public health problem. Am. J. Med. Sci. 2006, 331, 166–174. [Google Scholar] [CrossRef]
- Gudzune, K.A.; Kushner, R.F. Medications for obesity: A review. JAMA 2024, 332, 571–584. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P. Anti-obesity drugs: A review about their effects and their safety. Expert Opin. Drug Saf. 2012, 11, 459–471. [Google Scholar] [CrossRef]
- Wang, Y.; Kuang, Z.; Yu, X.; Ruhn, K.A.; Kubo, M.; Hooper, L.V. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science 2017, 357, 912–916. [Google Scholar] [CrossRef]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef]
- Turroni, F.; Ventura, M.; Buttó, L.F.; Duranti, S.; O’Toole, P.W.; Motherway, M.O.; van Sinderen, D. Molecular dialogue between the human gut microbiota and the host: A Lactobacillus and Bifidobacterium perspective. Cell. Mol. Life Sci. 2014, 71, 183–203. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Zhang, P.; Li, H.; Qu, K.; Xu, R.; Guo, N.; Zhu, H. Cordycepin alleviated metabolic inflammation in Western diet-fed mice by targeting intestinal barrier integrity and intestinal flora. Pharmacol. Res. 2022, 178, 106191. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kang, X.; Yang, H.; Liu, H.; Yang, X.; Liu, Q.; Tian, H.; Xue, Y.; Ren, P.; Kuang, X.; et al. Lactobacillus acidophilus ameliorates obesity in mice through modulation of gut microbiota dysbiosis and intestinal permeability. Pharmacol. Res. 2022, 175, 106020. [Google Scholar] [CrossRef] [PubMed]
- Molina-Tijeras, J.A.; Diez-Echave, P.; Vezza, T.; Hidalgo-García, L.; Ruiz-Malagón, A.J.; Rodríguez-Sojo, M.J.; Romero, M.; Robles-Vera, I.; García, F.; Plaza-Diaz, J.; et al. Lactobacillus fermentum CECT5716 ameliorates high fat diet-induced obesity in mice through modulation of gut microbiota dysbiosis. Pharmacol. Res. 2021, 167, 105471. [Google Scholar] [CrossRef] [PubMed]
- John, G.K.; Mullin, G.E. The gut microbiome and obesity. Curr. Oncol. Rep. 2016, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Relman, D.A. Clostridium difficile, aging, and the gut: Can microbiome rejuvenation keep us young and healthy? J. Infect. Dis. 2018, 217, 174–176. [Google Scholar] [CrossRef]
- Park, S.; Saravanakumar, K.; Sathiyaseelan, A.; Park, S.; Hu, X.; Wang, M.H. Cellular antioxidant properties of nontoxic exopolysaccharide extracted from Lactobacillales (Weissella cibaria) isolated from Korean kimchi. LWT 2022, 154, 112727. [Google Scholar] [CrossRef]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef]
- Yoo, H.; Rheem, I.; Rheem, S.; Oh, S. Optimizing medium components for the maximum growth of Lactobacillus plantarum JNU 2116 using response surface methodology. Korean J. Food Sci. Anim. Resour. 2018, 38, 240–250. [Google Scholar] [CrossRef]
- Vemuri, R.; Shinde, T.; Shastri, M.D.; Perera, A.P.; Tristram, S.; Martoni, C.J.; Gundamaraju, R.; Ahuja, K.D.K.; Ball, M.; Eri, R. A human origin strain Lactobacillus acidophilus DDS-1 exhibits superior in vitro probiotic efficacy in comparison to plant or dairy origin probiotics. Int. J. Med. Sci. 2018, 15, 840–848. [Google Scholar] [CrossRef]
- Mo, S.J.; Lee, K.; Hong, H.J.; Hong, D.K.; Jung, S.H.; Park, S.D.; Shim, J.J.; Lee, J.L. Effects of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 on overweight and the gut microbiota in humans: Randomized, double-blinded, placebo-controlled clinical trial. Nutrients 2022, 14, 2484. [Google Scholar] [CrossRef]
- Kim, D.; Choi, Y.; Kim, S.; Ha, J.; Oh, H.; Lee, Y.; Kim, Y.; Seo, Y.; Park, E.; Kang, J.; et al. Lactobacillus fermentum SMFM2017-NK4 isolated from kimchi can prevent obesity by inhibiting fat accumulation. Foods 2021, 10, 772. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wen, Z.; Li, X.; Meng, K.; Yang, P. Lactobacillus plantarum FRT10 alleviated high-fat diet-induced obesity in mice through regulating the PPARα signal pathway and gut microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 5959–5972. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, H.; Qi, X.; Sun, Y.; Ma, Y.; Li, Q. Lactobacillus plantarum HF02 alleviates lipid accumulation and intestinal microbiota dysbiosis in high-fat diet-induced obese mice. J. Sci. Food Agric. 2023, 103, 4625–4637. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yan, H.; Lu, Y.; Li, X.; Wang, X.; Shan, Y.; Yi, Y.; Liu, B.; Zhou, Y.; Lü, X. Anti-obesity effect of Lactobacillus rhamnosus LS-8 and Lactobacillus crustorum MN047 on high-fat and high-fructose diet mice base on inflammatory response alleviation and gut microbiota regulation. Eur. J. Nutr. 2020, 59, 2709–2728. [Google Scholar] [CrossRef]
- Kang, B.S.; Seo, J.G.; Lee, G.S.; Kim, J.H.; Kim, S.Y.; Han, Y.W.; Kang, H.; Kim, H.O.; Rhee, J.H.; Chung, M.J.; et al. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J. Microbiol. 2009, 47, 101–109. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ghosh, A.R. Probiotic Enterococcus faecalis AG5 mitigated high fat diet induced obesity and produced propionic acid stimulated apoptosis in 3T3-L1 pre-adipocyte. Life Sci. 2020, 261, 118292. [Google Scholar] [CrossRef]
- Fan, M.; Choi, Y.J.; Wedamulla, N.E.; Tang, Y.; Han, K.I.; Hwang, J.Y.; Kim, E.K. Heat-killed Enterococcus faecalis EF-2001 attenuate lipid accumulation in diet-induced obese (DIO) mice by activating AMPK signaling in liver. Foods 2022, 11, 575. [Google Scholar] [CrossRef]
- Quan, L.H.; Zhang, C.; Dong, M.; Jiang, J.; Xu, H.; Yan, C.; Liu, X.; Zhou, H.; Zhang, H.; Chen, L.; et al. Myristoleic acid produced by enterococci reduces obesity through brown adipose tissue activation. Gut 2020, 69, 1239–1247. [Google Scholar] [CrossRef]
- Boeder, A.M.; Spiller, F.; Carlstrom, M.; Izídio, G.S. Enterococcus faecalis: Implications for host health. World J. Microbiol. Biotechnol. 2024, 40, 190. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, Y.; Peng, Q.; Dia, V.P.; Shi, B. Probiotic activity of ropy Lactiplantibacillus plantarum NA isolated from Chinese northeast sauerkraut and comparative evaluation of its live and heat-killed cells on antioxidant activity and RAW 264.7 macrophage stimulation. Food Funct. 2023, 14, 2481–2495. [Google Scholar] [CrossRef]
- Apostolidis, E.; Kwon, Y.I.; Shetty, K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Food Sci. Emerg. Technol. 2007, 8, 46–54. [Google Scholar] [CrossRef]
- Lee, N.R.; Kwon, T.J.; Chung, E.C.; Bae, J.; Soung, S.H.; Tak, H.J.; Choi, J.Y.; Lee, Y.E.; Won Hwang, N.; Lee, J.S.; et al. Combination of Lacticaseibacillus paracasei BEPC22 and Lactiplantibacillus plantarum BELP53 attenuates fat accumulation and alters the metabolome and gut microbiota in mice with high-fat diet-induced obesity. Food Funct. 2024, 15, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Sahraoui, Y.; Fayolle, K.; Leriche, F.; Le Flèche-Matéos, A.; Sadoun, D. Antibacterial and technological properties of Lactococcus lactis ssp. lactis KJ660075 strain selected for its inhibitory power against Staphylococcus aureus for cheese quality improving. J. Food Sci. Technol. 2015, 52, 7133–7142. [Google Scholar] [CrossRef]
- Cai, H.; Wen, Z.; Zhao, L.; Yu, D.; Meng, K.; Yang, P. Lactobacillus plantarum FRT4 alleviated obesity by modulating gut microbiota and liver metabolome in high-fat diet-induced obese mice. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fang, R.; Lu, X.; Zhang, Y.; Yang, M.; Su, Y.; Jiang, Y.; Man, C. Lactobacillus reuteri J1 prevents obesity by altering the gut microbiota and regulating bile acid metabolism in obese mice. Food Func. 2022, 13, 6688–6701. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Din, A.U.; Hassan, A.; Wang, Y.; Wang, G. Beneficial effects of Enterococcus faecalis in hypercholesterolemic mice on cholesterol transportation and gut microbiota. Appl. Microbiol. Biotechnol. 2019, 103, 3181–3191. [Google Scholar] [CrossRef]
- Zeng, S.L.; Li, S.Z.; Xiao, P.T.; Cai, Y.Y.; Chu, C.; Chen, B.Z.; Li, P.; Li, J.; Liu, E.H. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci. Adv. 2020, 6, eaax6208. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Klaver, F.A.; van der Meer, R. The assumed assimilation of cholesterol by Lactobacilli and Bifidobacterium bifidum is due to their bile salt-deconjugating activity. Appl. Environ. Microbiol. 1993, 59, 1120–1124. [Google Scholar] [CrossRef]
- Pereira, D.I.; Gibson, G.R. Cholesterol assimilation by lactic acid bacteria and bifidobacteria isolated from the human gut. Appl. Environ. Microbiol. 2002, 68, 4689–4693. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, W.; Xia, Y.; Xiong, Z.; Ai, L. Cholesterol-lowering potentials of Lactobacillus strain overexpression of bile salt hydrolase on high cholesterol diet-induced hypercholesterolemic mice. Food Funct. 2019, 10, 1684–1695. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Hu, X.J.; Singh, W.; Geng, W.; Tikhonova, I.G.; Lin, J. The complex structure of bile salt hydrolase from Lactobacillus salivarius reveals the structural basis of substrate specificity. Sci. Rep. 2019, 9, 12438. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.S.; Joung, J.Y.; Lee, J.Y.; Kim, Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS ONE 2018, 13, e0192021. [Google Scholar] [CrossRef]
- Won, G.; Choi, S.I.; Park, N.; Kim, J.E.; Kang, C.H.; Kim, G.H. In vitro antidiabetic, antioxidant activity, and probiotic activities of Lactiplantibacillus plantarum and Lacticaseibacillus paracasei strains. Curr. Microbiol. 2021, 78, 3181–3191. [Google Scholar] [CrossRef]
- Sethi, J.K.; Vidal-Puig, A.J. Thematic review series: Adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J. Lipid Res. 2007, 48, 1253–1262. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Abarike, E.D.; Lu, Y.; Hlordzi, V.; Sakyi, M.E.; Afriyie, G.; Wang, Z.; Li, Y.; Xie, C.X. Mechanisms and the role of probiotic Bacillus in mitigating fish pathogens in aquaculture. Fish Physiol. Biochem. 2020, 46, 819–841. [Google Scholar] [CrossRef]
- Selvin, J.; Maity, D.; Sajayan, A.; Kiran, G.S. Revealing antibiotic resistance in therapeutic and dietary probiotic supplements. J. Glob. Antimicrob. Resist. 2020, 22, 202–205. [Google Scholar] [CrossRef]
- Motonaga, C.; Kondoh, M.; Hayashi, A.; Okamori, M.; Kitamura, Y.; Shimada, T. Effect of enterococcus faecalis FK-23 on anti-obesity in diet-induced obesity mice. Br. J. Nutr. 2014, 112, 868–875. [Google Scholar] [CrossRef]
- Dowman, J.K.; Tomlinson, J.W.; Newsome, P.N. Pathogenesis of non-alcoholic fatty liver disease. QJM 2010, 103, 71–83. [Google Scholar] [CrossRef]
- Choi, M.J.; Yu, H.; Kim, J.I.; Seo, H.; Kim, J.G.; Kim, S.K.; Lee, H.S.; Cheon, H.G. Anti-obesity effects of Lactiplantibacillus plantarum SKO-001 in high-fat diet-induced obese mice. Eur. J. Nutr. 2023, 62, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qiu, L.; Xu, X.; Liu, Z.; Zhan, H.; Tao, X.; Shah, N.P.; Wei, H. Beneficial effects of probiotic cholesterol-lowering strain of Enterococcus faecium WEFA23 from infants on diet-induced metabolic syndrome in rats. J. Dairy Sci. 2017, 100, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, J.; Yin, T.; Lv, H.; Zhang, P.; Li, H. Enterococcus faecium R0026 combined with Bacillus subtilis R0179 prevent obesity-associated hyperlipidemia and modulate gut microbiota in C57BL/6 Mice. J. Microbiol. Biotechnol. 2020, 31, 181–188. [Google Scholar] [CrossRef] [PubMed]
- van Beek, J.H.; de Moor, M.H.; de Geus, E.J.; Lubke, G.H.; Vink, J.M.; Willemsen, G.; Boomsma, D.I. The genetic architecture of liver enzyme levels: GGT, ALT and AST. Behav. Genet. 2013, 43, 329–339. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef]
- Panattoni, A.; Calvigioni, M.; Benvenuti, L.; D’Antongiovanni, V.; Pellegrini, C.; Di Salvo, C.; Mazzantini, D.; Celandroni, F.; Fornai, M.; Antonioli, L.; et al. The administration of Enterococcus faecium SF68 counteracts compositional shifts in the gut microbiota of diet-induced obese mice. Front. Microbiol. 2022, 13, 1054097. [Google Scholar] [CrossRef]
- Yang, J.Y.; Lee, Y.S.; Kim, Y.; Lee, S.H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.S.; Lim, H.S.; Kim, M.S.; et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef]
- Zagato, E.; Pozzi, C.; Bertocchi, A.; Schioppa, T.; Saccheri, F.; Guglietta, S.; Fosso, B.; Melocchi, L.; Nizzoli, G.; Troisi, J.; et al. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat. Microbiol. 2020, 5, 511–524. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, J.; Kim, M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 2014, 14, 277–288. [Google Scholar] [CrossRef]
- Chen, S.; Xu, M.; Zhou, M.; He, Y.; Li, Y.; Lang, H.; Wei, X.; Yan, L.; Xu, H. Hibiscus manihot L improves obesity in mice induced by a high-fat diet. J. Funct. Foods 2022, 89, 104953. [Google Scholar] [CrossRef]
- Hong, Y.; Sheng, L.; Zhong, J.; Tao, X.; Zhu, W.; Ma, J.; Yan, J.; Zhao, A.; Zheng, X.; Wu, G.; et al. Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, J.; Xu, Y.; Yang, H.; Wang, J.; Xue, C.; Yan, X.; Su, L. Anti-inflammation effects of fucosylated chondroitin sulphate from Acaudina molpadioides by altering gut microbiota in obese mice. Food Funct. 2019, 10, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, Y.; Li, X.; Sun, B. Dynamic balancing of intestinal short-chain fatty acids: The crucial role of bacterial metabolism. Trends Food Sci. Technol. 2020, 100, 118–130. [Google Scholar] [CrossRef]
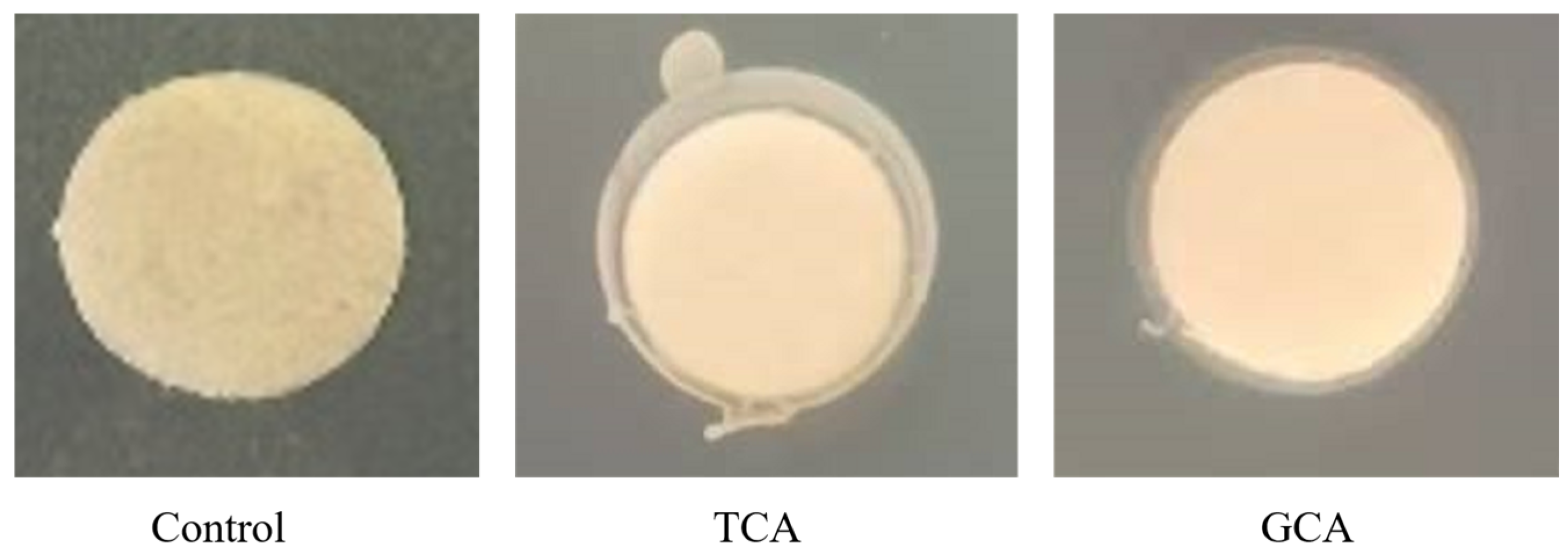



| Condition | Culture Time/h | Cell No. (log CFU/mL) | Survival Rate/% |
|---|---|---|---|
| Simulated gastric juice | 0 | 5.17 ± 0.55 c | |
| 1.5 | 5.33 ± 1.35 c | 103.23 ± 26.14 c | |
| 3 | 5.67 ± 0.85 c | 109.68 ± 16.46 c | |
| Simulated intestinal juice | 0 | 4.87 ± 1.10 c | |
| 2 | 9.6 ± 1.31 c | 197.25 ± 26.87 c | |
| 4 | 31.67 ± 11.93 a | 650.64 ± 245.13 b | |
| 8 | 43.3 ± 2.52 a | 890.35 ± 51.71 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Wang, Q.; Han, X.; Zhang, H.; Wang, N.; Huang, Y.; Yang, P.; Zhang, R.; Meng, K. In Vitro Evaluation of Probiotic Activities and Anti-Obesity Effects of Enterococcus faecalis EF-1 in Mice Fed a High-Fat Diet. Foods 2024, 13, 4095. https://doi.org/10.3390/foods13244095
Cai H, Wang Q, Han X, Zhang H, Wang N, Huang Y, Yang P, Zhang R, Meng K. In Vitro Evaluation of Probiotic Activities and Anti-Obesity Effects of Enterococcus faecalis EF-1 in Mice Fed a High-Fat Diet. Foods. 2024; 13(24):4095. https://doi.org/10.3390/foods13244095
Chicago/Turabian StyleCai, Hongying, Qingya Wang, Xiling Han, Haiou Zhang, Na Wang, Yuyin Huang, Peilong Yang, Rui Zhang, and Kun Meng. 2024. "In Vitro Evaluation of Probiotic Activities and Anti-Obesity Effects of Enterococcus faecalis EF-1 in Mice Fed a High-Fat Diet" Foods 13, no. 24: 4095. https://doi.org/10.3390/foods13244095
APA StyleCai, H., Wang, Q., Han, X., Zhang, H., Wang, N., Huang, Y., Yang, P., Zhang, R., & Meng, K. (2024). In Vitro Evaluation of Probiotic Activities and Anti-Obesity Effects of Enterococcus faecalis EF-1 in Mice Fed a High-Fat Diet. Foods, 13(24), 4095. https://doi.org/10.3390/foods13244095







