Amelioration of LPS-Induced Jejunum Injury and Mucus Barrier Damage in Mice by IgY Embedded in W/O/W Emulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of Embedded IgY Double Emulsion
2.2. Animal Ethics Statement
2.3. H&E and AB-PAS Staining
2.4. Immunohistochemistry
2.5. RNA Isolation and RT-qPCR Analysis
2.6. ELISA
2.7. Statistical Analysis
3. Results
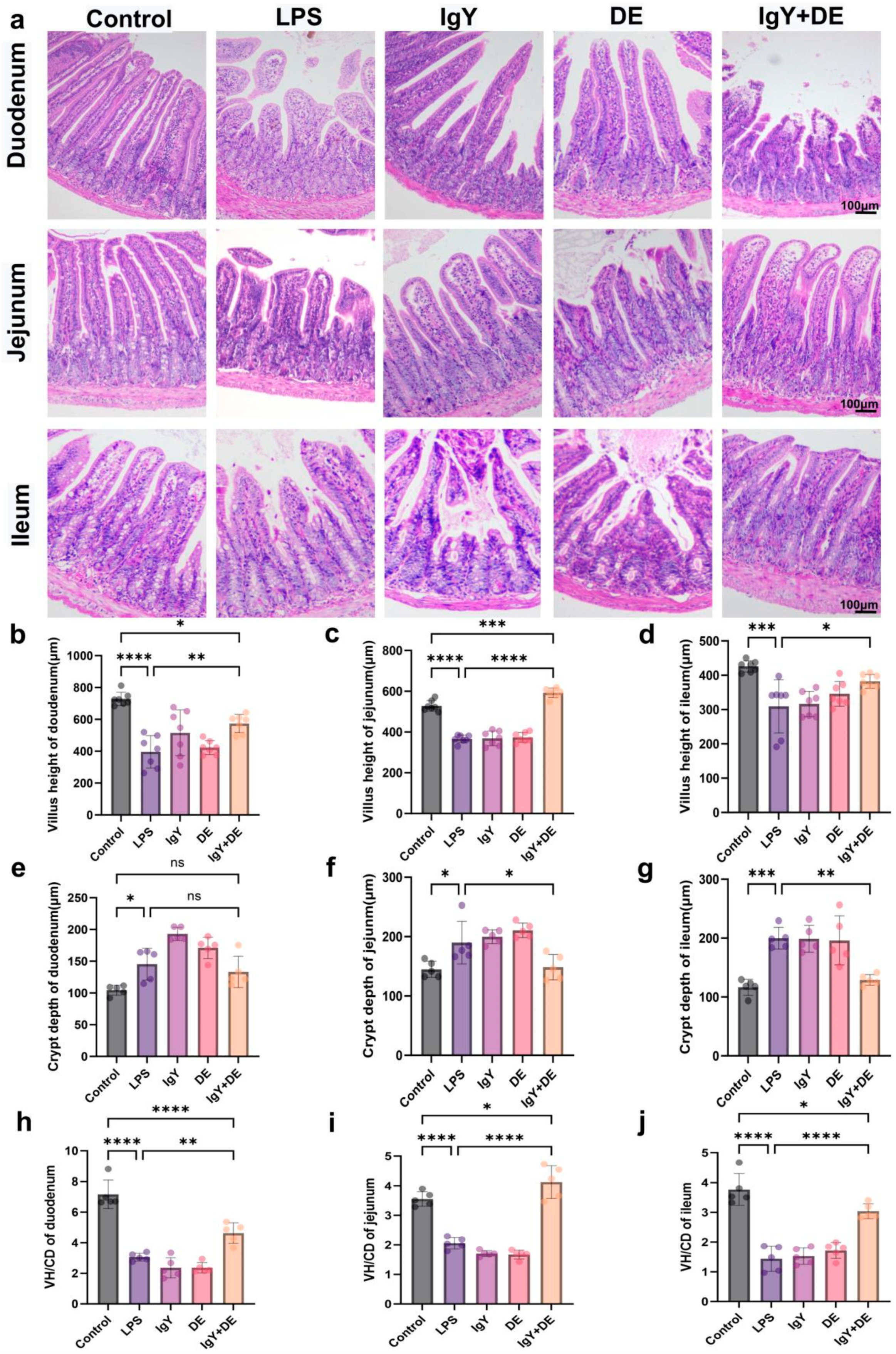
3.1. IgY + DE Alleviates LPS-Induced Structural Damage of Small Intestinal Tissues
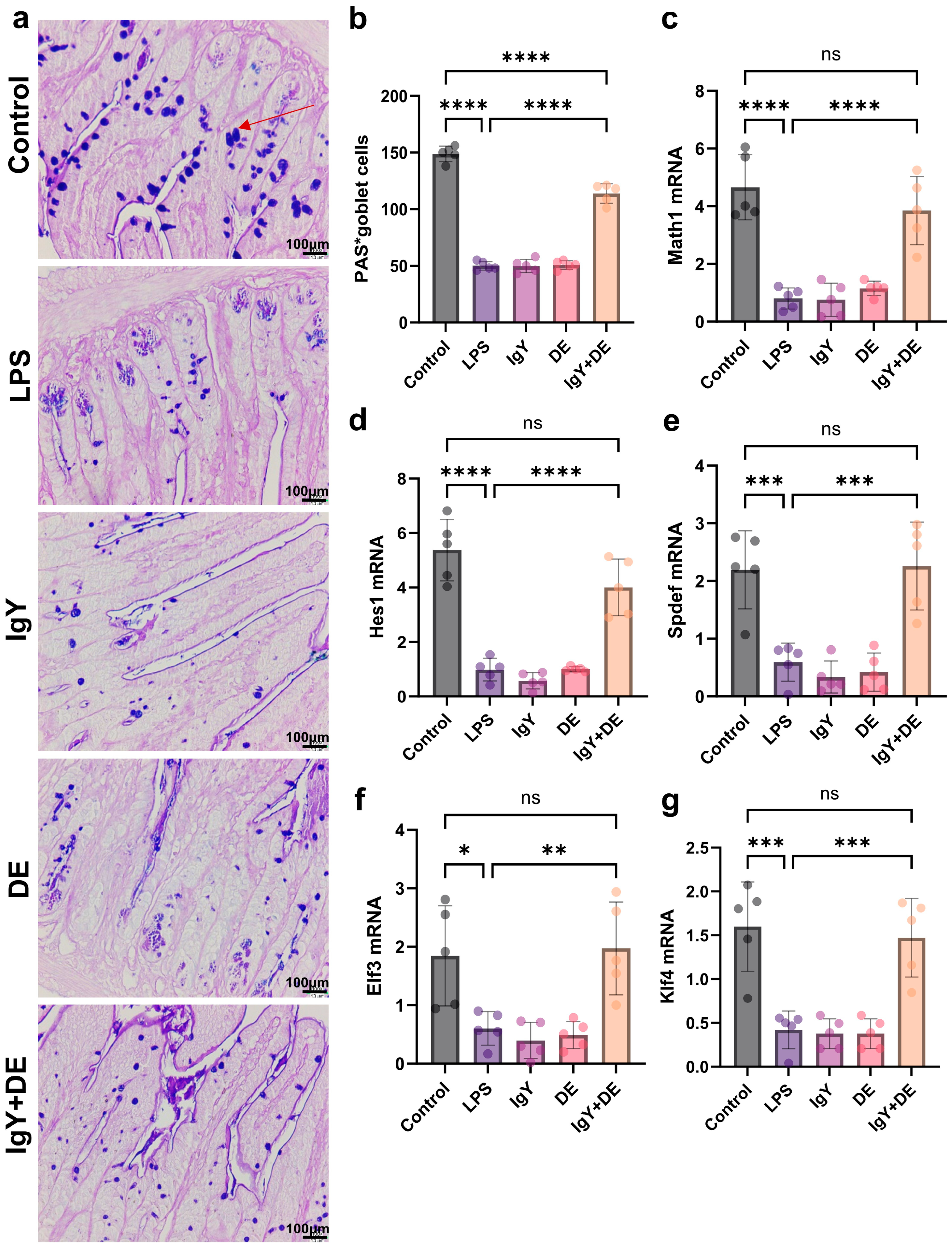
3.2. IgY + DE Increased Associated Transcription Factors and Goblet Cell Differentiation
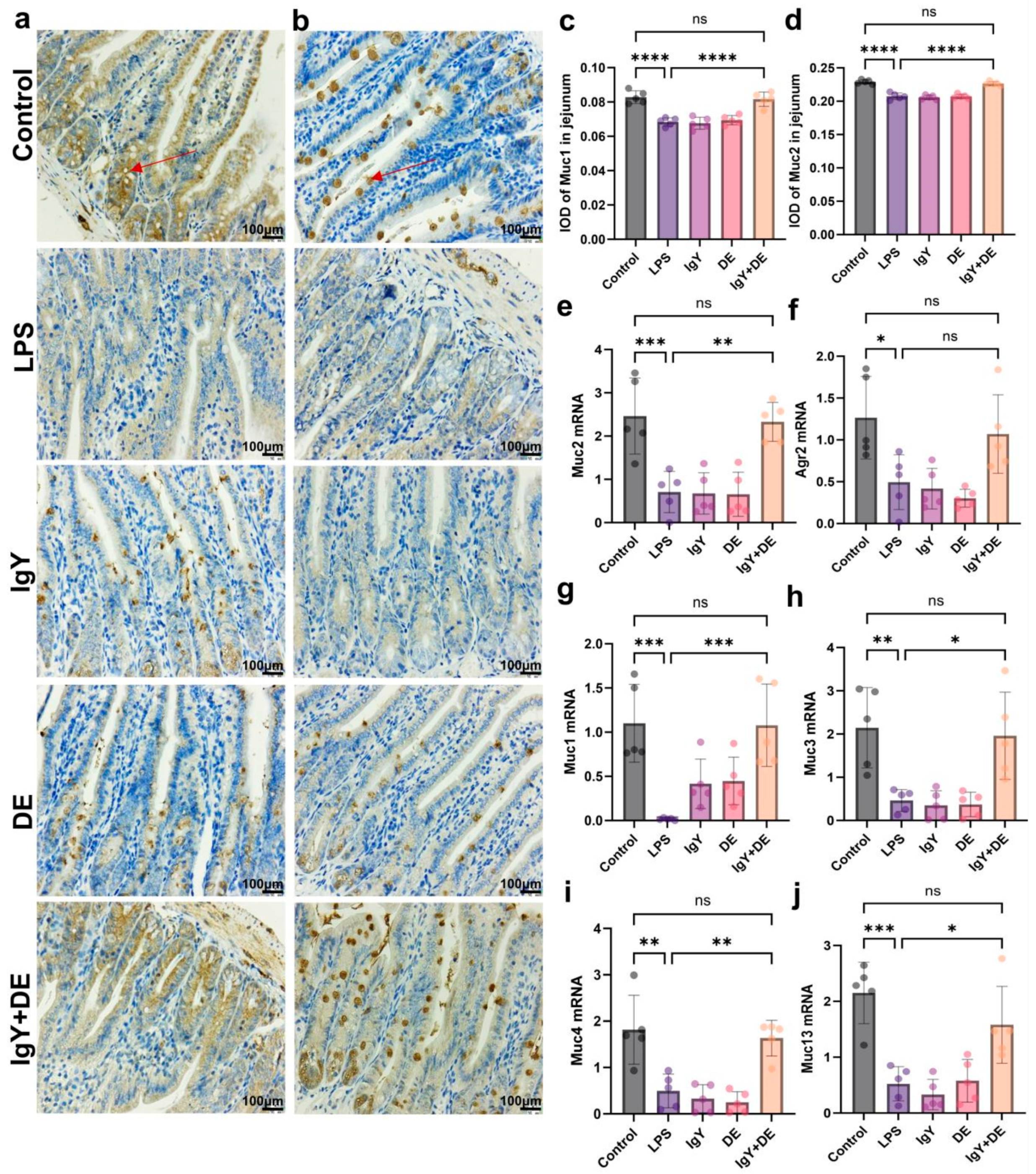
3.3. IgY + DE Ameliorated the LPS-Induced Reduction in Mucin Number and Markers
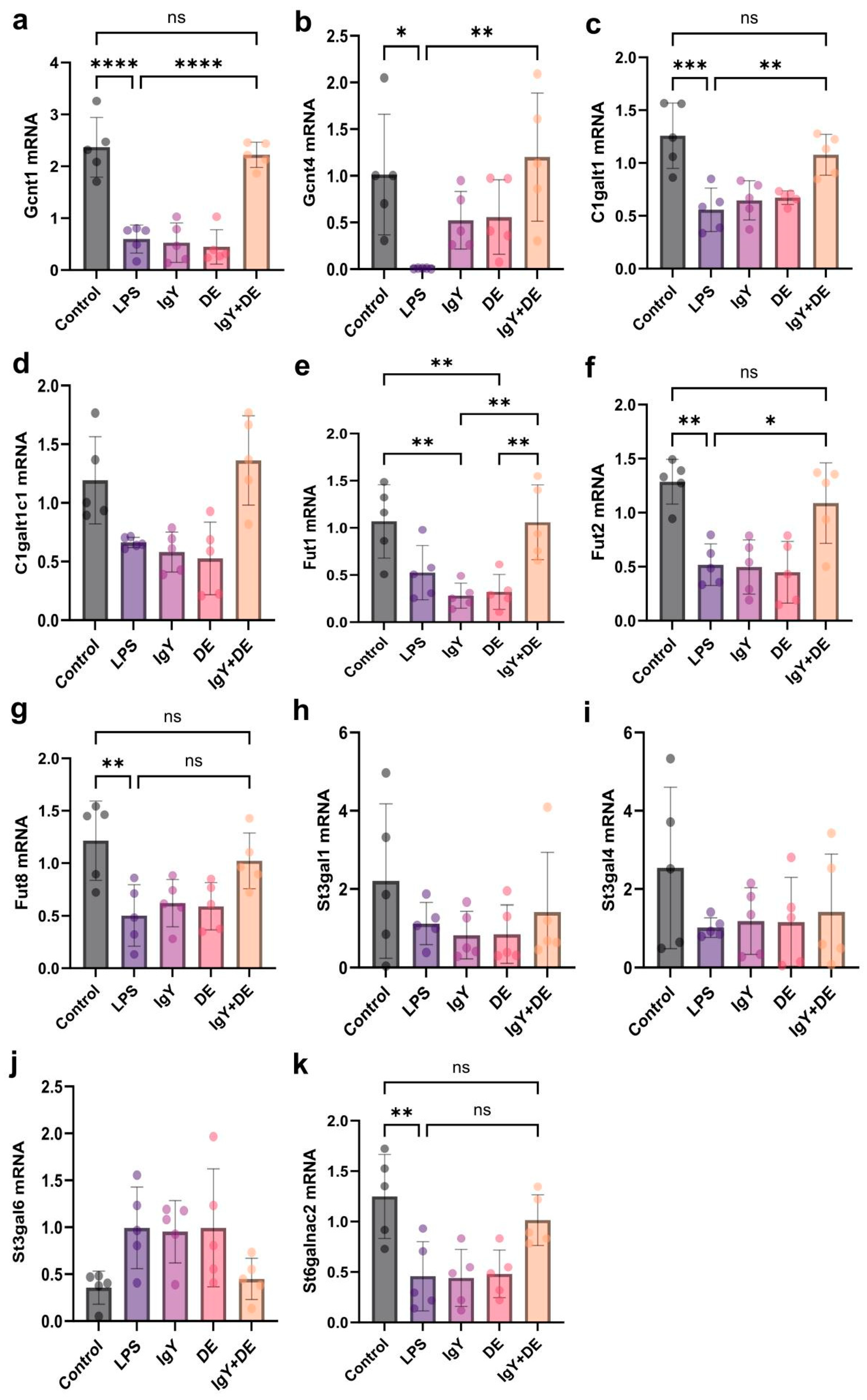
3.4. IgY + DE Increased the Expression of Glycosyltransferases Involved in Mucin Glycosylation
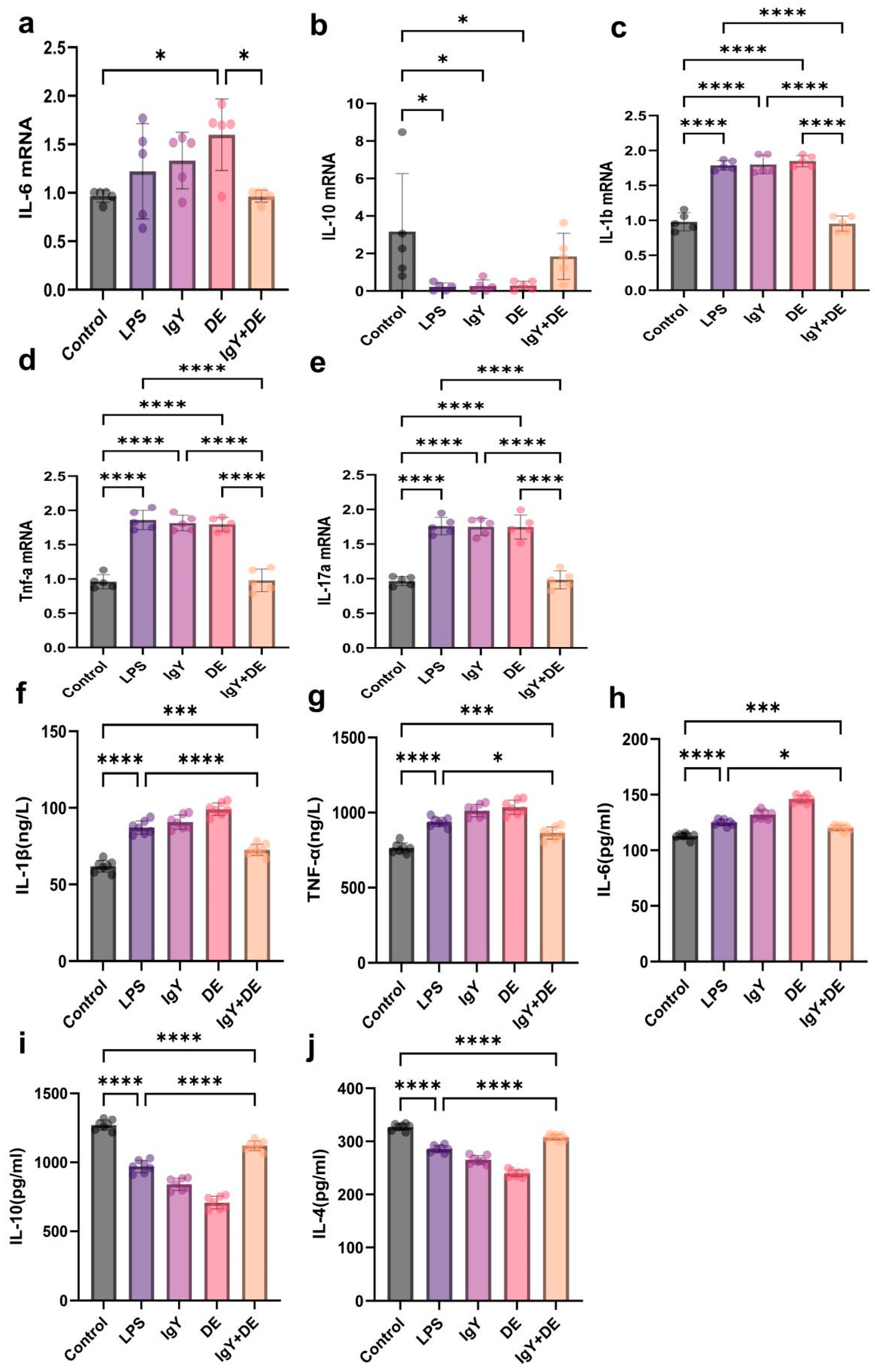
3.5. IgY + DE Inhibits LPS-Induced Expression of Inflammatory Factors in Mouse Jejunum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cox, C.M.; Lu, R.; Salcin, K.; Wilson, J.M. The endosomal protein endotubin is required for enterocyte differentiation. Cell Mol. Gastroenterol. Hepatol. 2018, 5, 145–156. [Google Scholar] [CrossRef]
- Guo, S.; Nighot, M.; Al-Sadi, R.; Alhmoud, T.; Nighot, P.; Ma, T.Y. Lipopolysaccharide regulation of intestinal tight junction permeability is mediated by TLR4 signal transduction pathway activation of FAK and MyD88. J. Immunol. 2015, 195, 4999–5010. [Google Scholar] [CrossRef]
- Tan, J.; Liu, S.; Guo, Y.; Applegate, T.J.; Eicher, S.D. Dietary L-arginine supplementation attenuates lipopolysaccharide-induced inflammatory response in broiler chickens. Br. J. Nutr. 2014, 111, 1394–1404. [Google Scholar] [CrossRef]
- Li, R.; Song, Z.; Zhao, J.; Huo, D.; Fan, Z.; Hou, D.X.; He, X. Dietary L-theanine alleviated lipopolysaccharide-induced immunological stress in yellow-feathered broilers. Anim. Nutr. 2018, 4, 265–272. [Google Scholar] [CrossRef]
- Abbas, A.T.; El-Kafrawy, S.A.; Sohrab, S.S.; Azhar, E. IgY antibodies for the immunoprophylaxis and therapy of respiratory infections. Hum. Vaccin. Immunother. 2019, 15, 264–275. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Jin, L.; Zhen, Y.; Lu, Y.; Li, S.; You, J. Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: A review. Biotechnol. Adv. 2011, 29, 860–868. [Google Scholar] [CrossRef]
- Leiva, C.L.; Gallardo, M.J.; Casanova, N.; Terzolo, H.; Chacana, P. IgY-technology (egg yolk antibodies) in human medicine: A review of patents and clinical trials. Int. Immunopharmacol. 2020, 81, 106269. [Google Scholar] [CrossRef]
- Marcus, E.A.; Scott, D.R. Cell lysis is responsible for the appearance of extracellular urease in Helicobacter pylori. Helicobacter 2001, 6, 93–99. [Google Scholar] [CrossRef]
- Schreiner, B.; Viertlboeck, B.C.; Gobel, T.W. A striking example of convergent evolution observed for the ggFcR:IgY interaction closely resembling that of mammalian FcR:IgG. Dev. Comp. Immunol. 2012, 36, 566–571. [Google Scholar] [CrossRef]
- Lee, W.; Atif, A.S.; Tan, S.C.; Leow, C.H. Insights into the chicken IgY with emphasis on the generation and applications of chicken recombinant monoclonal antibodies. J. Immunol. Methods 2017, 447, 71–85. [Google Scholar] [CrossRef]
- Larsson, A.; Balow, R.M.; Lindahl, T.L.; Forsberg, P.O. Chicken antibodies: Taking advantage of evolution—A review. Poult. Sci. 1993, 72, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Van Nguyen, S.; Icatlo, F.J.; Umeda, K.; Kodama, Y. Oral passive IgY-based immunotherapeutics: A novel solution for prevention and treatment of alimentary tract diseases. Hum. Vaccin. Immunother. 2013, 9, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Schubert, A.; Zajac, J.; Dyck, T.; Oelkrug, C. IgY antibodies in human nutrition for disease prevention. Nutr. J. 2015, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Shade, C.W. Liposomes as advanced delivery systems for nutraceuticals. Integr. Med. 2016, 15, 33–36. [Google Scholar]
- Cao, P.; Han, F.Y.; Grondahl, L.; Xu, Z.P.; Li, L. Enhanced oral vaccine efficacy of polysaccharide-coated calcium phosphate nanoparticles. ACS Omega 2020, 5, 18185–18197. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, R.; Xu, Z.; Zhang, S.; Liu, C.; Zhu, K.; Wang, P. Protective effect of IgY embedded in W/O/W emulsion on LPS enteritis-induced colonic injury in mice. Nutrients 2024, 16, 3361. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, J.; Park, I.; Huh, C.; Baek, Y.; Park, J. Protective effect of microencapsulation consisting of multiple emulsification and heat gelation processes on immunoglobulin in yolk. J. Food Sci. 2005, 70, E148–E151. [Google Scholar] [CrossRef]
- Johansson, M.E.; Jakobsson, H.E.; Holmen-Larsson, J.; Schutte, A.; Ermund, A. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe 2015, 18, 582–592. [Google Scholar] [CrossRef]
- Arike, L.; Holmen-Larsson, J.; Hansson, G.C. Intestinal Muc2 mucin O-glycosylation is affected by microbiota and regulated by differential expression of glycosyltranferases. Glycobiology 2017, 27, 318–328. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Khan, S.; Waliullah, S.; Godfrey, V.; Khan, M.; Ramachandran, R.A.; Cantarel, B.L.; Behrendt, C. Dietary simple sugars alter microbial ecology in the gut and promote colitis in mice. Sci. Transl. Med. 2020, 12, eaay6218. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin alleviates oxidative stress in sleep deprived mice: Involvement of small intestinal mucosa injury. Int. Immunopharmacol. 2020, 78, 106041. [Google Scholar] [CrossRef] [PubMed]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, C. The relationship between intestinal goblet cells and the immune response. Biosci. Rep. 2020, 40, BSR20201471. [Google Scholar] [CrossRef]
- McCauley, H.A.; Guasch, G. Three cheers for the goblet cell: Maintaining homeostasis in mucosal epithelia. Trends Mol. Med. 2015, 21, 492–503. [Google Scholar] [CrossRef]
- Yu, T.X.; Chung, H.K.; Xiao, L.; Piao, J.J.; Lan, S.; Jaladanki, S.K.; Turner, D.J. Long noncoding RNA H19 impairs the intestinal barrier by suppressing autophagy and lowering paneth and goblet cell function. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 611–625. [Google Scholar] [CrossRef]
- Stanger, B.Z.; Datar, R.; Murtaugh, L.C.; Melton, D.A. Direct regulation of intestinal fate by Notch. Proc. Natl. Acad. Sci. USA 2005, 102, 12443–12448. [Google Scholar] [CrossRef]
- Iiboshi, Y.; Nezu, R.; Cui, L.; Chen, K.; Khan, J.; Yoshida, H.; Sando, K. Adhesive mucous gel layer and mucus release as intestinal barrier in rats. JPEN J. Parenter. Enter. Nutr. 1996, 20, 98–104. [Google Scholar]
- Smirnova, M.G.; Guo, L.; Birchall, J.P.; Pearson, J.P. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol. 2003, 221, 42–49. [Google Scholar] [CrossRef]
- van Putten, J.; Strijbis, K. Transmembrane mucins: Signaling receptors at the intersection of inflammation and cancer. J. Innate Immun. 2017, 9, 281–299. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, G.; Li, Y.; Zhu, Y.; Yu, X.; Zhao, F.; Li, H. Intake of fish oil specifically modulates colonic muc2 expression in middle-aged rats by suppressing the glycosylation process. Mol. Nutr. Food Res. 2018, 62, 1700661. [Google Scholar] [CrossRef] [PubMed]
- Tardy, F.; Louisot, P.; Martin, A. Effect of dietary fiber at weaning on protein glycosylation in the rat small intestine. Int. J. Biochem. Cell Biol. 1995, 27, 403–413. [Google Scholar] [CrossRef]
- Mastrodonato, M.; Calamita, G.; Mentino, D.; Scillitani, G. High-fat diet alters the glycosylation patterns of duodenal mucins in a murine model. J. Histochem. Cytochem. 2020, 68, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Mastrodonato, M.; Mentino, D.; Portincasa, P.; Calamita, G.; Liquori, G.E.; Ferri, D. High-fat diet alters the oligosaccharide chains of colon mucins in mice. Histochem. Cell Biol. 2014, 142, 449–459. [Google Scholar]
- Gamage, H.; Chong, R.; Bucio-Noble, D.; Kautto, L.; Hardikar, A.A.; Ball, M.S.; Molloy, M.P. Changes in dietary fiber intake in mice reveal associations between colonic mucin O-glycosylation and specific gut bacteria. Gut Microbes 2020, 12, 1802209. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef]
- Fu, J.; Wei, B.; Wen, T.; Johansson, M.E.; Liu, X.; Bradford, E.; Thomsson, K.A. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J. Clin. Investig. 2011, 121, 1657–1666. [Google Scholar] [CrossRef]
- Wu, Y.; Murray, G.K.; Byrne, E.M.; Sidorenko, J.; Visscher, P.M.; Wray, N.R. GWAS of peptic ulcer disease implicates Helicobacter pylori infection, other gastrointestinal disorders and depression. Nat. Commun. 2021, 12, 1146. [Google Scholar] [CrossRef]
- Wang, D.; Du, Y.; Wang, S.; You, Z.; Liu, Y. Effects of sodium humate and glutamine combined supplementation on growth performance, diarrhea incidence, blood parameters, and intestinal microflora of weaned calves. Anim. Sci. J. 2021, 92, e13584. [Google Scholar] [CrossRef]
- Van Deventer, S.J. Tumour necrosis factor and Crohn’s disease. Gut 1997, 40, 443. [Google Scholar] [CrossRef]
- Schmitt, H.; Neurath, M.F.; Atreya, R. Role of the IL23/IL17 pathway in crohn’s disease. Front. Immunol. 2021, 12, 622934. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Cao, M.; Chen, P.; Cooper, D.K.; Zhao, Y.; Wei, L.; Xu, J. TNF-α promotes human antibody-mediated complement-dependent cytotoxicity of porcine endothelial cells through downregulating P38-mediated Occludin expression. Cell Commun. Signal. 2019, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Attanzio, A.; Allegra, M.; Gentile, C.; Livrea, M.A. Indicaxanthin inhibits NADPH oxidase (NOX)-1 activation and NF-κB-dependent release of inflammatory mediators and prevents the increase of epithelial permeability in IL-1β-exposed Caco-2 cells. Br. J. Nutr. 2014, 111, 415–423. [Google Scholar] [CrossRef]
- Yu, J.; Liu, T.; Gao, Z.; Liu, R.; Wang, Z.; Chen, Y.; Cao, J. Akkermansia muciniphila colonization alleviating high fructose and restraint stress-induced jejunal mucosal barrier disruption. Nutrients 2022, 14, 3164. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, J.; Kong, B.; Shuai, W.; Huang, H. Tirzepatide attenuates lipopolysaccharide-induced left ventricular remodeling and dysfunction by inhibiting the TLR4/NF-kB/NLRP3 pathway. Int. Immunopharmacol. 2023, 120, 110311. [Google Scholar] [CrossRef]
- Xiong, W.; Ma, H.; Zhang, Z.; Jin, M.; Wang, J.; Xu, Y.; Wang, Z. Retraction: Icariin enhances intestinal barrier function by inhibiting NF-κB signaling pathways and modulating gut microbiota in a piglet model. RSC Adv. 2023, 13, 2402. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Indhuprakash, S.T.; Gopal, G.; Ambi, S.V.; Krishnan, U.M.; Diraviyam, T. Passive immunotherapy using chicken egg yolk antibody (IgY) against diarrheagenic E. coli: A systematic review and meta-analysis. Int. Immunopharmacol. 2022, 102, 108381. [Google Scholar] [CrossRef]
- Majalekar, P.P.; Shirote, P.J. Fluoroquinolones: Blessings or curses. Curr. Drug Targets 2020, 21, 1354–1370. [Google Scholar] [CrossRef]
- Ye, R.; Huang, J.; Wang, Z.; Chen, Y.; Dong, Y. Trace element selenium effectively alleviates intestinal diseases. Int. J. Mol. Sci. 2021, 22, 11708. [Google Scholar] [CrossRef]
- Huang, X.; Yang, X.; Zhang, M.; Li, T.; Zhu, K.; Dong, Y.; Lei, X. SELENOI functions as a key modulator of ferroptosis pathway in colitis and colorectal cancer. Adv. Sci. 2024, 11, e2404073. [Google Scholar] [CrossRef]
- Huang, J.Q.; Jiang, Y.Y.; Ren, F.Z.; Lei, X.G. Novel role and mechanism of glutathione peroxidase-4 in nutritional pancreatic atrophy of chicks induced by dietary selenium deficiency. Redox Biol. 2022, 57, 102482. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, L.; Zhu, K.; Luo, Y.; Huang, X.; Dong, Y.; Huang, J. Biomimetic microRNAs-selenium-nanocomposites for targeted and combined hyperlipidemia therapy. Adv. Health Mater. 2024, 13, e2400064. [Google Scholar] [CrossRef] [PubMed]
- Davalos-Pantoja, L.; Ortega-Vinuesa, J.L.; Bastos-Gonzalez, D.; Hidalgo-Alvarez, R. A comparative study between the adsorption of IgY and IgG on latex particles. J. Biomater. Sci. Polym. Ed. 2000, 11, 657–673. [Google Scholar] [CrossRef] [PubMed]
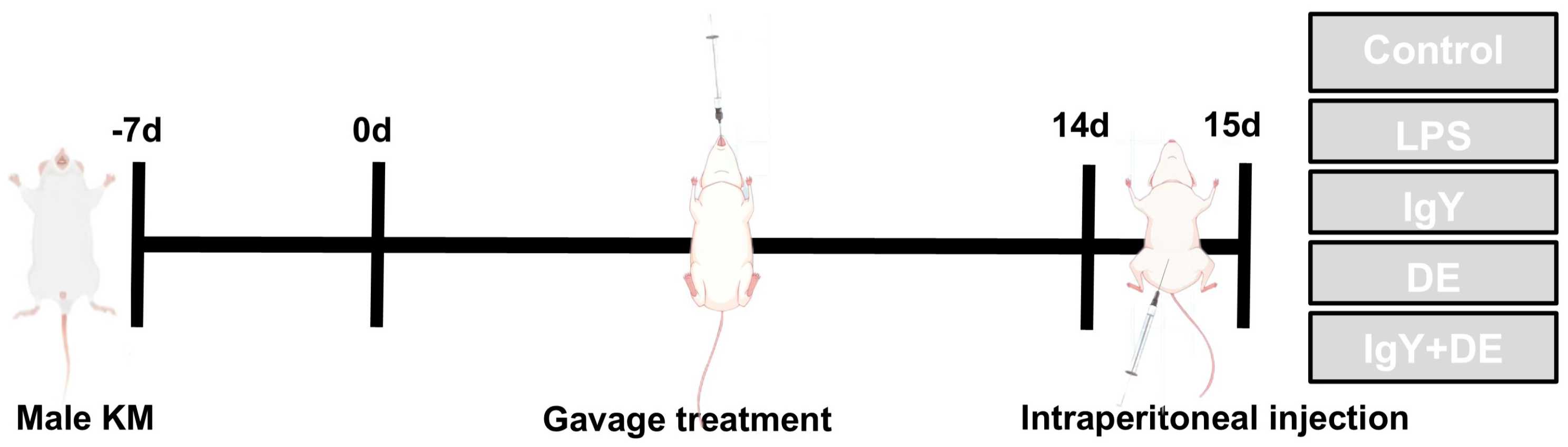
| Group Name | Gavage Treatment | Intraperitoneal Injection |
|---|---|---|
| Control | 0.5 mL 0.9% NaCl | 0.2 mL 0.9% NaCl |
| LPS | 0.5 mL 0.9% NaCl | 0.2 mL LPS (5 mg/kg) |
| IgY | 0.5 mL IgY (48 mg/mL) | 0.2 mL LPS (5 mg/kg) |
| DE | 0.5 mL double-emulsion | 0.2 mL LPS (5 mg/kg) |
| IgY + DE | 0.5 mL double-emulsion embedded with IgY (48 mg/mL) | 0.2 mL LPS (5 mg/kg) |
| Primers | Forward | Reverse |
|---|---|---|
| RPL19 | GAAGGTCAAAGGGAATGTGTTCA | CCTGTTGCTCACTTGT |
| Math1 | CAAGTGTGTCCAGCAGTGTG | TTGAGTTTCTTCAAGGCGGC |
| Spdef | AGGTGCAATCGATGGTTGTG | AGGGTCTGCTGTGATGTTCA |
| EIf3 | CCTATGAGAAGCTGAGCCGA | ACCTCTTCTTCCTTCCAGCC |
| KIf4 | GTGCCCCGACTAACCGTTG | GTCGTTGAACTCCTCGGTCT |
| Hes1 | CCGGCATTCCAAGCTAGAGA | GGTATTTCCCCAACACGCTC |
| Agr2 | GCCAAAGACACCACAGTCAA | CCATCAAGGGTCTGTTGCTT |
| Muc1 | GACATCTTTCCAACCCAGGACA | AAGAGAGACTGCTACTGCCATTAC |
| Muc2 | ATGCCCACCTCCTCAAAGAC | GTAGTTTCCGTTGGAACAGTGAA |
| Muc3 | CCGACACATTGCTGCTGAGAAT | GCTGTCGTCTTGGGTGCTATTT |
| Muc4 | CTGTGTCTGAGCTGCCTGTATT | GGGTGTCTGTGTTGATGTTGTTG |
| Muc13 | CCCTCATCCTCATCTTGCTGATT | CTCTGCTCTTCTCCATCCTTCTTT |
| Gcnt1 | ACAGATTCAGGCTTCCTGTGATT | GCCAGGTGAGATGCCAGTTTA |
| Gcnt4 | ATGTCCTGCAGTTCCATTGAGG | ATGTCCTGCAGTTCCATTGAGG |
| B 3gnt6 | GGCCAGATTCTCCTCTCTCAAAC | CAGTGTCGTGGGACTCTTGAAC |
| C1galt1 | ATGGACACAGTCACCTCAAAGG | GAGGTTCTCAGCAACGTCTATGT |
| C1galt1c1 | TCTCACGTCCAAGCCTCGT | TGTGGCCTAGCATAGTGATCAAG |
| Fut1 | AGAATTCGCTTGCACCACCA | AAGAAGGAGCCGGCAGAGA |
| Fut2 | TGAACTTTCGGCTAAGGTACATCT | GGAAGTGGGCCAGAGGAAAG |
| Fut8 | AGGCGAATGGCTGAGTCTCT | TGGCCTTAACAAGCTGTTCTTCT |
| St3gal1 | GCCCACTATGCCAGACACTT | TCAGCAGAGTCAAACCCAGC |
| St3gal3 | TGCTGCGGTCATGTAGGAAA | CAGCGGAGTCAAGGGAAAGA |
| St3gak4 | GGCTCTGGTCCTTGTTGTTG | TCCCTAGAACGGTTGCCAAAA |
| St3gal6 | CACCCCAAAAGCGCAGATTTATT | CCTGCCTGAAACAGAGTCCAA |
| St6galnac2 | CGGATGTTGTTGCTCGTTGC | AGTCGGCTCTTTCTGTTTTCC |
| IL-6 | CACTTCACAAGTCGGAGGCT | CTGCAAGTGCATCATCGTTGT |
| IL-1b | TCTTTGAAGTTGACGGACCC | TGAGTGATACTGCCTGCCTG |
| IL-10 | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| IL-17a | TGAGCTTCCCAGATCACAGA | TCCAGAAGGCCCTCAGACTA |
| Tnf-a | GCACAGAAAGCATGATCCGC | CCCCATCTTTTGGGGGAGTG |
| Mcp-1/Ccl2 | CAGGTCCCTGTCATGCTTCT | TCTGGACCCATTCCTTCTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Ye, R.; Zhang, S.; Liu, C.; Chen, K.; Zhu, K.; Wang, P.; Wang, F.; Huang, J. Amelioration of LPS-Induced Jejunum Injury and Mucus Barrier Damage in Mice by IgY Embedded in W/O/W Emulsion. Foods 2024, 13, 4138. https://doi.org/10.3390/foods13244138
Wang Z, Ye R, Zhang S, Liu C, Chen K, Zhu K, Wang P, Wang F, Huang J. Amelioration of LPS-Induced Jejunum Injury and Mucus Barrier Damage in Mice by IgY Embedded in W/O/W Emulsion. Foods. 2024; 13(24):4138. https://doi.org/10.3390/foods13244138
Chicago/Turabian StyleWang, Zhaohui, Ruihua Ye, Shidi Zhang, Chuanming Liu, Ke Chen, Kongdi Zhu, Pengjie Wang, Fuqing Wang, and Jiaqiang Huang. 2024. "Amelioration of LPS-Induced Jejunum Injury and Mucus Barrier Damage in Mice by IgY Embedded in W/O/W Emulsion" Foods 13, no. 24: 4138. https://doi.org/10.3390/foods13244138
APA StyleWang, Z., Ye, R., Zhang, S., Liu, C., Chen, K., Zhu, K., Wang, P., Wang, F., & Huang, J. (2024). Amelioration of LPS-Induced Jejunum Injury and Mucus Barrier Damage in Mice by IgY Embedded in W/O/W Emulsion. Foods, 13(24), 4138. https://doi.org/10.3390/foods13244138






