Purple Yampee Derivatives and Byproduct Characterization for Food Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material and Chemical Reagents
2.2. Technological Performance of the Fractions
2.3. Bioactives Assessment
2.4. Statistical Analysis
3. Results and Discussion
3.1. Resulting Technological Performance of the Fractions
3.1.1. Physical Characteristics
3.1.2. Technological Characteristics
3.2. Bioactives Found in Purple Yampee Derivatives and Byproducts
3.2.1. Phytochemicals Identification
3.2.2. Amino Acids Identification and Quantification
3.2.3. Anthocyanin Identification and Quantification
3.2.4. Sugars Quantification and Evolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Global Agriculture towards 2050. In Proceedings of the High Level Expert Forum, Rome, Italy, 12–13 October 2009. [Google Scholar]
- Toromade, A.S.; Soyombo, D.A.; Kupa, E.; Ijomah, T.I. Reviweing the Impact of Climate Change on Global Food Security: Challenges and Solutions. Int. J. Appl. Res. Soc. Sci. 2024, 6, 1403–1416. [Google Scholar] [CrossRef]
- Shin, Y.J.; Midgley, G.F.; Archer, E.R.M.; Arneth, A.; Barnes, D.K.A.; Chan, L.; Hashimoto, S.; Hoegh-Guldberg, O.; Insarov, G.; Leadley, P.; et al. Actions to Halt Biodiversity Loss Generally Benefit the Climate. Glob. Chang. Biol. 2022, 28, 2846–2874. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta-Cortijo, C.; Ford, J.D.; Arotoma-Rojas, I.; Lwasa, S.; Lancha-Rucoba, G.; García, P.J.; Miranda, J.J.; Namanya, D.B.; New, M.; Wright, C.J.; et al. Climate Change and COVID-19: Reinforcing Indigenous Food Systems. Lancet Planet Health 2020, 4, e381–e382. [Google Scholar] [CrossRef]
- Taylor, M.; Lebot, V.; McGregor, A.; Redden, R.J. Sustainable Production of Roots and Tuber Crops for Food Security under Climate Change. Food Secur. Clim. Chang. 2018, 15, 359–376. [Google Scholar] [CrossRef]
- Alzaabi, S.A.; Chia, W.Y.; Show, P.L. Exploring the Potential of Circular Economy in the Food Sector. Syst. Microbiol. Biomanufacturing 2024, 4, 620–630. [Google Scholar] [CrossRef]
- Chandrasekara, A. Roots and Tubers as Functional Foods. In Bioactive Molecules in Food; Springer: Cham, Switzerland, 2018; pp. 1–29. ISBN 9783319545288. [Google Scholar] [CrossRef]
- Sharma, H.K. Tropical Roots and Tubers: Production, Processing and Technology; Wiley-Blackwell: Hoboken, NJ, USA, 2016; ISBN 9781118992753. [Google Scholar]
- Medina-López, S.V.; Zuluaga-Domínguez, C.M.; Fernández-Trujillo, J.P.; Hernández-Gómez, M.S. Nonconventional Hydrocolloids’ Technological and Functional Potential for Food Applications. Foods 2022, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Lin, H.; Liu, Z.; Chen, K.; Lin, Y.; Xi, Y.; Chhuond, K. Starch Wastewater Treatment Technology. IOP Conf. Ser. Earth Environ. Sci. 2019, 358, 022054. [Google Scholar] [CrossRef]
- Forbes, H.; Peacock, E.; Abbot, N.; Jones, M. Food Waste Index Report. Think Eat Save. Tracking Progress to Halve Global Food Waste. United Nations Environment Programme. 2024. ISBN 9789280741391. Available online: https://knowledge4policy.ec.europa.eu/publication/food-waste-index-report-2024-think-eat-save-tracking-progress-halve-global-food-waste_en (accessed on 15 October 2024).
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 1–17. [Google Scholar] [CrossRef]
- Pradeepika, C.; Kalita, D.J.; Visalakshi, C.C.; Sankar, S.; Hanume, G.K. Yams and Aroid Crop Waste: Bio Valorization into Bioproducts and Platform Chemicals. In Roots, Tubers, and Bulb Crop Wastes: Management by Biorefinery Approaches; Ray, R.C., Ed.; Springer Nature: Singapore, 2024; pp. 149–181. ISBN 978-981-99-8266-0. [Google Scholar] [CrossRef]
- Ji, S.; Zeng, Q.; Xu, M.; Li, Y.; Xu, T.; Zhong, Y.; Liu, Y.; Wang, F.; Lu, B. Investigation of the Mechanism of Different 3D Printing Performance of Starch and Whole Flour Gels from Tuber Crops. Int. J. Biol. Macromol. 2023, 241, 124448. [Google Scholar] [CrossRef]
- de Maia Miamoto, J.B.; Pereira, J.; Vilela Bertolucci, S.K. Obtaining and Characterization of Freeze-Dried Whole Taro Root (Colocasia esculenta), Mucilage and Residue as Functional Food. Nutricao Brasil 2018, 17, 9–18. [Google Scholar] [CrossRef]
- Gu, X.; Yang, H.; Han, Y.; Niu, B.; Chen, H.; Gao, H. Analysis on the Morphology and Quality Characteristics of Different Varieties of Lotus Root Starch and Whole Powder. Sci. Technol. Food Ind. 2021, 42, 95–101. [Google Scholar] [CrossRef]
- Meng, X.; Hu, W.; Wu, S.; Zhu, Z.; Lu, R.; Yang, G.; Qin, C.; Yang, L.; Nie, G. Chinese Yam Peel Enhances the Immunity of the Common Carp (Cyprinus carpio L.) by Improving the Gut Defence Barrier and Modulating the Intestinal Microflora. Fish Shellfish Immunol. 2019, 95, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Amenaghawon, A.; Omoruyi, B.; Kenneth, I.; Okedi, M.; Esenogho, G.; Oyefolu, P.; Muojama, O.; Otuya, I.; Eshiemogie, S.; Okoh, R.; et al. Biotechnological Conversion of Yam Peels for Enhanced Citric Acid Production: Data-Driven Machine Learning Modeling and Global Sensitivity Analysis of the Impact of Metabolic Stimulants. Ind. Crops Prod. 2023, 191, 116022. [Google Scholar] [CrossRef]
- Teixeira, A.; Oliveira, I.; Lima, E.; Matsuura, T. The Use of Purple Yam (Dioscorea trifida) as a Health-Promoting Ingredient in Bread Making. J. Res. Biol. 2013, 3, 747–758. Available online: http://jresearchbiology.com/documents/RA0306.pdf (accessed on 15 October 2024).
- Gutiérrez, T.J.; Pérez, E.; Guzmán, R.; Tapia, M.S.; Famá, L. Physicochemical and Functional Properties of Native and Modified by Crosslinking, Dark-Cush-Cush Yam (Dioscorea trifida) and Cassava (Manihot esculenta) Starch. J. Polym. Biopolym. Phys. Chem. 2014, 2, 1–5. [Google Scholar]
- Pérez, E.; Gibert, O.; Rolland-Sabaté, A.; Jiménez, Y.; Sánchez, T.; Giraldo, A.; Pontoire, B.; Guilois, S.; Lahon, M.C.; Reynes, M.; et al. Physicochemical, Functional, and Macromolecular Properties of Waxy Yam Starches Discovered from “Mapuey” (Dioscorea trifida) Genotypes in the Venezuelan Amazon. J. Agric. Food Chem. 2011, 59, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Escudero, F.; Santos-Buelga, C.; Pérez-Alonso, J.J.; Yáñez, J.A.; Dueñas, M. HPLC-DAD-ESI/MS Identification of Anthocyanins in Dioscorea trifida L. Yam Tubers (Purple Sachapapa). Eur. Food Res. Technol. 2010, 230, 745–752. [Google Scholar] [CrossRef]
- da Silva Souza, D.A.; da Bandeira, D.R.; Peroni, N. Yams (Dioscorea Spp.) in Shellmounds and Swiddens: Ancient History in Babitonga Bay, Santa Catarina State, Southern Brazil. J. Ethnobiol. Ethnomed. 2024, 20, 13. [Google Scholar] [CrossRef]
- Ferrari, M.C.; Ferrari, R.A. Transformation of Fresh Yams (Dioscorea trifida) into Flours and Starches: Sustainable Options for the Food Industry Contributing to the Development of This Production Chain. Sci. Agric. 2024, 82, e20230290. [Google Scholar] [CrossRef]
- León-Bravo, V.; Moretto, A.; Cagliano, R.; Caniato, F. Innovation for sustainable development in the food industry: Retro and forward-looking innovation approaches to improve quality and healthiness. Corp. Soc. Responsib. Environ. Manag. 2019, 26, 1049–1062. [Google Scholar] [CrossRef]
- Espitia, J.; Salcedo, J.G.; Garcia, C. Functional Properties of Starch Yam (Dioscorea bulbífera, Dioscorea trífida y Dioscorea esculenta). Rev. Téc. Ing. Univ. Zulia 2016, 39, 31–36. [Google Scholar]
- Murgas, J.D.; Vasquez, M.A. Evaluación de La Obtención de Bioetanol a Partir de Almidón de Ñame (Dioscorea rotundata, Dioscorea alata y Dioscorea trífida) Mediante La Hidrolisis Enzimática y Posterior Fermentación. Univ. San Buenaventura 2015, 1, 1689–1699. Available online: https://bibliotecadigital.usb.edu.co/entities/publication/1c32929c-e799-4a70-8f59-6c04069c47f1/full (accessed on 15 October 2024).
- Gutiérrez, T.J.; Morales, N.J.; Pérez, E.; Tapia, M.S.; Famá, L. Physico-Chemical Properties of Edible Films Derived from Native and Phosphated Cush-Cush Yam and Cassava Starches. Food Packag. Shelf Life 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Quintero-Mendoza, W.; Orduz, L.; Lozano, K.; Cardona, J.; Carrillo, M.; Hernández-Gómez, M.S. Natural Derived Ingredients Development from Amazonic Purple Yam (Dioscorea trifida L.f Var. Morada). Acta Hortic. 2023, 1361, 83–91. [Google Scholar] [CrossRef]
- Ma, F.; Li, X.; Ren, Z.; Särkkä-Tirkkonen, M.; Zhang, Y.; Zhao, D.; Liu, X. Effects of Concentrations, Temperature, PH and Co-Solutes on the Rheological Properties of Mucilage from Dioscorea opposita Thunb. and Its Antioxidant Activity. Food Chem. 2021, 360, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Baeza, R.; Sánchez, V.; Salierno, G.; Molinari, F.; López, P.; Chirife, J. Storage Stability of Anthocyanins in Freeze-Dried Elderberry Pulp Using Low Proportions of Encapsulating Agents. Food Sci. Technol. Int. 2021, 27, 135–144. [Google Scholar] [CrossRef]
- Medina Lopez, S.V.; Fernandez-Trujillo, J.P.; Hernández Gomez, M.S. Technological Potential and Characterization of Underutilized South American Yam Flour with Food Applications. Acta Hortic. 2023, 119–128. [Google Scholar] [CrossRef]
- Delgado, N.; Albarracín, W. Microestructura y Propiedades Funcionales de Harinas de Quinua (Chenopodioum quinoa w.) y Chachafruto (Erythrina edulis): Potenciales Extensores Cárnicos. Vitae 2012, 19, S430–S432. Available online: https://www.redalyc.org/pdf/1698/169823914135.pdf (accessed on 15 October 2024).
- AOAC International. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Rodriguez, M.D.; Monsierra, L.; Mansilla, P.S.; Pérez, G.T.; de Pascual-Teresa, S. Phenolic Characterization of a Purple Maize (Zea mays Cv. “Moragro”) by HPLC-QTOF-MS and Study of Its Bioaccessibility Using a Simulated In Vitro Digestion/Caco-2 Culture Model. J. Agric. Food Chem. 2024, 72, 6327–6338. [Google Scholar] [CrossRef]
- Yu, Y.G.; Guo, X.Y.; Li, X.Y.; Dai, D.D.; Xu, X.R.; Ge, X.J.; Li, Y.J.; Yang, T.G. Organ- and Age-Specific Differences of Dioscorea polystachya Compounds Measured by UPLC-QTOF/MS. Chem. Biodivers. 2021, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- L’homme, C.; Peschet, J.L.; Puigserver, A.; Biagini, A. Evaluation of fructans in various fresh and stewed fruits by high-performance anion-exchange chromatography with pulsed amperometric detection. J. Chromat. A 2001, 920, 291–297. [Google Scholar] [CrossRef]
- Ratnaningsih; Richana, N.; Suzuki, S.; Fujii, Y. Effect of Soaking Treatment on Anthocyanin, Flavonoid, Phenolic Content and Antioxidant Activities of Dioscorea alata Flour. Indones. J. Chem. 2018, 18, 656–663. [Google Scholar] [CrossRef]
- Acurio, L.; Salazar, D.; García, M.E.; García-Segovia, P.; Martínez-Monzó, J.; Igual, M. Characterization, Mathematical Modeling of Moisture Sorption Isotherms and Bioactive Compounds of Andean Root Flours. Curr. Res. Food Sci. 2024, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khamidah, A.; Antarlina, S.S. Corn Flour Substitution at Pastry Production. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1107, p. 012058. [Google Scholar] [CrossRef]
- Chen, H.; Anderson, N.M.; Feng, Y.; Grasso-Kelley, E.M.; Harris, L.J.; Marks, B.P.; McGowen, L.; Scharff, R.L.; Subbiah, J.; Tang, J.; et al. Food Safety Research and Extension Needs for the U.S. Low-Moisture Food Industry. J. Food Prot. 2024, 87, 100358. [Google Scholar] [CrossRef]
- Wumsinwie, B.; Neba, N.; Kewir, F.V.; Nde, D.B.; Tambo, S.; Womeni, H. Production of Instant Cassava (Manihot esculenta Crantz) Tuber Flour for Water Fufu: Optimization of Process Conditions. J. Food Sci. Technol. 2024, 1–10. [Google Scholar] [CrossRef]
- Nofiani, R.; Ulta, S.; Safitri, D.; Destiarti, L. Physicochemical Properties of Flour and Starch of Purple Water Yam (Dioscorea alata) Tuber and the Difference on Sensory Acceptance of the Cookies Produced. Agrointek 2021, 15, 486–496. [Google Scholar] [CrossRef]
- Gborie, S.K.; Mugabi, R.; Byaruhanga, Y. Effect of Extrusion on the Functional and Pasting Properties of High-Quality Cassava Flour (HQCF). J. Food Res. 2022, 11, 1–12. [Google Scholar] [CrossRef]
- Kacou, M.A.; Elvis Ekissi, G.S.; Ebbah Djedji, B.C.; N’zué, B.; Kouamé, P.L. Assessment of Functional Properties Flours from Seven Local Varieties of Cassava (Manihot esculenta Crantz) Consumed in Côte d’Ivoire. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 815–826. [Google Scholar] [CrossRef]
- Akter, M.; Anjum, N.; Roy, F.; Yasmin, S.; Sohany, M.; Mahomud, M.S. Effect of Drying Methods on Physicochemical, Antioxidant and Functional Properties of Potato Peel Flour and Quality Evaluation of Potato Peel Composite Cake. J. Agric. Food Res. 2023, 11. [Google Scholar] [CrossRef]
- Ohuoba, A.N.; Onwuka, G.I.; Omodamiro, R.M. Effects of Drying Methods on Physico-Chemical Properties of Hydrocolloids Isolated from Peel Flour of Some Selected Root and Tuber Crops. Int. J. Biochem. Res. Rev. 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Lozano, E.; Padilla, K.; Salcedo, J.; Arrieta, A.; Andrade-Pizarro, R. Effects of Yam (Dioscorea rotundata) Mucilage on the Physical, Rheological and Stability Characteristics of Ice Cream. Polymers 2022, 14, 3142. [Google Scholar] [CrossRef]
- da Contado, E.W.N.F.; Pereira, J.; Evangelista, S.R.; Lima Júnior, F.A.; Romano, L.M.; Couto, E.M. Composição Centesimal Da Mucilagem Do Inhame (Dioscorea spp.) Liofilizado Comparado a de Um Melhorador Comercial Utilizado Na Panificação e Avaliação Sensorial de Pães de Forma. Cienc. E Agrotecnologia 2009, 33, 1813–1818. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, Y.; Liu, N.; Zhang, J.; Tan, G.; Kannan, B.; Liu, X.; Bell, A.E. Rheological Properties of Polysaccharides from Dioscorea opposita Thunb. Food Chem. 2017, 227, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Moriya, C.; Hosoya, T.; Agawa, S.; Sugiyama, Y.; Kozone, I.; Shin-Ya, K.; Terahara, N.; Kumazawa, S. New Acylated Anthocyanins from Purple Yam and Their Antioxidant Activity. Biosci. Biotechnol. Biochem. 2015, 79, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Carreño-Diaz, R.; Grau, N. Anthocyanin Pigments in Dioscorea tryphida L. J. Food Sci. 1977, 42, 615–617. [Google Scholar] [CrossRef]
- Liu, J.; Zhuang, Y.; Hu, Y.; Xue, S.; Li, H.; Chen, L.; Fei, P. Improving the Color Stability and Antioxidation Activity of Blueberry Anthocyanins by Enzymatic Acylation with P-Coumaric Acid and Caffeic Acid. LWT 2020, 130, 1–8. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Q.; Zhao, S.; Zhao, W.; Wang, P.; Zhao, X.; Wang, D. Determining Role of Structure in the Stability Increase of Purple Sweet Potato Anthocyanins Bound to Proteins. Food Front. 2024, 5, 722–734. [Google Scholar] [CrossRef]
- Tiwari, J.K.; Luthra, S.K.; Zinta, R.; Raigond, P.; Dalamu, D.; Buckseth, T. Transcriptomics Reveals Genes Involved in Purple Tuber Colour Development in Potato. Agric. Res. 2024, 1–15. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, S.; Zhao, G.; Ye, F. Destabilisation and Stabilisation of Anthocyanins in Purple-Fleshed Sweet Potatoes: A Review. Trends Food Sci. Technol. 2021, 116, 1141–1154. [Google Scholar] [CrossRef]
- Rosas, A.L.G.; Gonçalves, G.C.P.; da Silveira, T.F.F.; Barros, L.; Ramires, T.; de Sousa, R.C.; da Silva, W.P.; Meinhart, A.D. Food Extract of Purple Yam (Dioscorea trifida L.f.) from Brazil: Optimization of Extraction Method, Characterization, In Vivo Toxicity, and Antimicrobial Activity. Food Anal. Methods 2024, 17, 1254–1266. [Google Scholar] [CrossRef]
- Sivamanib, S.; Archanaa, K.; Santhosh, R.; Sivarajasekar, N.; Prasad, N. Synthesis and Characterization of Starch Nanoparticles from Cassava Peel. J. Bioresour. Bioprod. 2018, 3, 161–165. [Google Scholar] [CrossRef]
- Hasanin, M.S. Simple, Economic, Ecofriendly Method to Extract Starch Nanoparticles from Potato Peel Waste for Biological Applications. Starch/Staerke 2021, 73, 1–7. [Google Scholar] [CrossRef]
- He, X.L.; Li, X.L.; Lv, Y.P.; He, Q. Composition and Color Stability of Anthocyanin-Based Extract from Purple Sweet Potato. Food Sci. Technol. 2015, 35, 468–473. [Google Scholar] [CrossRef]
- Orava, S. Towards Myo-Inositol Based Hydrogelators. Master’s Thesis, University of Jyväskylä, Jyväskylä, Finland, 2024. [Google Scholar]
- Sánchez-Hidalgo, M.; León-González, A.J.; Gálvez-Peralta, M.; González-Mauraza, N.H.; Martin-Cordero, C. D-Pinitol: A Cyclitol with Versatile Biological and Pharmacological Activities. Phytochem. Rev. 2021, 20, 211–224. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Al-Suod, H.; Ligor, M.; Ligor, T.; Krakowska, A.; Górecki, R.; Buszewski, B. Simultaneous Determination of Cyclitols and Sugars Following a Comprehensive Investigation of 40 Plants. Food Anal. Methods 2019, 12, 1466–1478. [Google Scholar] [CrossRef]
- Concerto, C.; Chiarenza, C.; Di Francesco, A.; Natale, A.; Privitera, I.; Rodolico, A.; Trovato, A.; Aguglia, A.; Fisicaro, F.; Pennisi, M.; et al. Neurobiology and Applications of Inositol in Psychiatry: A Narrative Review. Curr. Issues Mol. Biol. 2023, 45, 1762–1778. [Google Scholar] [CrossRef]
- Chatree, S.; Thongmaen, N.; Tantivejkul, K.; Sitticharoon, C.; Vucenik, I. Role of Inositols and Inositol Phosphates in Energy Metabolism. Molecules 2020, 25, 5079. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Liu, L.; Chen, W.; Bai, J.; Ma, F.; Liu, X.; Kang, W. Preparation and Characterization of Edible Films Composed of Dioscorea opposita Thunb. Mucilage and Starch. Polym. Test. 2020, 90, 106708. [Google Scholar] [CrossRef]


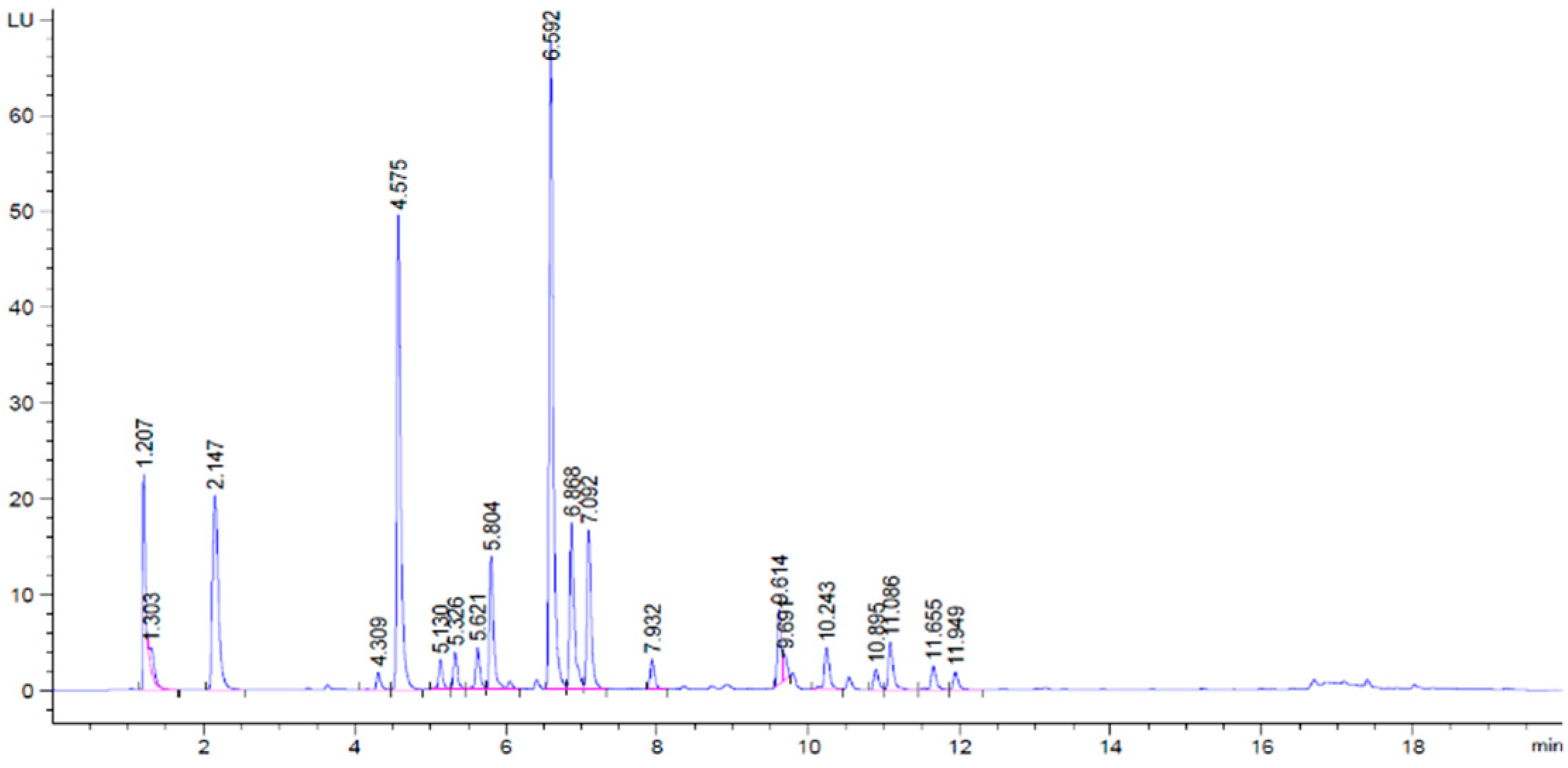


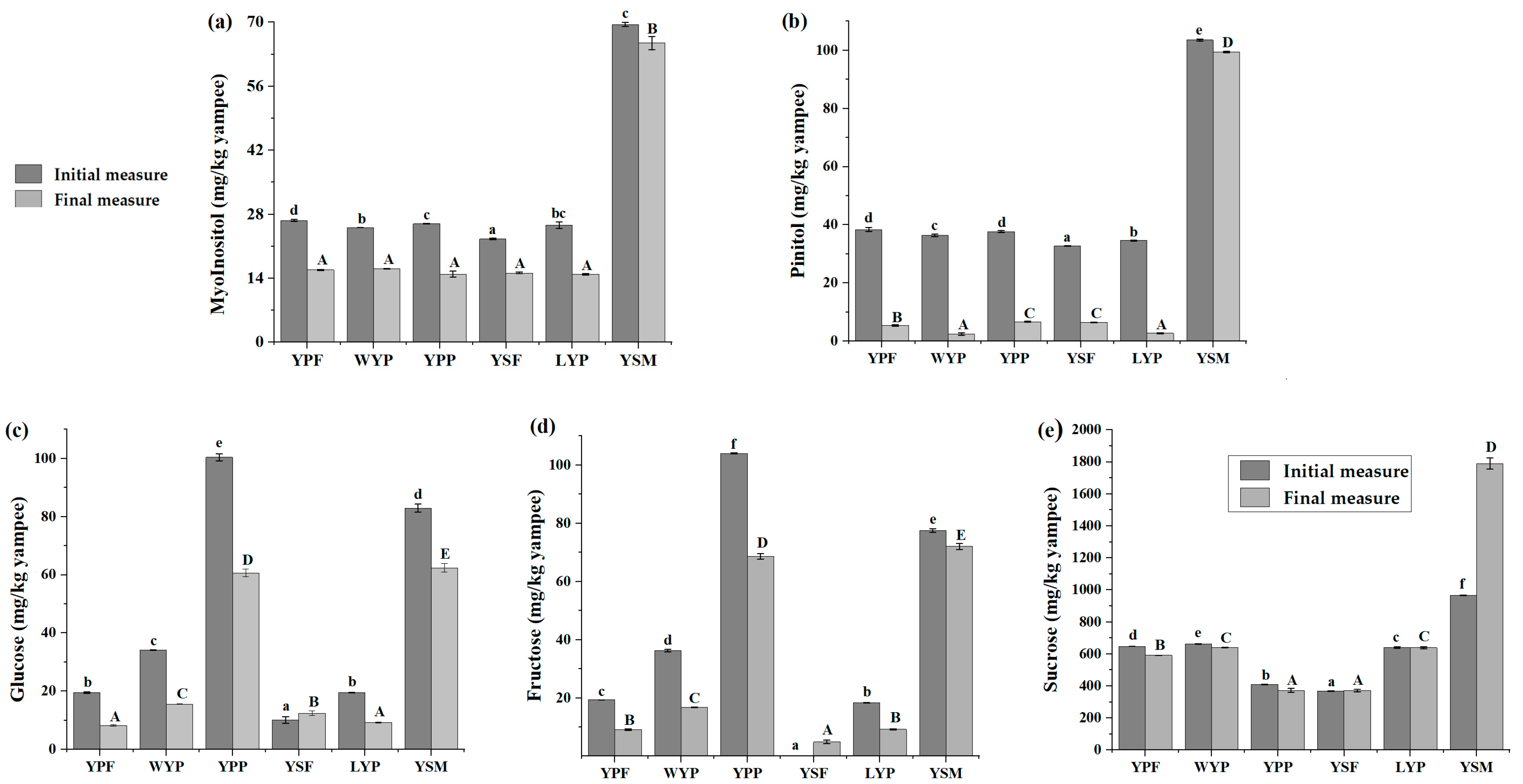
| YPF | WTP | YPP | YSF | YSM | LYP | |
|---|---|---|---|---|---|---|
| L* | 72.84 ± 0.52 d | 67.71 ± 0.49 c | 63.25 ± 8.69 b | 76.93 ± 0.13 e | 48.7 ± 0.09 a | 67.01 ± 0.10 c |
| a* | 8.69 ± 0.13 c | 7.72 ± 0.16 b | 6.57 ± 0.17 a | 6.31 ± 0.07 a | 10.34 ± 0.00 d | 10.33 ± 0.05 d |
| b* | −3.02 ± 0.02 b | 1.9 ± 0.16 d | 6.61 ± 0.14 f | 2.46 ± 0.08 e | −1.46 ± 0.05 c | −4.65 ± 0.03 a |
| C* | 9.19 ± 0.12 c | 7.95 ± 0.19 b | 9.32 ± 0.21 c | 6.78 ± 0.09 a | 10.44 ± 0.00 d | 11.32 ± 0.06 e |
| h* | 19.13 ± 0.44 c | 13.81 ± 0.90 b | 45.15 ± 0.51 f | 21.33 ± 0.46 d | 8.00 ± 0.28 a | 24.20 ± 0.12 e |
| Aw | 0.451 ± 0.004 c | 0.194 ± 0.015 a | 0.229 ± 0.009 a | 0.420 ± 0.002 c | 0.299 ± 0.037 b | 0.223 ± 0.011 a |
| Moist (%) | 9.59 ± 0.39 c | 4.99 ± 0.03 a | 5.35 ± 0.14 a | 9.74 ± 0.12 c | 8.65 ± 0.11 b | 5.22 ± 0.03 a |
| pH | 5.75 ± 0.01 a | 6.08 ± 0.01 b | 5.69 ± 0.13 a | 5.90 ± 0.39 ab | 6.07 ± 0.01 b | 6.43 ± 0.02 c |
| TTA (%) | 0.362 ± 0.006 a | 0.177 ± 0.047 a | 0.709 ± 0.049 a | 0.359 ± 0.007 a | 1.820 ± 0.495 b | 0.265 ± 0.227 a |
| YPF | WTP | YPP | YSF | YSM | LYP | |
|---|---|---|---|---|---|---|
| LBD * | 0.72 ± 0.04 c | 0.56 ± 0 b | 0.37 ± 0.01 a | 0.60 ± 0.01 b | 0.39 ± 0 a | 0.56 ± 0 b |
| PBD | 0.90 ± 0.02 e | 0.73 ± 0.02 c | 0.56 ± 0.02 b | 0.79 ± 0.02 d | 0.48 ± 0.01 a | 0.75 ± 0.02 cd |
| AD | 0.81 ± 0.03 b | 0.77 ± 0.03 b | 0.67 ± 0.02 a | 0.76 ± 0.00 b | 0.81 ± 0.00 b | 0.75 ± 0.02 b |
| WSI | 3.80 ± 0.08 a | 4.25 ± 0.11 a | 5.18 ± 0.13 a | 2.49 ± 0.29 a | 22.4 ± 5.46 b | 4.29 ± 0.08 a |
| WAI | 3.19 ± 0.12 a | 3.32 ± 0.08 a | 4.72 ± 0.1 b | 3.12 ± 0.12 a | 3.49 ± 0.63 a | 3.39 ± 0.25 a |
| SP | 3.27 ± 0.17 a | 3.42 ± 0.02 a | 4.89 ± 0.19 b | 3.17 ± 0.11 a | 4.14 ± 0.8 ab | 3.49 ± 0.17 a |
| WHC | 8.11 ± 0.02 c | 6.47 ± 1.21 b | 10.02 ± 0.08 d | 8.63 ± 1.04 c | 2.92 ± 0.55 a | 7.45 ± 0.21 bc |
| WAC | 1.86 ± 0.05 b | 1.59 ± 0.07 a | 3.30 ± 0.03 e | 2.21 ± 0.03 d | 1.99 ± 0.18 bc | 2.12 ± 0 cd |
| OAC | 0.86 ± 0.03 a | 1.09 ± 0.07 b | 1.85 ± 0.12 d | 1.07 ± 0.06 b | 1.57 ± 0.02 a | 1.10 ± 0.15 b |
| EA | 62.27 ± 3.41 a | 62.34 ± 1.5 a | 66.38 ± 4.08 ab | 64.86 ± 3.03 ab | 95.42 ± 1.43 c | 68.77 ± 1.33 b |
| Peak | Rt (min) | Tentative Assignment | CAS Number | Adduct Type | Observed m/z | Main MS/MS Fragment Ions (m/z) | |
|---|---|---|---|---|---|---|---|
| 1 | 3.064 | Allantoin | 97-59-6 | [M + H]- | 157.0354 | 114.0296/96.9588/71.0125/59.0143 | [12] |
| 2 | 3.211 | Quinic acid | 77-95-2 | [M + H]- | 191.058 | 173.0473/127.0413/93.0354/85.0303/59.0144 | [10] |
| 3 | 3.887 | Malic acid | 6915-15-7 | [M + H]- | 133.0155 | 115.0049/89.0253/72.0939/71.045/59.0143 | [13] |
| 4 | 4.385 | Isocitrate | 320-77-4 | [M + H]- | 191.0199 | 173.0091/154.9986/111.0087/101.02442/85.029/73.0295 | * |
| 5 | 7.723 | Citric acid | 77-92-9 | [M + H]- | 191.022 | 129.0195/111.009/87.0085/85.0295/67.0184/57.0340 | [12] |
| 6 | 9.035 | Citric acid derivate | N/A | [M + H]- | - | 191.0193/129.0192/87.0092/85.0298/59.0142/57.0349 | * |
| 7 | 9.072 | Succinic acid | 110-15-6 | [M + H]- | 117.0187 | 101.0238/99.0082/73.0287/55.0185 | [14] |
| 8 | 9.772 | Citric acid derivate | N/A | [M + H]- | - | 191.0196/129.0195/111.0089/87.0089/67.0197 | * |
| 9 | 13.33 | Adenosine | 58-61-7 | [M + H]- | 266.0894 | 134.0459/107.0363 | [12] |
| 10 | 17.345 | Glucogallic acid | 84274-52-2 | [M + H]- | 331.0685 | 164.0718 | [12] |
| 11 | 29.338 | Isopropylmalic acid | 3237-44-3 | [M + H]- | 175.0618 | 157.0509/131.0723/113.0611/ 101.0242/85.0663/72.9931/69.0706 | * |
| 12 | 33.666 | Benzoquinoneacetic acid | 10275-07-7 | [M + H]- | 166.0267 | 121.0298/140.0474/93.0346/77.0395 | * |
| 13 | 36.14 | Kaempferide derivative | N/A | [M + H]- | 623.1601 | 299.0556 | [15] |
| 14 | 36.82 | 2-O-Feruloylhydroxycitric acid | 62345-86-2 | [M + H]- | 383.0622 | 189.0040/134.0373/59.0138 | * |
| 15 | 38.1 | Rhamnetin 3-galactoside- 4′-glucoside | N/A | [M + H]- | 639.1577 | 315.0572 | * |
| 16 | 38.494 | Epigallocatechin 7-glucuronide | 569670-42-4 | [M + H]- + [H2O] | 463.0897/ 482.1075 | 169.043/125.0244/57.0345 | * |
| 17 | 39.338 | Rhamnocitrin-3-(5″-ferulylapiosyl)-(1 → 2)glucoside | 148210-00-8 | [M + H]- | 769.1987 | 299.0552/178.026 | * |
| 18 | 40.589 | Azelaic acid | 123-99-9 | [M + H]- | 187.0978 | 169.087/143.1077/125.0973/97.0659/57.0345 | [11] |
| 19 | 47.185 | Dihidrojasmonic acid derivative | N/A | [M + H]- | - | 211.1338/167.1445/59.0135 | * |
| 20 | 50.194 | Dihidrojasmonic acid derivative | N/A | [M + H]- | - | 211.1340/167.1441/59.0142 | * |
| Peak | Rt (min) | Fraction | Pulp | Mucilage |
|---|---|---|---|---|
| 1 | 1.203 | Aspartate | 101.91 ± 7.71 | 733.17 ± 72.99 |
| 2 | 4.559 | Serine | 101.45 ± 9.02 | 504.33 ± 28.03 |
| 3 | 5.313 | Histidine | <LOQ * | 103.14 ± 5.63 |
| 4 | 5.609 | Glycine | <LOQ | 38.71 ± 3.05 |
| 5 | 5.793 | Threonine | 30.5 ± 3.93 | 261.57 ± 11.97 |
| 6 | 6.585 | Arginine | 230.14 ± 10.85 | 1352.75 ± 35.19 |
| 7 | 6.857 | Alanine | 30.82 ± 4.16 | 217.75 ± 10.4 |
| 8 | 7.082 | Tyrosine | 59.78 ± 2.34 | 460.44 ± 34.77 |
| 9 | 9.607 | Valine | 17.26 ± 1.59 | 112.39 ± 10.94 |
| 10 | 11.081 | Isoleucine | <LOQ | 145.82 ± 9.01 |
| 11 | 11.651 | Leucine | <LOQ | 52.69 ± 9.32 |
| 12 | 11.951 | Lysine | 33.84 ± 4.15 | 138.4 ± 19.49 |
| Peak | Rt (min) | [M]+ (m/z) | Main MS2 Fragment Ions (m/z) | Tentative Identification |
|---|---|---|---|---|
| 1 | 19.279 | 625.1788 | 301.0726 | Peonidin-3-O-glucoside-5-O-glucoside * |
| 2 | 21.818 | 595.1696 | 271.0616 | Pelargonidin derivative |
| 3 | 24.13 | 787.2132 | 287.0570 | Cyanidin-3-O-feruloylglucoside-5-O-glucoside * |
| 4 | 24.923 | 757.20132 | 287.0567 | Cyanidin-3-O-p-coumaroylglucoside-5-O-glucoside * |
| 5 | 25.421 | 561.1589 | 271.0618 | Pelargonidin derivative |
| 6 | 26.366 | 831.2389 | 301.0728 | Peonidin derivative |
| 7 | 27.986 | 771.2176 | 271.0619 | Pelargonidin derivative |
| 8 | 28.613 | 801.2299 | 301.0733 | Peonidin 3-O-feruloylglucoside-5-O-glucoside * |
| 9 | 29.176 | 741.2067 | 271.0619 | Pelargonidin-3-O-p-coumaroylglucoside-5-O-glucoside * |
| 10 | 30.031 | 771.2187 | 301.0734 | Peonidin 3-O-p-cumaroylglucoside-5-O-glucoside * |
| 11 | 36.288 | 641.1754 | 317.0674 | Petunidin derivative |
| 12 | 38.963 | 625.1796 | 317.0678 | Petunidin derivative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-López, S.V.; Molina García, C.; Lizarazo-Aparicio, M.C.; Hernández-Gómez, M.S.; Fernández-Trujillo, J.P. Purple Yampee Derivatives and Byproduct Characterization for Food Applications. Foods 2024, 13, 4148. https://doi.org/10.3390/foods13244148
Medina-López SV, Molina García C, Lizarazo-Aparicio MC, Hernández-Gómez MS, Fernández-Trujillo JP. Purple Yampee Derivatives and Byproduct Characterization for Food Applications. Foods. 2024; 13(24):4148. https://doi.org/10.3390/foods13244148
Chicago/Turabian StyleMedina-López, Sandra V., Cristian Molina García, Maria Cristina Lizarazo-Aparicio, Maria Soledad Hernández-Gómez, and Juan Pablo Fernández-Trujillo. 2024. "Purple Yampee Derivatives and Byproduct Characterization for Food Applications" Foods 13, no. 24: 4148. https://doi.org/10.3390/foods13244148
APA StyleMedina-López, S. V., Molina García, C., Lizarazo-Aparicio, M. C., Hernández-Gómez, M. S., & Fernández-Trujillo, J. P. (2024). Purple Yampee Derivatives and Byproduct Characterization for Food Applications. Foods, 13(24), 4148. https://doi.org/10.3390/foods13244148








