Biopreservation of Fresh Sardines (Sardina pilchardus) Using Lactiplantibacillus plantarum OV50 Isolated from Traditional Algerian Green Olives Preparations
Abstract
:1. Introduction
2. Material and Methods
2.1. Isolation and Selection of Lactic Acid Bacteria Strains
2.2. Strains Identification by API 50 System
2.3. Strains Identification by Mass Spectrometry and 16S rDNA Gene Sequencing
2.4. Safety Aspects
2.4.1. Hemolytic Activity
2.4.2. Antibiotic Susceptibility
2.4.3. Cytotoxicity
2.5. Technological Properties
2.5.1. Proteolytic Activity
2.5.2. Lipolytic Activity
2.6. Application of Lactiplantibacillus plantarum OV50 for Biopreservation of Fresh Sardines
2.6.1. Coculture between Lactiplantibacillus plantarum OV50 Alongside Pathogenic Strains in Sardine Culture Medium
2.6.2. Application of Lactiplantibacillus plantarum OV50 as a Biopreservative Agent in Fresh Sardines
2.6.3. Measurement of Peroxide Value
2.6.4. Determination of Total Volatile Basic Nitrogen (TVB-N)
2.7. Statistical Analyzes
3. Results
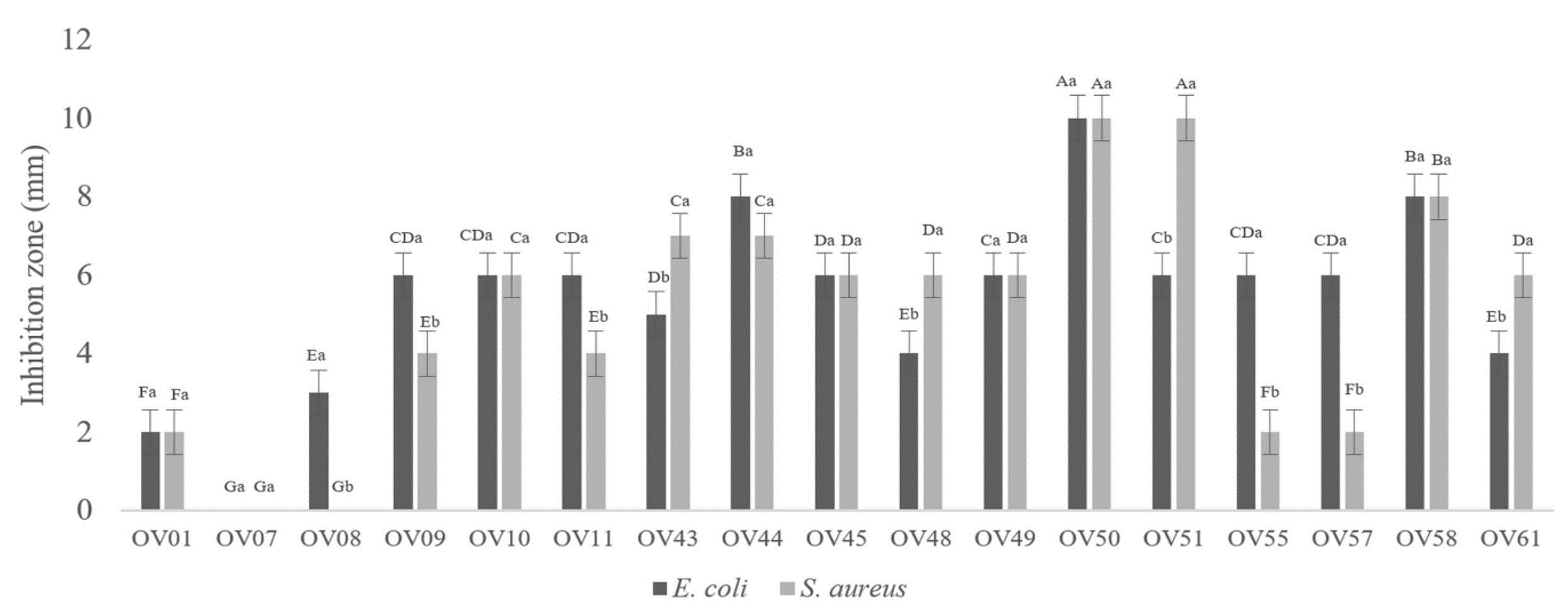
3.1. Lactiplantibacillus plantarum OV50 Revealed Highest Antibacterial Activity
3.2. Molecular Identification
3.3. Lactiplantibacillus plantarum OV50 Is Safe
3.4. Lactiplantibacillus plantarum OV50 Is Not Proteolytic Nor Lipolytic
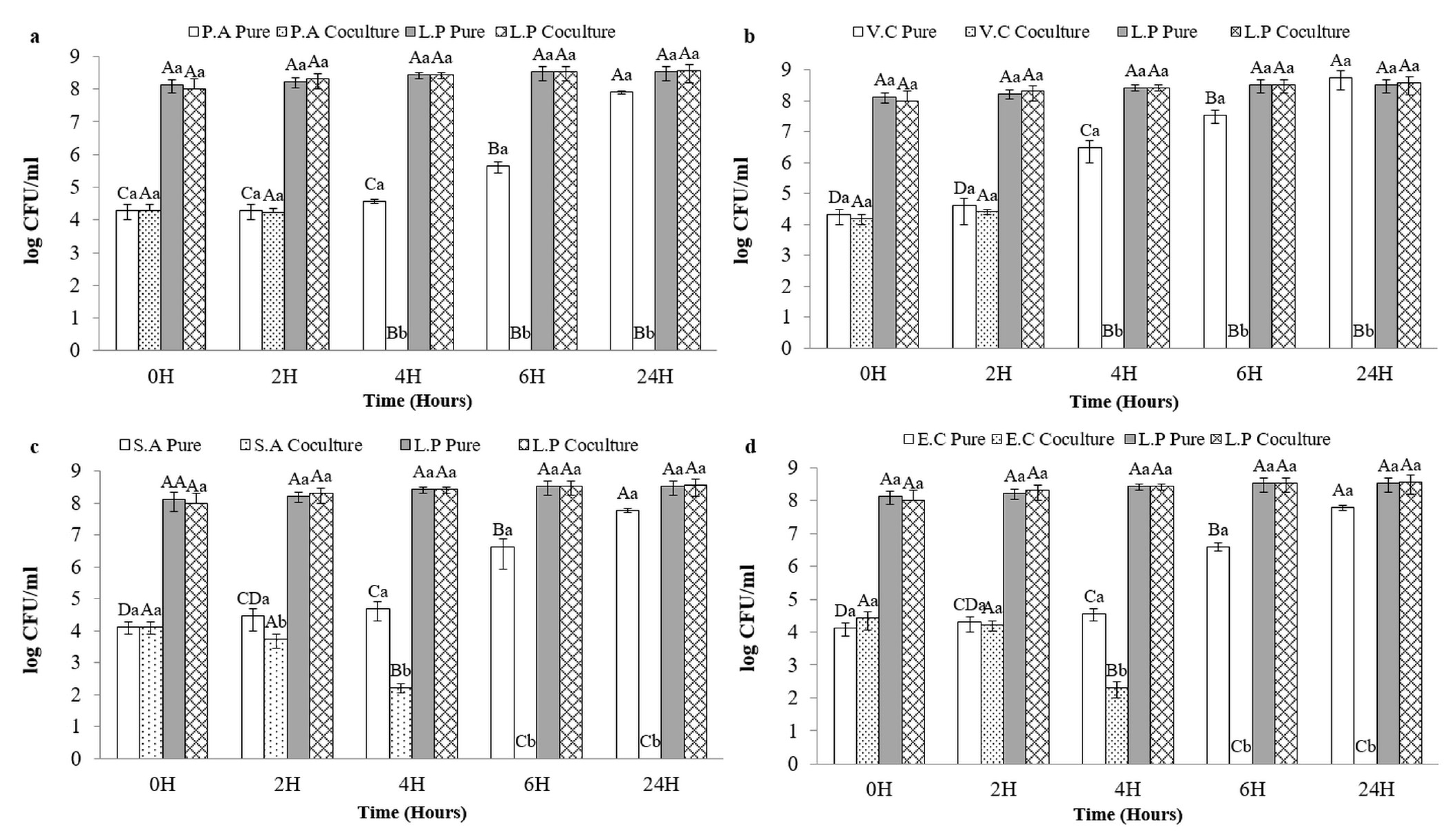
3.5. Coculture Results
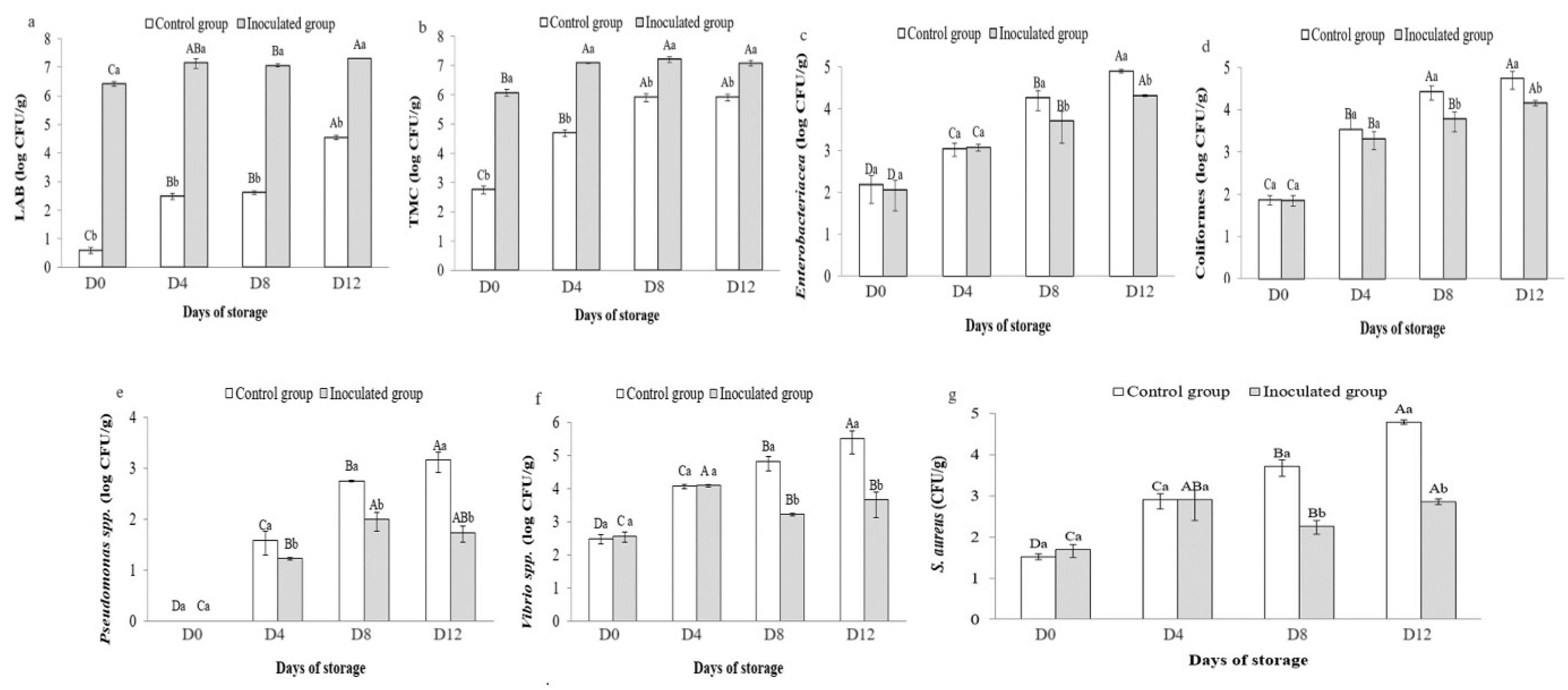
3.6. Microbial Counts in Preserved Sardines
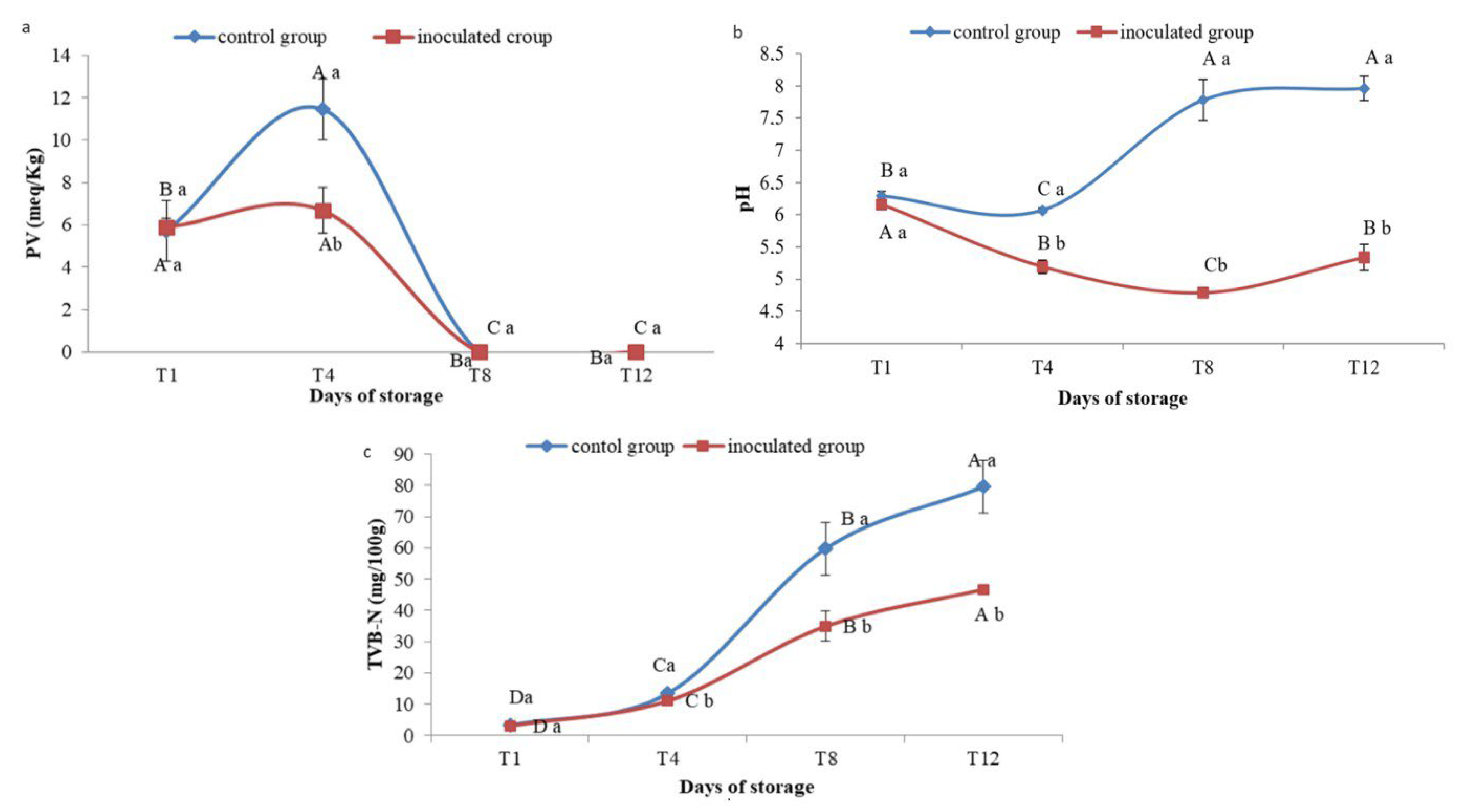
3.7. Physicochemical Results
3.7.1. Peroxide Value
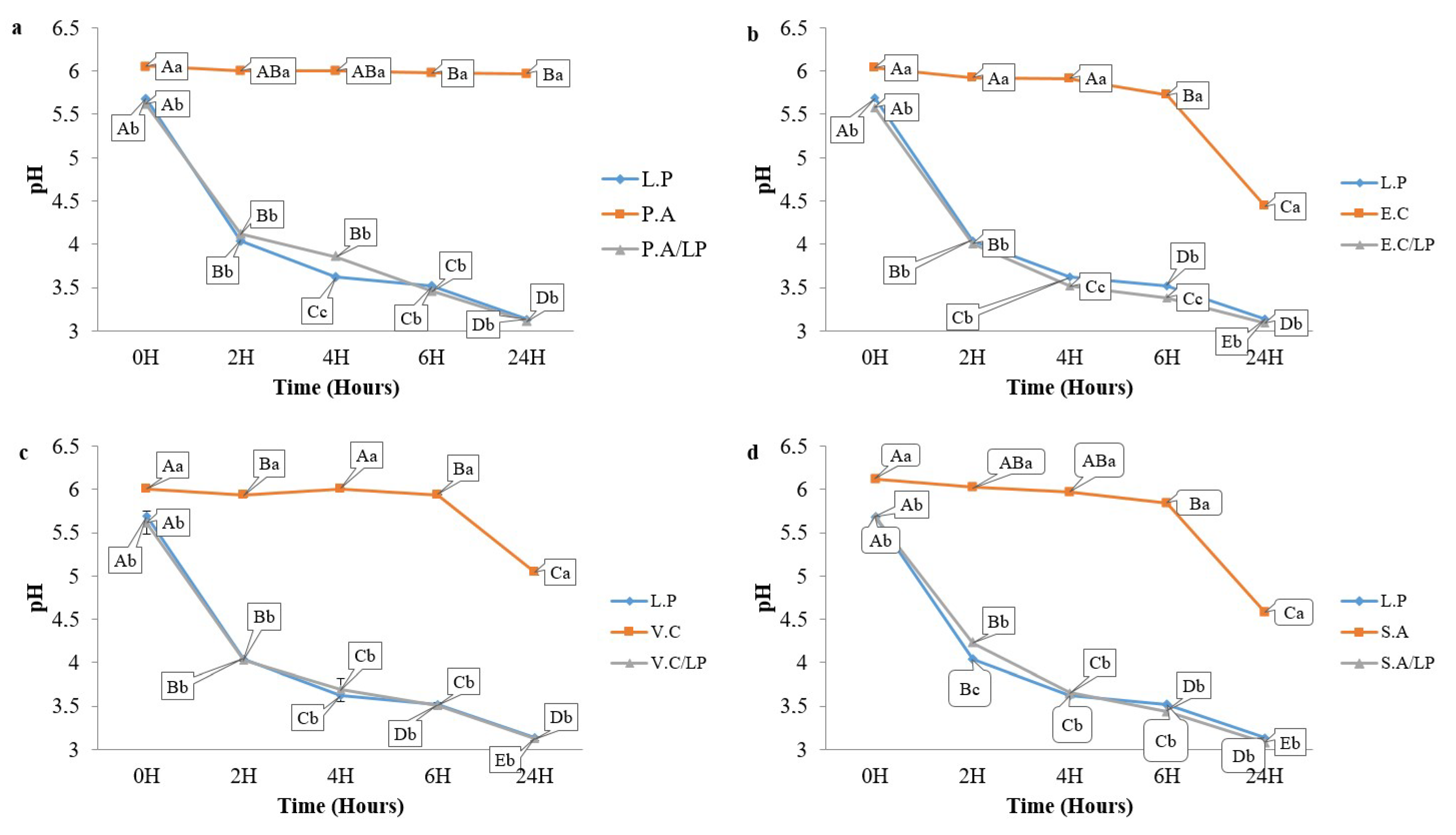
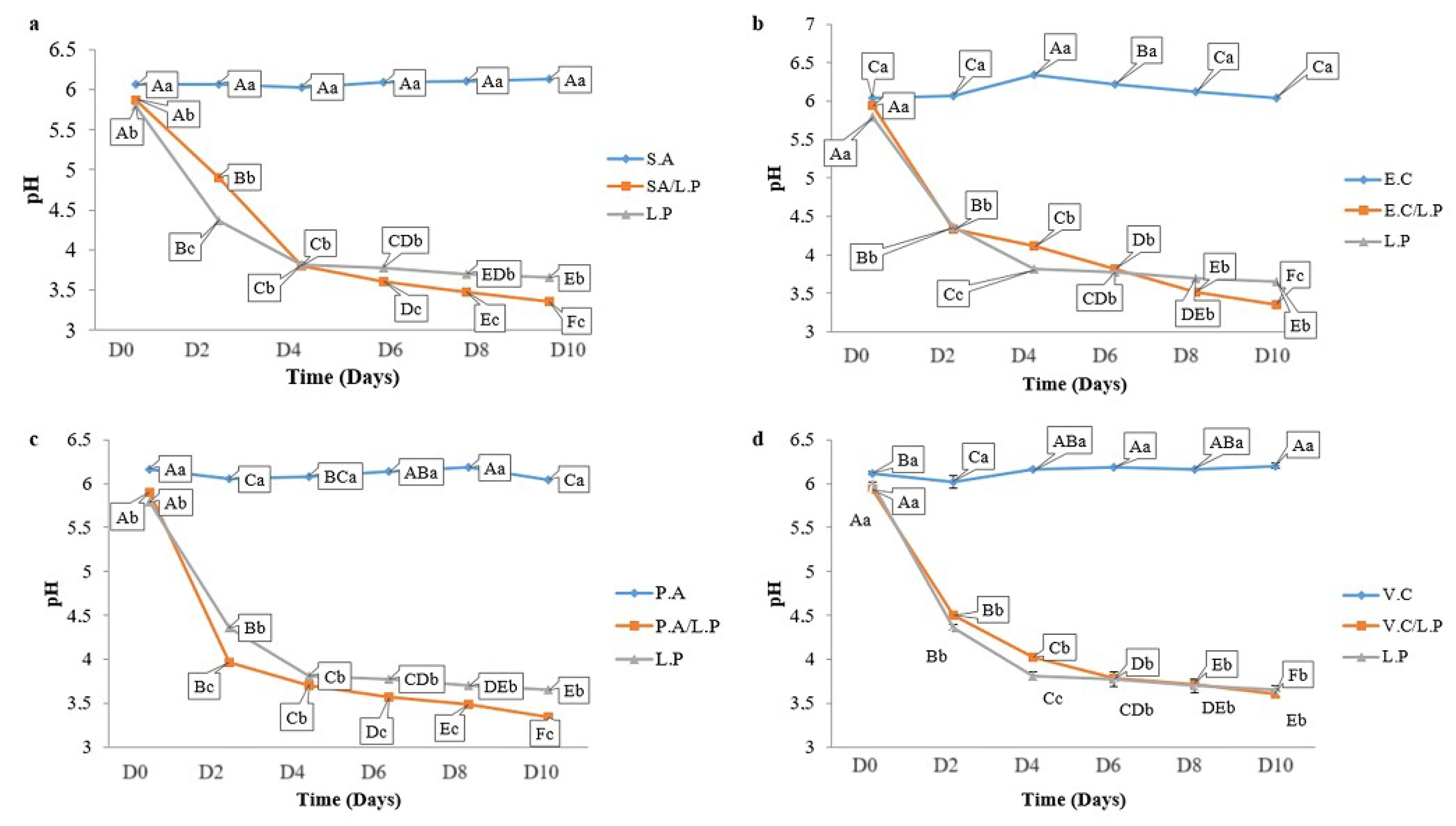
3.7.2. pH Monitoring
3.7.3. TVB-N
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Šimat, V.; Hamed, I.; Petričević, S.; Bogdanović, T. Seasonal Changes in Free Amino Acid and Fatty Acid Compositions of Sardines, Sardina pilchardus (Walbaum, 1792): Implications for Nutrition. Foods 2020, 9, 867. [Google Scholar] [CrossRef]
- Janči, T.; Gauta, T.; Putnik, P.; Kanski, D.; Lovrinov, M. Influence of Fish Handling Practices Onboard Purse Seiners on Quality Parameters of Sardines (Sardina pilchardus) during Cold Storage. Biomolecules 2023, 13, 192. [Google Scholar] [CrossRef]
- Reblová, Z.; Aubourg, S.P.; Pokorný, J. The Effect of Different Freshness of Raw Material on Lipid Quality and Sensory Acceptance of Canned Sardines. Foods 2022, 11, 1987. [Google Scholar] [CrossRef]
- Calanche, J.; Samayoa, S.; Alonso, V.; Provincial, L.; Roncalés, P.; Beltrán, J.A. Assessing the Effectiveness of a Cold Chain for Fresh Fish Salmon (Salmo salar) and Sardine (Sardina pilchardus) in a Food Processing Plant. Food Control 2013, 33, 126–135. [Google Scholar] [CrossRef]
- Maulu, S.; Hasimuna, O.J.; Monde, C.; Mweemba, M. An Assessment of Post-Harvest Fish Losses and Preservation Practices in Siavonga District, Southern Zambia. Fish. Aquat. Sci. 2020, 23, 25. [Google Scholar] [CrossRef]
- Gyan, W.R.; Alhassan, E.H.; Asase, A.; Akongyuure, D.N.; Qi-Hui, Y. Assessment of Postharvest Fish Losses: The Case Study of Albert Bosomtwi-Sam Fishing Harbour, Western Region, Ghana. Mar. Policy 2020, 120, 104120. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018 (SOFIA): Meeting the Sustainable Development Goals; The State of World Fisheries and Aquaculture (SOFIA); FAO: Rome, Italy, 2018. [Google Scholar]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Risius, A.; Janssen, M.; Hamm, U. Consumer Preferences for Sustainable Aquaculture Products: Evidence from in-Depth Interviews, Think Aloud Protocols and Choice Experiments. Appetite 2017, 113, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Román, S.; Sánchez-Siles, L.M.; Siegrist, M. The Importance of Food Naturalness for Consumers: Results of a Systematic Review. Trends Food Sci. Technol. 2017, 67, 44–57. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic Acid Bacteria as Antimicrobial Agents: Food Safety and Microbial Food Spoilage Prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.B.; Phadke, G.G.; Tabanelli, G.; Mane, A.; Ranveer, R.C.; Pagarkar, A.; Ozogul, F. Recent Advances in Bio-Preservatives Impacts of Lactic Acid Bacteria and Their Metabolites on Aquatic Food Products. Food Biosci. 2021, 44, 101440. [Google Scholar] [CrossRef]
- Hwanhlem, N.; H-Kittikun, A. Biopreservation of Seafood by Using Bacteriocins and Bacteriocinogenic Lactic Acid Bacteria as Potential Bio-Control Agents. In Beneficial Microorganisms in Agriculture, Aquaculture and Other Areas; Liong, M.-T., Ed.; Microbiology Monographs; Springer International Publishing: Cham, Switzerland, 2015; Volume 29, pp. 183–213. [Google Scholar] [CrossRef]
- Stupar, J.; Holøymoen, I.G.; Hoel, S.; Lerfall, J.; Rustad, T.; Jakobsen, A.N. Diversity and Antimicrobial Activity towards Listeria spp. and Escherichia coli among Lactic Acid Bacteria Isolated from Ready-to-Eat Seafood. Foods 2021, 10, 271. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A Medium for the Cultivation of Lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Barefoot, S.F.; Klaenhammer, T.R. Detection and Activity of Lactacin B, a Bacteriocin Produced by Lactobacillus acidophilus. Appl. Env. Microbiol. 1983, 45, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Matti, A.; Utami, T.; Hidayat, C.; Rahayu, E.S. Isolation, Screening, and Identification of Proteolytic Lactic Acid Bacteria from Indigenous Chao Product. J. Aquat. Food Prod. 2019, 28, 781–793. [Google Scholar] [CrossRef]
- Nacef, M.; Chevalier, M.; Chollet, S.; Drider, D.; Flahaut, C. MALDI-TOF Mass Spectrometry for the Identification of Lactic Acid Bacteria Isolated from a French Cheese: The Maroilles. Int. J. Food Microbiol. 2017, 247, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J. 16S/23S rRNA Sequencing. In Nucleic Acid Techniques in Bacterial Systematic; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1991; pp. 115–175. [Google Scholar]
- Barache, N.; Ladjouzi, R.; Belguesmia, Y.; Bendali, F.; Drider, D. Abundance of Lactobacillus plantarum Strains with Beneficial Attributes in Blackberries (Rubus sp.), Fresh Figs (Ficus carica), and Prickly Pears (Opuntia ficus-indica) Grown and Harvested in Algeria. Probiotics Antimicrob. Proteins 2020, 12, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Belguesmia, Y.; Domenger, D.; Caron, J.; Dhulster, P.; Ravallec, R.; Drider, D.; Cudennec, B. Novel Probiotic Evidence of Lactobacilli on Immunomodulation and Regulation of Satiety Hormones Release in Intestinal Cells. J. Funct. Foods 2016, 24, 276–286. [Google Scholar] [CrossRef]
- De Albuquerque, T.M.R.; Garcia, E.F.; De Oliveira Araújo, A.; Magnani, M.; Saarela, M.; De Souza, E.L. In Vitro Characterization of Lactobacillus Strains Isolated from Fruit Processing By-Products as Potential Probiotics. Probiotics Antimicrob. Proteins 2018, 10, 704–716. [Google Scholar] [CrossRef]
- Meradji, M.; Bachtarzi, N.; Mora, D.; Kharroub, K. Characterization of Lactic Acid Bacteria Strains Isolated from Algerian Honeybee and Honey and Exploration of Their Potential Probiotic and Functional Features for Human Use. Foods 2023, 12, 2312. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Lakshmanaperumalsamy, P.; Chandramohan, D. Fish Flesh Agar Medium—A Suitable Experimental Medium for the Detection of Spoilage Bacteria. Antonie Van Leeuwenhoek 1985, 51, 219–225. [Google Scholar] [CrossRef] [PubMed]
- AOAC Official Method 965.33. Peroxyde Value of Oils and Fats; AOAC: Rockville, MD, USA, 1969. [Google Scholar]
- European Commission Regulation. European Commission Regulation (EC) No 2074/2005 of 5 December 2005 Total Volatile Basic Nitrogen (TVB-N) Limit Values for Certain Categories of Fishery Products and Analysis Methods. Off. J. Eur. Union 2005, 36–39. [Google Scholar]
- Ghomi, M.R.; Nikoo, M.; Heshmatipour, Z.; Jannati, A.A.; Ovissipour, M.; Benjakul, S.; Hashemi, M.; Langroudi, H.F.; Hasandoost, M.; Jadiddokhani, D. Effect of Sodium Acetate and Nisin on Microbiological and Chemical Changes of Cultured Grass Carp (Ctenopharyngodon idella) during Refrigerated Storage. J. Food Saf. 2011, 31, 169–175. [Google Scholar] [CrossRef]
- European Commission guidelines. European Commission Decision 95/149/EC of 8 March 1995, Fixing the Total Volatile Basic Nitrogen (TVB-N) Limit Values for Certain Categories of Fishery Products and Specifying the Analysis Methods to Be Used. Off. J. Ser. L 1995, 97, 84–87. [Google Scholar]
- Barache, N.; Belguesmia, Y.; Ladjouzi, R.; Bendali, F.; Drider, D. Clusters of Lactobacillus Strains from Vegetal Origins Are Associated with Beneficial Functions: Experimental Data and Statistical Interpretations. Foods 2020, 9, 985. [Google Scholar] [CrossRef] [PubMed]
- Barache, N. Exploration de l’Ecosystème Végétal (Fruits): Recherche de Souches de Bactéries Lactiques et de Levures Antagonistes et/ou Probiotiques. Ph.D. Thesis, Université de Bejaia, Bejaia, Algérie, 2020. [Google Scholar]
- Nawaz, M.; Wang, J.; Zhou, A.; Ma, C.; Wu, X.; Moore, J.E.; Cherie Millar, B.; Xu, J. Characterization and Transfer of Antibiotic Resistance in Lactic Acid Bacteria from Fermented Food Products. Curr. Microbiol. 2011, 62, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Viji, P.; Jesmi, D.; Rao, B.M. Nutritional Significance of Seafood. In Training Manual on Value Addition of Seafood; 2018; pp. 1–5. Available online: https://krishi.icar.gov.in/jspui/bitstream/123456789/20806/1/1_nutritional%20significance.pdf (accessed on 10 October 2023).
- Chaula, D.; Jacobsen, C.; Laswai, H.S.; Chove, B.E.; Dalsgaard, A.; Mdegela, R.; Hyldig, G. Changes in Fatty Acids during Storage of Artisanal-Processed Freshwater Sardines (Rastrineobola argentea). Food Sci. Nutr. 2023, 11, 3040–3047. [Google Scholar] [CrossRef]
- Cebeci Avunca, S.; Öztürk Çetin, Ö.; Çağri Mehmetoğlu, A.; Öztürk, M. Identification of Lactic Acid Bacteria in Fermented Cornichon Pickles Produced with Acid Whey and Vinegar. Sak. Univ. J. Sci. 2022, 26, 273–282. [Google Scholar] [CrossRef]
- Pacheco-Aguilar, R.; Lugo-Sánchez, M.E.; Robles-Burgueño, M.R. Postmortem Biochemical and Functional Characteristic of Monterey Sardine Muscle Stored at 0 °C. J. Food Sci. 2000, 65, 40–47. [Google Scholar] [CrossRef]
- Sae-leaw, T.; Benjakul, S. Fatty Acid Composition, Lipid Oxidation, and Fishy Odour Development in Seabass (Lates calcarifer) Skin during Iced Storage. Eur. J. Lipid Sci. Technol. 2014, 116, 885–894. [Google Scholar] [CrossRef]
- Ringø, E.; Schillinger, U.; Holzapfel, W. Chapter 18 Antimicrobial Activity of Lactic Acid Bacteria Isolated from Aquatic Animals and the Use of Lactic Acid Bacteria in aquaculture11Financial Support from the Norwegian Research Council (Grant No. 122851/122) Is Gratefully Acknowledged. In Biology of Growing Animals; Holzapfel, W.H., Naughton, P.J., Pierzynowski, S.G., Zabielski, R., Salek, E., Eds.; Microbial Ecology in Growing Animals; Elsevier: Amsterdam, The Netherlands, 2005; Volume 2, pp. 418–453. [Google Scholar] [CrossRef]
- Gómez-Sala, B.; Muñoz-Atienza, E.; Sánchez, J.; Basanta, A.; Herranz, C.; Hernández, P.E.; Cintas, L.M. Bacteriocin Production by Lactic Acid Bacteria Isolated from Fish, Seafood and Fish Products. Eur. Food Res. Technol. 2015, 241, 341–356. [Google Scholar] [CrossRef]
- Calo-Mata, P.; Arlindo, S.; Boehme, K.; de Miguel, T.; Pascoal, A.; Barros-Velazquez, J. Current Applications and Future Trends of Lactic Acid Bacteria and Their Bacteriocins for the Biopreservation of Aquatic Food Products. Food Bioprocess Technol. 2008, 1, 43–63. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Sun, D.-W.; Wei, Q. Enhancing Visible and Near-Infrared Hyperspectral Imaging Prediction of TVB-N Level for Fish Fillet Freshness Evaluation by Filtering Optimal Variables. Food Anal. Methods 2017, 10, 1888–1898. [Google Scholar] [CrossRef]
- Orban, E.; Nevigato, T.; Di Lena, G.; Masci, M.; Casini, I.; Caproni, R.; Rampacci, M. Total Volatile Basic Nitrogen and Trimethylamine Nitrogen Levels during Ice Storage of European Hake (Merluccius merluccius): A Seasonal and Size Differentiation. Food Chem. 2011, 128, 679–682. [Google Scholar] [CrossRef]
- Wang, X.; Shan, J.; Han, S.; Zhao, J.; Zhang, Y. Optimization of Fish Quality by Evaluation of Total Volatile Basic Nitrogen (TVB-N) and Texture Profile Analysis (TPA) by Near-Infrared (NIR) Hyperspectral Imaging. Anal. Lett. 2019, 52, 1845–1859. [Google Scholar] [CrossRef]
- Fraqueza, M.J.; Ferreira, M.C.; Barreto, A.S. Spoilage of Light (PSE-like) and Dark Turkey Meat under Aerobic or Modified Atmosphere Package: Microbial Indicators and Their Relationship with Total Volatile Basic Nitrogen. Br. Poult. Sci. 2008, 49, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Paari, A.; Paulraj, K.; Ramraj, S.; Neelkandan, Y.; Vellaiyan, P.; Siva, P.; Venkatesan, A. Biopreservation of Sardinella longiceps and Penaeus Monodon Using Protective Culture Streptococcus phocae PI 80 Isolated from Marine Shrimp Penaeus indicus. Probiotics Antimicrob. Proteins 2011, 3, 103–111. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Kung, H.-F.; Huang, C.-Y.; Huang, T.-C.; Tsai, Y.-H. Reduction of Histamine and Biogenic Amines during Salted Fish Fermentation by Bacillus polymyxa as a Starter Culture. J. Food Drug. Anal. 2016, 24, 157–163. [Google Scholar] [CrossRef]
- Aguilar, C.; Vanegas, C.; Klotz, B. Antagonistic Effect of Lactobacillus Strains against Escherichia coli and Listeria monocytogenes in Milk. J. Dairy Res. 2011, 78, 136–143. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Rendueles, M.; Díaz, M. Microbial Amensalism in Lactobacillus casei and Pseudomonas taetrolens Mixed Culture. Bioprocess Biosyst. Eng. 2017, 40, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Daniela, C.; Speranza, B.; Bevilacqua, A.; Altieri, C.; Rosaria Corbo, M.; Sinigaglia, M. Industrial Validation of a Promising Functional Strain of Lactobacillus plantarum to Improve the Quality of Italian Sausages. Microorganisms 2020, 8, 116. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The Role of Lactic Acid Production by Probiotic Lactobacillus Species in Vaginal Health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. (Eds.) Intrinsic and Extrinsic Parameters of Foods That Affect Microbial Growth. In Modern Food Microbiology; Food Science Text Series; Springer: Boston, MA, USA, 2005; pp. 39–59. [Google Scholar] [CrossRef]
- Sun, J.-L.; Zhang, S.-K.; Chen, J.-Y.; Han, B.-Z. Metabolic Profiling of Staphylococcus Aureus Cultivated under Aerobic and Anaerobic Conditions with 1H NMR-Based Nontargeted Analysis. Can. J. Microbiol. 2012, 58, 709–718. [Google Scholar] [CrossRef]
- De Mey, M.; De Maeseneire, S.; Soetaert, W.; Vandamme, E. Minimizing Acetate Formation in E. coli Fermentations. J. Ind. Microbiol. Biotechnol. 2007, 34, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Nobechi, K. Contributions to the Knowledge of Vibrio Cholerae I. Fermentation of Carbohydrates and Polyatomic Alcohols by Vibrio Cholerae. J. Bacteriol. 1925, 10, 197–215. [Google Scholar] [CrossRef]
- Latour, X.; Lemanceau, P. Métabolisme Carboné et Énergétique Des Pseudomonas spp. Fluorescents Saprophytes à Oxydase Positive. Agronomie 1997, 17, 427–443. [Google Scholar] [CrossRef]
- Drago, L.; Gismondo, M.R.; Lombardi, A.; Haën, C.; Gozzini, L. Inhibition of in Vitro Growth of Enteropathogens by New Lactobacillus Isolates of Human Intestinal Origin. FEMS Microbiol. Lett. 2006, 153, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Patel, A.; Ambalam, P.; Holst, O.; Ljungh, A.; Prajapati, J. Determination of an Antimicrobial Activity of Weissella confusa, Lactobacillus fermentum, and Lactobacillus plantarum against Clinical Pathogenic Strains of Escherichia coli and Staphylococcus aureus in Co-Culture. Ann. Microbiol. 2016, 66, 1137–1143. [Google Scholar] [CrossRef]
- Kayode, M.; Alebiosu, A.; Adetoye, F.; Ayeni; Ayeni, F. Antimicrobial Activities of Lactic Acid Bacteria against Pseudomonas aeruginosa, Providencia vermicola, Alcaligenes faecalis and Methicillin Resistant S. aureus. West Afr. J. Pharm. 2017, 28, 132–142. [Google Scholar]
- Stiles, M.E.; Holzapfel, W.H. Lactic Acid Bacteria of Foods and Their Current Taxonomy. Int. J. Food Microbiol. 1997, 36, 1–29. [Google Scholar] [CrossRef]
- Govindaraj, K.; Samayanpaulraj, V.; Narayanadoss, V.; Uthandakalaipandian, R. Isolation of Lactic Acid Bacteria from Intestine of Freshwater Fishes and Elucidation of Probiotic Potential for Aquaculture Application. Probiotics Antimicrob. Proteins 2021, 13, 1598–1610. [Google Scholar] [CrossRef]
- Meruvu, H.; Harsa, S.T. Lactic Acid Bacteria: Isolation–Characterization Approaches and Industrial Applications. Crit. Rev. Food Sci. Nutr. 2022, 63, 8337–8356. [Google Scholar] [CrossRef]
- Françoise, L. Occurrence and Role of Lactic Acid Bacteria in Seafood Products. Food Microbiol. 2010, 27, 698–709. [Google Scholar] [CrossRef] [PubMed]
- ICMSF. Book—ICMSF | International Commission on Microbiological Specifications for Foods. 1986. Available online: https://www.icmsf.org/publications/books/ (accessed on 16 October 2023).
- Kuley, E.; Durmus, M.; Ucar, Y.; Kosker, A.R.; Aksun Tumerkan, E.T.; Regenstein, J.M.; Ozogul, F. Combined Effects of Plant and Cell-Free Extracts of Lactic Acid Bacteria on Biogenic Amines and Bacterial Load of Fermented Sardine Stored at 3 ± 1 °C. Food Biosci. 2018, 24, 127–136. [Google Scholar] [CrossRef]
- Faid, M.; Zouiten, A.; Elmarrakchi, A.; Achkari-Begdouri, A. Biotransformation of Fish Waste into a Stable Feed Ingredient. Food Chem. 1997, 60, 13–18. [Google Scholar] [CrossRef]
- Dyda, M.; Laudy, A.; Decewicz, P.; Romaniuk, K.; Ciezkowska, M.; Szajewska, A.; Solecka, D.; Dziewit, L.; Drewniak, L.; Skłodowska, A. Diversity of Biodeteriorative Bacterial and Fungal Consortia in Winter and Summer on Historical Sandstone of the Northern Pergola, Museum of King John III’s Palace at Wilanow, Poland. Appl. Sci. 2021, 11, 620. [Google Scholar] [CrossRef]
- Ndaw, A.; Zinedine, A.; Faid, M.; Bouseta, A. Effect of Controlled Lactic Acid Bacterial Fermentation on the Microbiological and Chemical Qualities of Moroccan Sardines (Sardina pilchardus). Acta Microbiol. Immunol. Hung. 2008, 55, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Chen, S.; Liu, G.; Yang, Q. Quality Enhancement in the Japanese Sea Bass (Lateolabrax japonicas) Fillets Stored at 4 °C by Chitosan Coating Incorporated with Citric Acid or Licorice Extract. Food Chem. 2014, 162, 156–160. [Google Scholar] [CrossRef] [PubMed]


| Strain | Antibiotic Susceptibility (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Ampi. | Amx. + Clavu. ac. | Imipen. | Cipro. | Vanco. | Erythro. | Clinda. | |
| OV50 | 22 | 29 | 30 | 0 | 0 | 35 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohellebi, N.; Hamma-Faradji, S.; Bendjeddou, K.; Ait Meddour, A.; Benchikh, Y.; Bendali, F.; Belguesmia, Y.; Drider, D. Biopreservation of Fresh Sardines (Sardina pilchardus) Using Lactiplantibacillus plantarum OV50 Isolated from Traditional Algerian Green Olives Preparations. Foods 2024, 13, 368. https://doi.org/10.3390/foods13030368
Mohellebi N, Hamma-Faradji S, Bendjeddou K, Ait Meddour A, Benchikh Y, Bendali F, Belguesmia Y, Drider D. Biopreservation of Fresh Sardines (Sardina pilchardus) Using Lactiplantibacillus plantarum OV50 Isolated from Traditional Algerian Green Olives Preparations. Foods. 2024; 13(3):368. https://doi.org/10.3390/foods13030368
Chicago/Turabian StyleMohellebi, Nassima, Samia Hamma-Faradji, Kamel Bendjeddou, Amel Ait Meddour, Yassine Benchikh, Farida Bendali, Yanath Belguesmia, and Djamel Drider. 2024. "Biopreservation of Fresh Sardines (Sardina pilchardus) Using Lactiplantibacillus plantarum OV50 Isolated from Traditional Algerian Green Olives Preparations" Foods 13, no. 3: 368. https://doi.org/10.3390/foods13030368






