Bioaccessibility, Intestinal Absorption and Anti-Inflammatory Activity of Curcuminoids Incorporated in Avocado, Sunflower, and Linseed Beeswax Oleogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Oleogels
2.2.2. Oleogel Characterization
2.2.2.1. Mechanical Strength
2.2.2.2. Oil Binding Capacity
2.2.2.3. Rheological Analysis
2.2.3. Oxidative Stability of Oleogels under Accelerated Conditions
2.2.4. In Vitro Gastrointestinal Digestion of Oleogels
2.2.4.1. Bioaccessibility of Total FFA, Individual FFA, and Curcumin from Digested Samples
2.2.5. Cell Model Assays
2.2.5.1. Cell Viability
2.2.5.2. CaCo-2 Cell Permeability Assay
2.2.5.3. Immune Stimulation Assay
2.2.6. Statistical Analysis
3. Results
3.1. Oleogel Characterization
3.2. Oxidative Stability of Oleogels under Accelerated Conditions
3.3. Bioaccessibility of Individual FFAs and Curcumin from Digested Samples
3.4. Cell Culture Assays
3.4.1. Cell Viability
3.4.2. CaCo-2 Cell Permeability Assay
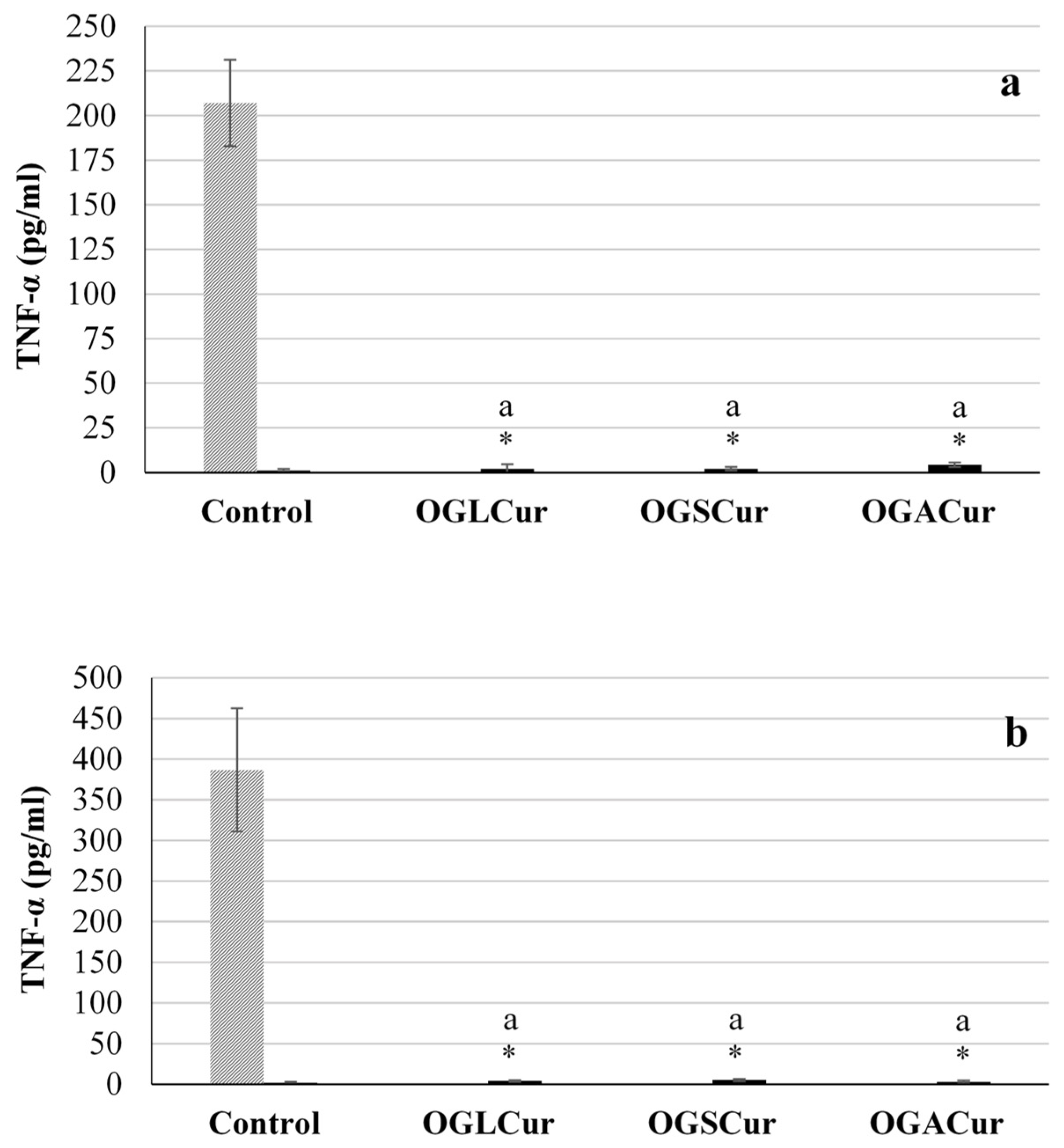
3.4.3. Immune Stimulation Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Co, E.D.; Marangoni, A.G. Organogels: An alternative edible oil-structuring method. J. Am. Oil Chem. Soc. 2012, 89, 749–780. [Google Scholar]
- Co, E.D.; Marangoni, A.G. Oleogels: An introduction. In Edible Oleogels: Structure and Health Implications; Marangoni, A.G., Garti, N., Eds.; AOCS Press: Boca Raton, FL, USA, 2018; pp. 1–29. [Google Scholar]
- Hwang, H.S. A critical review on structures, health effects, oxidative stability, and sensory properties of oleogels. Biocatal. Agric. Biotechnol. 2020, 26, 101657. [Google Scholar] [CrossRef]
- Doan, C.D.; Tavernier, I.; Okuro, P.K.; Dewettinck, K. Internal and external factors affecting the crystallization, gelation and applicability of wax-based oleogels in food industry. Innov. Food Sci. Emerg. Technol. 2018, 45, 42–52. [Google Scholar] [CrossRef]
- Pehlivanoğlu, H.; Demirci, M.; Toker, O.S.; Konar, N.; Karasu, S.; Sagdic, O. Oleogels, a promising structured oil for decreasing saturated fatty acid concentrations: Production and food-based applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 1330–1341. [Google Scholar] [CrossRef]
- Martini, S.; Tan, C.Y.; Jana, S. Physical characterization of wax/oil crystalline networks. J. Food Sci. 2015, 80, C989–C997. [Google Scholar] [CrossRef]
- Martins, A.J.; Cerqueira, M.A.; Fasolin, L.H.; Cunha, R.L.; Vicente, A.A. Beeswax organogels: Influence of gelator concentration and oil type in the gelation process. Food Res. Int. 2016, 84, 170–179. [Google Scholar] [CrossRef]
- Martins, A.J.; Vicente, A.A.; Pastrana, L.M.; Cerqueira, M.A. Oleogels for development of health-promoting food products. Food Sci. Hum. Well. 2020, 9, 31–39. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Pintado, T.; Jiménez-Colmenero, F.; Cofrades, S. The effect of household storage and cooking practices on quality attributes of pork burgers formulated with PUFA- and curcumin-loaded oleogels as healthy fat substitutes. LWT—Food Sci. Technol. 2020, 119, 108909. [Google Scholar] [CrossRef]
- Li, L.; Wan, W.; Cheng, W.; Liu, G.; Han, L. Oxidatively stable curcumin-loaded oleogels structured by β-sitosterol and lecithin: Physical characteristics and release behaviour in vitro. Int. J. Food Sci. Technol. 2019, 54, 2502–2510. [Google Scholar] [CrossRef]
- Ramírez-Carrasco, P.; Paredes-Toledo, J.; Romero-Hasler, P.; Soto-Bustamante, E.; Díaz-Calderón, P.; Robert, P.; Giménez, B. Effect of adding curcumin on the properties of linseed oil organogels used as fat replacers in pâtés. Antioxidants 2020, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Vellido-Pérez, J.A.; Rodríguez-Remacho, C.; Rodríguez-Rodríguez, J.; Ochando-Pulido, J.M.; de la Fuente, E.B.; Martínez-Férez, A. Optimization of oleogel formulation for curcumin vehiculization and lipid oxidation stability by multi-response surface methodology. Chem. Eng. Trans. 2019, 75, 427–432. [Google Scholar]
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol. 2020, 11, 1–23. [Google Scholar]
- Yu, H.; Huang, Q. Investigation of the absorption mechanism of solubilized curcumin using Caco-2 cell monolayers. J. Agric. Food Chem. 2011, 59, 9120–9126. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.M.; Barbut, S.; Marangoni, A.G. Edible oleogels for the oral delivery of lipid soluble molecules: Composition and structural design considerations. Trends Food Sci. Technol. 2016, 57, 59–73. [Google Scholar] [CrossRef]
- Pinto, T.C.; Martins, A.J.; Pastrana, L.; Pereira, M.C.; Cerqueira, M.A. Oleogel-based systems for the delivery of bioactive compounds in foods. Gels 2011, 7, 86. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Q. Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions. J. Agric. Food Chem. 2012, 60, 5373–5379. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.M.; Davidovich-Pinhas, M.; Wright, A.J.; Barbut, S.; Marangoni, A.G. Ethylcellulose oleogels for lipophilic bioactive delivery-effect of oleogelation on: In vitro bioaccessibility and stability of beta-carotene. Food Funct. 2017, 8, 1438–1451. [Google Scholar] [CrossRef]
- Fan, Y.; Gao, L.; Yi, J.; Zhang, Y.; Yokoyama, W. Development of β-carotene-loaded organogel-based nanoemulsion with improved in vitro and in vivo bioaccessibility. J. Agric. Food Chem. 2017, 65, 6188–6194. [Google Scholar] [CrossRef]
- Calligaris, S.; Alongi, M.; Lucci, P.; Anese, M. Effect of different oleogelators on lipolysis and curcuminoid bioaccessibility upon in vitro digestion of sunflower oil oleogels. Food Chem. 2020, 314, 126146. [Google Scholar] [CrossRef]
- AOCS Official Method Cd 8-53, Peroxide Value—Acetic Acid-Chloroform Method, Official Methods and Recommended Practices of the American Oil Chemist’s Society, 3rd ed.; American Oil Chemists Society: Champaign, IL, USA, 1993.
- ISO 3656:2011; Animal and Vegetable Fats and Oils—Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction. ISO: Geneva, Switzerland, 2011.
- Marczylo, T.; Steward, W.; Gescher, A. Rapid analysis of curcumin and curcumin metabolites in rat biomatrices using a novel ultraperformance liquid chromatography (UPLC) method. J. Agric. Food Chem. 2009, 57, 797–803. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Ng, N.; Chen, P.X.; Ghazani, S.M.; Wright, A.J.; Marangoni, A.; Goff, H.D.; Joye, I.J.; Rogers, M.A. Lipid digestion of oil-in-water emulsions stabilized with low molecular weight surfactants. Food Funct. 2019, 10, 8195–8207. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, R.; Giménez, B.; Mackie, A.; Torcello-Gómez, A.; Quintriqueo, A.; Oyarzun-Ampuero, F.; Robert, P. Influence of the particle size of encapsulated chia oil on the oil release and bioaccessibility during in vitro gastrointestinal digestion. Food Funct. 2022, 13, 1370–1379. [Google Scholar] [CrossRef]
- Alemán, A.; Marín-Peñalver, D.; Fernandez de Palencia, P.; Gómez-Guillén, C.; Montero, P. Anti-inflammatory properties, bioaccessibility and intestinal absorption of sea fennel (Crithmum maritimum) extract encapsulated in soy phosphatidylcholine liposomes. Nutrients 2022, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, L.S.T.; Rodali, D.R.; Ueno, S.; Sato, K. Crystallization kinetics of organogels prepared by rice bran wax and vegetable oils. J. Oleo Sci. 2012, 61, 1–9. [Google Scholar] [CrossRef]
- Patel, A.R. Natural waxes as oil structurants. In Alternative Routes to Oil Structuring; Hartel, R.W., Clark, P.J., Finley, J.W., Rodriguez-Lazaro, D., Roos, Y., Topping, D., Eds.; Springer Briefs in Food, Health, and Nutrition; Springer Publishing Company: New York, NY, USA, 2015; pp. 15–27. [Google Scholar]
- Gravelle, A.J.; Davidovich-Pinhas, M.; Zetzl, A.K.; Barbut, S.; Marangoni, A.G. Influence of solvent quality on the mechanical strength of ethylcellulose oleogels. Carbohydr. Polym. 2016, 135, 169–179. [Google Scholar] [CrossRef]
- Blake, A.; Co, E.D.; Marangoni, A.G. Structure and physical properties of plant wax crystal networks and their relationship to oil binding capacity. J. Am. Oil Chem. Soc. 2014, 91, 885–903. [Google Scholar] [CrossRef]
- Lim, J.; Hwang, H.S.; Lee, S. Oil-structuring characterization of natural waxes in canola oil oleogels: Rheological, thermal, and oxidative properties. Appl. Biol. Chem. 2017, 60, 17–22. [Google Scholar] [CrossRef]
- Hwang, H.S.; Fhaner, M.; Winkler-Moser, J.K.; Liu, S.X. Oxidation of fish oil oleogels formed by natural waxes in comparison with bulk oil. Eur. J. Lipid Sci. Technol. 2018, 120, 1700378. [Google Scholar] [CrossRef]
- Barclay, L.; Vinqvist, M.; Mukai, K.; Goto, H.; Hashimoto, Y.; Tokunaga, A.; Uno, H. On the antioxidant mechanism of curcumin: Classical methods are needed to determine antioxidant mechanism and activity. Org. Lett. 2000, 2, 2841–2843. [Google Scholar] [CrossRef]
- Barrera-Arellano, D.; Ruiz-Méndez, V.; Velasco, J.; Márquez-Ruiz, G.; Dobarganes, C. Loss of tocopherols and formation of degradation compounds at frying temperatures in oils differing in degree of unsaturation and natural antioxidant content. J. Sci. Food Agric. 2002, 82, 1696–1702. [Google Scholar] [CrossRef]
- Ye, Z.; Li, R.; Cao, C.; Xu, Y.-J.; Cao, P.; Li, Q.; Liu, Y. Fatty acid profiles of typical dietary lipids after gastrointestinal digestion and absorbtion: A combination study between in-vitro and in-vivo. Food Chem. 2019, 280, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, A.; Laufer, S.; Rosen-Kligvasser, J.; Lesmes, U. Impact of different oil gelators and oleogelation mechanisms on digestive lipolysis of canola oil oleogels. Food Hydrocoll. 2019, 97, 105218. [Google Scholar] [CrossRef]
- Dong, L.; Lv, M.; Xiangyang, G.; Zhang, L.; Rogers, M.; Cao, Y.; Lan, Y. In vitro gastrointestinal digestibility of phytosterol oleogels: Influence of self-assembled microstructures on emulsification efficiency and lipase activity. Food Funct. 2020, 11, 9503–9513. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Zhong, F.; Zhang, Y.; Yokoyama, W.; Zhao, L. Effects of lipids on in vitro release and cellular uptake of β-carotene in nanoemulsion-based delivery systems. J. Agric. Food Chem. 2015, 63, 10831–10837. [Google Scholar] [CrossRef]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Schiborr, C.; Kocher, A.; Meins, J.; Behnam, D.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Transepithelial transport of curcumin in caco-2 cells is significantly enhanced by micellar solubilisation. Plant Foods Hum. Nutr. 2017, 72, 48–53. [Google Scholar] [CrossRef]
- Wang, J.; Ma, W.; Tu, P. The mechanism of self-assembled mixed micelles in improving curcumin oral absorption: In vitro and in vivo. Colloids Surf. B Biointerfaces 2015, 133, 108–119. [Google Scholar] [CrossRef]
- Kim, K.B.; Nam, Y.A.; Kim, H.S.; Hayes, A.W.; Lee, B.M. α-Linolenic acid: Nutraceutical, pharmacological and toxicological evaluation. Food Chem. Toxicol. 2014, 70, 163–178. [Google Scholar] [CrossRef]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on anti-inflammatory molecular mechanisms induced by oleic acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef]
- Bisht, A.; Dickens, M.; Rutherfurd-Markwick, K.; Thota, R.; Mutukumira, A.N.; Singh, H. Chlorogenic acid potentiates the anti-inflammatory activity of curcumin in LPS-stimulated THP-1 cells. Nutrients 2020, 12, 2706. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-inflammatory effects of curcumin in the inflammatory diseases: Status, limitations and countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]
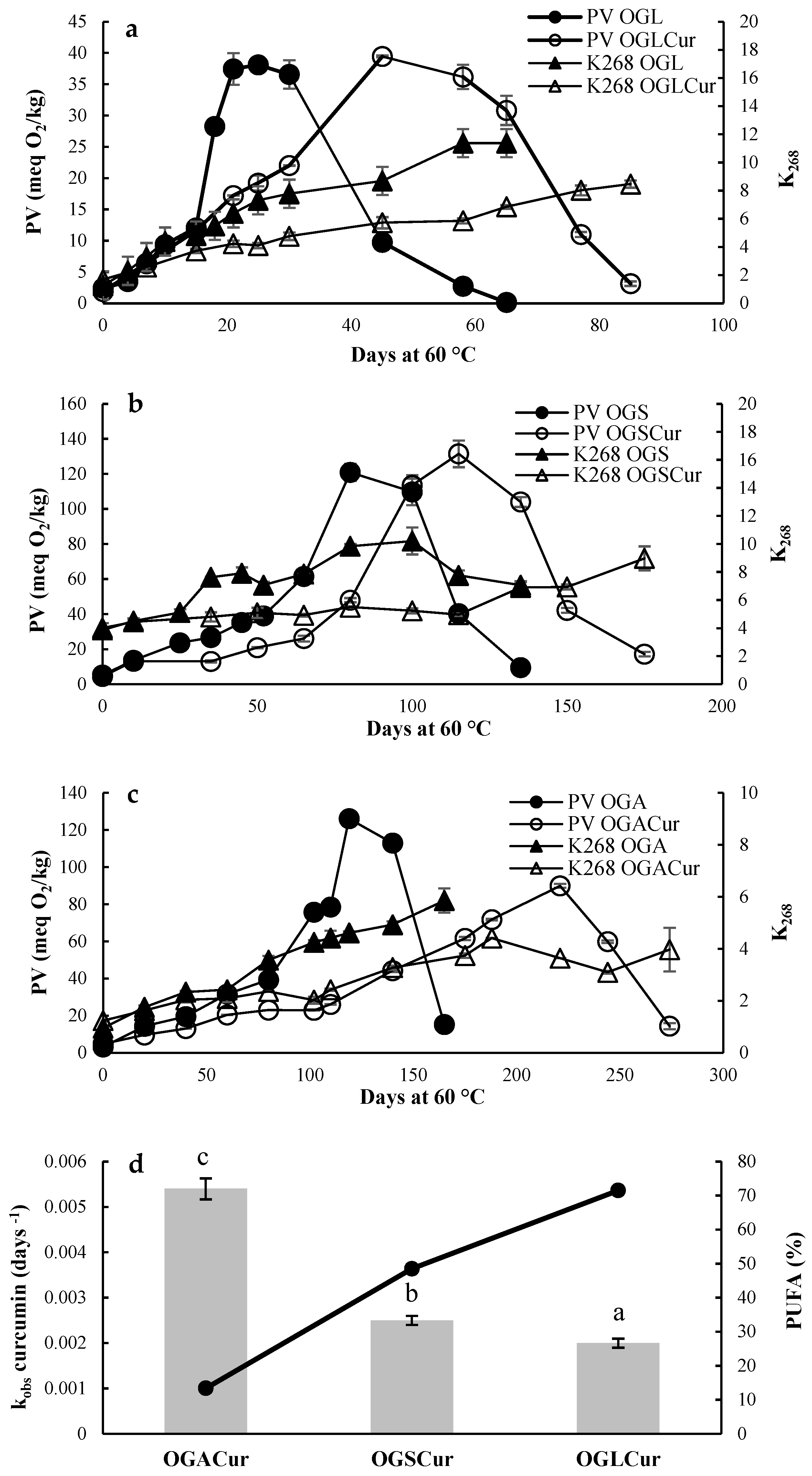
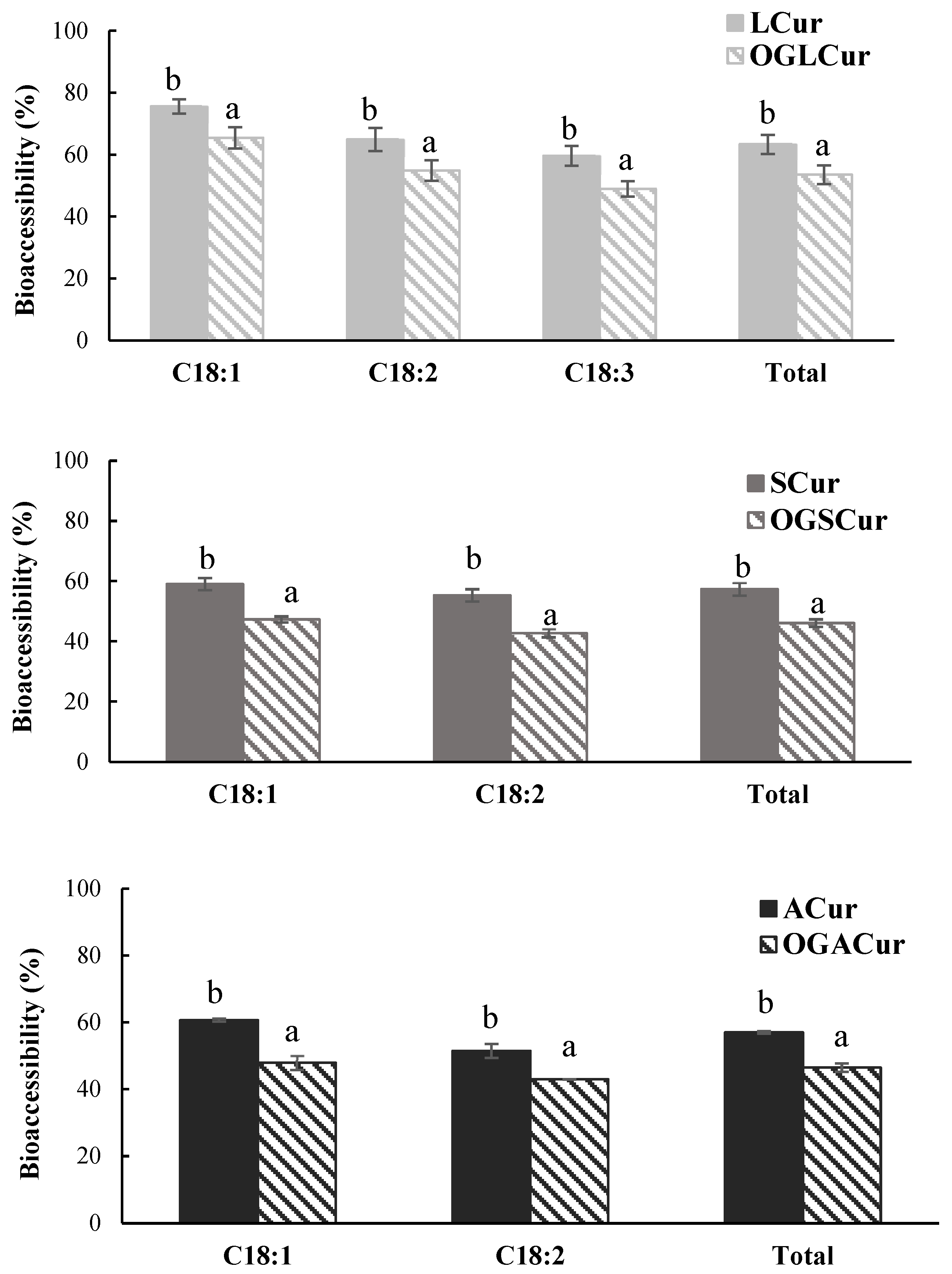
| Mechanical Strength (N) | OBC Day 1 (%) | OBC Day 7 (%) | |
|---|---|---|---|
| OGLCur | 19.5 ± 1.2 a | 91.4 ± 1.6 c/y | 80.4 ± 3.7 b/z |
| OGSCur | 18.9 ± 0.4 a | 86.0 ± 2.6 a/y | 73.7 ± 1.0 a/z |
| OGACur | 18.5 ± 1.6 a | 88.8 ± 1.1 b/y | 74.5 ± 1.7 a/z |
| Bisdemethoxycurcumin | Demethoxycurcumin | Curcumin | Total Curcuminoids | |
|---|---|---|---|---|
| LCur | 76.1 ± 1.3 a/x | 67.2 ± 1.3 a/y | 62.0 ± 1.6 a/z | 63.0 ± 1.5 a/z |
| SCur | 77.6 ± 1.9 a/x | 67.8 ± 3.5 a/y | 58.2 ± 3.7 a/z | 61.0 ± 3.7 a/y,z |
| ACur | 75.3 ± 5.7 a/x | 61.9 ± 2.8 a/y | 57.6 ± 5.2 a/y | 59.8 ± 5.5 a/y |
| OGLCur | 70.2 ± 2.0 a/x | 61.5 ± 2.2 a/y | 56.8 ± 2.5 a/y | 58.7 ± 2.4 a/y |
| OGSCur | 78.5 ± 0.8 a/x | 68.2 ± 2.1 a/y | 59.7 ± 2.2 a/z | 61.5 ± 2.2 a/y,z |
| OGACur | 71.7 ± 3.1 a/x | 61.7 ± 2.1 a/y | 53.7 ± 2.6 a/z | 55.9 ± 2.5 a/y,z |
| Curcumin (%, 1 h) | Curcumin (%, 2 h) | Papp (cm/s) | |
|---|---|---|---|
| LCur | 4.3 ± 0.1 a | 12.5 ± 0.5 a | 1.6 × 10−5 ± 0.2 × 10−6 a |
| SCur | 4.4 ± 0.4 a | 13.4 ± 0.6 a | 1.7 × 10−5 ± 1.3 × 10−6 a |
| ACur | 4.9 ± 0.3 a | 15.0 ± 0.5 a | 1.8 × 10−5 ± 1.4 × 10−6 a |
| OGLCur | 5.5 ± 0.3 b | 17.3 ± 0.1 b | 2.1 × 10−5 ± 1.1 × 10−6 b |
| OGSCur | 6.0 ± 0.1 b | 16.7 ± 0.1 b | 2.2 × 10−5 ± 0.4 × 10−6 b |
| OGACur | 6.0 ± 0.1 b | 17.3 ± 0.1 b | 2.3 × 10−5 ± 0.3 × 10−6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Carrasco, P.; Alemán, A.; González, E.; Gómez-Guillén, M.C.; Robert, P.; Giménez, B. Bioaccessibility, Intestinal Absorption and Anti-Inflammatory Activity of Curcuminoids Incorporated in Avocado, Sunflower, and Linseed Beeswax Oleogels. Foods 2024, 13, 373. https://doi.org/10.3390/foods13030373
Ramírez-Carrasco P, Alemán A, González E, Gómez-Guillén MC, Robert P, Giménez B. Bioaccessibility, Intestinal Absorption and Anti-Inflammatory Activity of Curcuminoids Incorporated in Avocado, Sunflower, and Linseed Beeswax Oleogels. Foods. 2024; 13(3):373. https://doi.org/10.3390/foods13030373
Chicago/Turabian StyleRamírez-Carrasco, Patricia, Ailén Alemán, Estefanía González, M. Carmen Gómez-Guillén, Paz Robert, and Begoña Giménez. 2024. "Bioaccessibility, Intestinal Absorption and Anti-Inflammatory Activity of Curcuminoids Incorporated in Avocado, Sunflower, and Linseed Beeswax Oleogels" Foods 13, no. 3: 373. https://doi.org/10.3390/foods13030373
APA StyleRamírez-Carrasco, P., Alemán, A., González, E., Gómez-Guillén, M. C., Robert, P., & Giménez, B. (2024). Bioaccessibility, Intestinal Absorption and Anti-Inflammatory Activity of Curcuminoids Incorporated in Avocado, Sunflower, and Linseed Beeswax Oleogels. Foods, 13(3), 373. https://doi.org/10.3390/foods13030373







