Quinoa (Chenopodium Quinoa Willd.) as Functional Ingredient for the Formulation of Gluten-Free Shortbreads
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Shortbreads Ingredients
2.2. Methods
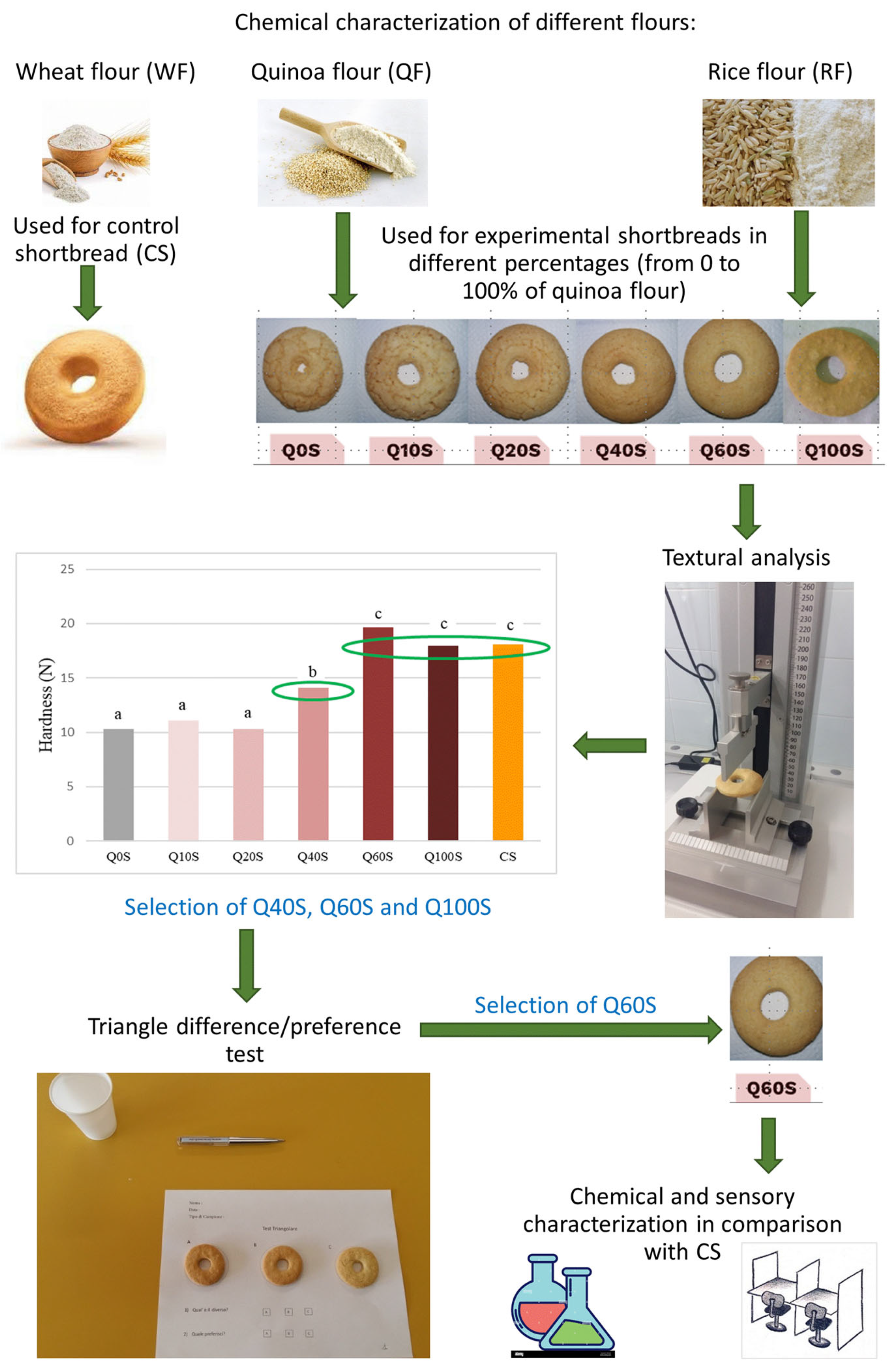
2.2.1. Experimental Design
2.2.2. Analytical Determination
2.2.3. Laboratory-Scale Shortbread Production
2.2.4. Shortbread Texture
2.2.5. Spread Factor and Weight Loss Determination
2.2.6. Sensory Analysis
Sensory Discrimination and Preference Test
Quantitative Descriptive Analysis
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Quality Parameters of Flour
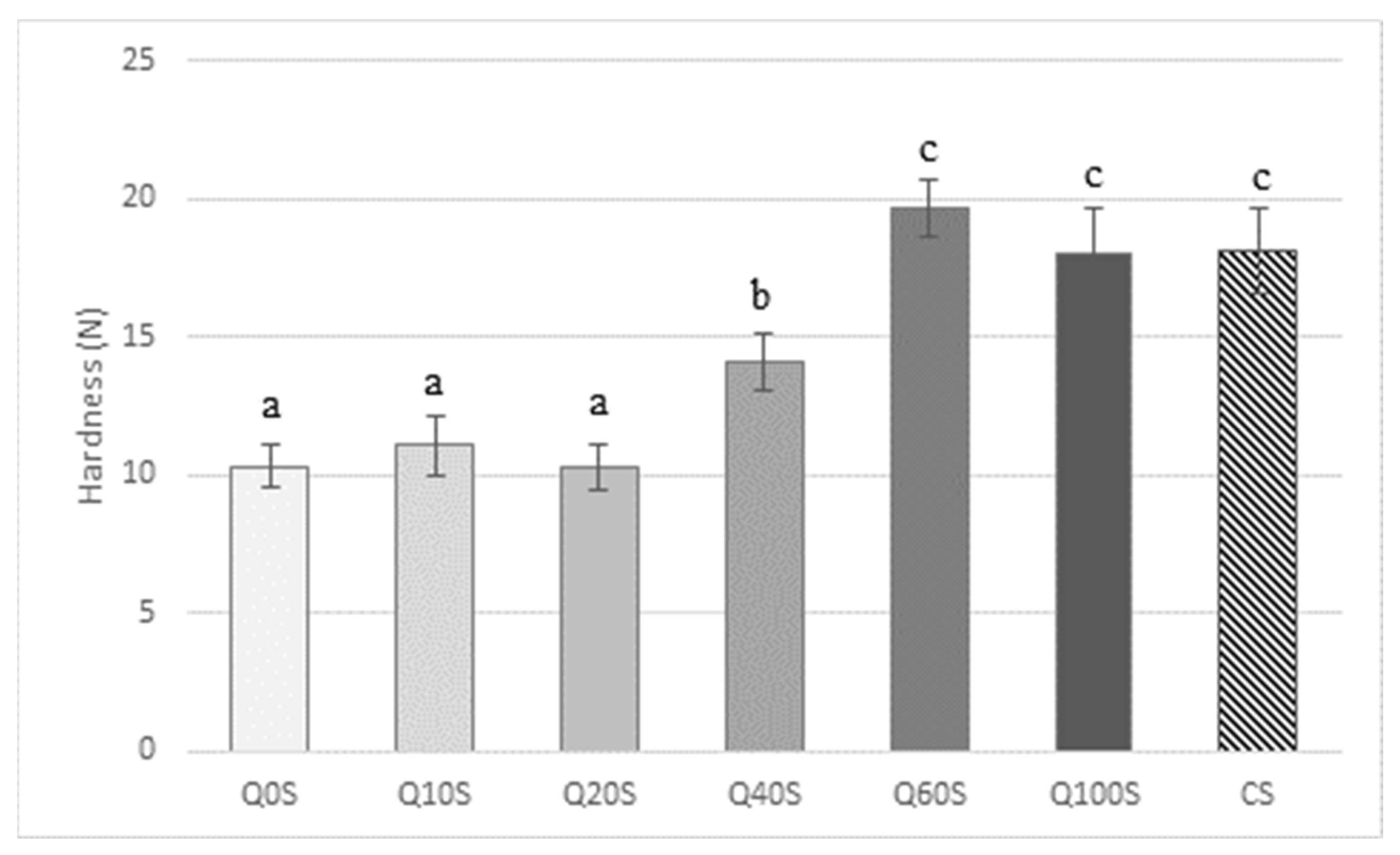
3.2. Control and Experimental Shortbread Textural Analysis
3.3. Sensory Discrimination and Preference between Q40S, Q60S, and Q100S
3.4. Quality Parameters of Selected Shortbreads
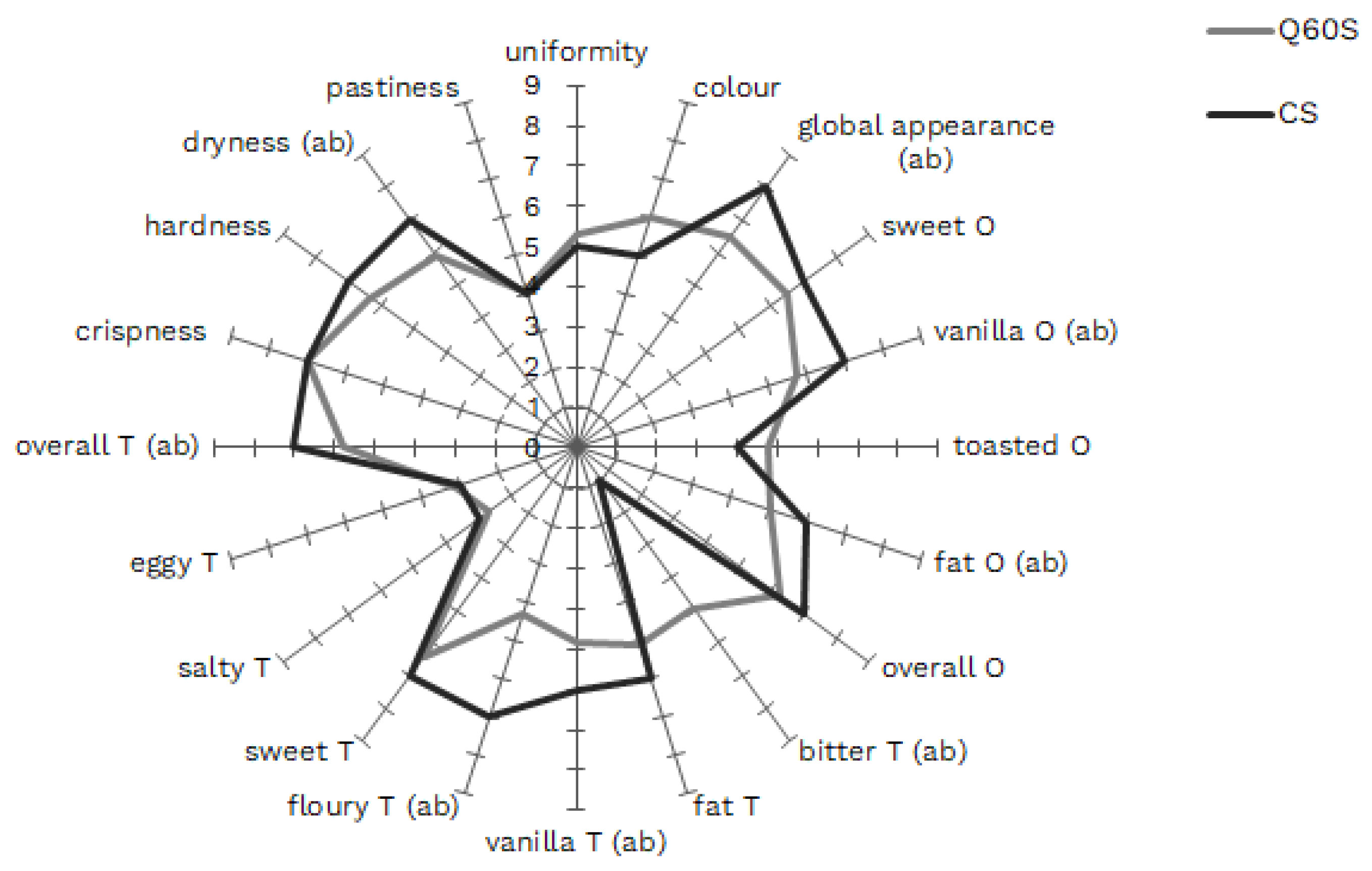
3.5. Sensory Analysis of Selected Shortbreads
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, R.A.; Srivastava, S.; Shahi, N.C. Formulation of quinoa incorporated protein-rich biscuits and numerical optimization of its process parameters. J. Food Process. Preserv. 2022, 46, e16209. [Google Scholar] [CrossRef]
- Gordillo-Bastidas, E.; Díaz-Rizzolo, D.A.; Roura, E.; Massanés, T.; Gomis, R. Quinoa (Chenopodium quinoa Willd) from Nutritional Value to Potential Health Benefits: An Integrative Review. J. Nutr. Food Sci. 2016, 6, 1000497. [Google Scholar]
- Angeli, V.; Silva, P.M.; Crispim Massuela, D.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An overview of the potentials of the “Golden Grain” and socio-economic and environmental aspects of its cultivation and marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef]
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusairi, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Otambekova, M.; et al. Worldwide Evaluations of Quinoa: Preliminary Results from Post International Year of Quinoa FAO Projects in Nine Countries. Front. Plant Sci. 2016, 7, 850. [Google Scholar] [CrossRef]
- Lazíková, J.; Takáč, I.; Schneir, E.R.; Rumanovská, L. Legal Aspects of the Quinoa Imports Into the EU. EU Agrar. Law. 2022, 11, 13–21. [Google Scholar] [CrossRef]
- King, J.A.; Jeong, J.; Underwood, F.E.; Quan, J.; Panaccione, N.; Windsor, J.W.; Coward, S.; deBruyn, J.; Ronksley, P.E.; Shaheen, A.A.; et al. Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2020, 115, 507–525. [Google Scholar] [CrossRef]
- Savarese, M.; Wismer, W.; Graffign, G. Conceptualizing “free-from” food consumption determinants: A systematic integrative literature review focused on gluten and lactose. Food Qual. Prefer. 2021, 90, 104170. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmed, M. A review on biscuit, a largest consumed processed product in India, its fortification and nutritional improvement. IJSIT 2014, 3, 169–186. [Google Scholar]
- Agrahar-Murugkar, D.; Gulati, P.; Kotwaliwale, N.; Gupta, C. Evaluation of nutritional, textural and particle size characteristics of dough and biscuits made from composite flours containing sprouted and malted ingredients. J. Food Sci. Technol. 2015, 52, 5129–5137. [Google Scholar] [CrossRef]
- Cayres, C.A.; Ascheri, J.L.R.; Peixoto-Gimenes Couto, M.A. Evaluation of nutritional characteristics and consumers’ acceptance of gluten-free sweet biscuits made from rice-based pregelatinized composite flours containing orange pomace and soy protein isolate. SN Appl. Sci. 2021, 3, 183. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 14th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1984; Available online: https://www.aoac.org/official-methods-of-analysis/ (accessed on 29 December 2023).
- Sileoni, V.; Alfeo, V.; Bravi, E.; Belardi, I.; Marconi, O. Upcycling of a by-product of the brewing production chain as an ingredient in the formulation of functional shortbreads. J. Funct. Foods 2022, 98, 105292. [Google Scholar] [CrossRef]
- McCleary, B.V.; Sloane, N.; Draga, A.; Lazewska, I. Measurement of total dietary fiber using AOAC method 2009.01 (AACC International approved method 32-45.01): Evaluation and updates. Cereal Chem. 2013, 90, 396–414. [Google Scholar] [CrossRef]
- Bravi, E.; Francesco, G.D.; Sileoni, V.; Perretti, G.; Galgano, F.; Marconi, O. Brewing by-product upcycling potential: Nutritionally valuable compounds and antioxidant activity evaluation. Antioxidants 2021, 10, 165. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Food Energy—Methods of Analysis and Conversion Factors; Food and Nutrition paper 77; FAO: Rome, Italy, 2003. [Google Scholar]
- Bravi, E.; Perretti, G.; Buzzini, P.; Della Sera, R.; Fantozzi, P. Technological Steps and Yeast Biomass as Factors Affecting the Lipid Content of Beer during the Brewing Process. J. Agric. Food Chem. 2009, 57, 6279–6284. [Google Scholar] [CrossRef]
- ACC. Approved Methods of the American Association of Cereal Chemists, 11th ed.; AACC International: St. Paul, MN, USA, 2000. [Google Scholar]
- Moawad, E.M.M.; Rizk, I.R.S.; Kishk, Y.F.M.; Youssif, M.R.G. Effect of substitution of wheat flour with quinoa flour on quality of pan bread and biscuit. Arab. Univ. J. Agric. Sci. 2019, 26, 2387–2400. [Google Scholar] [CrossRef]
- Jan, N.; Naik, H.R.; Gani, G.; Bashir, O.; Amin, T.; Wani, S.M.; Soi, S.A. Influence of replacement of wheat flour by rice flour on rheo-structural changes, in vitro starch digestibility and consumer acceptability of low-gluten pretzels. Food Prod. Process. Nutr. 2022, 4, 9. [Google Scholar] [CrossRef]
- Kowalska, S.; Szłyk, E.; Jastrzębsk, A. Simple extraction procedure for free amino acids determination in selected gluten-free flour samples. Eur. Food Res. Technol. 2022, 248, 507–517. [Google Scholar] [CrossRef]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Bravi, E.; Sileoni, V.; Perretti, G.; Marconi, O. Accelerated shelf-life model of gluten-free rusks by using oxidation indices. Food Chem. 2020, 326, 126971. [Google Scholar] [CrossRef]
- Regulation European Commission No 1924, of the European Parliament and the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32006R1924 (accessed on 29 December 2023).
- Kewuyemi, Y.O.; Kesa, H.; Meijboom, R.; Alimi, O.A.; Adebo, O.A. Comparison of nutritional quality, phenolic compounds, and antioxidant activity of conventional and 3D printed biscuits from wholegrain and multigrain flours. Innov. Food Sci. Emerg. Technol. 2023, 83, 103243. [Google Scholar] [CrossRef]
- Arslan, M.; Rakha, A.; Xiaobo, Z.; Arsalan Mahmood, M. Complimenting Gluten Free Bakery Products with Dietary Fiber: Opportunities and Constraints. Trends Food Sci. Technol. 2018, 83, 194–202. [Google Scholar] [CrossRef]
- Gasparre, N.; Pasqualone, A.; Mefleh, M.; Boukid, F. Nutritional Quality of Gluten-Free Bakery Products Labeled Ketogenic and/or Low-Carb Sold in the Global Market. Foods 2022, 11, 4095. [Google Scholar] [CrossRef]
- Beniwal, S.K.; Devi, A.; Sindhu, R. Effect of grain processing on nutritional and physicochemical, functional and pasting properties of amaranth and quinoa flours. IJTK 2019, 18, 500–507. [Google Scholar]
- Suárez-Estrella, D.; Marti, A.; Torri, L.; Pagani, M.A. Quinoa bitterness: Causes and solutions for improving product acceptability. J. Sci. Food Agric. 2018, 98, 4033–4041. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Galfo, M. A Cross-Sectional Survey of the Nutritional Quality of Quinoa Food Products Available in the Italian Market. Foods 2023, 12, 1562. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuna-Rodriguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar] [CrossRef]
| Ingredients (g) | CS | Q0S | Q10S | Q20S | Q40S | Q60S | Q100S |
|---|---|---|---|---|---|---|---|
| Wheat flour | 325 | - | - | - | - | - | - |
| Quinoa flour | - | - | 34.5 | 69 | 138 | 207 | 345 |
| Rice flour | - | 345 | 310.5 | 276 | 207 | 138 | - |
| Sucrose | 180 | 180 | 180 | 180 | 180 | 180 | 180 |
| NaCl | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 |
| (NH4)HCO3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| NaHCO3 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Butter | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Yolk | 24 | 24 | 24 | 24 | 24 | 24 | 24 |
| Egg white | 37 | 37 | 37 | 37 | 37 | 37 | 37 |
| Water | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Quality Parameter | WF | QF | RF |
|---|---|---|---|
| Moisture % | 10.60 ± 0.25 a | 9.22 ± 0.11 b | 2.59 ± 0.02 c |
| Carbohydrates (% dm) | 87.26 ± 0.38 a | 68.9 ± 1.3 b | 87.5 ± 0.5 a |
| Sugars (% dm) | 1.00 ± 0.01 b | 3.5 ± 0.3 a | nd |
| Ash (% dm) | 0.57 ± 0.01 b | 2.69 ± 0.01 a | 0.40 ± 0.01 c |
| Proteins (% dm) | 9.41 ± 0.08 b | 13.8 ± 0.1 a | 8.1 ± 0.1 c |
| Fat (% dm) | 1.01 ± 0.01 b | 7.0 ± 0.1 a | 0.4 ± 0.1 c |
| TDF (% dm) | 1.76 ± 0.33 c | 7.7 ± 1.4 a | 3.6 ± 0.5 b |
| aw | 0.57 ± 0.01 a | 0.44 ± 0.01 a | 0.50 ± 0.01 a |
| Total Polyphenols (GA mg/g) | 0.16 ± 0.01 c | 1.64 ± 0.08 a | 0.76 ± 0.04 b |
| ABTS (TE/g) | 0.15 ± 0.03 c | 0.69 ± 0.08 a | 0.29 ± 0.01 b |
| DPPH (TE/g) | 10.68 ± 1.00 c | 75.77 ± 0.80 a | 70.10 ± 0.91 b |
| FRAP (TE/g) | 0.98 ± 0.03 c | 3.58 ± 0.05 a | 1.12 ± 0.04 b |
| AAs (mg/Kg dm) | WF | QF | RF |
|---|---|---|---|
| Aspartic Acid | 105.8 ± 7.9 bI | 228.7 ± 15.9 aM | 17.6 ± 0.2 cH |
| Glutamic Acid | 60.9 ± 0.9 bG | 692.7 ± 0.7 aO | 43.6 ± 1.2 cL |
| Asparagine | 87.2 ± 0.3 aH | 50.0 ± 3.2 bDE | 52.8 ± 1.1 bM |
| Serine | 11.8 ± 3.0 bCD | 64.9 ± 11.4 aFG | 8.7 ± 0.2 bF |
| Glutamine | 44.5 ± 0.1 bF | 163.1 ± 7.1 aL | 6.9 ± 0.4 cE |
| Histidine | 8.3 ± 0.6 bABC | 254.1 ± 0.8 aN | 4.5 ± 0.1 cD |
| Arginine | 25.4 ± 0.2 bE | 736.1 ± 0.5 aP | 6.3 ± 0.5 cE |
| Glycine | 13.1 ± 0.9 bD | 69.4 ± 8.2 aG | 13.9 ± 0.2 bG |
| Alanine | 29.0 ± 1.1 bE | 146.4 ± 5.9 aI | 26.2 ± 1.5 bI |
| Tyrosine | 12.3 ± 0.5 bCD | 56.1 ± 3.7 aEF | 4.1 ± 0.4 bCD |
| Threonine | 8.4 ± 0.7 bABC | 37.6 ± 4.6 aABC | 4.8 ± 0.1 cD |
| Methionine | 4.8 ± 0.5 bA | 27.6 ± 0.1 aA | 3.3 ± 0.2 cBC |
| Tryptophan | 63.8 ± 0.7 bG | 74.4 ± 0.3 aGH | 2.8 ± 0.1 cB |
| Valine | 13.8 ± 1.2 bD | 85.5 ± 0.3 aH | 6.7 ± 0.1 cE |
| Phenylalanine | 11.3 ± 0.5 bCD | 45.2 ± 4.0 aCDE | 2.9 ± 0.2 cB |
| Isoleucine | 6.3 ± 0.3 bAB | 28.8 ± 2.0 aA | 1.4 ± 0.1 cA |
| Leucine | 10.3 ± 0.3 bBCD | 32.6 ± 2.4 aAB | 2.6 ± 0.2 cB |
| Lysine | 10.6 ± 1.0 bBCD | 42.9 ± 4.4 aBCD | 4.1 ± 0.3 cCD |
| Ʃ AAs | 527.6 ± 16.3 b | 2836.1 ± 40.7 a | 213.1 ± 2.9 c |
| Ʃ sweetness AAs | 62.2 ± 5.7 bA | 318.3 ± 30.1 aA | 53.6 ± 2.0 cB |
| Ʃ bitterness AAs | 103.2 ± 5.1 bB | 1308.9 ± 18.2 aC | 35.8 ± 2.1 cA |
| Ʃ neutral AAs | 365.2 ± 9.9 bC | 1208.9 ± 27.2 aB | 123.8 ± 3.9 cC |
| Fatty Acids (% dm) | WF | QF | RF |
|---|---|---|---|
| Butyric C4:0 | 0.21 ± 0.02 aC | nd | nd |
| Myristic C14:0 | 0.13 ± 0.01 bA | 0.12 ± 0.01 bB | 0.58 ± 0.01 aC |
| Palmitic C16:0 | 17.14 ± 0.01 aH | 8.72 ± 0.02 bI | 16.72 ± 0.01 aH |
| Palmitoleic C16:1 ω7 | 0.14 ± 0.01 cAB | 0.05 ± 0.01 aA | 0.22± 0.01 bA |
| Stearic C18:0 | 1.17 ± 0.01 bE | 0.79 ± 0.01 E | 2.25± 0.01 bG |
| Oleic C18:1 ω9 | 15.83 ± 0.01 cG | 29.75 ± 0.02 bL | 41.16 ± 0.01 aL |
| Linoleic C18:2 ω6 | 60.72 ± 0.08 I | 47.65 ± 0.03 bM | 34.88 ± 0.01 cI |
| γ-linolenic C18:3 ω6 | 3.30 ± 0.02 bF | 7.57 ± 0.01 bH | 1.29 ± 0.01 cF |
| Eicosenoic C20:1 ω9 | 0.19 ± 0.01 cBC | 0.59 ± 0.01 bD | 0.89 ± 0.02 aE |
| α-linolenic C18:3 ω3 | 0.74 ± 0.01 bD | 1.80 ± 0.01 G | 0.70 ± 0.01 bD |
| Arachidonic C20:4 ω6 | 0.21 ± 0.01 cC | 0.78 ± 0.01 aE | 0.42 ± 0.01 bB |
| Trycosilic C23:0 | nd | 1.58 ± 0.05 F | nd |
| Eicosapentaenoic C20:5 ω3 | 0.22 ± 0.01 bC | 0.30 ± 0.01 bC | 0.89 ± 0.01 aE |
| Nervonic C24:1 ω9 | nd | 0.30 ± 0.01 aC | nd |
| Ʃ Saturated | 18.65 ± 0.05 B | 11.21 ± 0.09 bA | 19.55 ± 0.08 aA |
| Ʃ Unsaturated | 81.35 ± 0.16 bD | 88.79± 0.12 aD | 80.45 ± 0.01 bD |
| Ʃ Monounsaturated | 16.16 ± 0.03 cA | 30.69 ± 0.05 bB | 42.27 ± 0.03 aC |
| Ʃ Polyunsaturated | 65.19 ± 0.13 C | 58.10 ± 0.07 bC | 38.18 ± 0.05 cB |
| Quality Parameter | CS | Q60S | |
|---|---|---|---|
| Energy value | (kcal/100 g) | 472 | 446 |
| (kJ/100 g) | 1975 | 1866 | |
| Moisture (% dm) | 1.56 ± 0.02 b | 2.51 ± 0.09 a | |
| Carbohydrates (% dm) | 73.28 ± 0.13 a | 67.30 ± 1.30 b | |
| Sugars (% dm) | 24.77 ± 0.22 a | 25.10 ± 0.10 a | |
| Ash (% dm) | 1.01 ± 0.05 a | 1.09 ± 0.03 a | |
| Proteins (% dm) | 7.13 ± 0.19 a | 7.60 ± 0.10 a | |
| Fat (% dm) | 15.99 ± 0.17 a | 16.30 ± 0.60 a | |
| TDF (% dm) | 1.03 ± 0.02 b | 5.20 ± 0.50 a | |
| aw | 0.28 ±0.01 a | 0.36 ± 0.05 a | |
| Spread factor | 48.89 ±0.82 b | 62.50 ± 1.08 a | |
| Weight loss | 1.67 ± 0.03 a | 1.33 ± 0.03 b | |
| Weight loss (%) | 16.06 ± 0.23 a | 13.40 ± 0.20 b | |
| T-Polyphenols (GA mg/g) | 1.25 ± 0.01 b | 5.73 ± 0.01 a | |
| ABTS (TE/g) | 0.10 ± 0.01 b | 0.37 ± 0.06 a | |
| DPPH (TE/g) | 25.07 ± 0.12 b | 70.03 ± 0.24 a | |
| FRAP (TE/g) | 2.77 ± 0.03 b | 8.37 ± 0.09 a | |
| AAs (mg/Kg dm) | CS | Q60S |
|---|---|---|
| Aspartic Acid | 44.8 ± 0.1 bO | 82.3 ± 6.0 aL |
| Glutamic Acid | 32.5 ± 0.6 bM | 239.0 ± 11.9 aN |
| Asparagine | 40.0 ± 0.3 aN | 31.7 ± 1.7 bEFG |
| Serine | 9.6 ± 0.5 bF | 30.0 ± 3.4 aDEF |
| Glutamine | 22.1 ± 0.2 bH | 40.2 ± 1.8 aG |
| Histidine | 5.1 ± 0.3 bC | 72.0 ± 3.4 aI |
| Arginine | 26.9 ± 0.1 bI | 217.8 ± 12.2 aM |
| Glycine | 7.3 ± 0.1 bD | 29.7 ± 0.9 aDEF |
| Alanine | 28.0 ± 0.5 bL | 59.6 ± 3.9 aH |
| Tyrosine | 9.1 ± 0.5 bF | 28.6 ± 1.8 aCDEF |
| Threonine | 5.3 ± 0.2 bC | 23.7 ± 2.6 aBCDE |
| Methionine | 2.7 ± 0.1 bA | 10.5 ± 0.1 aA |
| Tryptophan | 21.6 ± 0.2 aH | 21.0 ± 0.9 aBCD |
| Valine | 13.7 ± 0.3 bG | 35.4 ± 1.9 aFG |
| Phenylalanine | 8.2 ± 0.3 bE | 19.0 ± 0.6 aABC |
| Isoleucine | 7.1 ± 0.2 bD | 14.5 ± 0.7 aAB |
| Leucine | 9.7 ± 0.3 bF | 21.9 ± 1.3 aBCD |
| Lysine | 4.0 ± 0.2 bB | 23.4 ± 0.7 aBCDE |
| Ʃ AAs | 297.7 ± 5.0 b | 1000.3 ± 55.8 a |
| Ʃ sweetness AAs | 50.2 ± 1.3 bA | 143.0 ± 10.8 aA |
| Ʃ bitterness AAs | 86.5 ± 2.3 bB | 443.1 ± 22.7 aB |
| Ʃ neutral AAs | 161.0 ± 1.4 bC | 414.2 ± 22.3 aB |
| Fatty Acids (% dm) | CS | Q60S |
|---|---|---|
| Butyric C4:0 | 2.13 ± 0.03 aI | 2.01 ± 0.05 aH |
| Caproic C6:0 | 1.66 ± 0.01 aG | 1.64 ± 0.01 aG |
| Caprylic C8:0 | 1.13 ± 0.01 aF | 1.10 ± 0.01 aF |
| Capric C10:0 | 2.69 ± 0.01 aL | 2.62 ± 0.01 aI |
| Lauric C12:0 | 3.27 ± 0.01 aM | 3.17 ± 0.02 bL |
| Myristic C14:0 | 12.01 ± 0.04 aP | 10.12 ± 0.08 bO |
| Myristoleic C14:1 ω9 | 0.85 ± 0.01 bE | 0.94 ± 0.01 aE |
| Pentadecanoic C15:0 | 0.08 ± 0.01 bA | 1.15 ± 0.01 aF |
| Palmitic C16:0 | 31.08 ± 0.02 aR | 29.51 ± 0.01 bQ |
| Palmitoleic C16:1 ω7 | 1.79 ± 0.01 aH | 1.57 ± 0.02 bG |
| Heptadecanoic C17:0 | 0.1 ± 0.01 bA | 0.58 ± 0.01 aC |
| Heptadecenoic C17:1 | 0.1 ± 0.01 bA | 0.24 ± 0.01 aB |
| Stearic C18:0 | 10.37 ± 0.03 aO | 9.16 ± 0.04 bN |
| Oleic C18:1 ω9 | 25.55 ± 0.04 aQ | 25.15 ± 0.13 aP |
| Linoleic C18:2 ω6 | 5.14 ± 0.03 bN | 8.36 ± 0.02 aM |
| γ-linolenic C18:3 ω6 | 0.1 ± 0.01 bA | 1.17 ± 0.01 aF |
| Eicosenoic C20:1 ω9 | 0.65 ± 0.01 aC | 0.12 ± 0.01 bA |
| α-linolenic C18:3 ω3 | 0.77 ± 0.01 bD | 0.85 ± 0.01 aD |
| Behenic C22:0 | 0.10 ± 0.01 aA | 0.11 ± 0.01 aA |
| Eicosatrienoic C20:3 ω6 | nd | 0.11 ± 0.01 A |
| Arachidonic C20:4 ω6 | 0.2 ± 0.01 aB | 0.13 ± 0.01 bA |
| Trycosilic C23:0 | 0.23 ± 0.01 aB | 0.22 ± 0.04 aB |
| Ʃ Saturated | 64.95 ± 0,20 aA | 61.36 ± 0,30 bD |
| Ʃ Unsaturated | 35.15± 0,14 bB | 38.64 ± 0,23 aC |
| Ʃ Monounsaturated | 28.94 ± 0,08 aC | 28.02 ± 0,17 bB |
| Ʃ Polyunsaturated | 6.21 ± 0.06 bD | 10.62 ± 0.06 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravi, E.; Sileoni, V.; Marconi, O. Quinoa (Chenopodium Quinoa Willd.) as Functional Ingredient for the Formulation of Gluten-Free Shortbreads. Foods 2024, 13, 377. https://doi.org/10.3390/foods13030377
Bravi E, Sileoni V, Marconi O. Quinoa (Chenopodium Quinoa Willd.) as Functional Ingredient for the Formulation of Gluten-Free Shortbreads. Foods. 2024; 13(3):377. https://doi.org/10.3390/foods13030377
Chicago/Turabian StyleBravi, Elisabetta, Valeria Sileoni, and Ombretta Marconi. 2024. "Quinoa (Chenopodium Quinoa Willd.) as Functional Ingredient for the Formulation of Gluten-Free Shortbreads" Foods 13, no. 3: 377. https://doi.org/10.3390/foods13030377
APA StyleBravi, E., Sileoni, V., & Marconi, O. (2024). Quinoa (Chenopodium Quinoa Willd.) as Functional Ingredient for the Formulation of Gluten-Free Shortbreads. Foods, 13(3), 377. https://doi.org/10.3390/foods13030377








