Environmentally Friendly Approach to Pectin Extraction from Grapefruit Peel: Microwave-Assisted High-Pressure CO2/H2O
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Grapefruit Peel Pectin Extraction
2.2.1. Conventional Extraction
2.2.2. Microwave-Assisted High-Pressure CO2/H2O (MW-HPCO2) Extraction
2.3. Experimental Design and Optimization by Response Surface Methodology
2.4. Physicochemical Properties of the Extracted Pectin
2.4.1. Moisture, Ash, and Protein Content
2.4.2. GalA Content
2.4.3. Equivalent Weight and Methoxyl Content
2.4.4. Degree of Esterification
2.4.5. Reducing Sugar Content
2.4.6. Color
2.4.7. Viscosity
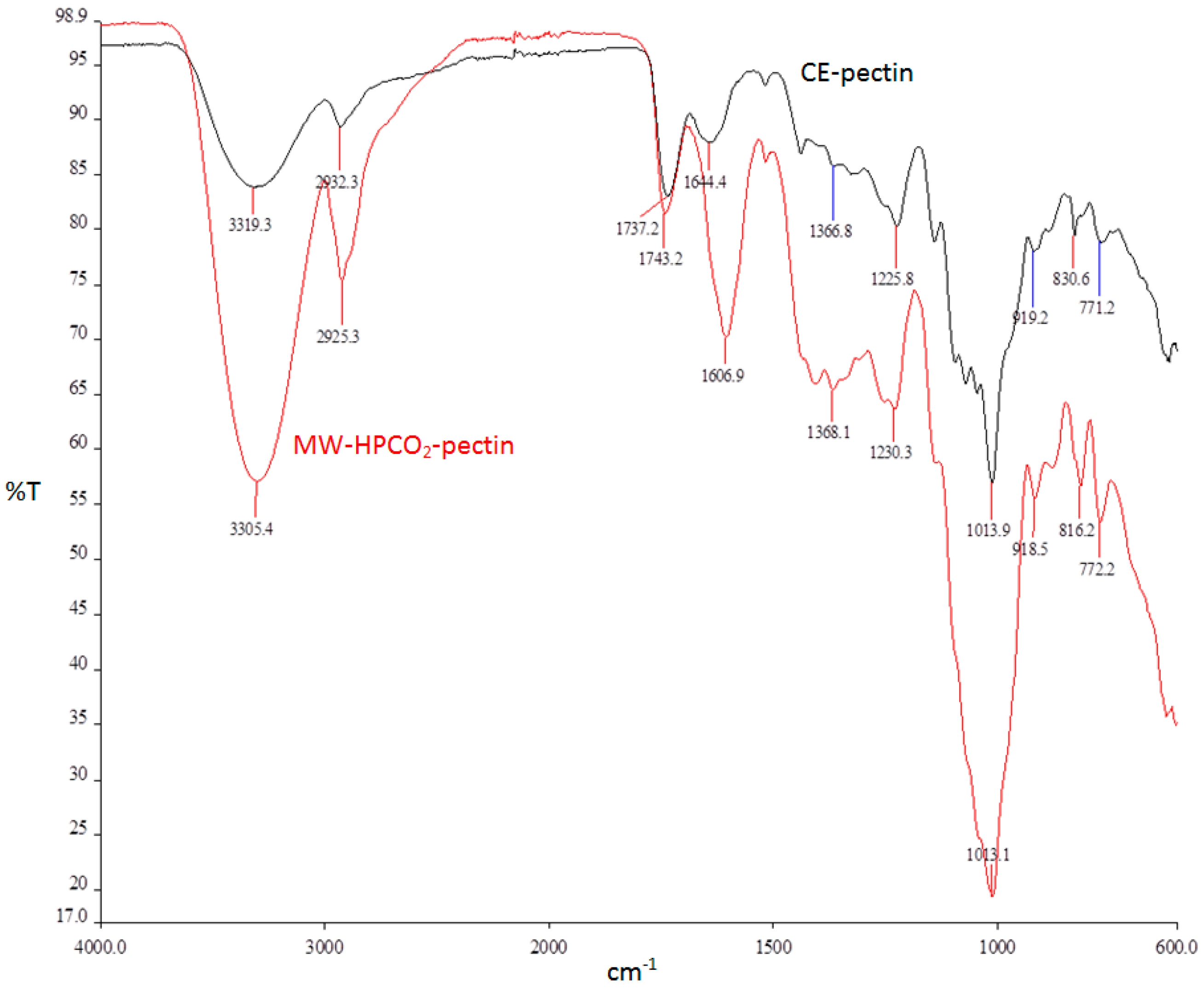
2.4.8. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
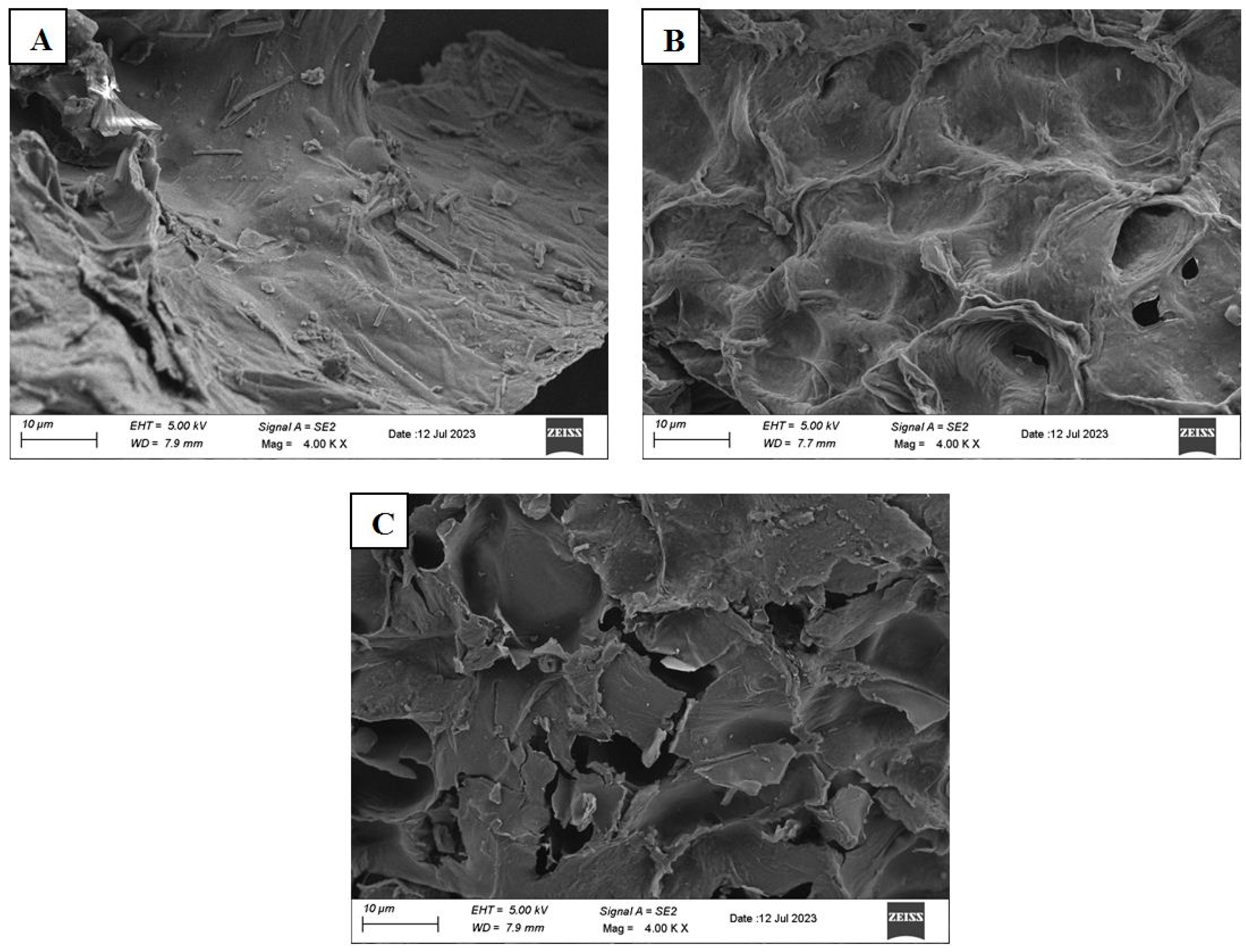
2.5. Morphology
2.6. Preparation of Apricot Jam with MW-HPCO2 Pectin
2.7. Textural Properties of Jam
2.8. Statistical Analysis
3. Results and Discussion
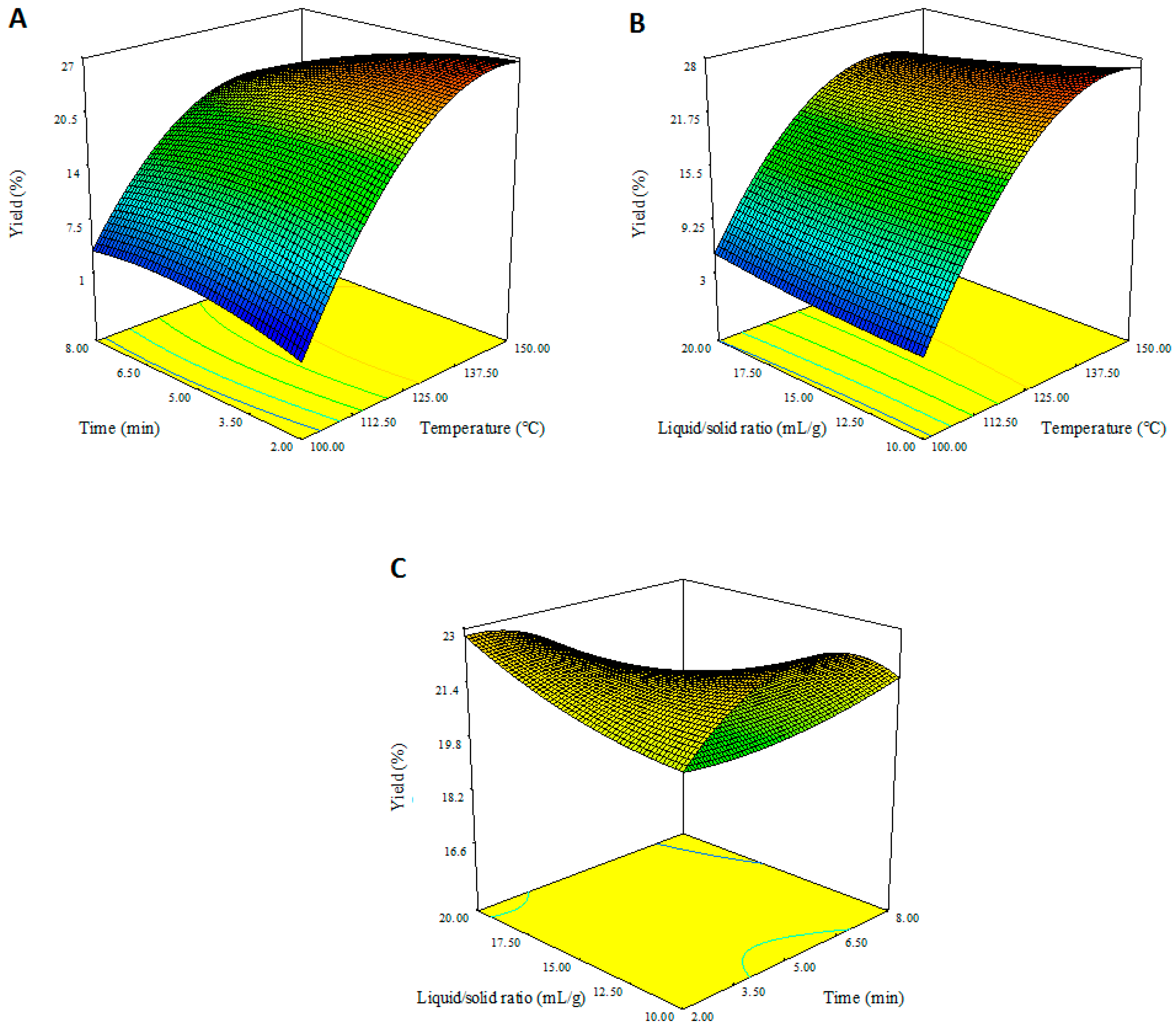
3.1. Modeling of Pectin Yield in MW-HPCO2 Extraction
3.2. Effect of MW-HPCO2 Extraction Parameters on Pectin Yield
3.3. Optimization and Validation of Model
3.4. Comparison of MW-HPCO2 and CE Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ukom, A.N.; Ezenwigbo, M.J.C.; Ugwuona, F.U. Grapefruit peel powder as a functional ingredient in cake production: Effect on the physicochemical properties, antioxidant activity and sensory acceptability of cakes during storage. Int. J. Gastron. Food Sci. 2022, 28, 100517. [Google Scholar] [CrossRef]
- Goksu, A.; Duran, G.; Çilingir, S.; Çevik, M.; Sabanci, S. Performance evaluation of pectin extraction from grapefruit peel powder by ohmic heating. J. Food Process. Preserv. 2022, 46, e16813. [Google Scholar] [CrossRef]
- Roman-Benn, A.; Contador, C.A.; Li, M.W.; Lam, H.M.; Ah-Hen, K.; Ulloa, P.E.; Ravanal, M.C. Pectin: An overview of sources, extraction and applications in food products and health. Food Chem. Adv. 2023, 2, 100192. [Google Scholar] [CrossRef]
- Suri, S.; Singh, A.; Nema, P.K. Current applications of citrus fruit processing waste: A scientific outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Turunçgil Kabuklarından Elde Edilen Pektinlerin Karakterizasyonu ve Karşılaştırılması. Akad. Gıda 2017, 15, 17. [Google Scholar] [CrossRef]
- Cui, J.; Ren, W.; Zhao, C.; Gao, W.; Tian, G.; Bao, Y. The structure—Property relationships of acid- and alkali-extracted grapefruit peel pectins. Carbohydr. Polym. 2020, 229, 115524. [Google Scholar] [CrossRef]
- Pasandide, B.; Khodaiyan, F.; Mousavi, Z.; Saeid, S. Pectin extraction from citron peel: Optimization by Box—Behnken response surface design. Food Sci. Biotechnol. 2018, 27, 997–1005. [Google Scholar] [CrossRef]
- Singhal, S.; Rachayya, N.; Hulle, S. Citrus pectins: Structural properties, extraction methods, modifications and applications in food systems—A review. Appl. Food Res. 2022, 2, 100215. [Google Scholar] [CrossRef]
- Belkheiri, A.; Forouhar, A.; Ursu, A.V.; Dubessay, P.; Pierre, G.; Delattre, C.; Djelveh, G.; Abdelkafi, S.; Hamdami, N.; Michaud, P. Extraction, Characterization, and Applications of Pectins from Plant By-Products. Appl. Sci. 2021, 11, 6596. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, S.; Sun-waterhouse, D.; Zhou, T.; Xu, F.; Waterhouse, G.I.N.; Wu, P. Physicochemical, structural and emulsifying properties of RG-I enriched pectin extracted from unfermented or fermented cherry pomace. Food Chem. 2023, 405, 134985. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, L.; Li, H.; Zhu, Y.; Pan, C.; Gao, X.; Liu, W. Structural characterization and rheological properties of a pectin with anti-constipation activity from the roots of Arctium lappa L. Carbohydr Polym. 2019, 215, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Phaiphan, A. Ultrasound Assisted Extraction of Pectin from Banana Peel Waste as a Potential Source for Pectin Production. Acta Sci. Pol. Technol. Aliment. 2022, 21, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Baker-Fales, M.; Chen, T.Y.; Vlachos, D.G. Scale-up of Microwave-Assisted, Continuous flow, Liquid phase reactors: Application to 5-Hydroxymethylfurfural production. Chem. Eng. J. 2023, 454, 139985. [Google Scholar] [CrossRef]
- Paulus, R.M.; Erdmenger, T.; Becer, C.R.; Hoogenboom, R.; Schubert, U.S. Scale-Up of Microwave-Assisted Polymerizations in Continuous-Flow Mode: Cationic Ring-Opening Polymerization of 2-Ethyl-2-oxazoline. Macromol. Rapid Commun. 2007, 28, 484–491. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C. Assessment of Scale-Up Parameters of Microwave-Assisted Extraction via the Extraction of Flavonoids from Cocoa Leaves. Chem. Eng. Technol. 2015, 38, 489–496. [Google Scholar] [CrossRef]
- Hazal, F.; Özbek, H.N.; Göğüş, F.; Yanık, D.K. A Comprehensive Review on Valuable Components in Food Processing Waste and Their Recovery by Microwave Assisted Extraction. Euroasia J. Math. Eng. Nat. Med. Sci. 2023, 10, 164–184. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Optimization of Microwave Assisted Extraction of Pectin from Orange Peel. Carbohydr. Polym. 2013, 97, 703–709. [Google Scholar] [CrossRef]
- Karbuz, P.; Tugrul, N. Microwave and Ultrasound Assisted Extraction of Pectin from Various Fruit Peel. JFST 2021, 58, 641–650. [Google Scholar] [CrossRef]
- Tien, N.N.T.; Le, N.L.; Khoi, T.T.; Richel, A. Optimization of Microwave-Ultrasound-Assisted Extraction (MUAE) of Pectin from Dragon Fruit Peels using Natural Deep Eutectic Solvents (NADES). J. Food Process. Preserv. 2022, 46, e16117. [Google Scholar] [CrossRef]
- Xu, S.Y.; Liu, J.P.; Huang, X.; Du, L.P.; Shi, F.L.; Dong, R.; Huang, X.T.; Zheng, K.; Liu, Y.; Cheong, K.L. Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. LWT—Food Sci. Technol. 2018, 90, 577–582. [Google Scholar] [CrossRef]
- Lal, A.M.N.; Prince, M.V.; Kothakota, A.; Pandiselvam, R.; Thirumdas, R.; Kumar, N.; Sreeja, R. Pulsed electric field combined with microwave-assisted extraction of pectin polysaccharide from jackfruit waste. Innov. Food Sci. Emerg. Technol. 2021, 74, 102844. [Google Scholar] [CrossRef]
- Su, D.; Li, P.; Young, S.; Huang, Z.; Yuan, Y. Efficient extraction and characterization of pectin from orange peel by a combined surfactant and microwave assisted process. Food Chem. 2019, 286, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, S.; Ye, X.; Zheng, X.; Ahmadi, S.; Hu, W.; Yu, C.; Cheng, H.; Linhardt, R.J.; Chen, J. Enzyme-extracted raspberry pectin exhibits a high-branched structure and enhanced anti-inflammatory properties than hot acid-extracted pectin. Food Chem. 2022, 383, 132387. [Google Scholar] [CrossRef] [PubMed]
- El Fihry, N.; El, K.; Eeckhout, M.; Schols, H.A.; Filali-zegzouti, Y.; Hajjaj, H. Physicochemical and functional characterization of pectin extracted from Moroccan citrus peels. LWT—Food Sci. Technol. 2022, 16, 113508. [Google Scholar] [CrossRef]
- Peng, X.; Yang, G.; Shi, Y.; Zhou, Y.; Zhang, M.; Li, S. Box-Behnken Design Based Statistical Modeling for the Extraction and Physicochemical Properties of Pectin from Sunflower Heads and the Comparison with Commercial Low-methoxyl Pectin. Sci. Rep. 2020, 10, 3595. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chem. 2019, 278, 364–372. [Google Scholar] [CrossRef]
- Barış, S.; Elik, A.; Göğüş, F.; Yanık, D.K. Pistachio hull as an alternative pectin source: Its extraction and use in oil in water emulsion system. Prep. Biochem. Biotechnol. 2023, 53, 433–442. [Google Scholar] [CrossRef]
- Özbek, H.N.; Yanık, D.K.; Fadıloğlu, S.; Göğüş, F. Optimization of microwave-assisted extraction of bioactive compounds from pistachio (Pistacia vera L.) hull. Sep. Sci. Technol. 2020, 55, 289–299. [Google Scholar] [CrossRef]
- Nourmohammadi, A.; Ahmadi, E.; Heshmati, A. Optimization of physicochemical, textural, and rheological properties of sour cherry jam containing stevioside by using response surface methodology. Food Sci. Nutr. 2021, 9, 2483–2496. [Google Scholar] [CrossRef]
- Bagherian, H.; Zokaee, F.; Fouladitajar, A. Comparisons Between Conventional, Microwave and Ultrasound-Assisted Methods for Extraction of Pectin from Grapefruit. Chem. Eng. Process. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Wang, S.; Chen, F.; Wu, J.; Wang, Z.; Liao, X.; Hu, X. Optimization of Pectin Extraction Assisted by Microwave from Apple Pomace using Response Surface Methodology. J. Food Eng. 2007, 78, 693–700. [Google Scholar] [CrossRef]
- Taşan, N.T.; Akpınar, Ö. Microwave Assisted Extraction of Pectin from Grapefruit Peel and Optimization of Extraction Conditions. Turk. J. Agric.-Food Sci. Technol. 2020, 8, 1528–1535. [Google Scholar] [CrossRef]
- Pérez, J.; Gómez, K.; Vega, L. Optimization and Preliminary Physicochemical Characterization of Pectin Extraction from Watermelon Rind (Citrullus lanatus) with Citric Acid. Int. J. Food Sci. 2022, 2022, 3068829. [Google Scholar] [CrossRef] [PubMed]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Osama, K.; Varjani, S.; Farooqui, A.; Younis, K. Microwave-assisted valorization and characterization of Citrus limetta peel waste into pectin as a perspective food additive. J. Food Sci. Technol. 2023, 60, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Liao, J.; Qi, J.R.; Jiang, W.; Yang, X. Physicochemical characteristics and functional properties of high methoxyl pectin with different degree of esterification. Food Chem. 2022, 375, 131806. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, L.; Chen, J.; Du, X.; Lu, Z.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; et al. Systematic evaluation of a series of pectic polysaccharides extracted from apple pomace by regulation of subcritical water conditions. Food Chem. 2022, 368, 130833. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Utilization of food processing wastes of eggplant as a high potential pectin source and characterization of extracted pectin. Food Chem. 2019, 294, 339–346. [Google Scholar] [CrossRef]
- Wongkaew, M.; Sommano, S.R.; Tangpao, T.; Rachtanapun, P.; Jantanasakulwong, K. Mango Peel Pectin by Microwave-Assisted Extraction. Foods 2020, 9, 450. [Google Scholar] [CrossRef]
- Dranca, F.; Tal, E.; Vargas, M.; Oroian, M. Microwave vs. Conventional Extraction of Pectin from Malus domestica ‘Fâlticeni’ Pomace and Its Potential Use in Hydrocolloid-based Films. Food Hydrocoll. 2021, 121, 107026. [Google Scholar] [CrossRef]
- Liu, C.M.; Liang, L.; Shuai, X.X.; Liang, R.H.; Chen, J. Dynamic high-pressure microfluidization-treated pectin under different ethanol concentrations. Polymers 2018, 10, 1410. [Google Scholar] [CrossRef]
- Basak, S.; Annapure, U.S. Trends in “green” and novel methods of pectin modification—A review. Carbohydr. Polym. 2022, 278, 118967. [Google Scholar] [CrossRef]
- Einhorn-stoll, U.; Archut, A.; Eichhorn, M.; Kastner, H. Pectin—Plant protein systems and their application. Food Hydrocoll. 2021, 118, 106783. [Google Scholar] [CrossRef]
- Çavdaroğlu, E.; Yemenicioğlu, A. Utilization of stalk waste separated during processing of sun-dried figs (Ficus carica) as a source of pectin: Extraction and determination of molecular and functional properties. LWT—Food Sci. Technol. 2022, 154, 112624. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Wu, D.; Zhu, K.; Ye, X. Manosonication assisted extraction and characterization of pectin from different citrus peel wastes. Food Hydrocoll. 2021, 121, 106952. [Google Scholar] [CrossRef]
- Zainudin, B.H.; Wong, T.W.; Hamdan, H. Design of low molecular weight pectin and its nanoparticles through combination treatment of pectin by microwave and inorganic salts. Polym. Degrad. Stab. 2018, 147, 35–40. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Bekele, M.; Satheesh, N.; Sadik, J.A. Screening of Ethiopian mango cultivars for suitability for preparing jam and determination of pectin, sugar, and acid effects on physico-chemical and sensory properties of mango jam. Sci. Afr. 2020, 7, e00277. [Google Scholar] [CrossRef]
| Run Numbers | Factors | Response | ||
|---|---|---|---|---|
| A: Temperature (°C) | B: Time (min) | C: Liquid/Solid Ratio (mL/g) | Pectin Yield (%) | |
| 1 | 150 | 2 | 15 | 27.00 |
| 2 | 100 | 2 | 15 | 2.70 |
| 3 | 125 | 5 | 15 | 20.90 |
| 4 | 125 | 8 | 10 | 22.00 |
| 5 | 100 | 5 | 10 | 3.80 |
| 6 | 150 | 5 | 10 | 28.00 |
| 7 | 125 | 2 | 10 | 19.60 |
| 8 | 100 | 5 | 20 | 4.40 |
| 9 | 125 | 5 | 15 | 23.00 |
| 10 | 125 | 5 | 15 | 22.66 |
| 11 | 150 | 8 | 15 | 17.00 |
| 12 | 150 | 5 | 20 | 22.20 |
| 13 | 125 | 5 | 15 | 20.50 |
| 14 | 125 | 8 | 20 | 18.00 |
| 15 | 125 | 5 | 15 | 21.90 |
| 16 | 100 | 8 | 15 | 3.53 |
| 17 | 125 | 2 | 20 | 22.40 |
| R2 Value | Response Pectin Yield (Y1) |
|---|---|
| R2 | 0.9860 |
| Adjusted R2 | 0.9721 |
| Predicted R2 | 0.8963 |
| Adeq. Precision | 24.686 |
| Lack of fit | 0.2086 |
| Sample | CE-GPP | MW-HPCO2-GPP |
|---|---|---|
| Yield (%) | 24.74 ± 0.07 a | 27.69 ± 0.07 b |
| GalA (%) | 73.79 ± 0.23 a | 83.90 ± 0.06 b |
| DE (%) | 69.30 ± 0.92 a | 71.76 ± 0.81 b |
| Eq.W | 2383.8 ± 2.19 a | 5049 ± 1.41 b |
| Methoxyl content (%) | 8.93 ± 0.05 a | 10.44 ± 0.08 b |
| Reducing sugar (%) | 8.43 ± 0.23 a | 7.68 ± 0.22 b |
| Color | ||
| L* | 74.69 ± 0.35 a | 90.68 ± 0.22 b |
| a* | 1.43 ± 0.09 a | 1.42 ± 0.11 a |
| b* | 23.36 ± 0.49 a | 21.10 ± 0.10 b |
| Viscosity (mPa.s) | 16.67 ± 0.43 a | 31.37 ± 0.63 b |
| Apricot Jam Samples | Firmness | Consistency | Cohesiveness |
|---|---|---|---|
| Control | 398.72 ± 2.54 a | 883.30 ± 4.77 a | 321.51 ± 0.78 a |
| MW-HPCO2-GPP | 459.47 ± 8.37 b | 1009.29 ± 13.13 b | 393.23 ± 1.44 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öztürk, T.; Özbek, H.N.; Koçak Yanık, D. Environmentally Friendly Approach to Pectin Extraction from Grapefruit Peel: Microwave-Assisted High-Pressure CO2/H2O. Foods 2024, 13, 476. https://doi.org/10.3390/foods13030476
Öztürk T, Özbek HN, Koçak Yanık D. Environmentally Friendly Approach to Pectin Extraction from Grapefruit Peel: Microwave-Assisted High-Pressure CO2/H2O. Foods. 2024; 13(3):476. https://doi.org/10.3390/foods13030476
Chicago/Turabian StyleÖztürk, Tuğba, Hatice Neval Özbek, and Derya Koçak Yanık. 2024. "Environmentally Friendly Approach to Pectin Extraction from Grapefruit Peel: Microwave-Assisted High-Pressure CO2/H2O" Foods 13, no. 3: 476. https://doi.org/10.3390/foods13030476
APA StyleÖztürk, T., Özbek, H. N., & Koçak Yanık, D. (2024). Environmentally Friendly Approach to Pectin Extraction from Grapefruit Peel: Microwave-Assisted High-Pressure CO2/H2O. Foods, 13(3), 476. https://doi.org/10.3390/foods13030476








