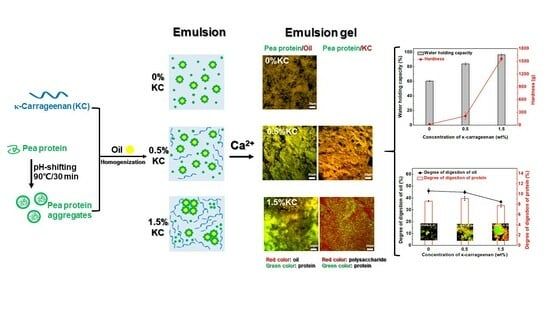
Impact of κ-Carrageenan on the Cold-Set Pea Protein Isolate Emulsion-Filled Gels: Mechanical Property, Microstructure, and In Vitro Digestive Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pea Protein Isolate (PPI) Extraction
2.3. Emulsion Preparation
2.4. Emulsion Size Distribution and ζ-Potential Measurement
2.5. Cold-Set Emulsion-Filled Gel Preparation
2.6. Emulsion-Filled Gel Properties Characterization
2.6.1. Water Holding Capacity (WHC)
2.6.2. Gel Texture Profile Analysis
2.7. Emulsion Gel Microstructure Characterization
2.7.1. Confocal Laser Scanning Microscopy (CLSM)
2.7.2. Scanning Electron Microscopy (SEM)
2.8. Fourier Infrared Spectroscopy (FT-IR)
2.9. Measurement of Protein Solubility
2.10. In Vitro Digestion of Emulsion Gels
2.10.1. Simulated Gastrointestinal Tract Digestion
2.10.2. Measurement of Oil Droplet Size
2.10.3. Degree of Protein Hydrolysis
2.11. Statistical Analysis
3. Results and Discussion
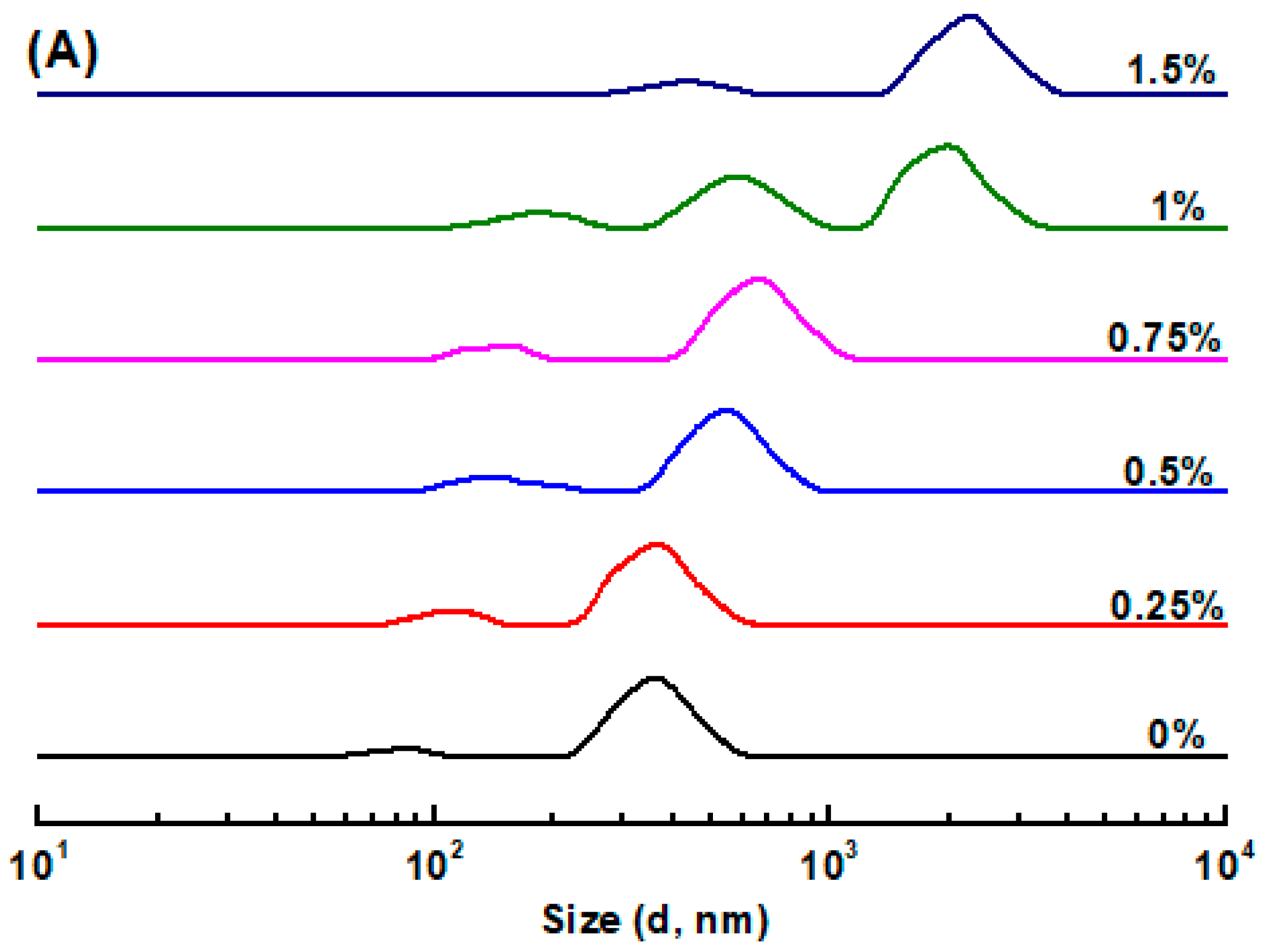
3.1. Size Distribution and ζ-Potential of Emulsions
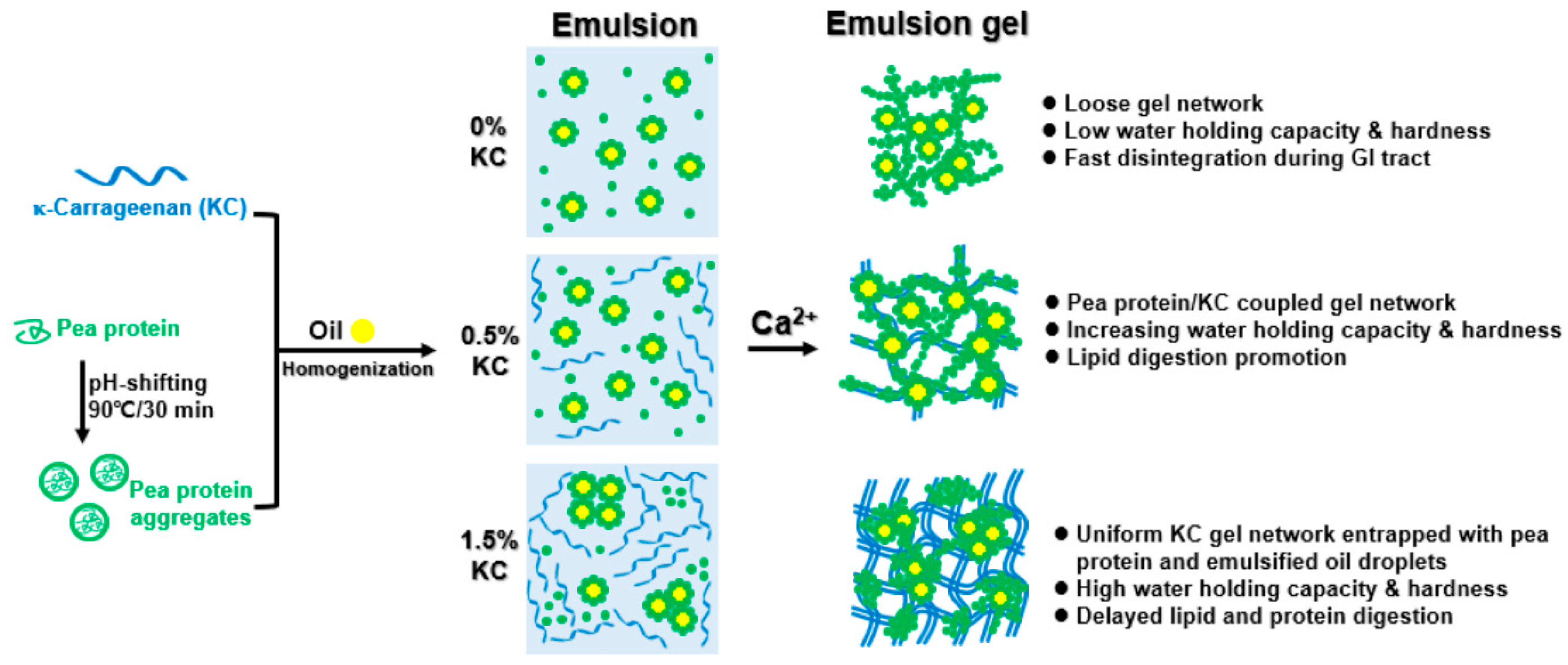
3.2. Emulsion Gel Formation and Microstructure
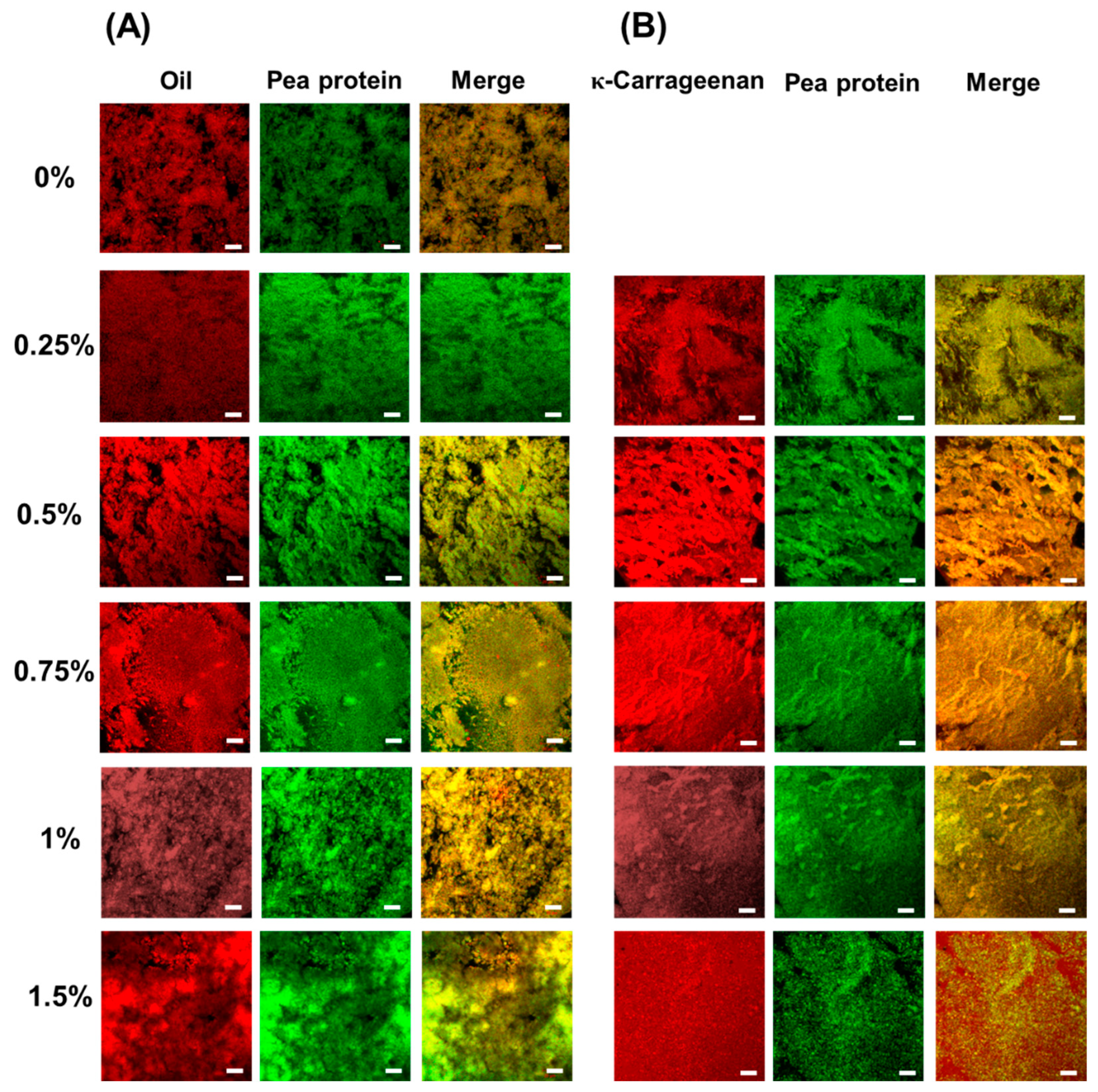
3.2.1. Confocal Laser Scanning Microscopy (CLSM)
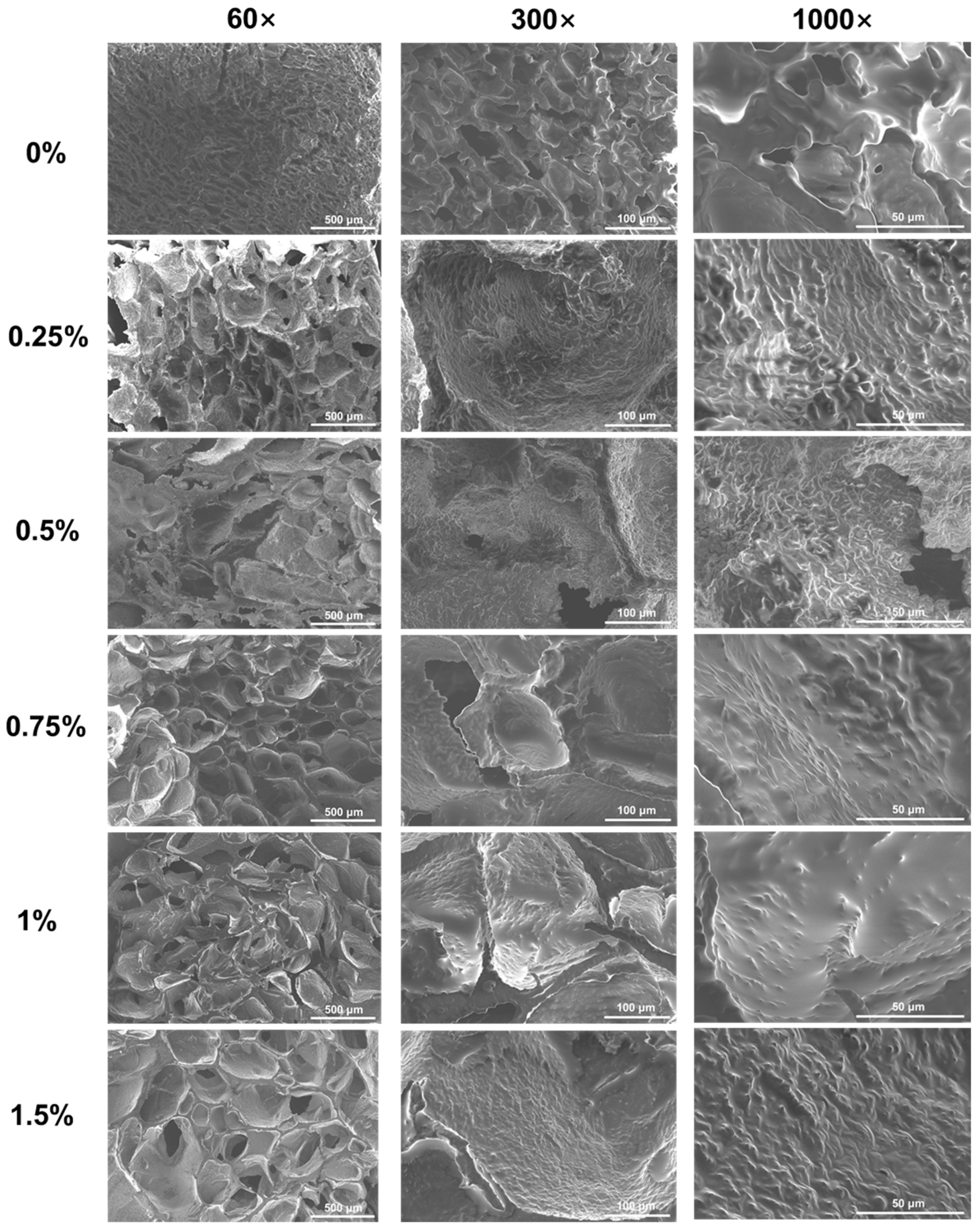
3.2.2. Scanning Electron Microscopy (SEM)
3.3. Pea Protein Conformational Change
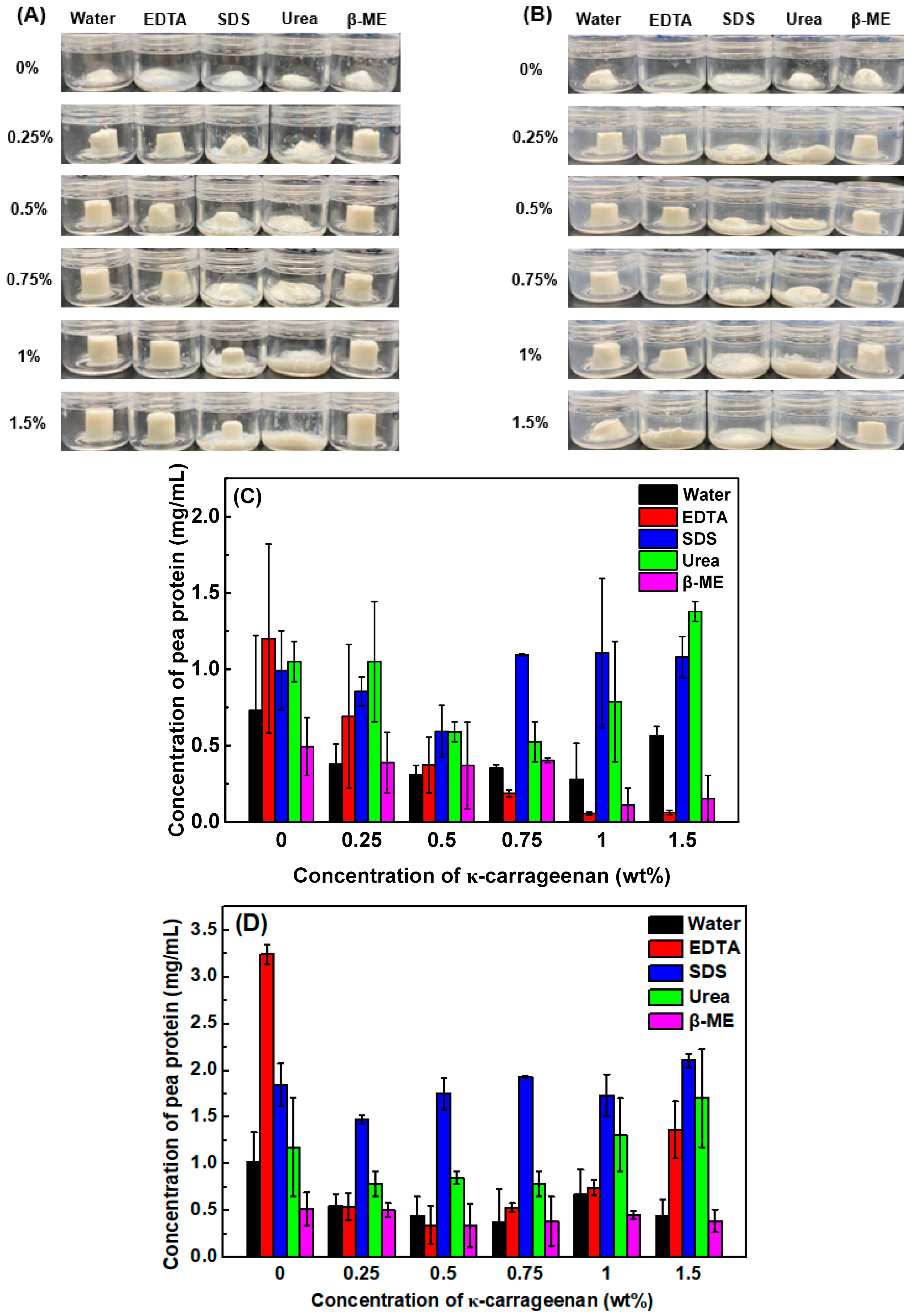
3.4. Emulsion Gel Dissociation Test
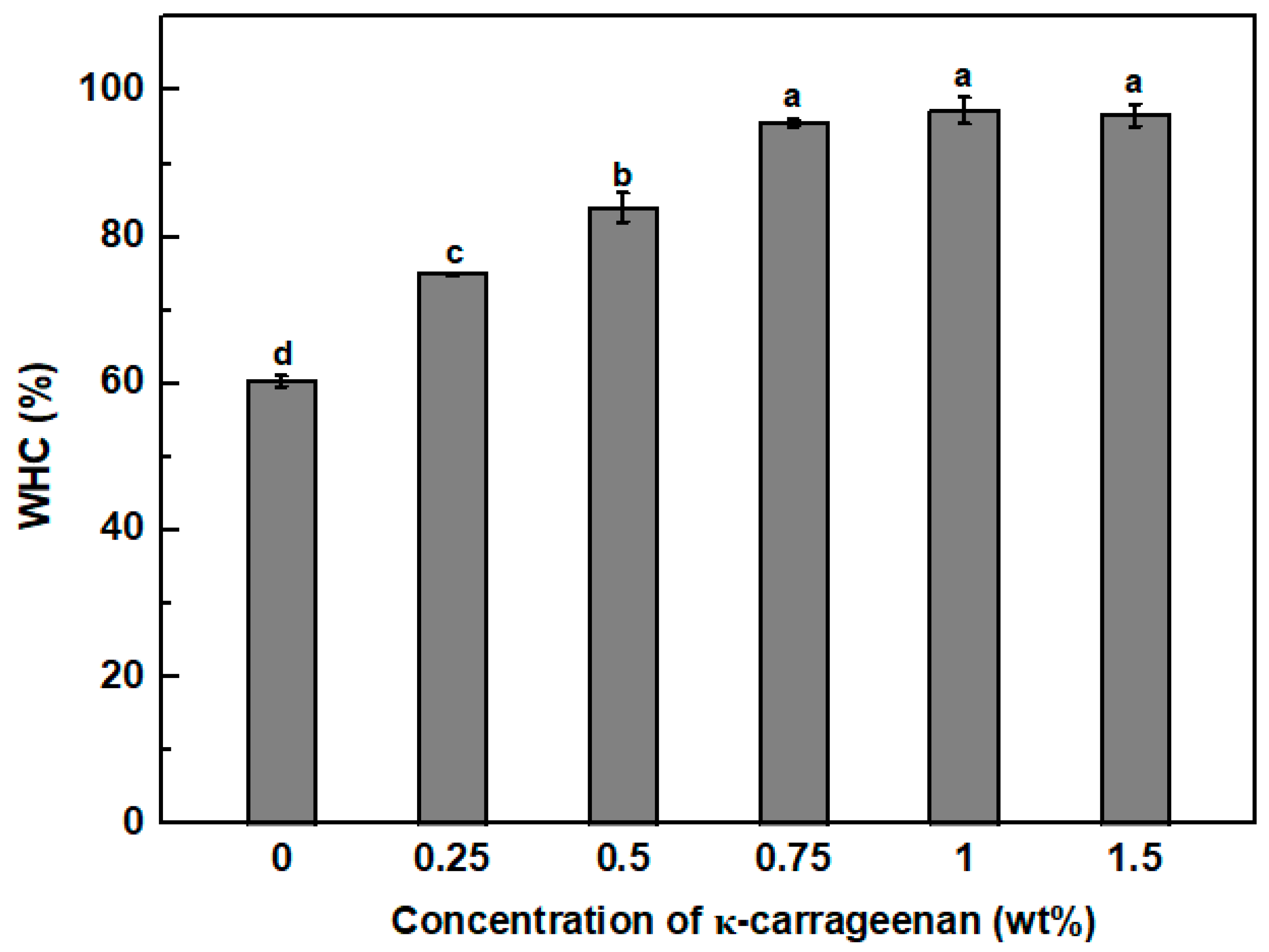
3.5. Water Holding Capacity (WHC)
3.6. Textural Properties
3.7. Gastrointestinal Fate of Emulsion Gels
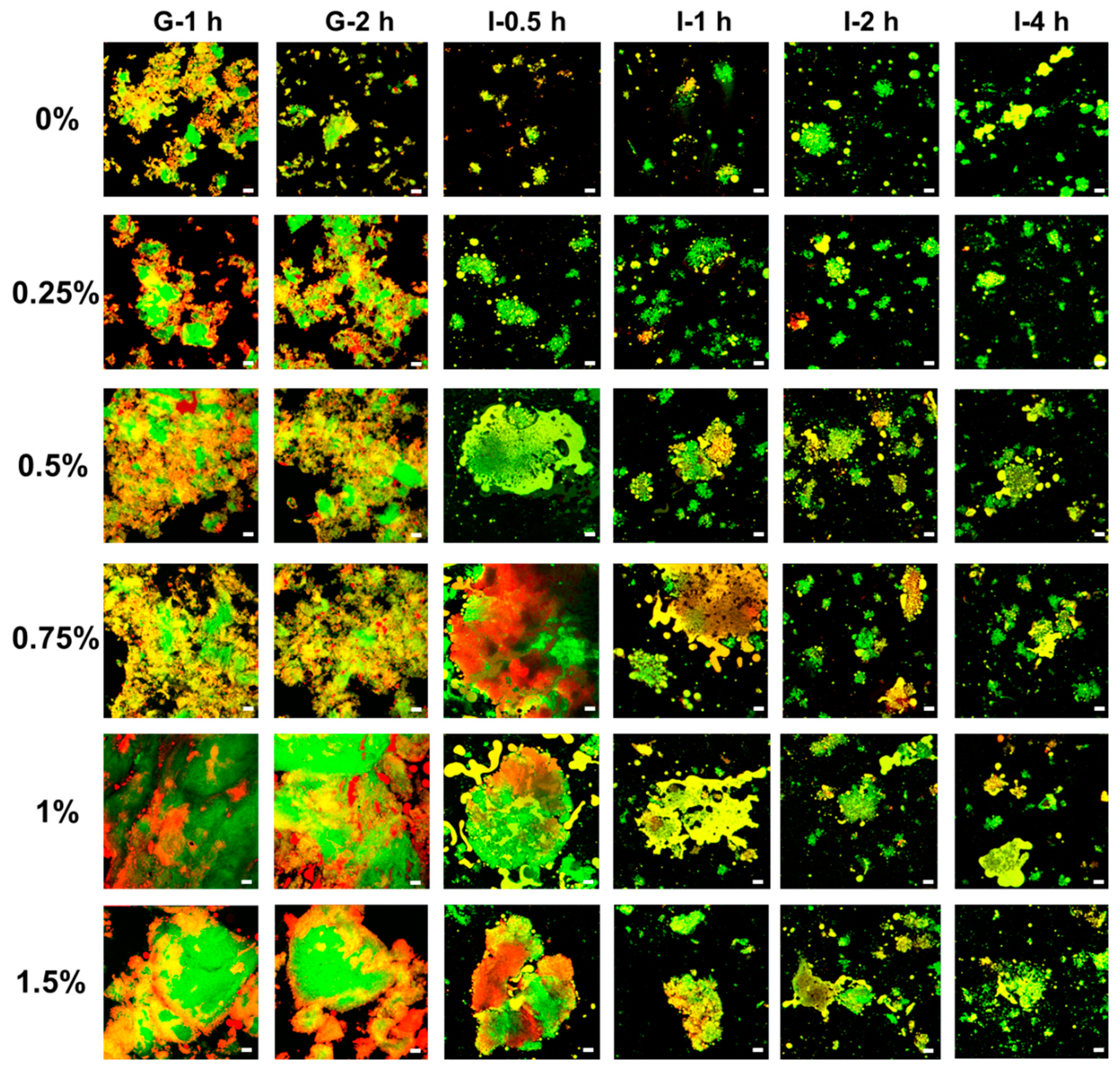
3.7.1. Microstructure of Digesta
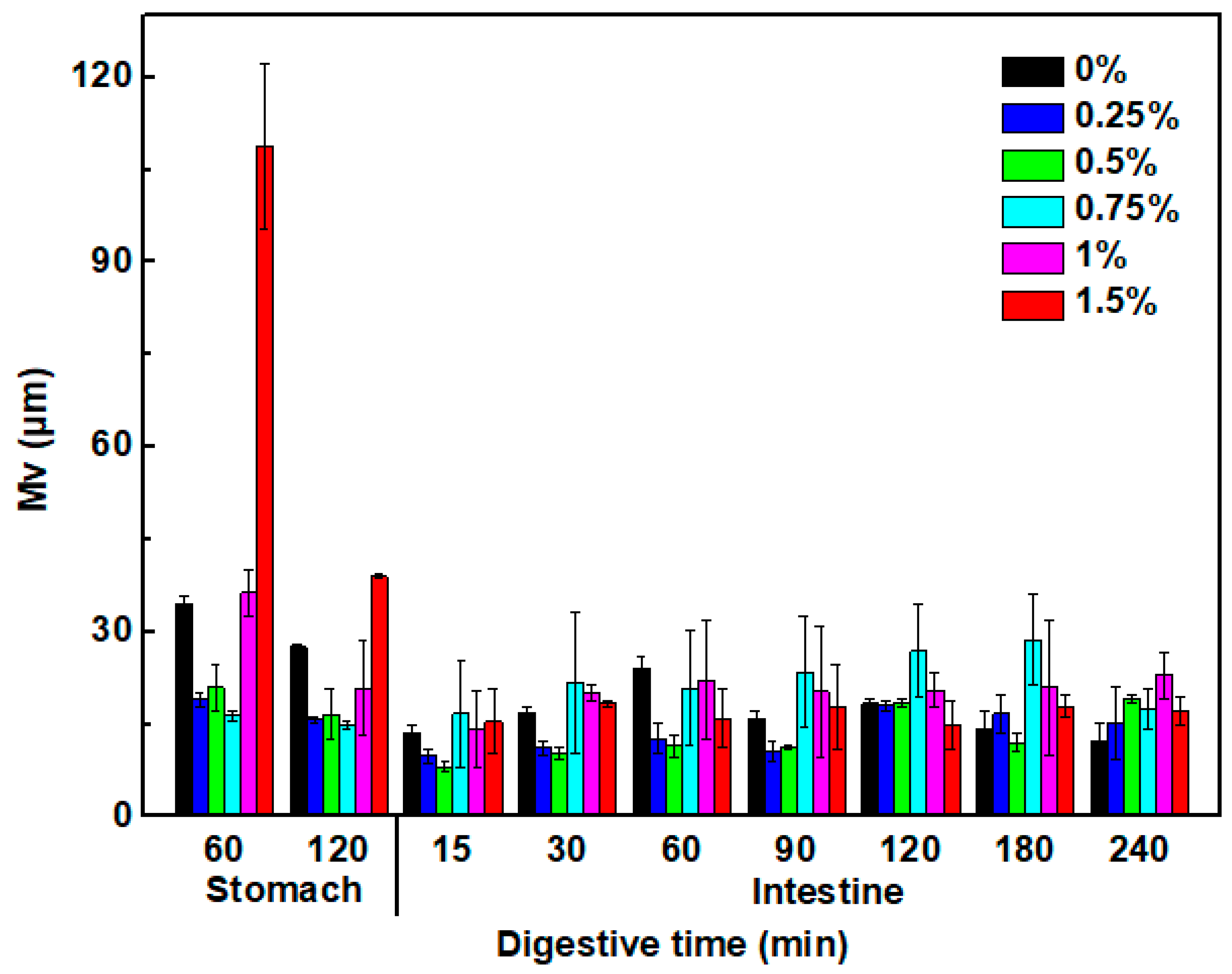
3.7.2. Size of Oil Droplet in the Digestive Fluids
3.7.3. Protein Hydrolysis
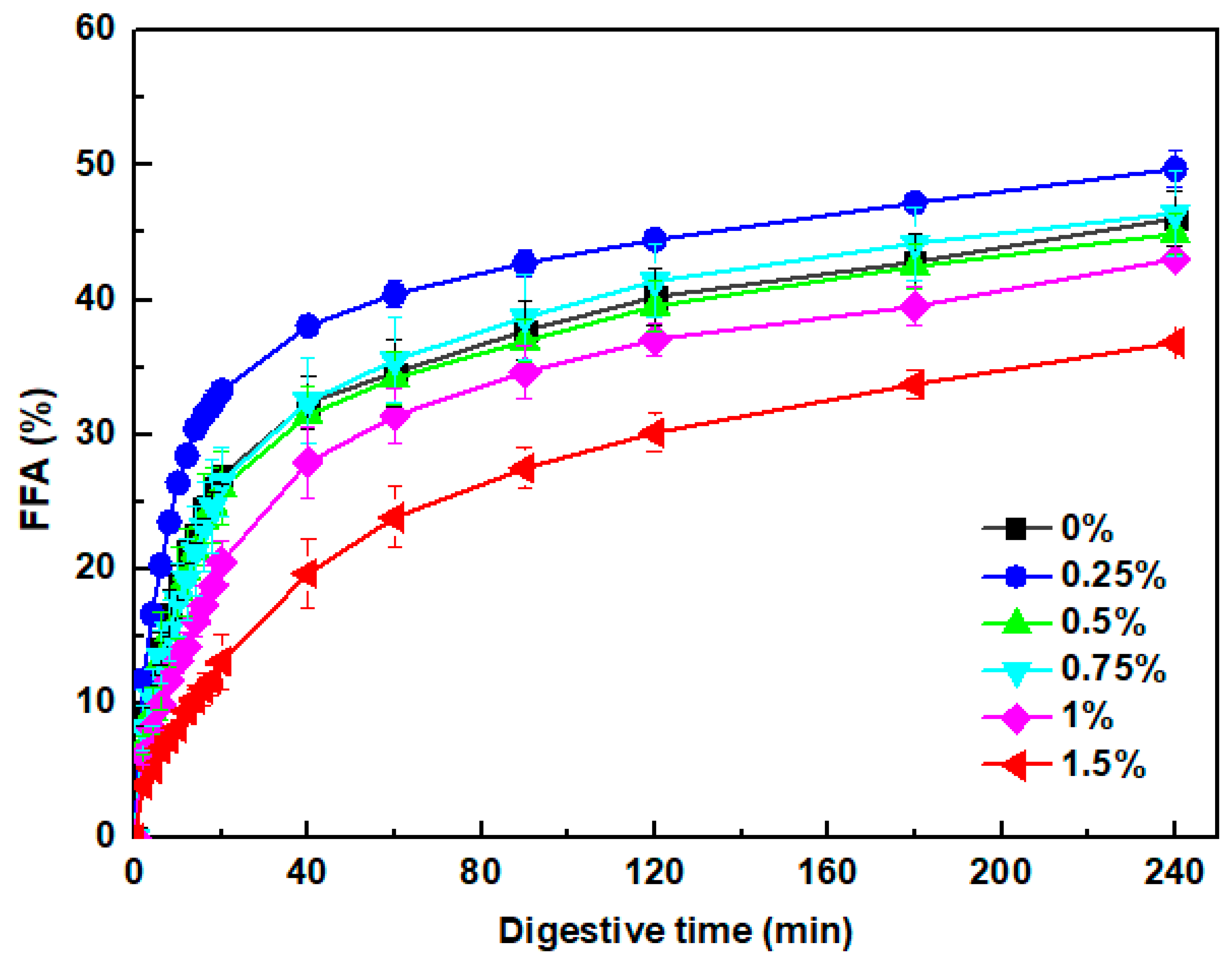
3.7.4. Free Fatty Acids Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farjami, T.; Madadlou, A. An overview on preparation of emulsion-filled gels and emulsion particulate gels. Trends Food Sci. Technol. 2019, 86, 85–94. [Google Scholar] [CrossRef]
- Mao, L.K.; Lu, Y.; Cui, M.N.; Miao, S.; Gao, Y.X. Design of gel structures in water and oil phases for improved delivery of bioactive food ingredients. Crit. Rev. Food Sci. Nutr. 2020, 60, 1651–1666. [Google Scholar] [CrossRef]
- Wang, X.F.; Luo, K.Y.; Liu, S.T.; Zeng, M.M.; Adhikari, B.; He, Z.Y.; Chen, J. Textural and rheological properties of soy protein isolate tofu-type emulsion gels: Influence of soybean variety and coagulant type. Food Biophys. 2018, 13, 324–332. [Google Scholar] [CrossRef]
- Yang, X.; Li, A.Q.; Yu, W.Y.; Li, X.X.; Sun, L.J.; Xue, J.; Guo, Y.R. Structuring oil-in-water emulsion by forming egg yolk/alginate complexes: Their potential application in fabricating low-fat mayonnaise-like emulsion gels and redispersible solid emulsions. Int. J. Biol. Macromol. 2020, 147, 595–606. [Google Scholar] [CrossRef]
- Lu, Y.; Mao, L.K.; Hou, Z.Q.; Miao, S.; Gao, Y.X. Development of emulsion gels for the delivery of functional food ingredients: From structure to functionality. Food Eng. Rev. 2019, 11, 245–258. [Google Scholar] [CrossRef]
- Ren, Y.Q.; Huang, L.; Zhang, Y.X.; Li, H.; Zhao, D.; Cao, J.N.; Liu, X.Q. Application of emulsion gels as fat substitutes in meat products. Foods 2022, 11, 1950. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.J.; Guo, J.X.; Meng, Z. A review on food-grade-polymer-based O/W emulsion gels: Stabilization mechanism and 3D printing application. Food Hydrocoll. 2023, 139, 108588. [Google Scholar] [CrossRef]
- Cao, Y.P.; Mezzenga, R. Design principles of food gels. Nat. Food 2020, 1, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.G.; Liang, X.P.; Yan, J.; Zhao, S.L.; Li, S.Q.; Liu, X.B.; Ngai, T.; McClements, D.J. Tailoring the properties of double-crosslinked emulsion gels using structural design principles: Physical characteristics, stability, and delivery of lycopene. Biomaterials 2022, 280, 121265. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.X.; Corke, H.; Gul, K.; Gan, R.Y.; Fang, Y.P. The health benefits; functional properties, modifications, and applications of pea (Pisum sativum L.) protein: Current status, challenges, and perspectives. Compr. Rev. Food. Sci. Food Saf. 2020, 19, 1835–1876. [Google Scholar] [CrossRef]
- Hadidi, M.; Boostani, S.; Jafari, S.M. Pea proteins as emerging biopolymers for the emulsification and encapsulation of food bioactives. Food Hydrocoll. 2022, 126, 107474. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Molecular forces involved in heat-induced pea protein gelation: Effects of various reagents on the rheological properties of salt-extracted pea protein gels. Food Hydrocoll. 2012, 28, 325–332. [Google Scholar] [CrossRef]
- Shand, P.J.; Ya, H.; Pietrasik, Z.; Wanasundara, P. Physicochemical and textural properties of heat-induced pea protein isolate gels. Food Chem. 2007, 102, 1119–1130. [Google Scholar] [CrossRef]
- O’Kane, F.E.; Happe, R.P.; Vereijken, J.M.; Gruppen, H.; Van Boekel, M. Heat-induced gelation of pea legumin: Comparison with soybean glycinin. J. Agric. Food Chem. 2004, 52, 5071–5078. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Arntfield, S.D. Gelation properties of salt-extracted pea protein isolate induced by heat treatment: Effect of heating and cooling rate. Food Chem. 2011, 124, 1011–1016. [Google Scholar] [CrossRef]
- Kornet, R.; Penris, S.; Venema, P.; van der Goot, A.J.; Meinders, M.B.J.; van der Linden, E. How pea fractions with different protein composition and purity can substitute WPI in heat-set gels. Food Hydrocoll. 2021, 120, 106891. [Google Scholar] [CrossRef]
- Zhu, P.N.; Huang, W.J.; Chen, L.Y. Develop and characterize thermally reversible transparent gels from pea protein isolate and study the gel formation mechanisms. Food Hydrocoll. 2022, 125, 107373. [Google Scholar] [CrossRef]
- Zhu, P.N.; Huang, W.J.; Guo, X.J.; Chen, L.Y. Strong and elastic pea protein hydrogels formed through pH-shifting method. Food Hydrocoll. 2021, 117, 106705. [Google Scholar] [CrossRef]
- Mession, J.L.; Chihi, M.L.; Sok, N.; Saurel, R. Effect of globular pea proteins fractionation on their heat-induced aggregation and acid cold-set gelation. Food Hydrocoll. 2015, 46, 233–243. [Google Scholar] [CrossRef]
- Liu, Q.Z.; Li, Z.W.; Pan, X.X.; Dai, Y.Y.; Hou, H.X.; Wang, W.T.; Ding, X.Z.; Zhang, H.; Li, X.Y.; Dong, H.Z. Effect of grinding on the structure of pea protein isolate and the rheological properties of its acid-induced gels. Int. J. Food Sci. Technol. 2021, 56, 3455–3462. [Google Scholar] [CrossRef]
- Mession, J.L.; Blanchard, C.; Mint-Dah, F.V.; Lafarge, C.; Assifaoui, A.; Saurel, R. The effects of sodium alginate and calcium levels on pea proteins cold-set gelation. Food Hydrocoll. 2013, 31, 446–457. [Google Scholar] [CrossRef]
- Bryant, C.M.; McClements, D.J. Molecular basis of protein functionality with special consideration of cold-set gels derived from heat-denatured whey. Trends Food Sci. Technol. 1998, 9, 143–151. [Google Scholar] [CrossRef]
- Line, V.L.S.; Remondetto, G.E.; Subirade, M. Cold gelation of β-lactoglobulin oil-in-water emulsions. Food Hydrocoll. 2005, 19, 269–278. [Google Scholar] [CrossRef]
- Yi, J.; Huang, H.M.; Liu, Y.X.; Lu, Y.J.; Fan, Y.T.; Zhang, Y.Z. Fabrication of curcumin-loaded pea protein-pectin ternary complex for the stabilization and delivery of β-carotene emulsions. Food Chem. 2020, 313, 126118. [Google Scholar] [CrossRef]
- Musampa, R.M.; Alves, M.M.; Maia, J.M. Phase separation, rheology and microstructure of pea protein-kappa-carrageenan mixtures. Food Hydrocoll. 2007, 21, 92–99. [Google Scholar] [CrossRef]
- Diener, M.; Adamcik, J.; Sanchez-Ferrer, A.; Jaedig, F.; Schefer, L.; Mezzenga, R. Primary, secondary, tertiary and quaternary structure levels in linear polysaccharides: From random coil, to single helix to supramolecular assembly. Biomacromolecules 2019, 20, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.; Raymundo, A.; Sousa, I. Rheological behaviour and microstructure of pea protein/κ-carrageenan/starch gels with different setting conditions. Food Hydrocoll. 2006, 20, 106–113. [Google Scholar] [CrossRef]
- Cai, W.H.; Huang, W.J.; Chen, L.Y. Soluble pea protein aggregates form strong gels in the presence of κ-carrageenan. ACS Food Sci. Technol. 2021, 1, 1605–1614. [Google Scholar] [CrossRef]
- Li, X.M.; Meng, R.; Xu, B.C.; Zhang, B. Investigation of the fabrication, characterization, protective effect and digestive mechanism of a novel Pickering emulsion gels. Food Hydrocoll. 2021, 117, 106708. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Qi, B.K.; Han, L.; Wang, D.Q.; Zhang, S.; Jiang, L.Z.; Xie, F.Y.; Li, Y. Study on the gel properties, interactions, and pH stability of pea protein isolate emulsion gels as influenced by inulin. LWT–Food Sci. Technol. 2021, 137, 110421. [Google Scholar] [CrossRef]
- Kornet, R.; Sridharan, S.; Venema, P.; Sagis, L.M.C.; Nikiforidis, C.V.; van der Goot, A.J.; Meinders, M.B.J.; van der Linden, E. Fractionation methods affect the gelling properties of pea proteins in emulsion-filled gels. Food Hydrocoll. 2022, 125, 107427. [Google Scholar] [CrossRef]
- Yang, J.Q.; Zamani, S.; Liang, L.; Chen, L.Y. Extraction methods significantly impact pea protein composition, structure and gelling properties. Food Hydrocoll. 2021, 117, 106678. [Google Scholar] [CrossRef]
- Bao, H.Y.; Ni, Y.Z.; Wusigale; Dong, H.H.; Liang, L. alpha-Tocopherol and resveratrol in emulsion-filled whey protein gels: Co-encapsulation and in vitro digestion. Int. Dairy J. 2020, 104, 104649. [Google Scholar] [CrossRef]
- Choi, M.; Choi, H.W.; Kim, H.; Hahn, J.; Choi, Y.J. Mimicking animal adipose tissue using a hybrid network-based solid-emulsion gel with soy protein isolate, agar, and alginate. Food Hydrocoll. 2023, 145, 109043. [Google Scholar] [CrossRef]
- Heilig, A.; Gögerle, A.; Hinrichs, J. Multiphase visualisation of fat containing β-lactoglobulin-κ-carrageenan gels by confocal scanning laser microscopy, using a novel dye, V03-01136, for fat staining. LWT–Food Sci. Technol. 2009, 42, 646–653. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef]
- Shimada, K.; Cheftel, J.C. Sulfhydryl group/disulfide bond interchange reactions during heat-induced gelation of whey protein isolate. J. Agric. Food Chem. 1989, 37, 161–168. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Zhang, R.J.; Zhang, Z.P.; Zhang, H.; Decker, E.A.; McClements, D.J. Influence of emulsifier type on gastrointestinal fate of oil-in-water emulsions containing anionic dietary fiber (pectin). Food Hydrocoll. 2015, 45, 175–185. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.Q.; Lad, M.; Dalgleish, D.; Singh, H. The breakdown properties of heat-set whey protein emulsion gels in the human mouth. Food Hydrocoll. 2013, 33, 215–224. [Google Scholar] [CrossRef]
- Abernathy, D.G.; Spedding, G.; Starcher, B. Analysis of protein and total usable nitrogen in beer and wine using a microwell ninhydrin assay. J. Inst. Brew. 2009, 115, 122–127. [Google Scholar] [CrossRef]
- Sarkar, A.; Murray, B.; Holmes, M.; Ettelaie, R.; Abdalla, A.; Yang, X.Y. In vitro digestion of Pickering emulsions stabilized by soft whey protein microgel particles: Influence of thermal treatment. Soft Matter 2016, 12, 3558–3569. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Khan, M.A.; Xie, Z.F.; Tao, S.N.; Li, Y.X.; Liang, L. A peppermint oil emulsion stabilized by resveratrol-zein-pectin complex particles: Enhancing the chemical stability and antimicrobial activity in combination with the synergistic effect. Food Hydrocoll. 2020, 103, 105675. [Google Scholar] [CrossRef]
- McClements, D.J. Comments on viscosity enhancement and depletion flocculation by polysaccharides. Food Hydrocoll. 2000, 14, 173–177. [Google Scholar] [CrossRef]
- Liu, L.Y.; Zhao, Q.Z.; Liu, T.X.; Kong, J.; Long, Z.; Zhao, M.M. Sodium caseinate/carboxymethylcellulose interactions at oil-water interface: Relationship to emulsion stability. Food Chem. 2012, 132, 1822–1829. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Surh, J.; Decker, E.A.; McClements, D.J. Influence of pH and pectin type on properties and stability of sodium-caseinate stabilized oil-in-water emulsions. Food Hydrocoll. 2006, 20, 607–618. [Google Scholar] [CrossRef]
- Perrechil, F.A.; Cunha, R.L. Stabilization of multilayered emulsions by sodium caseinate and κ-carrageenan. Food Hydrocoll. 2013, 30, 606–613. [Google Scholar] [CrossRef]
- Schefer, L.; Adamcik, J.; Diener, M.; Mezzenga, R. Supramolecular chiral self-assembly and supercoiling behavior of carrageenans at varying salt conditions. Nanoscale 2015, 7, 16182–16188. [Google Scholar] [CrossRef]
- Yan, J.X.; Yin, L.J.; Qu, Y.Y.; Yan, W.J.; Zhang, M.H.; Su, J.Q.; Jia, X. Effect of calcium ions concentration on the properties and microstructures of doubly induced sorghum arabinoxylan/soy protein isolate mixed gels. Food Hydrocoll. 2022, 133, 107997. [Google Scholar] [CrossRef]
- Tang, C.H.; Chen, L.; Foegeding, E.A. Mechanical and water-holding properties and microstructures of soy protein isolate emulsion gels induced by CaCl2, glucono-δ-lactone (GDL), and transglutaminase: Influence of thermal treatments before and/or after emulsification. J. Agric. Food Chem. 2011, 59, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Bellissimo, N.; Rousseau, D. Role of gel structure in controlling in vitro intestinal lipid digestion in whey protein emulsion gels. Food Hydrocoll. 2017, 69, 264–272. [Google Scholar] [CrossRef]
- Yan, J.; Liang, X.P.; Ma, C.C.; McClements, D.J.; Liu, X.B.; Liu, F.G. Design and characterization of double-cross-linked emulsion gels using mixed biopolymers: Zein and sodium alginate. Food Hydrocoll. 2021, 113, 106473. [Google Scholar] [CrossRef]
- Zhao, H.B.; Chen, J.; Hemar, Y.; Cui, B. Improvement of the rheological and textural properties of calcium sulfate-induced soy protein isolate gels by the incorporation of different polysaccharides. Food Chem. 2020, 310, 125983. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Li, L. Thermoreversible gelation and scaling behavior of Ca2+-induced κ-carrageenan hydrogels. Food Hydrocoll. 2016, 61, 793–800. [Google Scholar] [CrossRef]
- Li, P.Y.; Guo, C.; Li, X.F.; Yuan, K.; Yang, X.D.; Guo, Y.R.; Yang, X. Preparation and structural characteristics of composite alginate/casein emulsion gels: A microscopy and rheology study. Food Hydrocoll. 2021, 118, 106792. [Google Scholar] [CrossRef]
- Jiang, S.S.; Ma, Y.Y.; Wang, Y.H.; Wang, R.; Zeng, M.Y. Effect of k-carrageenan on the gelation properties of oyster protein. Food Chem. 2022, 382, 132329. [Google Scholar] [CrossRef]
- Deng, C.N.; Liu, Y.; Li, J.L.; Yadav, M.P.; Yin, L.J. Diverse rheological properties, mechanical characteristics and microstructures of corn fiber gum/soy protein isolate hydrogels prepared by laccase and heat treatment. Food Hydrocoll. 2018, 76, 113–122. [Google Scholar] [CrossRef]
- Man, H.; Jia, Y.J.; Song, H.Y.; Yan, X.Y.; Zhang, D.M.; Huang, Y.Y.; Qi, B.K.; Zhang, S.; Li, Y. Effects of κ-carrageenan addition and chlorogenic acid covalent crosslinking on protein conformation and gelling properties of soy protein hydrogels. LWT–Food Sci. Technol. 2023, 174, 114434. [Google Scholar] [CrossRef]
- Ren, Z.Y.; Li, Z.M.; Chen, Z.Z.; Zhang, Y.Y.; Lin, X.R.; Weng, W.Y.; Yang, H.S.; Li, B. Characteristics and application of fish oil-in-water pickering emulsions structured with tea water-insoluble proteins/κ-carrageenan complexes. Food Hydrocoll. 2021, 114, 106562. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.R.; Li-Sha, Y.J.; Chen, H.Q. The effects of basil seed gum on the physicochemical and structural properties of arachin gel. Food Hydrocoll. 2021, 110, 106189. [Google Scholar] [CrossRef]
- Sen, M.; Erboz, E.N. Determination of critical gelation conditions of kappa-carrageenan by viscosimetric and FT-IR analyses. Food Res. Int. 2010, 43, 1361–1364. [Google Scholar] [CrossRef]
- Liang, X.P.; Ma, C.C.; Yan, X.J.; Zeng, H.H.; McClements, D.J.; Liu, X.B.; Liu, F.G. Structure, rheology and functionality of whey protein emulsion gels: Effects of double cross-linking with transglutaminase and calcium ions. Food Hydrocoll. 2020, 102, 105569. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Z.; Guo, P.P.; Guo, Q.N.; Zhang, H.J.; Jiang, L.W.; Xia, N.; Xiao, B.W. Tuning egg yolk granules/sodium alginate emulsion gel structure to enhance β-carotene stability and in vitro digestion property. Int. J. Biol. Macromol. 2023, 232, 123444. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.Q.; Ouyang, H.; Peng, W.; Yu, X.W.; Jin, L.; Li, S.G. Effect of NaCl on the rheological, structural, and gelling properties of walnut protein isolate-κ-carrageenan composite gels. Gels 2022, 8, 259. [Google Scholar] [CrossRef]
- Kwon, H.C.; Shin, D.M.; Yune, J.H.; Jeong, C.H.; Han, S.G. Evaluation of gels formulated with whey proteins and sodium dodecyl sulfate as a fat replacer in low-fat sausage. Food Chem. 2021, 337, 127682. [Google Scholar] [CrossRef]
- Salahi, M.R.; Razavi, S.M.A.; Mohebbi, M. Physicochemical, rheological and structural properties of cold-set emulsion-filled gels based on whey protein isolate-basil seed gum mixed biopolymers. Food Biophys. 2022, 17, 635–649. [Google Scholar] [CrossRef]
- Lin, D.Q.; Kelly, A.L.; Miao, S. Preparation, structure-property relationships and applications of different emulsion gels: Bulk emulsion gels, emulsion gel particles, and fluid emulsion gels. Trends Food Sci. Technol. 2020, 102, 123–137. [Google Scholar] [CrossRef]
- Tezuka, M.; Taira, H.; Igarashi, Y.; Yagasaki, K.; Ono, T. Properties of tofus and soy milks prepared from soybeans having different subunits of glycinin. J. Agric. Food Chem. 2000, 48, 1111–1117. [Google Scholar] [CrossRef]
- Cakir, E.; Foegeding, E.A. Combining protein micro-phase separation and protein-polysaccharide segregative phase separation to produce gel structures. Food Hydrocoll. 2011, 25, 1538–1546. [Google Scholar] [CrossRef]
- Hou, W.B.; Long, J.; Hua, Y.F.; Chen, Y.M.; Kong, X.Z.; Zhang, C.M.; Li, X.F. Formation and characterization of solid fat mimetic based on pea protein isolate/polysaccharide emulsion gels. Front. Nutr. 2022, 9, 1053469. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.Q.; Lad, M.; Dalgleish, D.; Singh, H. Effect of gel structure on the gastric digestion of whey protein emulsion gels. Soft Matter 2014, 10, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ye, A.Q.; Lad, M.; Dalgleish, D.; Singh, H. Impact of colloidal structure of gastric digesta on in-vitro intestinal digestion of whey protein emulsion gels. Food Hydrocoll. 2016, 54, 255–265. [Google Scholar] [CrossRef]
- Usuelli, M.; Germerdonk, T.; Cao, Y.P.; Peydayesh, M.; Bagnani, M.; Handschin, S.; Nyström, G.; Mezzenga, R. Polysaccharide-reinforced amyloid fibril hydrogels and aerogels. Nanoscale 2021, 13, 12534–12545. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.Q.; Kelly, A.L.; Maidannyk, V.; Miao, S. Effect of structuring emulsion gels by whey or soy protein isolate on the structure, mechanical properties, and in-vitro digestion of alginate-based emulsion gel beads. Food Hydrocoll. 2021, 110, 106165. [Google Scholar] [CrossRef]
- Sarkar, A.; Zhang, S.N.; Holmes, M.; Ettelaie, R. Colloidal aspects of digestion of Pickering emulsions: Experiments and theoretical models of lipid digestion kinetics. Adv. Colloid Interface Sci. 2019, 263, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ye, A.Q.; Bellissimo, N.; Singh, H.; Rousseau, D. Modulating fat digestion through food structure design. Prog. Lipid Res. 2017, 68, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.G.; McClements, D.J. Influence of emulsifier type on the in vitro digestion of fish oil-in-water emulsions in the presence of an anionic marine polysaccharide (fucoidan): Caseinate, whey protein, lecithin, or Tween 80. Food Hydrocoll. 2016, 61, 92–101. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, Q.; Liu, T.C.; Wusigale; Liang, L. Co-encapsulation of alpha-tocopherol and resveratrol in oil-in-water emulsion stabilized by sodium caseinate: Impact of polysaccharide on the stability and bioaccessibility. J. Food Eng. 2020, 264, 109685. [Google Scholar] [CrossRef]
- Qiu, C.Y.; Zhao, M.M.; Decker, E.A.; McClements, D.J. Influence of anionic dietary fibers (xanthan gum and pectin) on oxidative stability and lipid digestibility of wheat protein-stabilized fish oil-in-water emulsion. Food Res. Int. 2015, 74, 131–139. [Google Scholar] [CrossRef]
- Yan, W.J.; Zhang, B.Y.; Yadav, M.P.; Feng, L.P.; Yan, J.X.; Jia, X.; Yin, L.J. Corn fiber gum-soybean protein isolate double network hydrogel as oral delivery vehicles for thermosensitive bioactive compounds. Food Hydrocoll. 2020, 107, 105865. [Google Scholar] [CrossRef]
- Wilde, P.J.; Chu, B.S. Interfacial & colloidal aspects of lipid digestion. Adv. Colloid Interface Sci. 2011, 165, 14–22. [Google Scholar] [PubMed]
- Verrijssen, T.A.J.; Balduyck, L.G.; Christiaens, S.; Van Loey, A.M.; Van Buggenhout, S.; Hendrickx, M.E. The effect of pectin concentration and degree of methyl-esterification on the in vitro bioaccessibility of β-carotene-enriched emulsions. Food Res. Int. 2014, 57, 71–78. [Google Scholar] [CrossRef]



| κ-Carrageenan Concentration (%) | Hardness (g) | Springiness | Cohesiveness | Chewiness (g) |
|---|---|---|---|---|
| 0 | 20.956 ± 4.406 d | 0.366 ± 0.044 c | 0.307 ± 0.038 bc | 3.140 ± 0.440 c |
| 0.25 | 43.804 ± 3.404 cd | 0.531 ± 0.007 ab | 0.421 ± 0.003 a | 7.060 ± 3.710 c |
| 0.5 | 214.437 ± 88.419 c | 0.530 ± 0.078 ab | 0.370 ± 0.016 ab | 25.010 ± 2.150 c |
| 0.75 | 215.681 ± 13.635 c | 0.560 ± 0.001 a | 0.261 ± 0.021 c | 31.199 ± 4.029 c |
| 1 | 767.646 ± 211.026 b | 0.442 ± 0.077 bc | 0.396 ± 0.100 ab | 125.333 ± 24.603 b |
| 1.5 | 1560.162 ± 51.637 a | 0.506 ± 0.026 ab | 0.270 ± 0.021 c | 215.448 ± 35.271 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chen, X.; Cheng, H. Impact of κ-Carrageenan on the Cold-Set Pea Protein Isolate Emulsion-Filled Gels: Mechanical Property, Microstructure, and In Vitro Digestive Behavior. Foods 2024, 13, 483. https://doi.org/10.3390/foods13030483
Li X, Chen X, Cheng H. Impact of κ-Carrageenan on the Cold-Set Pea Protein Isolate Emulsion-Filled Gels: Mechanical Property, Microstructure, and In Vitro Digestive Behavior. Foods. 2024; 13(3):483. https://doi.org/10.3390/foods13030483
Chicago/Turabian StyleLi, Xiaojiao, Xing Chen, and Hao Cheng. 2024. "Impact of κ-Carrageenan on the Cold-Set Pea Protein Isolate Emulsion-Filled Gels: Mechanical Property, Microstructure, and In Vitro Digestive Behavior" Foods 13, no. 3: 483. https://doi.org/10.3390/foods13030483
APA StyleLi, X., Chen, X., & Cheng, H. (2024). Impact of κ-Carrageenan on the Cold-Set Pea Protein Isolate Emulsion-Filled Gels: Mechanical Property, Microstructure, and In Vitro Digestive Behavior. Foods, 13(3), 483. https://doi.org/10.3390/foods13030483