Effects of Phenolic Evolution on Color Characteristics of Single-Cultivar Vitis vinifera L. Marselan and Merlot Wines during Vinification and Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Winemaking and Sampling
2.3. Measurement of Physicochemical Parameters
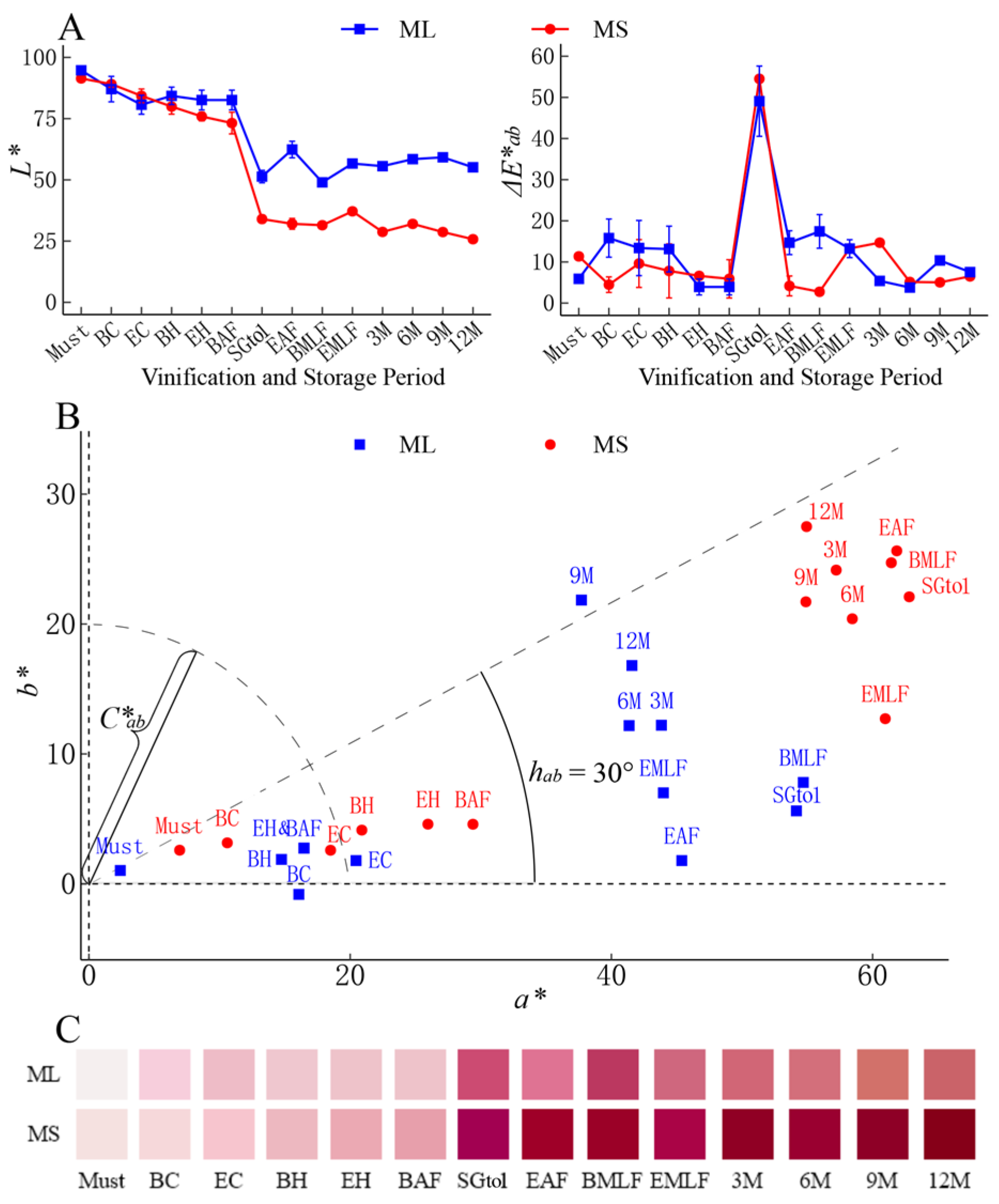
2.4. Measurement of Chromatic Parameters
2.5. Extraction of Phenols from Grape Skins
2.6. Analysis of Phenolic Compounds in Grape and Wine
2.7. Statistical Analysis
3. Results
3.1. Phenolic Profiles of the Grapes of Two Varieties
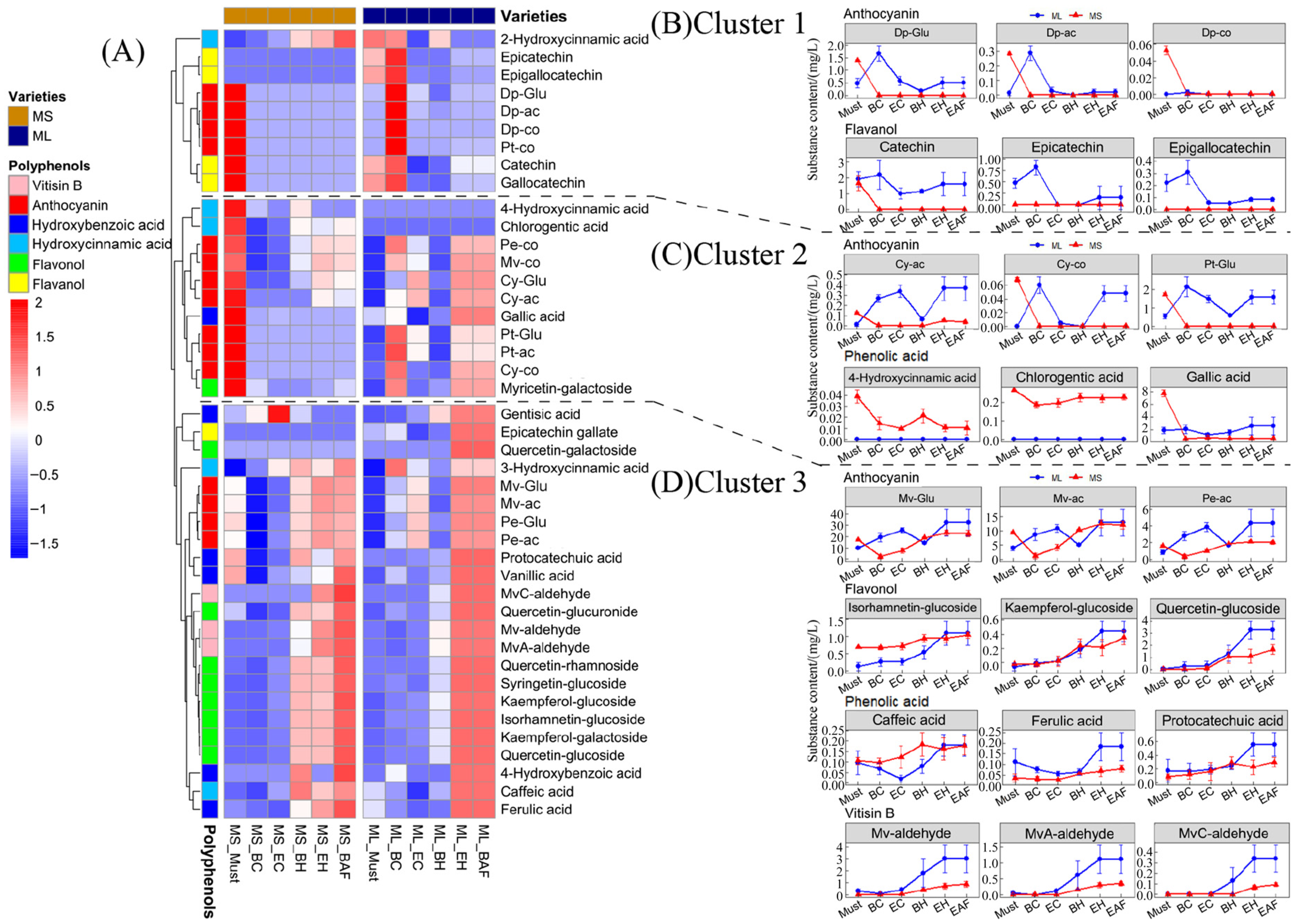
3.1.1. Anthocyanins
3.1.2. Non-Anthocyanin Phenolics
3.2. Physicochemical Indices and Chromaticity Index
3.2.1. Physicochemical Indices
3.2.2. Chromaticity Index
3.3. Phenolic Profiles of the Wines of Two Varieties
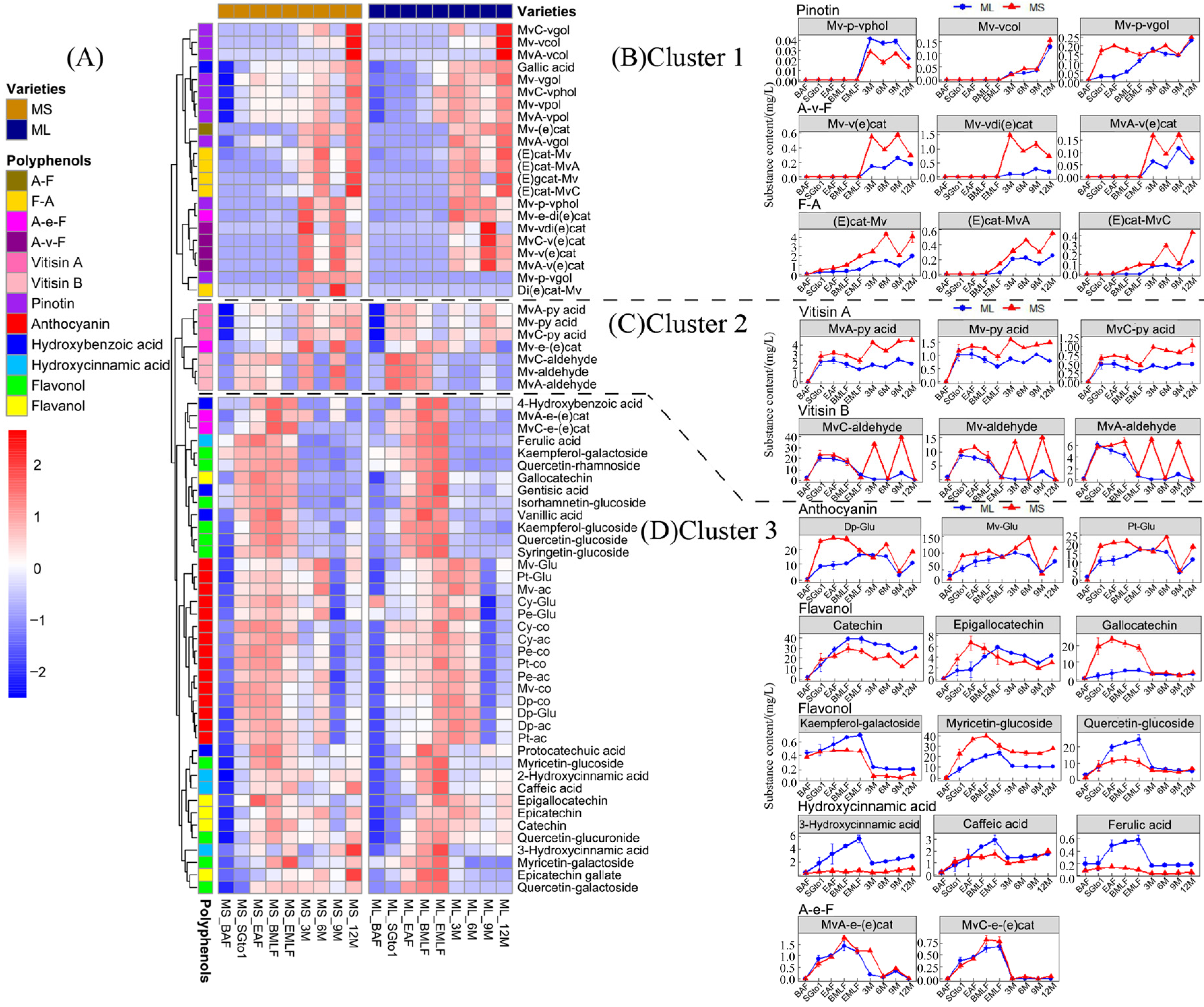
3.3.1. Anthocyanins and Their Derivatives in Wine
3.3.2. Non-Anthocyanin Phenolics in Wine
3.4. Leaching and Evolution of Phenolics from Grape to Wine
3.4.1. Leaching of Phenolics during Cold Maceration
3.4.2. Evolution of Phenolics during Fermentation and Bottle Storage
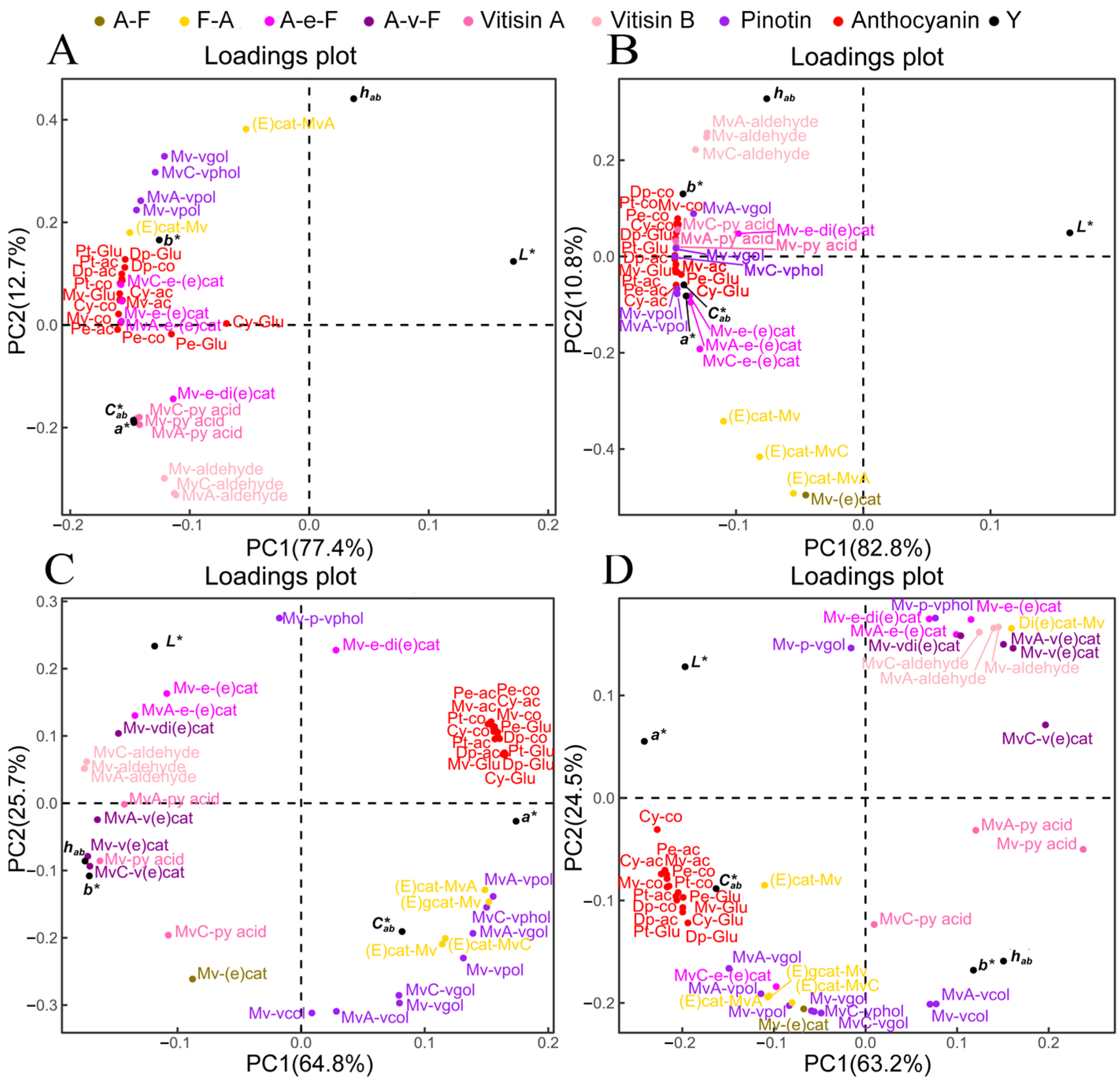
3.5. Key Components for Wine Color Expression
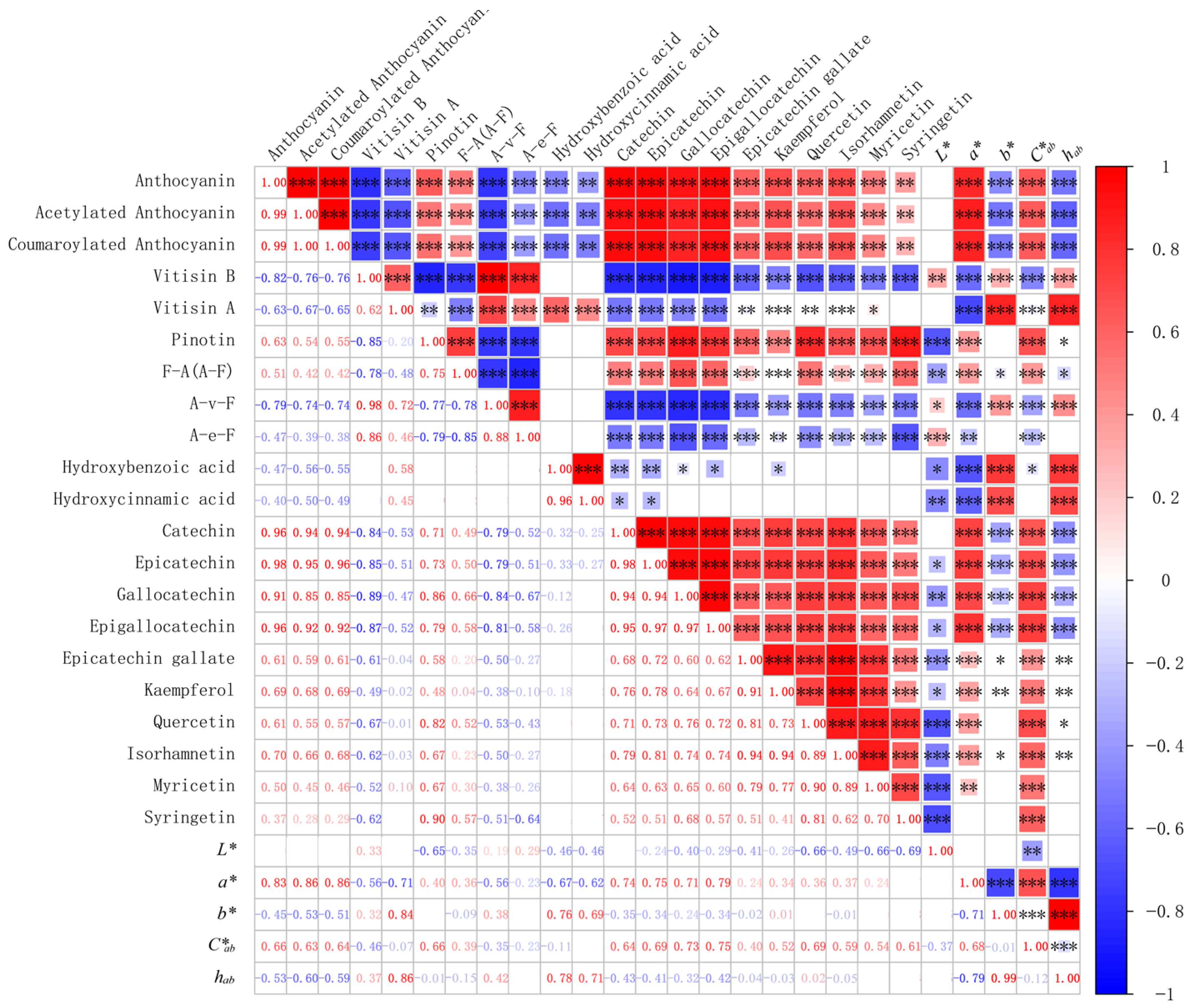
3.6. Correlation among Changes of Phenolic Compounds in Wine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A-e-F | Anthocyanin ethyl-linked flavanols bridged polymerized pigments |
| A-F | Direct anthocyanin-flavanol polymerized pigments (A type) |
| ANOVA | Analysis of variance |
| A-v-F | Flavanyl-pyranoanthocyanins |
| BAF | Before alcohol fermentation |
| BC | Before cooling |
| BH | Before heating |
| BMLF | Before malolactic fermentation |
| Cy-ac | Cyanidin-3-O-acetylglucoside |
| Cy-co | Cyanidin-3-O-coumarylglucoside |
| Cy-Glu | Cyanidin-3-O-glucoside |
| Di(e)cat-Mv | Di(epi)catechin-malvidin-3-O-glucoside (B type) |
| Dp-ac | Delphinidin-3-O-acetylglucoside |
| Dp-co | Delphinidin-3-O-coumarylglucoside |
| Dp-Glu | Delphinidin-3-O-glucoside |
| EAF | End of alcohol fermentation |
| EC | End of cooling |
| (E)cat-Mv | (Epi)catechin-malvidin-3-O-Glucoside (B type) |
| (E)cat-MvA | (Epi)catechin-malvidin-3-O-acetylglucoside (B type) |
| (E)cat-MvC | (Epi)catechin-malvidin-3-O-coumaroylglucoside (B type) |
| (E)gcat-Mv | (Epi)gallocatechin-malvidin-3-O-glucoside (B type) |
| EH | End of heating |
| EMLF | End of malolactic fermentation |
| F-A | Direct flavanol-anthocyanin polymerized pigments (B type) |
| HPLC-QqQ | High-performance liquid chromatography tandem triple-quadrupole |
| MvA-aldehyde | Malvidin-3-O-acetylglucoside-acetaldehyde |
| Mv-ac | Malvidin-3-O-acetylglucoside |
| MvA-e-(e)cat | Malvidin-3-O-acetylglucoside-ethyl-(epi)cate |
| Mv-aldehyde | Malvidin-3-O-glucoside-acetaldehyde |
| MvA-py acid | Malvidin-3-O-acetylglucoside-pyruvic acid |
| MvA-vcol | Malvidin-3-O-acetylglucoside-4-vinylcatechol |
| MvA-v(e)cat | Malvidin-3-O-acetylglucoside-4-vinyl(epi)catechin |
| MvA-vgol | Malvidin-3-O-acetylglucoside-4-vinylguaiacol |
| MvA-vpol | Malvidin-3-O-acetylglucoside-4-vinylphenol |
| MvC-aldehyde | Malvidin-3-O-coumaroylglucoside-acetaldehyde |
| MvC-e-(e)cat | Malvidin-3-O-coumaroylglucoside-ethyl-(epi)catechin |
| Mv-co | Malvidin-3-O-coumarylglucoside |
| MvC-py acid | Malvidin-3-O-coumaroylglucoside-pyruvic acid |
| MvC-v(e)cat | Malvidin-3-O-coumaroylglucoside-4-vinyl(epi)catechin |
| MvC-vgol | Malvidin-3-O-coumaroylglucoside-4-vinylguaiacol |
| MvC-vphol | Malvidin-3-O-coumaroylglucoside-4-vinylphenol |
| Mv-(e)cat | Malvidin-3-O-glucoside-(epi)catechin (A type) |
| Mv-e-di(e)cat | Malvidin-3-O-glucoside-ethyl-di(epi)catechin |
| Mv-e-(e)cat | Malvidin-3-O-glucoside-ethyl—(epi)catechin |
| Mv-Glu | Malvidin-3-O-glucoside |
| Mv-p-vphol | Malvidin-3-O-glucoside-pyrano-vinylphenol |
| Mv-py acid | Malvidin-3-O-glucoside-pyruvic acid |
| Mv-vcol | Malvidin-3-O-glucoside-4-vinylcatechol |
| Mv-vdi(e)cat | Malvidin-3-O-glucoside-4-vinyl-di(epi)catechin |
| Mv-v(e)cat | Malvidin-3-O-glucoside-4-vinyl(epi)catechin |
| Mv-vgol | Malvidin-3-O-glucoside-4-vinylguaiacol |
| Mv-vpol | Malvidin-3-O-glucoside-4-vinylphenol |
| Pe-ac | Peonidin-3-O-acetylglucoside |
| Pe-co | Peonidin-3-O-coumarylglucoside |
| Pe-Glu | Peonidin-3-O-glucoside |
| PLSR | Partial Least Squares Regression Analysis |
| Pt-ac | Petunidin-3-O-acetylglucoside |
| Pt-co | Petunidin-3-O-coumarylglucoside |
| Pt-Glu | Petunidin-3-O-glucoside |
| SGto1 | Specific gravity reduced to 1 |
| 3M | Storage for 3 months |
| 6M | Storage for 6 months |
| 9M | Storage for 9 months |
| 12M | Storage for 12 months |
References
- Cohen, S.D.; Tarara, J.M.; Kennedy, J.A. Assessing the impact of temperature on grape phenolic metabolism. Anal. Chim. Acta 2008, 621, 57–67. [Google Scholar] [CrossRef]
- Solovchenko, A.; Schmitz-Eiberger, M. Significance of skin flavonoids for UV-B-protection in apple fruits. J. Exp. Bot. 2003, 54, 1977–1984. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, J.; Meng, J.; Shi, P.; Fang, Y.; Zhang, Z.; Sun, X. Harvesting at the Right Time: Maturity and Its Effects on the Aromatic Characteristics of Cabernet Sauvignon Wine. Molecules 2019, 24, 2777. [Google Scholar] [CrossRef]
- Zhang, B.; He, F.; Liu, Y.; Cai, J.; Duan, C.Q. Impact of adding Ellagic acid to red grapes on the phenolic composition and chromatic quality of cabernet sauvignon wines from a warm climate. J. Food Process. Preserv. 2017, 41, e13080. [Google Scholar] [CrossRef]
- Liu, Y.; He, F.; Shi, Y.; Zhang, B.; Duan, C.Q. Effect of the high pressure treatments on the physicochemical properties of the young red wines supplemented with pyruvic acid. Innov. Food Sci. Emerg. Technol. 2018, 48, 56–65. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhao, P.R.; Ling, M.Q.; Qi, M.Y.; García-Estévez, I.; Escribano-Bailón, M.T.; Chen, X.J.; Shi, Y.; Duan, C.Q. Blending strategies for wine color modification Ⅰ: Color improvement by blending wines of different phenolic profiles testified under extreme oxygen exposures. Food Res. Int. 2020, 130, 108885. [Google Scholar] [CrossRef]
- Rivas, E.G.P.; Alcalde-Eon, C.; Santos-Buelga, C.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Behaviour and haracterization of the colour during red wine making and maturation. Anal. Chim. Acta 2006, 563, 215–222. [Google Scholar] [CrossRef]
- Chen, X.; Guan, Y.M.; Zeng, M.M.; Wang, Z.J.; Qin, F.; Chen, J.; He, Z.Y. Effect of whey protein isolate and phenolic copigments in the thermal stability of mulberry anthocyanin extract at an acidic pH. Food Chem. 2022, 377, 132005. [Google Scholar] [CrossRef] [PubMed]
- González-Manzano, S.; Dueñas, M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T.; Santos-Buelga, C. Studies on the copigmentation between anthocyanins and flavan-3-ols and their influence in the colour expression of red wine. Food Chem. 2009, 114, 649–656. [Google Scholar] [CrossRef]
- Price, S.F.; Breen, P.J.; Valladao, M.; Watson, B.T. Cluster sun exposure and quercetin in Pinot Noir grapes and wines. Am. J. Enol. Vitic. 1995, 46, 187–194. [Google Scholar] [CrossRef]
- Ghasemifar, E.; Saeidian, S. The effects catechin on stability of grape anthocyanin-copigment complex. Int. J. Sci. Res. Environ. Sci. 2014, 2, 150–155. [Google Scholar] [CrossRef]
- Zhang, X.K.; He, F.; Zhang, B.; Reeves, M.J.; Liu, Y.; Zhao, X.; Duan, C.Q. The effect of prefermentative addition of gallic acid and ellagic acid on the red wine color, copigmentation and phenolic profiles during wine aging. Food Res. Int. 2018, 106, 568–579. [Google Scholar] [CrossRef]
- Aleixandre-Tudó, J.L.; Alvarez, I.; Lizama, V.; García, M.J.; Aleixandre, J.L.; Du Toit, W.J. Impact of caffeic acid addition on phenolic composition of tempranillo wines from different winemaking techniques. J. Agric. Food Chem. 2013, 61, 11900–11912. [Google Scholar] [CrossRef]
- Parenti, A.; Spugnoli, P.; Calamai, L.; Ferrari, S.; Gori, C. Effects of cold maceration on red wine quality from Tuscan Sangiovese grape. Eur. Food Res. Technol. 2004, 218, 360–366. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez-Cutillas, A.; Fernández-Fernández, J.I. Phenolic compounds and color stability of red wines. Effect of skin maceration time. Am. J. Enol. Vitic. 2001, 52, 271–275. [Google Scholar] [CrossRef]
- Koyama, K.; Goto-Yamamoto, N.; Hashizume, K. Influence of maceration temperature in red wine vinification on extraction of phenolics from berry skins and seeds of grape (Vitis vinifera). Biosci. Biotechnol. Biochem. 2007, 71, 958–965. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Moreno-Pérez, A.; Vila-López, R.; Fernández-Fernández, J.I.; Martínez-Cutillas, A.; Gómez-Plaza, E. Influence of low temperature prefermentative techniques on chromatic and phenolic characteristics of Syrah and Cabernet Sauvignon wines. Eur. Food Res. Technol. 2009, 228, 777–788. [Google Scholar] [CrossRef]
- Setford, P.C.; Jeffery, D.W.; Grbin, P.R.; Muhlack, R.A. Modelling the Mass Transfer Process of Malvidin-3-Glucoside during Simulated Extraction from Fresh Grape Solids under Wine-Like Conditions. Molecules 2018, 23, 2159. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Timberlake, C.F. Isolation, identification, and characterization of new color-stable anthocyanins occurring in some red wines. J. Agric. Food Chem. 1997, 45, 35–43. [Google Scholar] [CrossRef]
- Fulcrand, H.; Benabdeljalil, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. A new class of wine pigments generated by reaction between pyruvic acid and grape anthocyanins. Phytochemistry 1998, 47, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Remy-Tanneau, S.; Le Guernevé, C.; Meudec, E.; Cheynier, V. Characterization of a colorless anthocyanin-flavan-3-ol dimer containing both carbon-carbon and ether interflavanoid linkages by NMR and mass spectrometry. J. Agric. Food Chem. 2003, 51, 3592–3597. [Google Scholar] [CrossRef] [PubMed]
- FranciaAricha, E.M.; Guerra, M.T.; RivasGonzalo, J.C.; SantosBuelga, C. New anthocyanin pigments formed after condensation with flavanols. J. Agric. Food Chem. 1997, 45, 2262–2266. [Google Scholar] [CrossRef]
- Yao, X.C.; Zhang, H.L.; Ma, X.R.; Xia, N.Y.; Duan, C.Q.; Yang, W.M.; Pan, Q.H. Leaching and evolution of anthocyanins and aroma compounds during Cabernet Sauvignon wine fermentation with whole-process skin-seed contact. Food Chem. 2024, 436, 137727. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.N.; Zhu, B.Q.; Han, S.; Wang, J.H.; Pan, Q.H.; Reeves, M.J.; Duan, C.Q.; He, F. Regional characteristics of anthocyanin and flavonol compounds from grapes of four Vitis vinifera varieties in five wine regions of China. Food Res. Int. 2014, 64, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Wang, Y.; Cheng, C.F.; Chen, W.; Li, S.D.; He, F.; Duan, C.Q.; Wang, J. Distal leaf removal made balanced source-sink vines, delayed ripening, and increased flavonol composition in Cabernet Sauvignon grapes and wines in the semi-arid Xinjiang. Food Chem. 2022, 366, 130582. [Google Scholar] [CrossRef] [PubMed]
- GB/T 15038—2006; Analysis Method of Wine and Fruit Wine; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Standards Press of China: Beijing, China, 2006.
- Brill, M.H. Acquisition and reproduction of color images: Colorimetric and multispectral approaches. Color Res. Appl. 2010, 27, 304–305. [Google Scholar] [CrossRef]
- Tian, M.B.; Liu, Y.; Lu, H.C.; Hu, L.; Wang, Y.; Cheng, C.F.; Chen, W.; Li, S.D.; He, F.; Duan, C.Q.; et al. Cluster spatial positions varied the phenolics profiles of ‘Cabernet Sauvignon’ grapes and wines under a fan training system with multiple trunks. Food Chem. 2022, 387, 132930. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Yao, X.C.; Xia, N.Y.; Sun, Q.; Duan, C.Q.; Pan, Q.H. Evolution of seed-soluble and insoluble tannins during grape berry maturation. Molecules 2023, 28, 3050. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.T.; Li, H.Q.; Lu, H.C.; He, L.; Peng, W.T.; Chen, W.; Li, S.D.; Li, S.P.; Duan, C.Q.; et al. Microclimate changes caused by black inter-row mulch decrease flavonoids concentrations in grapes and wines under semi-arid climate. Food Chem. 2021, 361, 130064. [Google Scholar] [CrossRef]
- Liang, N.N.; Pan, Q.H.; He, F.; Wang, J.; Reeves, M.J.; Duan, C.Q. Phenolic profiles of Vitis davidii and Vitis quinquangularis species native to China. J. Agric. Food Chem. 2013, 61, 6016–6027. [Google Scholar] [CrossRef]
- Goupy, P.; Bautista-Ortin, A.B.; Fulcrand, H.; Dangles, O. Antioxidant activity of wine pigments derived from anthocyanins: Hydrogen transfer reactions to the DPPH radical and inhibition of the heme-induced peroxidation of linoleic acid. J. Agric. Food Chem. 2009, 57, 5762–5770. [Google Scholar] [CrossRef]
- De Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef]
- Edwin, H. In vino veritas: Oligomeric procyanidins and the ageing of red wines. Phytochemistry 1980, 19, 2577–2582. [Google Scholar] [CrossRef]
- Reshef, N.; Walbaum, N.; Agam, N.; Fait, A. Sunlight modulates fruit metabolic profile and shapes the spatial pattern of compound accumulation within the grape cluster. Front. Plant Sci. 2017, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Tofalo, R.; Suzzi, G.; Perpetuini, G. Discovering the Influence of Microorganisms on Wine Color. Front. Microbiol. 2021, 12, 790935. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, I.; Aleixandre, J.L.; García, M.J.; Lizama, V. Impact of prefermentative maceration on the phenolic and volatile compounds in Monastrell red wines. Anal. Chim. Acta 2006, 563, 109–115. [Google Scholar] [CrossRef]
- Sacchi, K.; Bisson, L.; Adams, D. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar] [CrossRef]
- Ivanova-Petropulos, V.; Hermosín-Gutiérrez, I.; Boros, B.; Stefova, M.; Stafilov, T.; Vojnoski, B.; Dörnyei, A.; Kilár, F. Phenolic compounds and antioxidant activity of Macedonian red wines. J. Food Compos. Anal. 2015, 41, 1–14. [Google Scholar] [CrossRef]
- De Freitas, V.; Mateus, N. Chemical transformations of anthocyanins yielding a variety of colours. Environ. Chem. Lett. 2006, 4, 175–183. [Google Scholar] [CrossRef]
- Quaglieri, C.; Jourdes, M.; Waffo-Teguo, P.; Teissedre, P.L. Updated knowledge about pyranoanthocyanins: Impact of oxygen on their contents, and contribution in the winemaking process to overall wine color. Trends Food Sci. Technol. 2017, 67, 139–149. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Studies on the acetaldehyde-induced condensation of (−)-epicatechin and malvidin-3-O-glucoside in a model solution system. J. Agric. Food Chem. 1999, 47, 2096–2102. [Google Scholar] [CrossRef]
- He, J.R.; Santos-Buelga, C.; Mateus, N.; de Freitas, V. Isolation and quantification of oligomeric pyranoanthocyanin-flavanol pigments from red wines by combination of column chromatographic techniques. J. Chromatogr. A 2006, 1134, 215–225. [Google Scholar] [CrossRef]
- Zhang, X.K.; Lan, Y.B.; Huang, Y.; Zhao, X.; Duan, C.Q. Targeted metabolomics of anthocyanin derivatives during prolonged wine aging: Evolution, color contribution and aging prediction. Food Chem. 2021, 339, 127795. [Google Scholar] [CrossRef] [PubMed]
- Salas, E.; Dueñas, M.; Schwarz, M.; Winterhalter, P.; Cheynier, W.; Fulcrand, H. Characterization of pigments from different high speed countercurrent chromatography wine fractions. J. Agric. Food Chem. 2005, 53, 4536–4546. [Google Scholar] [CrossRef] [PubMed]
- Salas, E.; Atanasova, V.; Poncet-Legrand, C.; Meudec, E.; Mazauric, J.P.; Cheynier, V. Demonstration of the occurrence of flavanol-anthocyanin adducts in wine and in model solutions. Anal. Chim. Acta 2004, 513, 325–332. [Google Scholar] [CrossRef]
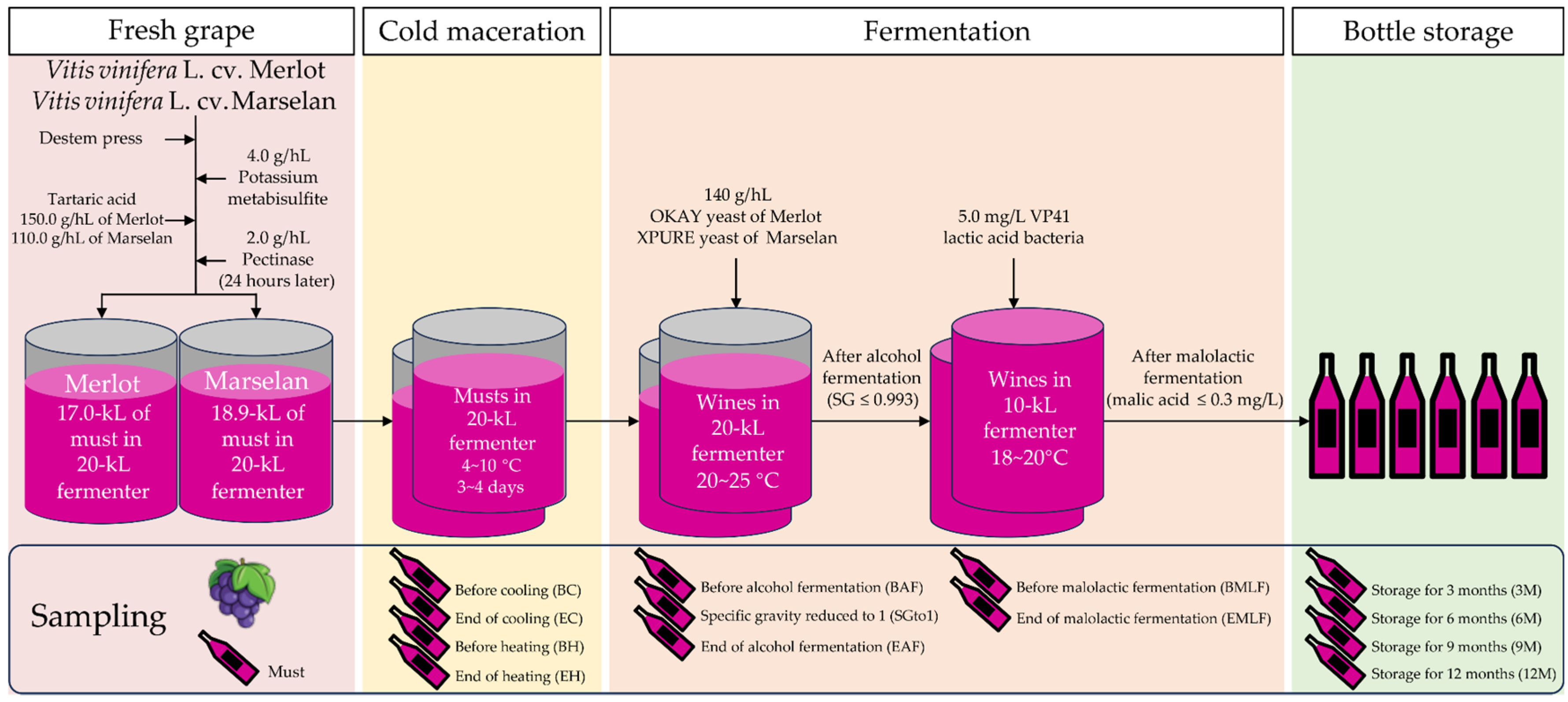
| Category | Phenolic Compounds | ‘Merlot’ | ‘Marselan’ |
|---|---|---|---|
| Anthocyanins | Cyanidin-3-O-glucoside | 28.18 ± 7.67 * | 9.18 ± 3.39 |
| Cyanidin-3-O-acetylglucoside | 4.89 ± 1.32 * | 1.1 ± 0.27 | |
| Cyanidin-3-O-coumarylglucoside | 3.35 ± 1.18 * | 0.87 ± 0.38 | |
| Peonidin-3-O-glucoside | 79.96 ± 17.36 * | 37.8 ± 7.37 | |
| Peonidin-3-O-acetylglucoside | 16.78 ± 1.47 | 22.72 ± 1.38 * | |
| Peonidin-3-O-coumarylglucoside | 25.1 ± 3.34 * | 15.98 ± 0.97 | |
| Delphinidin-3-O-glucoside | 44.85 ± 7.62 | 65.8 ± 6.46 * | |
| Delphinidin-3-O-acetylglucoside | 8.97 ± 1.36 | 10.03 ± 1.31 | |
| Delphinidin-3-O-coumarylglucoside | 1.86 ± 0.55 | 6.06 ± 0.39 * | |
| Petunidin-3-O-glucoside | 36.46 ± 4.56 | 62.86 ± 3.42 * | |
| Petunidin-3-O-acetylglucoside | 29.23 ± 3.52 * | 13.01 ± 2.01 | |
| Petunidin-3-O-coumarylglucoside | 4.54 ± 0.7 | 13.77 ± 0.75 * | |
| Malvidin-3-O-glucoside | 99.07 ± 0.63 | 237.6 ± 10.24 * | |
| Malvidin-3-O-acetylglucoside | 69.5 ± 5.2 | 135.21 ± 8.77 * | |
| Malvidin-3-O-coumarylglucoside | 29.19 ± 1.09 | 99.04 ± 8.41 * | |
| Flavonols | Kaempferol-galactoside | 7.55 ± 0.83 | 16.1 ± 2.4 * |
| Kaempferol-glucoside | 3.46 ± 0.38 | 4.88 ± 0.35 * | |
| Quercetin-galactoside | 30.79 ± 5.62 * | 19.7 ± 2.49 | |
| Quercetin-glucoside | 43.51 ± 1.8 | 48.71 ± 2.73 | |
| Quercetin-glucuronide | 37.06 ± 1.8 | 74.01 ± 8.95 * | |
| Isorhamnetin-glucoside | 8.46 ± 0.73 | 18.01 ± 1.43 * | |
| Myricetin-galactoside | 14.75 ± 0.93 * | 11.11 ± 0.81 | |
| Myricetin-glucoside | 9.23 ± 0.32 | 9.46 ± 0.47 | |
| Syringetin-glucoside | 26.98 ± 2.21 * | 10.27 ± 0.56 | |
| Flavan-3-ols | Epicatechin-free unit | 0.57 ± 0.01 | 0.7 ± 0.01 * |
| Catechin-end unit | 36.74 ± 5.96 * | 27.15 ± 2.18 | |
| Epicatechin-end unit | 5.24 ± 0.63 * | 3.51 ± 0.61 | |
| Epigallocatechin-end unit | 15.26 ± 2.45 * | 6.23 ± 0.36 | |
| Epicatechin gallate-end unit | 1.34 ± 0.25 | 1.43 ± 0.3 | |
| Catechin-extension unit | 821.53 ± 52.57 | 1473.51 ± 54.93 * | |
| Epicatechin-extension unit | 24.02 ± 1.15 | 28.65 ± 1.12 * | |
| Epigallocatechin-extension unit | 134.09 ± 4.56 | 160.71 ± 2.86 * | |
| Epicatechin gallate-extension unit | 1.67 ± 0.16 | 4.15 ± 0.41 * | |
| Mean degree of polymerization | 13.05 ± 1.24 | 32.19 ± 3.37 * |
| Samples | Parameters | ‘Merlot’ | ‘Marselan’ |
|---|---|---|---|
| Juice | Total soluble solid (°Brix) | 25.7 ± 0.1 | 26.2 ± 0.2 * |
| Titratable acidity (g/L) | 4.2 ± 0.06 | 4.7 ± 0.04 * | |
| pH | 3.67 ± 0.01 * | 3.21 ± 0.02 | |
| Wine | Residual sugar (g/L) | 3.63 ± 0.29 | 4.87 ± 0.31 * |
| Total acid (g/L) | 6.80 ± 0.01 | 7.80 ± 0.01 * | |
| Volatile acid (g/L) | 0.67 ± 0.01 | 0.67 ± 0.02 | |
| Ethanol (%, v/v) | 14.31 ± 0.01 | 14.88 ± 0.04 * | |
| Free SO2/(mg/L) | 2.57 ± 0.03 | 3.50 ± 0.06 * | |
| pH | 3.54 ± 0.01 * | 3.28 ± 0.01 |
| Category | Phenolic Compounds | ‘Merlot’ | ‘Marselan’ | ||
|---|---|---|---|---|---|
| Amount of Components | Concentration of Each Category | Amount of Components | Concentration of Each Category | ||
| Anthocyanins | Cyanidin | 3 | 8.53 ± 0.50 * | 3 | 6.19 ± 0.31 |
| Peonidin | 3 | 50.75 ± 3.54 * | 3 | 25.68 ± 1.18 | |
| Delphinidin | 3 | 22.16 ± 1.25 | 3 | 25.02 ± 1.69 * | |
| Petunidin | 3 | 28.33 ± 1.85 | 3 | 29.48 ± 1.57 | |
| Malvidin | 3 | 160.27 ± 9.49 | 3 | 157.09 ± 2.52 | |
| Pyranoanthocyanins | Vitisin A | 3 | 2.19 ± 0.20 | 3 | 3.68 ± 0.31 * |
| Vitisin B | 3 | 7.25 ± 0.46 * | 3 | 4.47 ± 0.17 | |
| Pinotin | 4 | 3.72 ± 0.12 * | 4 | 1.93 ± 0.19 | |
| Polymeric pigments | F-A (A-F) | 2 | 0.54 ± 0.08 | 4 | 2.16 ± 0.22 * |
| A-e-F | 4 | 4.30 ± 0.49 | 4 | 5.03 ± 0.50 | |
| Non-anthocyanin phenols | Flavonol | 10 | 54.30 ± 3.62 * | 10 | 34.35 ± 4.23 |
| Favan-3-ol | 5 | 105.81 ± 9.12 * | 5 | 66.32 ± 9.02 | |
| Phenolic acid | 9 | 95.73 ± 8.10 * | 11 | 76.67 ± 5.51 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.-L.; Xia, N.-Y.; Yao, X.-C.; Duan, C.-Q.; Pan, Q.-H. Effects of Phenolic Evolution on Color Characteristics of Single-Cultivar Vitis vinifera L. Marselan and Merlot Wines during Vinification and Aging. Foods 2024, 13, 494. https://doi.org/10.3390/foods13030494
Zhang H-L, Xia N-Y, Yao X-C, Duan C-Q, Pan Q-H. Effects of Phenolic Evolution on Color Characteristics of Single-Cultivar Vitis vinifera L. Marselan and Merlot Wines during Vinification and Aging. Foods. 2024; 13(3):494. https://doi.org/10.3390/foods13030494
Chicago/Turabian StyleZhang, Hua-Lin, Nong-Yu Xia, Xue-Chen Yao, Chang-Qing Duan, and Qiu-Hong Pan. 2024. "Effects of Phenolic Evolution on Color Characteristics of Single-Cultivar Vitis vinifera L. Marselan and Merlot Wines during Vinification and Aging" Foods 13, no. 3: 494. https://doi.org/10.3390/foods13030494
APA StyleZhang, H.-L., Xia, N.-Y., Yao, X.-C., Duan, C.-Q., & Pan, Q.-H. (2024). Effects of Phenolic Evolution on Color Characteristics of Single-Cultivar Vitis vinifera L. Marselan and Merlot Wines during Vinification and Aging. Foods, 13(3), 494. https://doi.org/10.3390/foods13030494








