Mango Peels as an Industrial By-Product: A Sustainable Source of Compounds with Antioxidant, Enzymatic, and Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Microorganisms
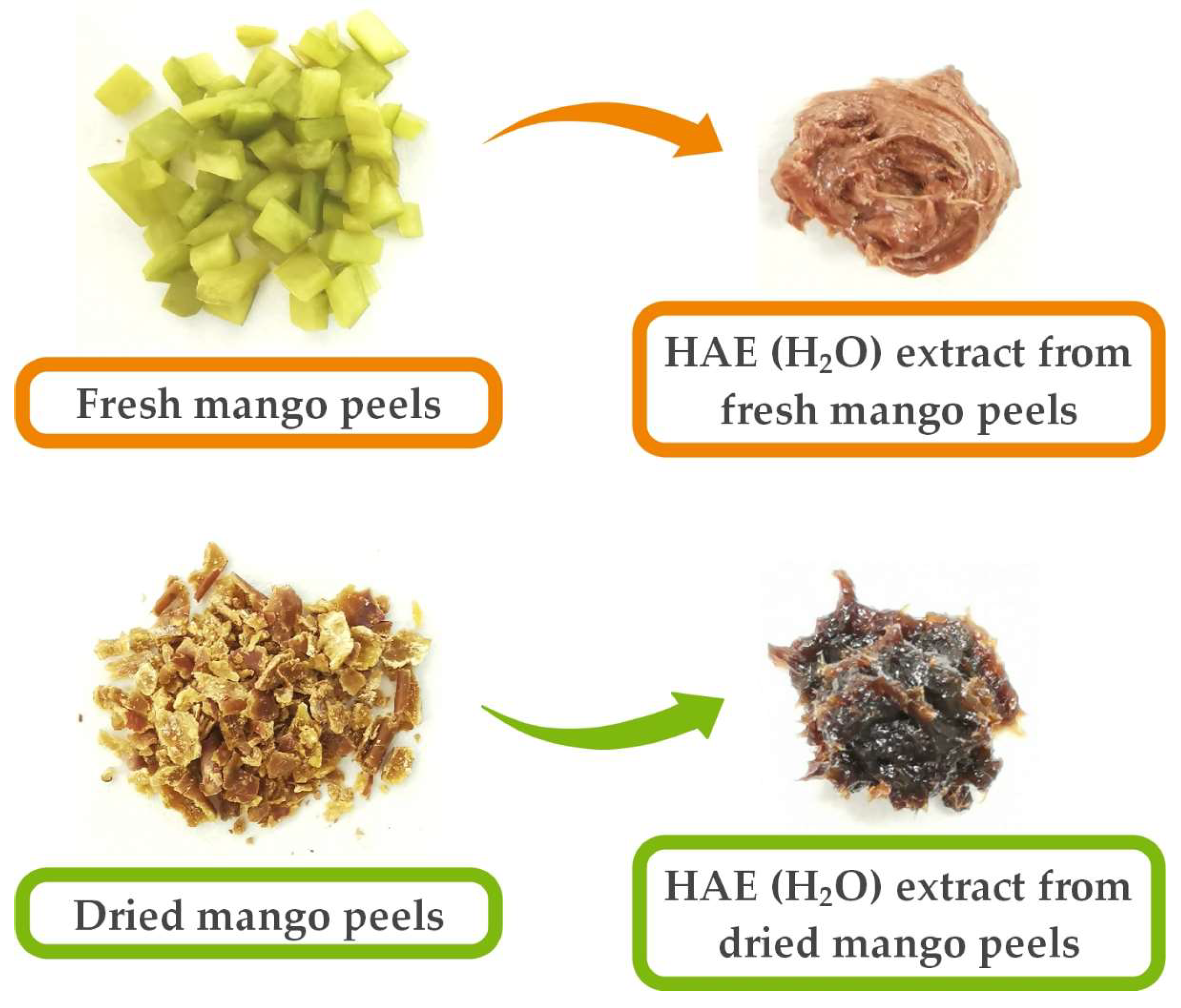
2.3. Preparation of Plant Material
2.4. Moisture Content
2.5. Thermogravimetric Analysis/Differential Scanning Calorimetry
2.6. Extraction Procedures
2.6.1. Soxhlet Extraction
2.6.2. Ultrasound-Assisted Extraction
2.6.3. Homogenization-Assisted Extraction
2.6.4. Supercritical Fluid Extraction
2.6.5. Extraction Yield Determination
2.7. Quantitative Determination of Total Phenols and Proanthocyanidins
2.7.1. Determination of Total Phenolic Content (TPC)
2.7.2. Determination of Total Proanthocyanidin Content (PAC)
2.8. Determination of Antioxidant Activity
2.9. Liquid Chromatography with Tandem Mass Spectrometry Analysis
2.10. Determination of Total Protein (TP) Content
2.11. Determination of Enzyme Activities
2.12. Determination of Antimicrobial Activity
2.12.1. Qualitative Disk Diffusion Method
2.12.2. Quantitative Microbroth Dilution Method
2.13. Statistical Analysis
3. Results and Discussion
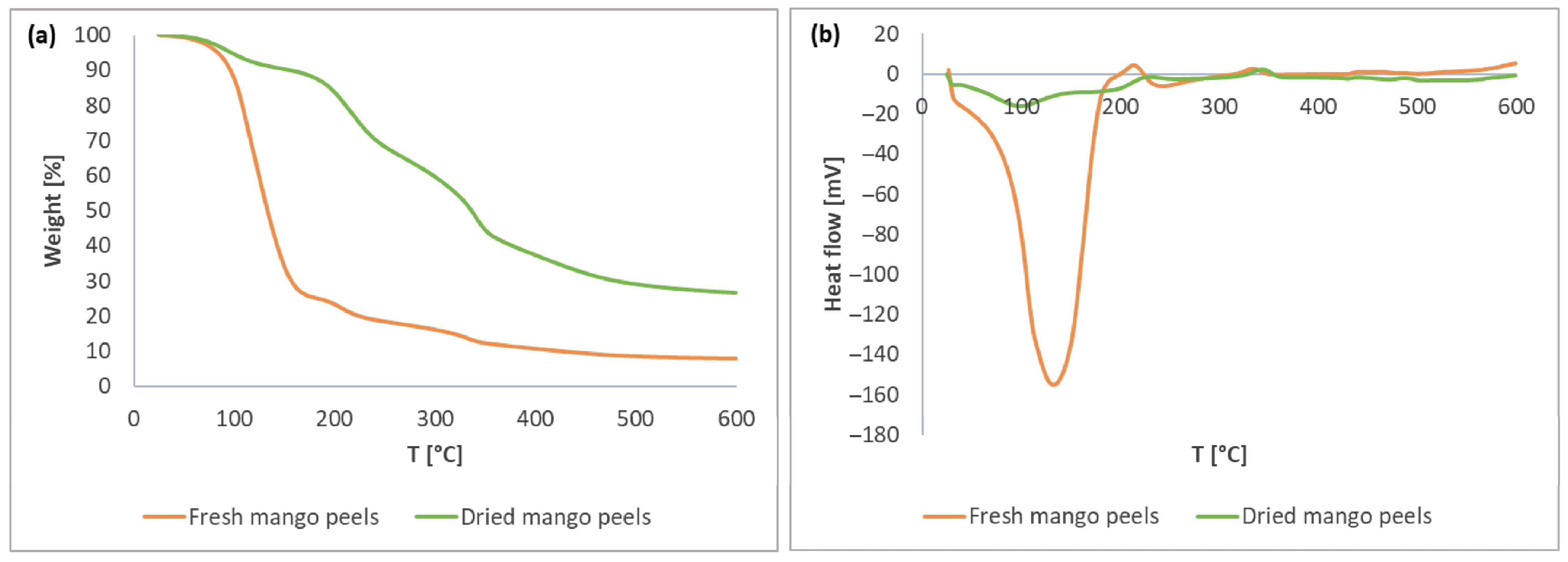
3.1. Thermal Stability of Mango Peels
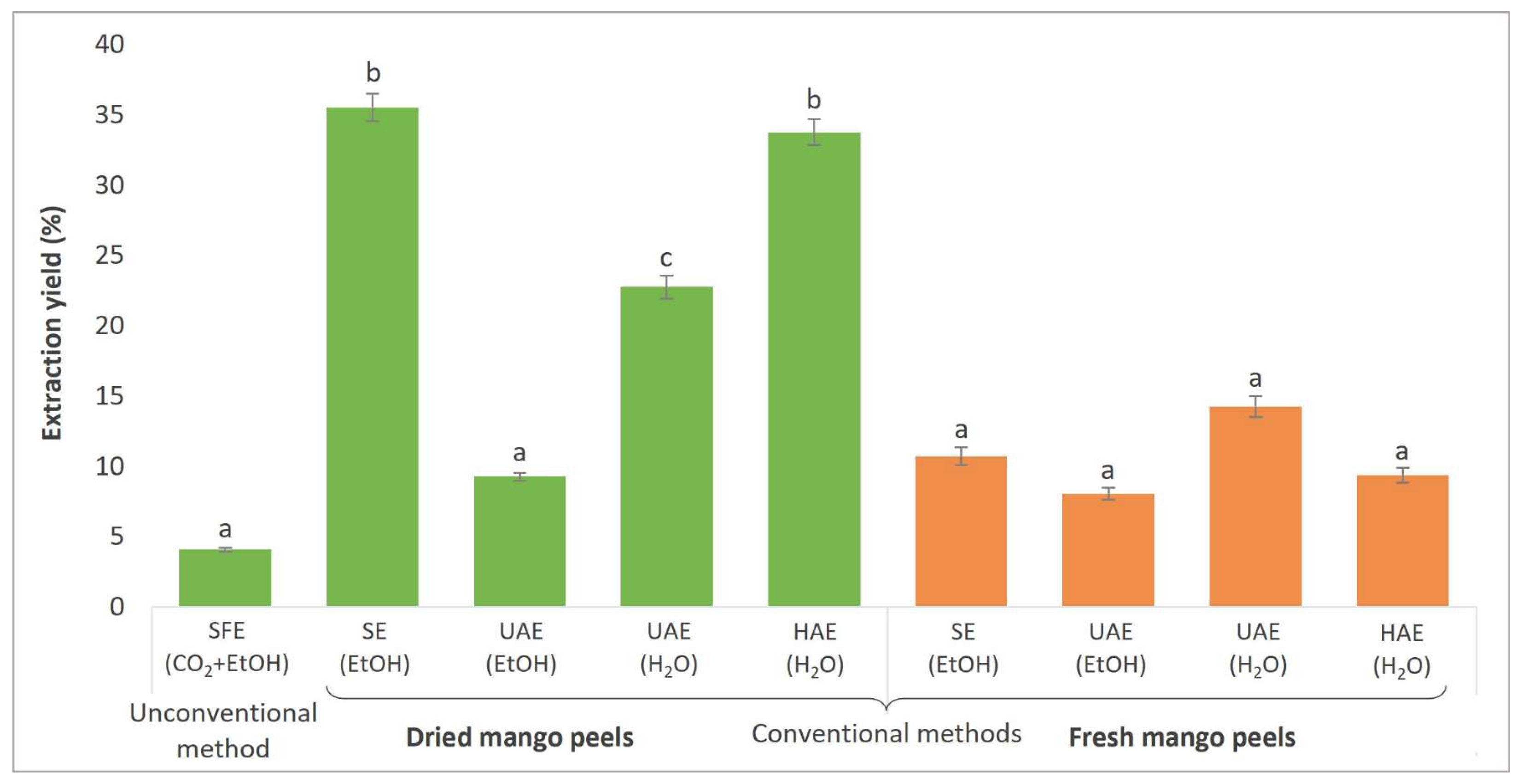
3.2. Extraction Yield Determination
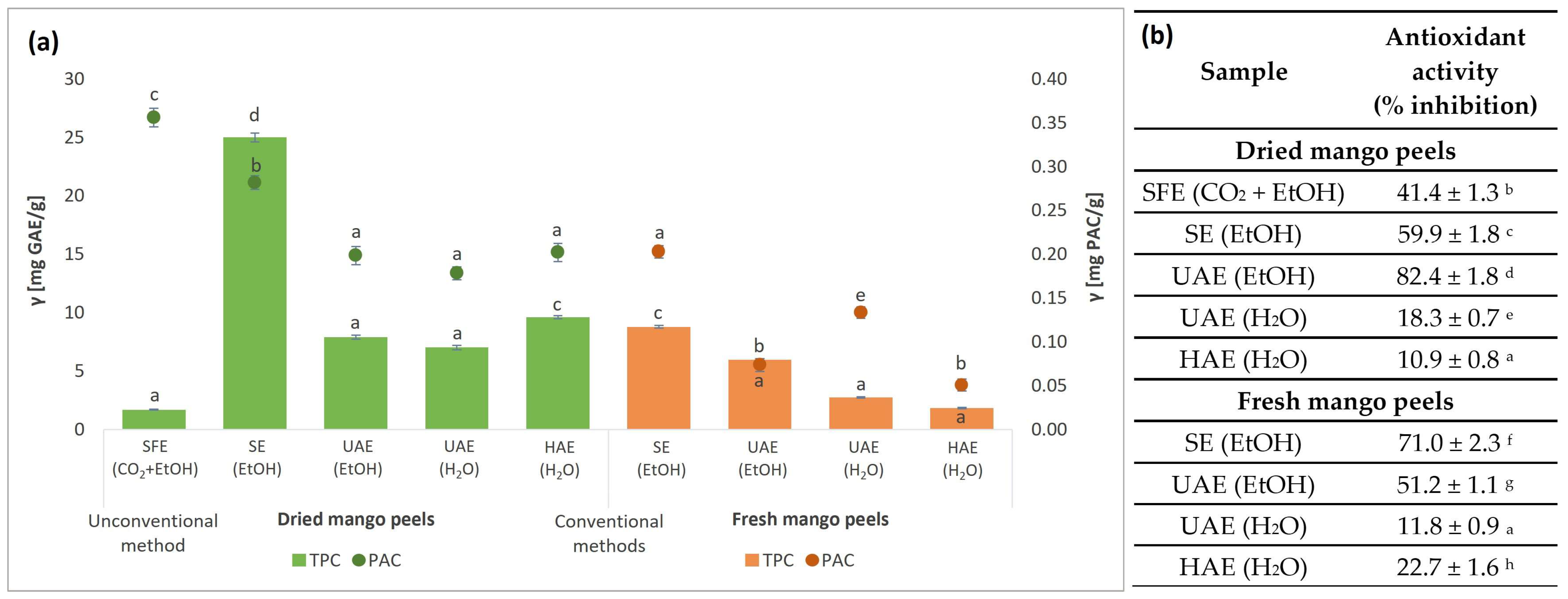
3.3. Content of Secondary Metabolites in Mango Peel Extracts and Their Antioxidant Activity
3.4. Content of Important Phenolic Compounds in Mango Peel Extracts
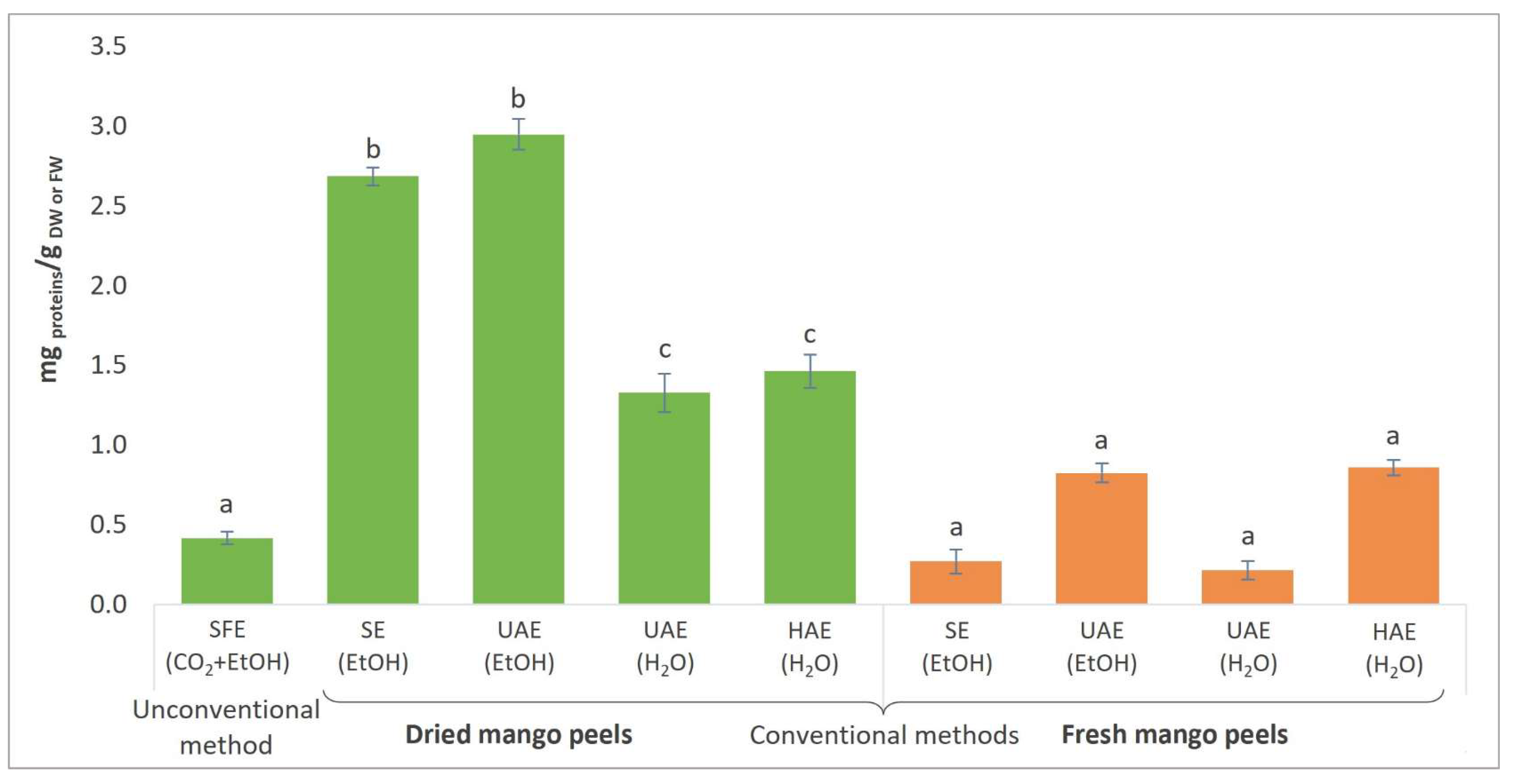
3.5. Total Protein Content and Activities of Certain Enzymes in Mango Peel Extracts
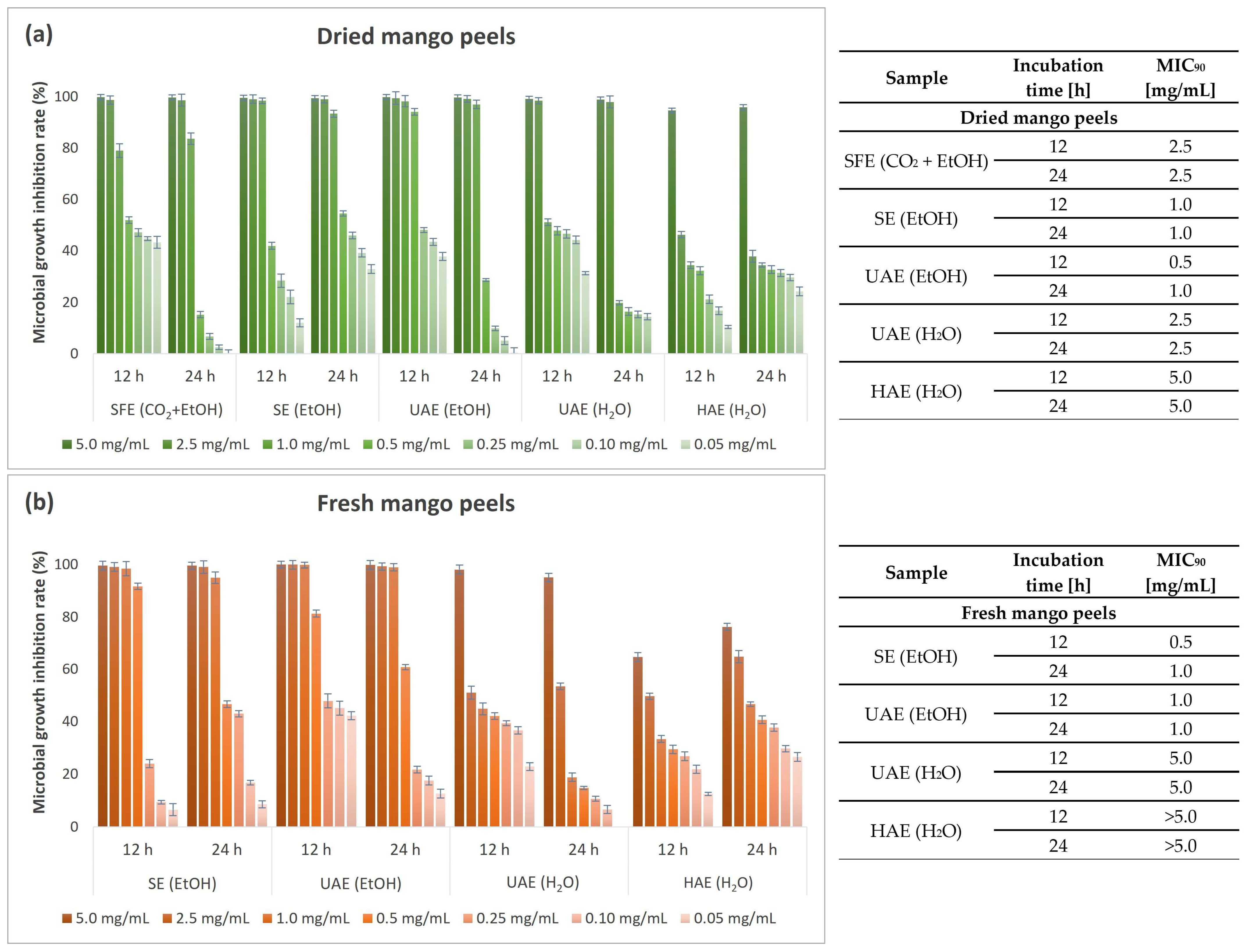
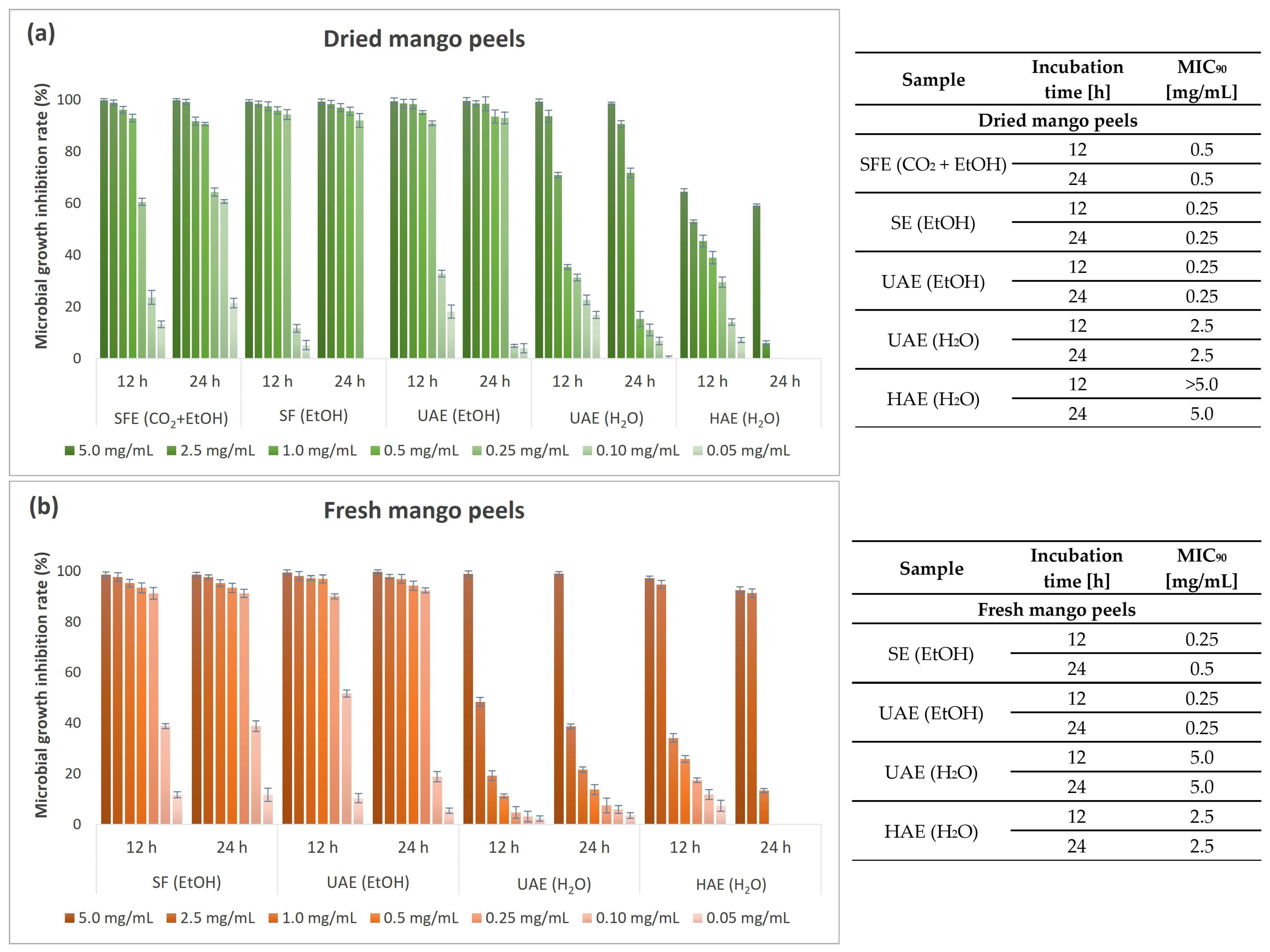
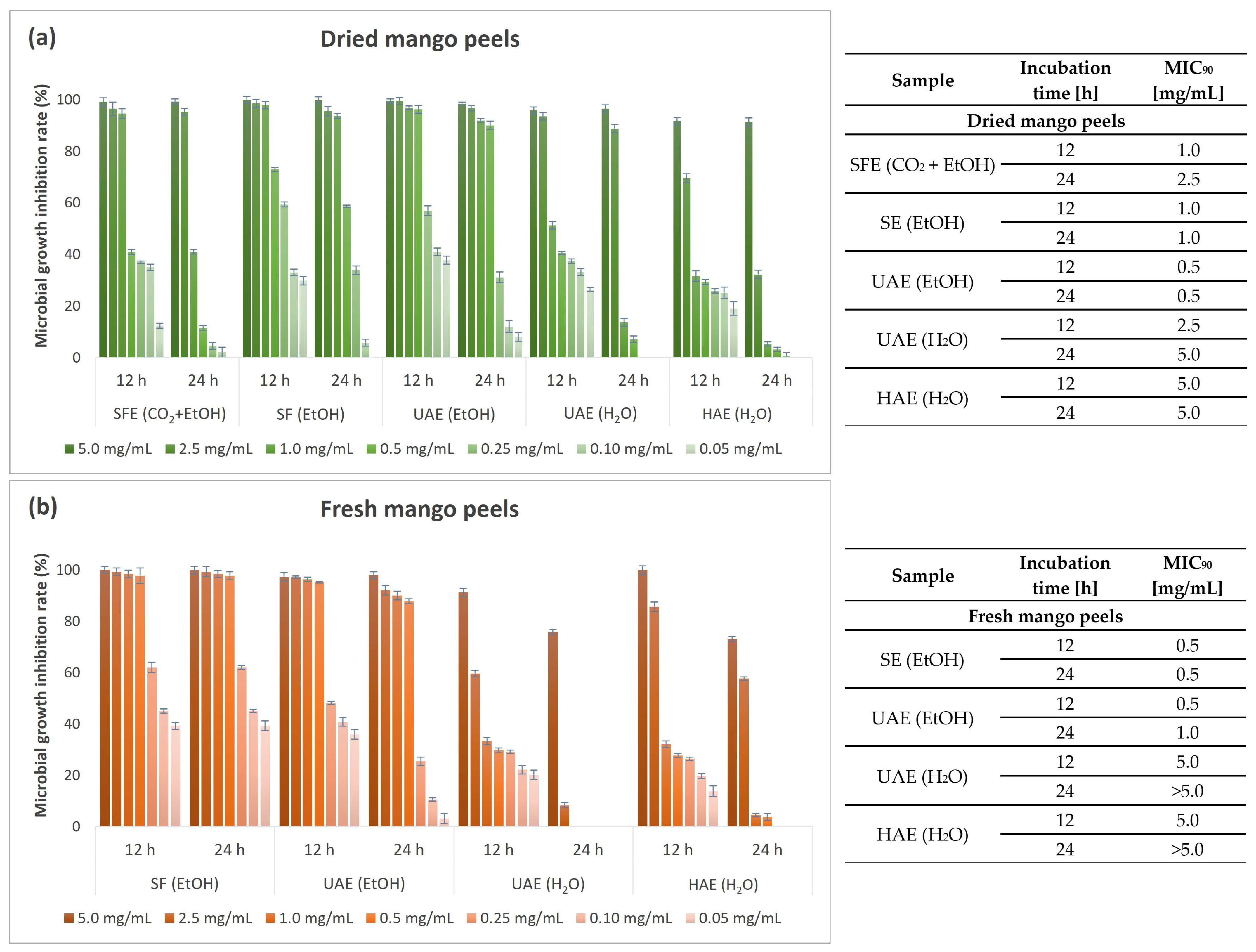
3.6. Antimicrobial Activity of Mango Peel Extracts
3.6.1. Qualitative Antimicrobial Determination of Mango Peel Extracts
3.6.2. Quantitative Antimicrobial Determination of Mango Peel Extracts
3.7. The Most Promising Mango Peel Extracts for Various Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diaconeasa, Z.; Iuhas, C.I.; Ayvaz, H.; Mortas, M.; Farcaş, A.; Mihai, M.; Danciu, C.; Stanilă, A. Anthocyanins from Agro-Industrial Food Waste: Geographical Approach and Methods of Recovery—A Review. Plants 2023, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Chang, J.-S. Valorization of Fruit Wastes for Circular Bioeconomy: Current Advances, Challenges, and Opportunities. Bioresour. Technol. 2022, 359, 127459. [Google Scholar] [CrossRef]
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent Advances in Extraction Technologies for Recovery of Bioactive Compounds Derived from Fruit and Vegetable Waste Peels: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 719–752. [Google Scholar] [CrossRef]
- Hussain, H.; Mamadalieva, N.Z.; Hussain, A.; Hassan, U.; Rabnawaz, A.; Ahmed, I.; Green, I.R. Fruit Peels: Food Waste as a Valuable Source of Bioactive Natural Products for Drug Discovery. CIMB 2022, 44, 1960–1994. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Verma, R.; Bhardwaj, P.; Sharma, S.; Kumar, D. Fruit and Vegetable Peels: Utilization of High Value Horticultural Waste in Novel Industrial Applications. Molecules 2020, 25, 2812. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Luan, Y.; Zhao, Y.; Liu, J.; Duan, Z.; Ruan, R. Current Technologies and Uses for Fruit and Vegetable Wastes in a Sustainable System: A Review. Foods 2023, 12, 1949. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Dubey, K.K.; Marathe, S.J.; Singhal, R. Supercritical Fluid Extraction of Bioactives from Fruit Waste and Its Therapeutic Potential. Food Biosci. 2023, 52, 102418. [Google Scholar] [CrossRef]
- Espinosa-Espinosa, L.; Garduño-Siciliano, L.; Rodriguez-Canales, M.; Hernandez-Portilla, L.B.; Canales-Martinez, M.M.; Rodriguez-Monroy, M.A. The Wound-Healing Effect of Mango Peel Extract on Incision Wounds in a Murine Model. Molecules 2022, 27, 259. [Google Scholar] [CrossRef] [PubMed]
- García-Mahecha, M.; Soto-Valdez, H.; Carvajal-Millan, E.; Madera-Santana, T.J.; Lomelí-Ramírez, M.G.; Colín-Chávez, C. Bioactive Compounds in Extracts from the Agro-Industrial Waste of Mango. Molecules 2023, 28, 458. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Simancas, R.; Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Alañón, M.E. Viability of Pre-Treatment Drying Methods on Mango Peel by-Products to Preserve Flavouring Active Compounds for Its Revalorisation. J. Food Eng. 2020, 279, 109953. [Google Scholar] [CrossRef]
- Marçal, S.; Pintado, M. Mango Peels as Food Ingredient/Additive: Nutritional Value, Processing, Safety and Applications. Trends Food Sci. Technol. 2021, 114, 472–489. [Google Scholar] [CrossRef]
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; Hanani, Z.A.N. Utilization of Mango Peel Extracts on the Biodegradable Films for Active Packaging. Food Packag. Shelf Life 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Ahmad, R.; Aldholmi, M.; Mostafa, A.; Alqathama, A.; Aldarwish, A.; Abuhassan, A.; Alateeq, L.; Bubshait, S.; Aljaber, M.; Aldossary, S. A Novel Green Extraction and Analysis Technique for the Comprehensive Characterization of Mangiferin in Different Parts of the Fresh Mango Fruit (Mangifera Indica). LWT 2022, 159, 113176. [Google Scholar] [CrossRef]
- Maenpuen, S.; Mee-udorn, P.; Pinthong, C.; Athipornchai, A.; Phiwkaow, K.; Watchasit, S.; Pimviriyakul, P.; Rungrotmongkol, T.; Tinikul, R.; Leartsakulpanich, U.; et al. Mangiferin Is a New Potential Antimalarial and Anticancer Drug for Targeting Serine Hydroxymethyltransferase. Arch. Biochem. Biophys. 2023, 745, 109712. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Lv, Q.; Zhao, Y.; Hu, G.; Huang, G.; Zhang, J.; Sun, C.; Li, X.; Chen, K. Quantification and Purification of Mangiferin from Chinese Mango (Mangifera indica L.) Cultivars and Its Protective Effect on Human Umbilical Vein Endothelial Cells under H2O2-Induced Stress. Int. J. Mol. Sci. 2012, 13, 11260–11274. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.-J.; Ye, W.; Korivi, M. Nutritional Composition and Bioactive Compounds in Three Different Parts of Mango Fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef]
- Tokas, J.; Punia, H.; Baloda, S.; Sheokand, R.N. Mango Peel: A Potential Source of Bioactive Compounds and Phytochemicals. Austin Food Sci. 2020, 5, id1035. [Google Scholar]
- Gupta, A.K.; Gurjar, P.S.; Beer, K.; Pongener, A.; Ravi, S.C.; Singh, S.; Verma, A.; Singh, A.; Thakur, M.; Tripathy, S.; et al. A Review on Valorization of Different Byproducts of Mango (Mangifera indica L.) for Functional Food and Human Health. Food Biosci. 2022, 48, 101783. [Google Scholar] [CrossRef]
- Alaiya, M.A.; Odeniyi, M.A. Utilisation of Mangifera indica Plant Extracts and Parts in Antimicrobial Formulations and as a Pharmaceutical Excipient: A Review. Future J. Pharm. Sci. 2023, 9, 29. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Kuo, C.-H.; Wu, C.-H.; Kuan, A.-W.; Guo, H.-R.; Lin, Y.-H.; Wang, P.-K. Free Radical-Scavenging, Anti-Inflammatory, and Antibacterial Activities of Water and Ethanol Extracts Prepared from Compressional-Puffing Pretreated Mango (Mangifera indica L.) Peels. J. Food Qual. 2018, 2018, e1025387. [Google Scholar] [CrossRef]
- Thambi, P.A.; John, S.; Lydia, E.; Iyer, P.; Jane, S. Antimicrobial Efficacy of Mango Peel Powder and Formulation of Recipes Using Mango Peel Powder (Mangifera indica L.). Int. J. Home Sci. 2016, 2, 155–161. [Google Scholar]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between Medical Plant-Derived Bioactive Compounds: Focus on Antimicrobial Combination Effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern Methods of Pre-Treatment of Plant Material for the Extraction of Bioactive Compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef] [PubMed]
- Bar, M.; Binduga, U.E.; Szychowski, K.A. Methods of Isolation of Active Substances from Garlic (Allium sativum L.) and Its Impact on the Composition and Biological Properties of Garlic Extracts. Antioxidants 2022, 11, 1345. [Google Scholar] [CrossRef] [PubMed]
- Grández-Yoplac, D.E.; Mori-Mestanza, D.; Muñóz-Astecker, L.D.; Cayo-Colca, I.S.; Castro-Alayo, E.M. Kinetics Drying of Blackberry Bagasse and Degradation of Anthocyanins and Bioactive Properties. Antioxidants 2021, 10, 548. [Google Scholar] [CrossRef] [PubMed]
- El Gamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; El Masry, G. Thermal Degradation of Bioactive Compounds during Drying Process of Horticultural and Agronomic Products: A Comprehensive Overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Inyang, U.; Oboh, I.; Etuk, B. Drying and the Different Techniques. Int. J. Food Nutr. Saf. 2017, 8, 45–72. [Google Scholar]
- Mbondo, N.N.; Owino, W.O.; Ambuko, J.; Sila, D.N. Effect of Drying Methods on the Retention of Bioactive Compounds in African Eggplant. Food Sci. Nutr. 2018, 6, 814–823. [Google Scholar] [CrossRef]
- Hasan, M.U.; Malik, A.U.; Ali, S.; Imtiaz, A.; Munir, A.; Amjad, W.; Anwar, R. Modern Drying Techniques in Fruits and Vegetables to Overcome Postharvest Losses: A Review. J. Food Process. Preserv. 2019, 43, e14280. [Google Scholar] [CrossRef]
- Roshanak, S.; Rahimmalek, M.; Goli, S.A.H. Evaluation of Seven Different Drying Treatments in Respect to Total Flavonoid, Phenolic, Vitamin C Content, Chlorophyll, Antioxidant Activity and Color of Green Tea (Camellia sinensis or C. assamica) Leaves. J. Food Sci. Technol. 2016, 53, 721–729. [Google Scholar] [CrossRef]
- Guiné, R.P.F. Influence of Drying Method on Some Physical and Chemical Properties of Pears. Int. J. Fruit Sci. 2011, 11, 245–255. [Google Scholar] [CrossRef]
- Yao, J.; Chen, W.; Fan, K. Novel Efficient Physical Technologies for Enhancing Freeze Drying of Fruits and Vegetables: A Review. Foods 2023, 12, 4321. [Google Scholar] [CrossRef]
- Rybak, K.; Parniakov, O.; Samborska, K.; Wiktor, A.; Witrowa-Rajchert, D.; Nowacka, M. Energy and Quality Aspects of Freeze-Drying Preceded by Traditional and Novel Pre-Treatment Methods as Exemplified by Red Bell Pepper. Sustainability 2021, 13, 2035. [Google Scholar] [CrossRef]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, Proanthocyanidins, Flavones and Flavonols in Some Plant Materials and Their Antioxidant Activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Leitgeb, M.; Kupnik, K.; Knez, Ž.; Primožič, M. Enzymatic and Antimicrobial Activity of Biologically Active Samples from Aloe arborescens and Aloe barbadensis. Biology 2021, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Hrnčič, M.K.; Cör, D.; Kotnik, P.; Knez, Ž. Extracts of White and Red Grape Skin and Rosehip Fruit: Phenolic Compounds and Their Antioxidative Activity. Acta Chim. Slov. 2019, 66, 751–761. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, M.; Knez, Ž.; Hojnik Podrepšek, G. Effect of Green Food Processing Technology on the Enzyme Activity in Spelt Flour. Foods 2022, 11, 3832. [Google Scholar] [CrossRef]
- Primožič, M.; Čolnik, M.; Knez, Ž.; Leitgeb, M. Advantages and Disadvantages of Using SC CO2 for Enzyme Release from Halophilic Fungi. J. Supercrit. Fluids 2019, 143, 286–293. [Google Scholar] [CrossRef]
- Bermeyer, J.; Bergmeyer, E.-C.H.U. Methods of Enzymatic Analysis, Methods of Enzymatic Analysis: Volume 1: Fundamentals; Bergmeyer, H.-U., Ed.; Wiley-Blackwell: Weinheim, Germany, 1983; Volume 1, ISBN 978-3-527-26041-6. [Google Scholar]
- Primozic, M.; Vasic, K.; Kravanja, G.; Knez, Z.; Leitgeb, M. Immobilized Laccase for Sustainable Technological Processes. Chem. Eng. Trans. 2019, 76, 91–96. [Google Scholar] [CrossRef]
- Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. The Influence of Supercritical Carbon Dioxide on Graham Flour Enzyme Polyphenol Oxidase Activity. Molecules 2020, 25, 5981. [Google Scholar] [CrossRef]
- Kupnik, K.; Primožič, M.; Kokol, V.; Knez, Ž.; Leitgeb, M. Enzymatic, Antioxidant, and Antimicrobial Activities of Bioactive Compounds from Avocado (Persea americana L.) Seeds. Plants 2023, 12, 1201. [Google Scholar] [CrossRef]
- Gajšek, M.; Jančič, U.; Vasić, K.; Knez, Ž.; Leitgeb, M. Enhanced Activity of Immobilized Transglutaminase for Cleaner Production Technologies. J. Clean. Prod. 2019, 240, 118218. [Google Scholar] [CrossRef]
- Kupnik, K.; Primožič, M.; Knez, Ž.; Leitgeb, M. Antimicrobial Efficiency of Aloe arborescens and Aloe barbadensis Natural and Commercial Products. Plants 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Marsiglia-Fuentes, R.; Chiralt, A.; García-Zapateiro, L.A. Investigating the Water Relations in Aqueous Extract Powders of Mango (Mangifera indica) Peel and Seed Waste for Their Use in Food Matrices as a Value-Added By-Product. Foods 2023, 12, 3497. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Shah, N.G.; Mahajani, S.M. A Novel Technique to Characterize and Quantify Crystalline and Amorphous Matter in Complex Sugar Mixtures. Food Anal. Methods 2020, 13, 2087–2101. [Google Scholar] [CrossRef]
- Wongkaew, M.; Kittiwachana, S.; Phuangsaijai, N.; Tinpovong, B.; Tiyayon, C.; Pusadee, T.; Chuttong, B.; Sringarm, K.; Bhat, F.M.; Sommano, S.R.; et al. Fruit Characteristics, Peel Nutritional Compositions, and Their Relationships with Mango Peel Pectin Quality. Plants 2021, 10, 1148. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Blay, V.; Taberner, V.; Pérez-Gago, M.B.; Palou, L. Postharvest Treatments with Sulfur-Containing Food Additives to Control Major Fungal Pathogens of Stone Fruits. Foods 2021, 10, 2115. [Google Scholar] [CrossRef] [PubMed]
- Dorta, E.; Lobo, M.G.; González, M. Using Drying Treatments to Stabilise Mango Peel and Seed: Effect on Antioxidant Activity. LWT Food Sci. Technol. 2012, 45, 261–268. [Google Scholar] [CrossRef]
- Souza, M.; Mezzomo, N.; Corrêa, L.; Lima, M.; Azevêdo, L.; Ferreira, S. Recovery of Antioxidant Compounds from Mango Peel by Green Extraction Processes. Int. Food Res. J. 2019, 26, 1845–1859. [Google Scholar]
- Sánchez-Camargo, A.d.P.; Gutiérrez, L.-F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.-E. Valorisation of Mango Peel: Proximate Composition, Supercritical Fluid Extraction of Carotenoids, and Application as an Antioxidant Additive for an Edible Oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential Extraction of Phenolics and Pectin from Mango Peel Assisted by Ultrasound. Food Res. Int. 2019, 119, 455–461. [Google Scholar] [CrossRef]
- Jirasuteeruk, C.; Theerakulkait, C. Ultrasound-Assisted Extraction of Phenolic Compounds from Mango (Mangifera indica Cv. Chok Anan) Peel and Its Inhibitory Effect on Enzymatic Browning of Potato Puree. Food Technol. Biotechnol. 2019, 57, 350–357. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Nadeem, M. Comparison of Ultrasound and Maceration Techniques for the Extraction of Polyphenols from the Mango Peel. J. Food Process. Preserv. 2017, 41, e13028. [Google Scholar] [CrossRef]
- Kaur, B.; Panesar, P.S.; Anal, A.K. Standardization of Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Mango Peels. J. Food Sci. Technol. 2022, 59, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Enríquez, A.J.; Reyes-Ventura, E.; Villanueva-Rodríguez, S.J.; Moreno-Vilet, L. Effect of Ultrasound-Assisted Extraction Parameters on Total Polyphenols and Its Antioxidant Activity from Mango Residues (Mangifera indica L. Var. Manililla). Separations 2021, 8, 94. [Google Scholar] [CrossRef]
- Tunchaiyaphum, S.; Eshtiaghi, M.N.; Yoswathana, N. Extraction of Bioactive Compounds from Mango Peels Using Green Technology. IJCEA 2013, 4, 194–198. [Google Scholar] [CrossRef]
- Mannino, G.; Chinigò, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and Where to Find Them: A Meta-Analytic Approach to Investigate Their Chemistry, Biosynthesis, Distribution, and Effect on Human Health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Tan, L.; Jin, Z.; Ge, Y.; Nadeem, H.; Cheng, Z.; Azeem, F.; Zhan, R. Comprehensive ESI-Q TRAP-MS/MS Based Characterization of Metabolome of Two Mango (Mangifera indica L.) Cultivars from China. Sci. Rep. 2020, 10, 20017. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of Mango Byproducts: Study of the Effect of Extraction Solvent and Temperature on Their Antioxidant Properties. J. Food Sci. 2012, 77, C80–C88. [Google Scholar] [CrossRef] [PubMed]
- Dorta, E.; González, M.; Lobo, M.G.; Laich, F. Antifungal Activity of Mango Peel and Seed Extracts against Clinically Pathogenic and Food Spoilage Yeasts. Nat. Prod. Res. 2016, 30, 2598–2604. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, M.; Verghese, A.; Shivashankar, S.; Chakravarthy, A.; Sumathi, M.; Kandakoor, S. Does Change in Tannin Content in Mango (Mangifera indica) Fruits Influence the Extent of Fruit Fly (Bactrocera Dorsalis Hendel) Herbivory. J. Entomol. Zool. Stud. 2017, 381, 381–385. [Google Scholar]
- Hangun-Balkir, Y.; McKenney, M.L. Determination of Antioxidant Activities of Berries and Resveratrol. Green Chem. Lett. Rev. 2012, 5, 147–153. [Google Scholar] [CrossRef]
- Barreto, J.C.; Trevisan, M.T.S.; Hull, W.E.; Erben, G.; de Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and Quantitation of Polyphenolic Compounds in Bark, Kernel, Leaves, and Peel of Mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef]
- Thakare, V.B.; Jadeja, G.C.; Desai, M.A. Extraction of Mangiferin and Pectin from Mango Peels Using Process Intensified Tactic: A Step towards Waste Valorization. Chem. Eng. Res. Des. 2023, 192, 280–288. [Google Scholar] [CrossRef]
- Kaur, P.; Gupta, R.C.; Dey, A.; Malik, T.; Pandey, D.K. Optimization of Harvest and Extraction Factors by Full Factorial Design for the Improved Yield of C-Glucosyl Xanthone Mangiferin from Swertia Chirata. Sci. Rep. 2021, 11, 16346. [Google Scholar] [CrossRef]
- Lerma-Torres, J.M.; Navarro-Ocaña, A.; Calderón-Santoyo, M.; Hernández-Vázquez, L.; Ruiz-Montañez, G.; Ragazzo-Sánchez, J.A. Preparative Scale Extraction of Mangiferin and Lupeol from Mango (Mangifera indica L.) Leaves and Bark by Different Extraction Methods. J. Food Sci. Technol. 2019, 56, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Tayana, N.; Inthakusol, W.; Duangdee, N.; Chewchinda, S.; Pandith, H.; Kongkiatpaiboon, S. Mangiferin Content in Different Parts of Mango Tree (Mangifera indica L.) in Thailand. Songklanakarin J. Sci. Technol. 2019, 41, 522–528. [Google Scholar]
- Ruiz-Montañez, G.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; Velázquez-de la Cruz, G.; Ramírez de León, J.A.; Navarro-Ocaña, A. Evaluation of Extraction Methods for Preparative Scale Obtention of Mangiferin and Lupeol from Mango Peels (Mangifera indica L.). Food Chem. 2014, 159, 267–272. [Google Scholar] [CrossRef]
- Zuin, V.G.; Segatto, M.L.; Zanotti, K. Towards a Green and Sustainable Fruit Waste Valorisation Model in Brazil: Optimisation of Homogenizer-Assisted Extraction of Bioactive Compounds from Mango Waste Using a Response Surface Methodology. Pure Appl. Chem. 2020, 92, 617–629. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; López-Cobo, A.; Verardo, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-q-TOF-MS as a Powerful Platform for the Determination of Phenolic and Other Polar Compounds in the Edible Part of Mango and Its by-Products (Peel, Seed, and Seed Husk). Electrophoresis 2016, 37, 1072–1084. [Google Scholar] [CrossRef]
- Marcillo-Parra, V.; Anaguano, M.; Molina, M.; Tupuna-Yerovi, D.S.; Ruales, J. Characterization and Quantification of Bioactive Compounds and Antioxidant Activity in Three Different Varieties of Mango (Mangifera indica L.) Peel from the Ecuadorian Region Using HPLC-UV/VIS and UPLC-PDA. NFS J. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Ochocka, R.; Hering, A.; Stefanowicz–Hajduk, J.; Cal, K.; Barańska, H. The Effect of Mangiferin on Skin: Penetration, Permeation and Inhibition of ECM Enzymes. PLoS ONE 2017, 12, e0181542. [Google Scholar] [CrossRef]
- Montes, A.; Wehner, L.; Pereyra, C.; de la Ossa, E.J.M. Mangiferin Nanoparticles Precipitation by Supercritical Antisolvent Process. J. Supercrit. Fluids 2016, 112, 44–50. [Google Scholar] [CrossRef]
- Montes, A.; Wehner, L.; Pereyra, C.; Martínez de la Ossa, E.J. Generation of Microparticles of Ellagic Acid by Supercritical Antisolvent Process. J. Supercrit. Fluids 2016, 116, 101–110. [Google Scholar] [CrossRef]
- Liu, X.; Yan, X.; Bi, J.; Wu, X.; Liu, J.; Zhou, M. Identification of Phenolic Compounds and Antioxidant Activity of Guava Dehydrated by Different Drying Methods. Dry. Technol. 2020, 38, 987–1000. [Google Scholar] [CrossRef]
- Ojeda, G.A.; Sgroppo, S.C.; Sánchez-Moreno, C.; de Ancos, B. Mango ‘Criollo’ by-Products as a Source of Polyphenols with Antioxidant Capacity. Ultrasound Assisted Extraction Evaluated by Response Surface Methodology and HPLC-ESI-QTOF-MS/MS Characterization. Food Chem. 2022, 396, 133738. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Torres-Moreno, H.; Villegas-Ochoa, M.A.; Ayala-Zavala, J.F.; Robles-Zepeda, R.E.; Wall-Medrano, A.; González-Aguilar, G.A. Gallic Acid Content and an Antioxidant Mechanism Are Responsible for the Antiproliferative Activity of ‘Ataulfo’ Mango Peel on LS180 Cells. Molecules 2018, 23, 695. [Google Scholar] [CrossRef]
- Daneshfar, A.; Ghaziaskar, H.S.; Homayoun, N. Solubility of Gallic Acid in Methanol, Ethanol, Water, and Ethyl Acetate. J. Chem. Eng. Data 2008, 53, 776–778. [Google Scholar] [CrossRef]
- Cháfer, A.; Fornari, T.; Stateva, R.P.; Berna, A.; García-Reverter, J. Solubility of the Natural Antioxidant Gallic Acid in Supercritical CO2 + Ethanol as a Cosolvent. J. Chem. Eng. Data 2007, 52, 116–121. [Google Scholar] [CrossRef]
- Çoklar, H.; Akbulut, M. Effect of Sun, Oven and Freeze-Drying on Anthocyanins, Phenolic Compounds and Antioxidant Activity of Black Grape (Ekşikara) (Vitis vinifera L.). S. Afr. J. Enol. Vitic. 2017, 38, 264–272. [Google Scholar] [CrossRef]
- Alañón, M.E.; Pimentel-Moral, S.; Arráez-Román, D.; Segura-Carretero, A. Profiling Phenolic Compounds in Underutilized Mango Peel By-Products from Cultivars Grown in Spanish Subtropical Climate over Maturation Course. Food Res. Int. 2021, 140, 109852. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Ramos, M.J.; Razola-Díaz, M.d.C.; Verardo, V.; Gómez-Caravaca, A.M. Comparison between Ultrasonic Bath and Sonotrode Extraction of Phenolic Compounds from Mango Peel By-Products. Horticulturae 2022, 8, 1014. [Google Scholar] [CrossRef]
- Peng, D.; Zahid, H.F.; Ajlouni, S.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Profiling of Australian Mango Peel By-Product Polyphenols and Their Potential Antioxidant Activities. Processes 2019, 7, 764. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.M.; Guo, X.; Fu, X.; Zhou, L.; Chen, Y.; Zhu, Y.; Yan, H.; Liu, R.H. Comparative Assessment of Phenolic Content and in Vitro Antioxidant Capacity in the Pulp and Peel of Mango Cultivars. Int. J. Mol. Sci. 2015, 16, 13507–13527. [Google Scholar] [CrossRef]
- Gąsecka, M.; Siwulski, M.; Magdziak, Z.; Budzyńska, S.; Stuper-Szablewska, K.; Niedzielski, P.; Mleczek, M. The Effect of Drying Temperature on Bioactive Compounds and Antioxidant Activity of Leccinum scabrum (Bull.) Gray and Hericium erinaceus (Bull.) Pers. J. Food Sci. Technol. 2020, 57, 513–525. [Google Scholar] [CrossRef]
- Miletić, N.; Mitrović, O.; Popović, B.; Nedović, V.; Zlatković, B.; Kandić, M. Polyphenolic Content and Antioxidant Capacity in Fruits of Plum (Prunus domestica L.) Cultivars “Valjevka” and “Mildora” as Influenced by Air Drying. J. Food Qual. 2013, 36, 229–237. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Ji, W.; Meng, Q.; Ding, L.; Wang, F.; Dong, J.; Zhou, G.; Wang, B. Measurement and Correlation of the Solubility of Caffeic Acid in Eight Mono and Water + ethanol Mixed Solvents at Temperatures from (293.15 to 333.15) K. J. Mol. Liq. 2016, 224, 1275–1281. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Kustrin, E.; Morton, D.W. Phenolic Acids Contribution to Antioxidant Activities and Comparative Assessment of Phenolic Content in Mango Pulp and Peel. S. Afr. J. Bot. 2018, 116, 158–163. [Google Scholar] [CrossRef]
- Cuevas-Valenzuela, J.; González-Rojas, Á.; Wisniak, J.; Apelblat, A.; Pérez-Correa, J.R. Solubility of (+)-Catechin in Water and Water-Ethanol Mixtures within the Temperature Range 277.6–331.2K: Fundamental Data to Design Polyphenol Extraction Processes. Fluid Ph. Equilibria 2014, 382, 279–285. [Google Scholar] [CrossRef]
- Soetaredjo, F.E.; Ismadji, S.; Yuliana Liauw, M.; Angkawijaya, A.E.; Ju, Y.-H. Catechin Sublimation Pressure and Solubility in Supercritical Carbon Dioxide. Fluid Ph. Equilibria 2013, 358, 220–225. [Google Scholar] [CrossRef]
- Kawamoto, Y.; Suidou, Y.; Suetsugu, T.; Takamizu, A.; Tanaka, M.; Hoshino, M.; Diono, W.; Kanda, H.; Goto, M. Functional Ingredients Extraction from Citrus Genkou by Supercritical Carbon Dioxide. Asian J. Appl. Sci. 2016, 4, 833–838. [Google Scholar]
- Abdel-Aty, A.M.; Salama, W.H.; Hamed, M.B.; Fahmy, A.S.; Mohamed, S.A. Phenolic-Antioxidant Capacity of Mango Seed Kernels: Therapeutic Effect against Viper Venoms. Rev. Bras. Farmacogn. 2018, 28, 594–601. [Google Scholar] [CrossRef]
- Ajila, C.M.; Bhat, S.G.; Prasada Rao, U.J.S. Valuable Components of Raw and Ripe Peels from Two Indian Mango Varieties. Food Chem. 2007, 102, 1006–1011. [Google Scholar] [CrossRef]
- Umbreen, H.; Arshad, M.U.; Saeed, F.; Bhatty, N.; Hussain, A.I. Probing the Functional Potential of Agro-Industrial Wastes in Dietary Interventions. J. Food Process. Preserv. 2015, 39, 1665–1671. [Google Scholar] [CrossRef]
- Abdul Aziz, N.A.; Wong, L.M.; Bhat, R.; Cheng, L.H. Evaluation of Processed Green and Ripe Mango Peel and Pulp Flours (Mangifera indica Var. Chokanan) in Terms of Chemical Composition, Antioxidant Compounds and Functional Properties. J. Sci. Food Agric. 2012, 92, 557–563. [Google Scholar] [CrossRef]
- Serna-Cock, L.; García-Gonzales, E.; Torres-León, C. Agro-Industrial Potential of the Mango Peel Based on Its Nutritional and Functional Properties. Food Rev. Int. 2016, 32, 364–376. [Google Scholar] [CrossRef]
- Adiletta, G.; Zampella, L.; Coletta, C.; Petriccione, M. Chitosan Coating to Preserve the Qualitative Traits and Improve Antioxidant System in Fresh Figs (Ficus carica L.). Agriculture 2019, 9, 84. [Google Scholar] [CrossRef]
- Sáez, G.T.; Están-Capell, N. Antioxidant Enzymes. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 288–294. ISBN 978-3-662-46875-3. [Google Scholar]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A Comprehensive Review on Tyrosinase Inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic Potentials of Superoxide Dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Contesini, F.J.; De Alencar Figueira, J.; Kawaguti, H.Y.; De Barros Fernandes, P.C.; De Oliveira Carvalho, P.; Da Graça Nascimento, M.; Sato, H.H. Potential Applications of Carbohydrases Immobilization in the Food Industry. Int. J. Mol. Sci. 2013, 14, 1335–1369. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, U.; Sohail, M.; Ghanemi, A. Cellulases: From Bioactivity to a Variety of Industrial Applications. Biomimetics 2021, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.B.; Abraham, A.; Mathew, A.K.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of Microbial Enzymes in Food Industry. Food Technol. Biotechnol. 2018, 56, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Brugnari, T.; Braga, D.M.; dos Santos, C.S.A.; Torres, B.H.C.; Modkovski, T.A.; Haminiuk, C.W.I.; Maciel, G.M. Laccases as Green and Versatile Biocatalysts: From Lab to Enzyme Market—An Overview. Bioresour. Bioprocess. 2021, 8, 131. [Google Scholar] [CrossRef]
- Eckert, R.L.; Kaartinen, M.T.; Nurminskaya, M.; Belkin, A.M.; Colak, G.; Johnson, G.V.W.; Mehta, K. Transglutaminase Regulation of Cell Function. Physiol. Rev. 2014, 94, 383–417. [Google Scholar] [CrossRef] [PubMed]
- Amirdivani, S.; Khorshidian, N.; Fidelis, M.; Granato, D.; Koushki, M.R.; Mohammadi, M.; Khoshtinat, K.; Mortazavian, A.M. Effects of Transglutaminase on Health Properties of Food Products. Curr. Opin. Food Sci. 2018, 22, 74–80. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental Antimicrobial Resistance and Its Drivers: A Potential Threat to Public Health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Braz, V.S.; Melchior, K.; Moreira, C.G. Escherichia Coli as a Multifaceted Pathogenic and Versatile Bacterium. Front. Cell. Infect. Microbiol. 2020, 10, 548492. [Google Scholar] [CrossRef]
- Wu, X.; Yang, L.; Wu, Y.; Li, H.; Shao, B. Spread of Multidrug-Resistant Pseudomonas Aeruginosa in Animal-Derived Foods in Beijing, China. Int. J. Food Microbiol. 2023, 403, 110296. [Google Scholar] [CrossRef]
- Bottone, E.J. Bacillus cereus, a Volatile Human Pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Mousavi, B.; Hedayati, M.; Hedayati, N.; Ilkit, M.; Syedmousavi, S. Aspergillus Species in Indoor Environments and Their Possible Occupational and Public Health Hazards. Curr. Med. Mycol. 2016, 2, 36–42. [Google Scholar] [CrossRef]
- Jawhara, S. Healthy Diet and Lifestyle Improve the Gut Microbiota and Help Combat Fungal Infection. Microorganisms 2023, 11, 1556. [Google Scholar] [CrossRef] [PubMed]
- Allemailem, K.S. Antimicrobial Potential of Naturally Occurring Bioactive Secondary Metabolites. J. Pharm. Bioallied Sci. 2021, 13, 155–162. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef]
- Jabin, T.; Kamal, S.; Islam, S.; Hasan Razu, M.; Kumar Paul, G.; Karmaker, P.; Huda, M.; Rahman, M.; Moniruzzaman, M.; Salah Uddin, M.; et al. Effect of Gamma Irradiation on Chemical Composition, Antioxidant Activity, Antibacterial Activity, Shelf Life, and Cytotoxicity in the Peels of Two Mango Varieties Grown in Bangladesh. Arab. J. Chem. 2023, 16, 104708. [Google Scholar] [CrossRef]
- Falusi, V.; Adesina, I.; Aladejimokun, A.; Elehinafe, T. Phytochemical Screening and Antibacterial Activity of Methanolic Extracts of Ripe and Unripe Peels of Mango (Mangifera indica L.). JALSI 2017, 14, 1–7. [Google Scholar] [CrossRef]
- El-Desoukey, R.M.A.; Aljor, N.M.; Alaotibi, A.D. The Phytochemical and Antimicrobial Effect of Mango (Mangifera indica L.) Peel Extracts on Some Animal Pathogens as Eco-Friendly. Acta Sci. Microbiol. 2020, 3, 34–39. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.-E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.-C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing Microbial Infections with Natural Phenolic Compounds. Future Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus aureus Clinical Strains. BioMed Res. Int. 2018, 2018, e7413504. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.-M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef] [PubMed]
- Kabir, F.; Katayama, S.; Tanji, N.; Nakamura, S. Antimicrobial Effects of Chlorogenic Acid and Related Compounds. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 359–365. [Google Scholar] [CrossRef]
- Ghudhaib, K.K.; Hanna, E.R.; Jawad, A.H. Effect of Ellagic Acid on Some Types of Pathogenic Bacteria. JNUS 2010, 13, 79–85. [Google Scholar] [CrossRef]
- Hassan, S.; Hamed, S.; Almuhayawi, M.; Hozzein, W.; Selim, S.; AbdElgawad, H. Bioactivity of Ellagic Acid and Velutin: Two Phenolic Compounds Isolated from Marine Algae. Egypt. J. Bot. 2021, 61, 219–231. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Keyvani-Ghamsari, S.; Rahimi, M.; Khorsandi, K. An Update on the Potential Mechanism of Gallic Acid as an Antibacterial and Anticancer Agent. Food Sci. Nutr. 2023, 11, 5856–5872. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Brown, A.C. Applications of Catechins in the Treatment of Bacterial Infections. Pathogens 2021, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ding, S.; Fei, Y.; Liu, G.; Jang, H.; Fang, J. Antimicrobial Activity of Anthocyanins and Catechins against Foodborne Pathogens Escherichia coli and Salmonella. Food Control 2019, 106, 106712. [Google Scholar] [CrossRef]
- Iranshahi, M.; Rezaee, R.; Parhiz, H.; Roohbakhsh, A.; Soltani, F. Protective Effects of Flavonoids against Microbes and Toxins: The Cases of Hesperidin and Hesperetin. Life Sci. 2015, 137, 125–132. [Google Scholar] [CrossRef]
- Choi, S.-S.; Lee, S.-H.; Lee, K.-A. A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef]
- Al-Majmaie, S.; Nahar, L.; Sharples, G.P.; Wadi, K.; Sarker, S.D. Isolation and Antimicrobial Activity of Rutin and Its Derivatives from Ruta chalepensis (Rutaceae) Growing in Iraq. Rec. Nat. Prod. 2019, 13, 64–70. [Google Scholar] [CrossRef]
- Singh, M.; Govindarajan, R.; Rawat, A.K.S.; Khare, P.B. Antimicrobial Flavonoid Rutin from Pteris vittata L. Against Pathogenic Gastrointestinal Microflora. Am. Fern J. 2008, 98, 98–103. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.-H.; Arshad, M.U.; Sultan, M.T. Mangiferin: A Natural Miracle Bioactive Compound against Lifestyle Related Disorders. Lipids Health Dis. 2017, 16, 84. [Google Scholar] [CrossRef]
- Zafra Ciprián, D.I.; Nevárez Moorillón, G.V.; Soto Simental, S.; Guzmán Pantoja, L.E.; López Hernández, L.H.; Santiago Castro, J.T.; Villalobos Delgado, L.H. Ataulfo Mango (Mangifera indica L.) Peel Extract as a Potential Natural Antioxidant in Ground Beef. Processes 2023, 11, 1772. [Google Scholar] [CrossRef]
- Parvez, G.M.; Rana, M.M.; Jahan, E.N.; Mosaddik, D.A. Alternation of Antimicrobial Potential of Mango Peel and Pulp after Formalin Treatment against Six Bacteria. J. Pharmacogn. Phytochem. 2016, 5, 158–161. [Google Scholar]
- Ribeiro, A.C.B.; Cunha, A.P.; da Silva, L.M.R.; Mattos, A.L.A.; de Brito, E.S.; de Souza Filho, M.d.S.M.; de Azeredo, H.M.C.; Ricardo, N.M.P.S. From Mango By-Product to Food Packaging: Pectin-Phenolic Antioxidant Films from Mango Peels. Int. J. Biol. Macromol. 2021, 193, 1138–1150. [Google Scholar] [CrossRef]
- Vega-Vega, V.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Bernal-Mercado, A.T.; Gonzalez-Aguilar, G.A.; Ruiz-Cruz, S.; Moctezuma, E.; Siddiqui, M.W.; Ayala-Zavala, J.F. Antimicrobial and Antioxidant Properties of Byproduct Extracts of Mango Fruit. JABFQ 2013, 86, 205–211. [Google Scholar] [CrossRef]
- Rakholiya, K.; Kaneria, M.; Desai, D.; Chanda, S. Antimicrobial Activity of Decoction Extracts of Residual Parts (Seed and Peels) of Mangifera indica L. Var. Kesar against Pathogenic and Food Spoilage Microorganism; Formatex Research Center: Torremolinos-Malaga, Spain, 2013; pp. 850–856. [Google Scholar]
- Oliveira, S.M.S.D.; Falcão-Silva, V.S.; Siqueira-Junior, J.P.; Costa, M.J.D.C.; Diniz, M.D.F.F.D.M. Modulation of Drug Resistance in Staphylococcus aureus by Extract of Mango (Mangifera indica L., Anacardiaceae) Peel. Rev. Bras. Farmacogn. 2011, 21, 190–193. [Google Scholar] [CrossRef]

| Phenolic Compound | Content (µg PC/g DW) | Content (µg PC/g FW) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SFE (CO2 + EtOH) | SE (EtOH) | UAE (EtOH) | UAE (H2O) | HAE (H2O) | SE (EtOH) | UAE (EtOH) | UAE (H2O) | HAE (H2O) | ||
| Xanthones | ||||||||||
| Mangiferin | 0.15 ± 0.01 a | 1.73 ± 0.03 c | 0.24 ± 0.01 a | 39.74 ± 0.54 d | 60.43 ± 0.72 b | 3.86 ± 0.06 c | 24.87 ± 0.41 d | - | 1.36 ± 0.02 c | |
| Phenolic acids | ||||||||||
| Caffeic acid | 2.80 ± 0.12 c | 6.60 ± 0.24 a | 2.86 ± 0.01 c | 5.11 ± 0.12 a | 1.81 ± 0.03 c | 0.56 ± 0.01 d | 0.25 ± 0.01 d | - | 0.08 ± 0.00 b | |
| Chlorogenic acid | 2.54 ± 0.06 a | 26.54 ± 0.17 c | 4.69 ± 0.04 a | 10.82 ± 0.09 b | - | 3.06 ± 0.02 a | 3.04 ± 0.04 a | - | - | |
| Ellagic acid | 37.00 ± 0.17 a | 310.00 ± 1.32 c | 96.94 ± 0.65 d | 97.23 ± 1.28 d | 50.37 ± 0.64 e | 531.98 ± 4.09 b | 268.89 ± 1.41c | 539.42 ± 3.50 b | 324.49 ± 3.02 c | |
| Gallic acid | 58.24 ± 1.23 c | 373.53 ± 0.42 a | 71.31 ± 0.53 d | 405.72 ± 0.75 a | 370.12 ± 2.72 a | 92.75 ± 0.51 d | 37.59 ± 0.55 c | 8.03 ± 0.12 b | 10.68 ± 0.04 b | |
| Flavonoids | ||||||||||
| Catechin | 15.35 ± 0.73 c | 109.39 ± 0.6 a | 67.59 ± 0.27 d | 100.13 ± 0.44 a | 46.59 ± 0.16 d | 31.91 ± 0.12 d | 1.95 ± 0.02 e | - | 0.38 ± 0.01 b | |
| Hesperidin/Neohesperidin | 0.23 ± 0.01 a | 2.12 ± 0.04 b | 0.59 ± 0.01 c | 0.88 ± 0.02 c | 2.38 ± 0.03 b | 1.45 ± 0.02 d | 2.67 ± 0.01 b | 0.62 ± 0.02 c | 0.69 ± 0.02 c | |
| Rutin | - | - | - | 0.80 ± 0.03 | - | - | - | - | - | |
| Total content of analyzed phenolic acids | 101.08 ± 1.58 | 716.67 ± 2.14 | 175.80 ± 1.23 | 518.89 ± 2.24 | 422.30 ± 3.38 | 628.35 ± 4.63 | 309.77 ± 2.00 | 547.45 ± 3.62 | 335.24 ± 3.07 | |
| Total content of analyzed flavonoids | 15.57 ± 0.74 | 111.51 ± 0.40 | 68.18 ± 0.28 | 101.81 ± 0.49 | 48.98 ± 0.19 | 33.37 ± 0.15 | 4.62 ± 0.03 | 0.62 ± 0.02 | 1.07 ± 0.03 | |
| Total content of analyzed phenolic compounds | 116.80 ± 2.32 | 829.92 ± 2.57 | 244.22 ± 1.52 | 660.43 ± 3.24 | 531.71 ± 4.29 | 665.58 ± 4.84 | 339.26 ± 2.44 | 548.07 ± 3.36 | 337.67 ± 3.12 | |
| Activity (U/g protein ± SD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | α-Amylase | Cellulase | Glucoamylase | Laccase | Lipase | Peroxidase | PPO | Protease | SOD | TGM |
| Dried mango peels | ||||||||||
| SFE (CO2 + EtOH) | 272.78 ± 7.91 d | 37.93 ± 2.69 a | 303.04 ± 15.12 a | 154.23 ± 9.65 b | 2155.15 ± 34.84 d | 0.17 ± 0.00 a | 329,109.98 ± 370.77 d | 5.43 ± 0.08 a | 12,400.67 ± 185.61 d | 1.05 ± 0.00 a |
| SE (EtOH) | 66.92 ± 4.85 c | 72.63 ± 4.23 a | 206.00 ± 16.3 a | 36.08 ± 1.74 a | 5502.79 ± 102.31 b | 0.01 ± 0.00 a | 99,992.16 ± 143.32 c | 6.19 ± 0.31 a | 9368.46 ± 42.63 b | 3.88 ± 0.02 d |
| UAE (EtOH) | 429.08 ± 12.88 e | 124.15 ± 9.63d | 260.77 ± 6.33 a | 173.23 ± 5.32 b | 1505.90 ± 34.65 a | 0.19 ± 0.01 a | 14,036.01 ± 125.32 a | 3.08 ± 0.51b | 106,502.73 ± 421.05 a | 1.85 ± 0.01 a |
| UAE (H2O) | 110.36 ± 5.64 d | 132.86 ± 9.61d | 232.99 ± 8.51 a | 35.05 ± 0.95 a | 2885.41 ± 38.21 d | 0.06 ± 0.00 a | 122,938.17 ± 531.61 c | 2.42 ± 0.09 b | 17,302.85 ± 105.92 d | 1.27 ± 0.04 a |
| HAE (H2O) | 9.94 ± 0.52 a | 122.51 ± 10.32 d | 592.60 ± 22.16 b | 30.78 ± 0.75 a | 18,643.62 ± 86.96 c | - | 407,622.96 ± 540.49 b | - | 47,844.09 ± 103.61 c | 9.76 ± 0.02 b |
| Fresh mango peels | ||||||||||
| SE (EtOH) | 1372.05 ± 53.65 b | 22.97 ± 3.51 a | 1375.13 ± 81.19 c | 41.41 ± 2.36 a | 3551.50 ± 58.45 e | 0.01 ± 0.00 a | 106,299.10 ± 654.61 c | 8.05 ± 0.51 c | 31,349.82 ± 205.15 c | 10.06 ± 0.08 b |
| UAE (EtOH) | 226.39 ± 9.71 d | 38.98 ± 2.15 a | 584.46 ± 27.77 b | 124.74 ± 5.54 b | 1160.53 ± 44.99 a | 0.14 ± 0.01 a | 27,350.05 ± 259.28 a | 2.56 ± 0.25 b | 95,360.96 ± 329.58 a | 1.86 ± 0.03 a |
| UAE (H2O) | 414.98 ± 25.91 e | 238.14 ± 12.19 b | 606.13 ± 32.84 b | 228.88 ± 8.13 c | 1847.06 ± 21.62 a | 0.91 ± 0.05 b | 342,415.39 ± 456.32 d | 1.69 ± 0.23 b | 7445.10 ± 89.51 b | 2.99 ± 0.03 d |
| HAE (H2O) | 230.26 ± 21.71 d | 460.42 ± 18.21 c | 665.78 ± 25.48 b | 53.24 ± 0.58 a | 5189.92 ± 44.21 b | - | 430,837.99 ± 653.94 b | - | 30,210.78 ± 213.62 c | 120.31 ± 0.21 c |
| Sample | Zone of Inhibition (mm ± SD) | |||
|---|---|---|---|---|
| Gram-Positive Bacteria | Gram-Negative Bacteria | |||
| E. coli | P. aeruginosa | B. cereus | S. aureus | |
| Dried mango peel | ||||
| SFE (CO2 + EtOH) | 17 ± 1 | 10 ± 2 | 14 ± 1 | 10 ± 2 |
| SE (EtOH) | 18 ± 1 | 14 ± 1 | 18 ± 0 | 15 ± 1 |
| UAE (EtOH) | 19 ± 1 | 14 ± 2 | 16 ± 2 | 14 ± 1 |
| UAE (H2O) | 14 ± 2 | 7 ± 1 | 10 ± 1 | 9 ± 1 |
| HAE (H2O) | 12 ± 1 | 9 ± 1 | 12 ± 1 | 7 ± 1 |
| Fresh mango peel | ||||
| SE (EtOH) | 18 ± 2 | 16 ± 2 | 19 ± 1 | 16 ± 1 |
| UAE (EtOH) | 21 ± 1 | 16 ± 1 | 22 ± 2 | 15 ± 2 |
| UAE (H2O) | 17 ± 2 | 10 ± 1 | 15 ± 1 | 11 ± 1 |
| HAE (H2O) | 10 ± 1 | 8 ± 1 | 13 ± 1 | 7 ± 0 |
| Ciprofloxacin | 43 ± 1 | 55 ± 0 | 52 ± 1 | 41 ± 1 |
| SFE (CO2 + EtOH) | SE (EtOH) | UAE (EtOH) | |||
|---|---|---|---|---|---|
| Total phenolic content (mg GAE/g DW) | 1.7 ± 0.0 | 25.0 ± 0.4 | 7.9 ± 0.2 | ||
| Proanthocyanidin content (mg PAC/g DW) | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.0 | ||
| Phenolic compounds present in high concentrations | Chlorogenic acid, ellagic acid, gallic acid, catechin | ||||
| Antioxidant activity (% inhibition) | 41.4 ± 1.3 | 59.9 ± 1.8 | 82.4 ± 1.8 | ||
| Antibacterial potential (MIC90 after 24 h) | Gram-negative bacteria | E. coli | 2.5 | 1.0 | 1.0 |
| P. aeruginosa | 2.5 | 2.5 | 2.5 | ||
| Gram-positive bacteria | B. cereus | 0.5 | 0.25 | 0.25 | |
| S. aureus | 2.5 | 1.0 | 0.5 | ||
| Total protein content (mg protein/g DW) | 0.41 ± 0.04 | 2.68 ± 0.06 | 2.95 ± 0.10 | ||
| Enzymes present in their highly active form | α-Amylase, cellulase, glucoamylase, laccase, lipase, PPO, SOD | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kučuk, N.; Primožič, M.; Kotnik, P.; Knez, Ž.; Leitgeb, M. Mango Peels as an Industrial By-Product: A Sustainable Source of Compounds with Antioxidant, Enzymatic, and Antimicrobial Activity. Foods 2024, 13, 553. https://doi.org/10.3390/foods13040553
Kučuk N, Primožič M, Kotnik P, Knez Ž, Leitgeb M. Mango Peels as an Industrial By-Product: A Sustainable Source of Compounds with Antioxidant, Enzymatic, and Antimicrobial Activity. Foods. 2024; 13(4):553. https://doi.org/10.3390/foods13040553
Chicago/Turabian StyleKučuk, Nika, Mateja Primožič, Petra Kotnik, Željko Knez, and Maja Leitgeb. 2024. "Mango Peels as an Industrial By-Product: A Sustainable Source of Compounds with Antioxidant, Enzymatic, and Antimicrobial Activity" Foods 13, no. 4: 553. https://doi.org/10.3390/foods13040553
APA StyleKučuk, N., Primožič, M., Kotnik, P., Knez, Ž., & Leitgeb, M. (2024). Mango Peels as an Industrial By-Product: A Sustainable Source of Compounds with Antioxidant, Enzymatic, and Antimicrobial Activity. Foods, 13(4), 553. https://doi.org/10.3390/foods13040553







