Sheep’s Second Cheese Whey Edible Coatings with Oregano and Clary Sage Essential Oils Used as Sustainable Packaging Material in Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Sheep Second Cheese Whey Powders
2.2. Manufacture of the Coatings with Sheep Second Cheese Whey Powder
2.3. Manufacture of Cheeses
- C: control cheese without coating;
- N: control cheese with natamycin;
- WC: cheese with SCW:WPI coating;
- WCO: cheese with SCW:WPI coating containing oregano essential oil;
- WCS: cheese with SCW:WPI coating containing clary sage essential oil.
2.4. Physicochemical Analysis
2.4.1. pH and Titratable Acidity
2.4.2. Water Activity
2.4.3. Color Parameters
2.4.4. Texture Parameters
2.5. Microbiological Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madureira, A.; Pereira, C.; Truszkowska, K.; Gomes, A.; Pintado, M.; Malcata, F. Survival of probiotic bacteria in a whey cheese vector submitted to environmental conditions prevailing in the gastrointestinal tract. Int. Dairy J. 2005, 15, 921–927. [Google Scholar] [CrossRef]
- Maragkoudakis, P.; Vendramin, V.; Bovo, B.; Treu, L.; Corich, V.; Giacomini, A. Potential use of scotta, the by-product of the ricotta cheese manufacturing process, for the production of fermented drinks. J. Dairy Res. 2016, 83, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy by-products: A review on the valorization of whey and second cheese whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Secchi, N.; Giunta, D.; Pretti, L.; García, M.R.; Roggio, T.; Mannazzu, I.; Catzeddu, P. Bioconversion of ovine scotta into lactic acid with pure and mixed cultures of lactic acid bacteria. J. Ind. Microbiol. Biotechnol. 2012, 39, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445, 385–396. [Google Scholar] [CrossRef]
- Pereira, C.D.; Diaz, O.; Cobos, A. Valorization of by-products from ovine cheese manufacture: Clarification by thermocalcic precipitation/microfiltration before ultrafiltration. Int. Dairy J. 2002, 12, 773–783. [Google Scholar] [CrossRef]
- Mariotti, M.; Fratini, F.; Cerri, D.; Andreuccetti, V.; Giglio, R.; Angeletti, F.G.S.; Turchi, B. Use of fresh scotta whey as an additive for alfalfa silage. Agronomy 2020, 10, 365. [Google Scholar] [CrossRef]
- Minhalma, M.; Magueijo, V.; Queiroz, D.P.; de Pinho, M.N. Optimization of “Serpa” cheese whey nanofiltration for efluent minimization and by-products recovery. J. Environ. Manag. 2007, 82, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Monti, L.; Donati, E.; Zambrini, A.V.; Contarini, G. Application of membrane technologies to bovine Ricotta cheese exhausted whey (scotta). Int. Dairy J. 2018, 85, 121–128. [Google Scholar] [CrossRef]
- Sansonetti, S.; Curcio, S.; Calabro, V.; Iorio, G. Bio-ethanol production by fermentation of ricotta cheese whey as an effective alternative non-vegetable source. Biomass Bioenergy 2009, 33, 1687–1692. [Google Scholar] [CrossRef]
- Macedo, A.; Duarte, E.; Fragoso, R. Assessment of the performance of three ultrafiltration membranes for fractionation of ovine second cheese whey. Int. Dairy J. 2015, 48, 31–37. [Google Scholar] [CrossRef]
- Zoppellari, F.; Bardi, L. Production of bioethanol from effluents of the dairy industry by Kluyveromyces marxianus. New Biotechnol. 2013, 30, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Tesfaw, A. The current trends of bioethanol production from cheese whey using yeasts: Biological and economical perspectives. Front. Energy Res. 2023, 11, 1183035. [Google Scholar] [CrossRef]
- de Giorgi, S.; Raddadi, N.; Fabbri, A.; Gallina Toschi, T.; Fava, F. Potential use of ricotta cheese whey for the production of lactobionic acid by Pseudomonas taetrolens strains. New Biotechnol. 2018, 42, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Bosco, F.; Cirrincione, S.; Carletto, R.; Marmo, L.; Chiesa, F.; Mazzoli, R.; Pessione, E. PHA production from cheese whey and “scotta”: Comparison between a consortium and a pure culture of leuconostoc mesenteroides. Microorganisms 2021, 9, 2426. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pepe, G.; Ventre, G.; Pagano, F.; Conte, G.M.; Ostacolo, C.; Manfra, M.; Tenore, G.C.; Russo, M.; Novellino, E.; et al. Detailed peptide profiling of “Scotta”: From a dairy waste to a source of potential health-promoting compounds. Dairy Sci. Technol. 2016, 96, 763–771. [Google Scholar] [CrossRef]
- Monari, S.; Ferri, M.; Russo, C.; Prandi, B.; Tedeschi, T.; Bellucci, P.; Zambrini, A.V.; Donati, E.; Tassoni, A. Enzymatic production of bioactive peptides from scotta, an exhausted by-product of ricotta cheese processing. PLoS ONE 2019, 14, e0226834. [Google Scholar] [CrossRef]
- Coronas, R.; Zara, G.; Gallo, A.; Rocchetti, G.; Lapris, M.; Petretto, G.L.; Zara, S.; Fancello, F.; Mannazzu, I. Propionibacteria as promising tools for the production of pro-bioactive scotta: A proof-of-concept study. Front. Microbiol. 2023, 14, 1223741. [Google Scholar] [CrossRef]
- Chessa, L.; Paba, A.; Daga, E.; Caredda, M.; Comunian, R. Optimization of scotta as growth medium to preserve biodiversity and maximise bacterial cells concentration of natural starter cultures for Pecorino Romano PDO cheese. FEMS Microbiol. Lett. 2020, 367, fnaa110. [Google Scholar] [CrossRef]
- Naziri, E.; Papadaki, E.; Savvidis, I.; Botsaris, G.; Gkatzionis, K.; Mugampoza, E.; Mantzouridou, F.T. Exploring the potential of halloumi second cheese whey for the production of lactic acid cultures for the dairy industry. Sustainability 2023, 15, 9082. [Google Scholar] [CrossRef]
- Vasilakis, G.; Karayannis, D.; Massouras, T.; Politis, I.; Papanikolaou, S. Biotechnological conversions of mizithra second cheese whey by wild-type non-conventional yeast strains: Production of yeast cell biomass, single-cell oil and polysaccharides. Appl. Sci. 2022, 12, 11471. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Oliviero, M.; Baselice, M.; Sacchi, R.; Masi, P. Valorization of second cheese whey through cultivation of extremophile microalga Galdieria sulphuraria. AIMS Environ. Sci. 2021, 8, 435–448. [Google Scholar] [CrossRef]
- Tsolcha, O.N.; Tekerlekopoulou, A.G.; Akratos, C.S.; Bellou, S.; Aggelis, G.; Katsiapi, M.; Moustaka-Gouni, M.; Vayenas, D.V. Treatment of second cheese whey effluents using a Choricystis-based system with simultaneous lipid production. J. Chem. Technol. Biotechnol. 2016, 91, 2349–2359. [Google Scholar] [CrossRef]
- Pires, A.; Gomes, D.; Noronha, J.; Díaz, O.; Cobos, A.; Pereira, C.D. Evaluation of the characteristics of sheep’s and goat’s ice cream, produced with UF concentrated second cheese whey and different starter cultures. Foods 2022, 11, 4091. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.M.E.; Pereira, C.J.D.; Freitas, A.C.; Gomes, A.M.P.; Pintado, M.M.E. Development and characterization of a novel sustainable probiotic goat whey cheese containing second cheese whey powder and stabilized with thyme essential oil and sodium citrate. Foods 2022, 11, 2698. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.R.; Pires, A.F.; Marnotes, N.G.; Gomes, D.G.; Henriques, M.F.; Pereira, C.D. Dairy by-products concentrated by ultrafiltration used as ingredients in the production of reduced fat washed curd cheese. Foods 2020, 9, 1020. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.C.A.; Eda, S.H.; Andrade, R.P.; Amorim, J.C.; Duarte, W.F. New alcoholic fermented beverages-Potentials and challenges. In Fermented Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 577–603. [Google Scholar]
- Díaz, O.; Pereira, C.D.; Cobos, A. Functional properties of ovine whey protein concentrates produced by membrane technology after clarification of cheese manufacture by-products. Food Hydrocoll. 2004, 18, 601–610. [Google Scholar] [CrossRef]
- Cruz-Diaz, K.; Cobos, Á.; Fernández-Valle, M.E.; Díaz, O.; Cambero, M.I. Characterization of edible films from whey proteins treated with heat, ultrasounds and/or transglutaminase. Application in cheese slices packaging. Food Packag. Shelf Life 2019, 22, 100397. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Optical, mechanical, and moisture sorption properties of whey protein edible films. J. Food Proc. Eng. 2019, 42, e13245. [Google Scholar] [CrossRef]
- Pérez, L.M.; Piccirilli, G.N.; Delorenzi, N.J.; Verdini, R.A. Effect of different combinations of glycerol and/or trehalose on physical and structural properties of whey protein concentrate-based edible films. Food Hydrocoll. 2016, 56, 352–359. [Google Scholar] [CrossRef]
- Papadaki, A.; Manikas, A.C.; Papazoglou, E.; Kachrimanidou, V.; Lappa, I.; Galiotis, C.; Mandala, I.; Kopsahelis, N. Whey protein films reinforced with bacterial cellulose nanowhiskers: Improving edible film properties via a circular economy approach. Food Chem. 2022, 385, 132604. [Google Scholar] [CrossRef]
- Rosseto, M.; Rigueto, C.V.T.; Alessandretti, I.; de Oliveira, R.; Raber Wohlmuth, D.A.; Loss, R.A.; Dettmer, A.; Richards, N.S.P.D.S. Whey-based polymeric films for food packaging applications: A review of recent trends. J. Sci. Food Agric. 2023, 103, 3217–3229. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.B.; Seol, K.H.; Kim, H.W.; Ham, J.S. Application of whey protein-based edible films and coatings in food industries: An updated overview. Coatings 2021, 11, 1056. [Google Scholar] [CrossRef]
- Nemati, V.; Hashempour-Baltork, F.; Sadat Gharavi-Nakhjavani, M.; Feizollahi, E.; Marangoni Júnior, L.; Mirza Alizadeh, A. Application of a whey protein edible film incorporated with cumin essential oil in cheese preservation. Coatings 2023, 13, 1470. [Google Scholar] [CrossRef]
- Fahrullah, M.; Ervandi, M.; Rosyidi, D. Characterization and antimicrobial activity of whey edible film composite enriched with clove essential oil. Trop. Anim. Sci. J. 2021, 44, 369–376. [Google Scholar] [CrossRef]
- Çakmak, H.; Özselek, Y.; Turan, O.Y.; Fıratlıgil, E.; Karbancioğlu-Güler, F. Whey protein isolate edible films incorporated with essential oils: Antimicrobial activity and barrier properties. Polym. Degrad. Stab. 2020, 179, 109285. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus-Tutal, G.; Sogut, E. Effect of whey protein edible films containing plant essential oils on microbial inactivation of sliced Kasar cheese. Food Packag. Shelf Life 2020, 26, 100567. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Socaciu, M.I.; Fogarasi, M.; Semeniuc, C.A.; Socaci, S.A.; Rotar, M.A.; Mureşan, V.; Pop, O.L.; Vodnar, D.C. Formulation and characterization of antimicrobial edible films based on whey protein isolate and tarragon essential oil. Polymers 2020, 12, 1748. [Google Scholar] [CrossRef]
- Bahram, S.; Rezaei, M.; Soltani, M.; Kamali, A.; Ojagh, S.M.; Abdollahi, M. Whey protein concentrate edible film activated with cinnamon essential oil. J. Food Proc. Pres. 2014, 38, 1251–1258. [Google Scholar] [CrossRef]
- Barba, C.; Eguinoa, A.; Maté, J.I. Preparation and characterization of β-cyclodextrin inclusion complexes as a tool of a controlled antimicrobial release in whey protein edible films. LWT 2015, 64, 1362–1369. [Google Scholar] [CrossRef]
- Robalo, J.; Lopes, M.; Cardoso, O.; Silva, A.S.; Ramos, F. Efficacy of whey protein film incorporated with portuguese green tea (Camellia sinensis L.) extract for the preservation of latin-style fresh cheese. Foods 2022, 11, 1158. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Kosma, I.; Mataragas, M.; Pappa, E.; Badeka, A.V.; Bosnea, L. Innovative intelligent cheese packaging with whey protein-based edible films containing spirulina. Sustainability 2023, 15, 13909. [Google Scholar] [CrossRef]
- Pluta-Kubica, A.; Jamróz, E.; Kawecka, A.; Juszczak, L.; Krzyściak, P. Active edible furcellaran/whey protein films with yerba mate and white tea extracts: Preparation, characterization and its application to fresh soft rennet-curd cheese. Int. J. Biol. Macromol. 2020, 155, 1307–1316. [Google Scholar] [CrossRef]
- Yangilar, F. Chitosan/whey protein (CWP) edible films efficiency for controlling mould growth and on microbiological, chemical and sensory properties during storage of göbek kashar cheese. Korean J. Food Sci. Anim. Res. 2015, 35, 216–224. [Google Scholar] [CrossRef]
- Ramos, O.L.; Silva, S.I.; Soares, J.C.; Fernandes, J.C.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Features and performance of edible films, obtained from whey protein isolate formulated with antimicrobial compounds. Food Res. Int. 2012, 45, 351–361. [Google Scholar] [CrossRef]
- Silva, S.P.M.; Teixeira, J.A.; Silva, C.C.G. Application of enterocin-whey films to reduce Listeria monocytogenes contamination on ripened cheese. Food Microbiol. 2023, 109, 104134. [Google Scholar] [CrossRef]
- Dopazo, V.; Luz, C.; Calpe, J.; Vila-Donat, P.; Rodríguez, L.; Meca, G. Antifungal properties of whey fermented by lactic acid bacteria in films for the preservation of cheese slices. Int. J. Dairy Technol. 2022, 75, 619–629. [Google Scholar] [CrossRef]
- Fierro-Corona, G.; Ruiz-López, I.R.; Ochoa-Velasco, C.E.; Hernández-Carranza, P. Effect of edible films’ application on the quality characteristics of Manchego-type cheese during storage. Food Bioprocess Technol. 2023, 16, 2910–2920. [Google Scholar] [CrossRef]
- Guimarães, A.; Ramos, Ó.; Cerqueira, M.; Venâncio, A.; Abrunhosa, L. Active whey protein edible films and coatings incorporating Lactobacillus buchneri for Penicillium nordicum control in cheese. Food Bioprocess Technol. 2020, 13, 1074–1086. [Google Scholar] [CrossRef]
- Hernández-Carranza, P.; Fierro-Corona, G.; Tapia-Maruri, D.; Ruiz-Martínez, I.; Ávila-Reyes, S.V.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. Bioactive edible films based on lab-fermented whey solution and potato starch: Characterization and storage behavior. Food Bioprocess Technol. 2023, 16, 3045–3056. [Google Scholar] [CrossRef]
- Odila Pereira, J.; Soares, J.; Sousa, S.; Madureira, A.R.; Gomes, A.; Pintado, M. Edible films as carrier for lactic acid bacteria. LWT 2016, 73, 543–550. [Google Scholar] [CrossRef]
- Sáez-Orviz, S.; Marcet, I.; Rendueles, M.; Díaz, M. Preparation of edible films with lactobacillus plantarum and lactobionic acid produced by sweet whey fermentation. Membranes 2022, 12, 115. [Google Scholar] [CrossRef]
- Cosentino, S.; Viale, S.; Deplano, M.; Fadda, M.E.; Pisano, M.B. Application of autochthonous lactobacillus strains as biopreservatives to control fungal spoilage in caciotta cheese. BioMed Res. Int. 2018, 2018, 3915615. [Google Scholar] [CrossRef]
- Gagliarini, N.; Diosma, G.; Garrote, G.L.; Abraham, A.G.; Piermaria, J. Whey protein-kefiran films as driver of probiotics to the gut. LWT 2019, 105, 321–328. [Google Scholar] [CrossRef]
- Dinika, I.; Verma, D.K.; Balia, R.; Utama, G.L.; Patel, A.R. Potential of cheese whey bioactive proteins and peptides in the development of antimicrobial edible film composite: A review of recent trends. Trends Food Sci. Technol. 2020, 103, 57–67. [Google Scholar] [CrossRef]
- Alfano, A.; D’ambrosio, S.; Cimini, D.; Falco, L.; D’Agostino, M.; Finamore, R.; Schiraldi, C. No waste from waste: Membrane-based fractionation of second cheese whey for potential nutraceutical and cosmeceutical applications, and as renewable substrate for fermentation processes development. Fermentation 2022, 8, 514. [Google Scholar] [CrossRef]
- Ramos, O.L.; Pereira, J.O.; Silva, S.I.; Fernandes, J.C.; Franco, M.I.; Lopes-da-Silva, J.A.; Pintado, M.E.; Malcata, F.X. Evaluation of antimicrobial edible coatings from a whey protein isolate base to improve the shelf life of cheese. J. Dairy Sci. 2012, 95, 6282–6292. [Google Scholar] [CrossRef]
- Ramos, O.L.; Santos, A.C.; Leão, M.V.; Pereira, J.O.; Silva, S.I.; Fernandes, J.C.; Franco, M.I.; Pintado, M.E.; Malcata, F.X. Antimicrobial activity of edible coatings prepared from whey protein isolate and formulated with various antimicrobial agents. Int. Dairy J. 2012, 25, 132–141. [Google Scholar] [CrossRef]
- Mileriene, J.; Serniene, L.; Henriques, M.; Gomes, D.; Pereira, C.; Kondrotiene, K.; Kasetiene, N.; Lauciene, L.; Sekmokiene, D.; Malakauskas, M. Effect of liquid whey protein concentrate–based edible coating enriched with cinnamon carbon dioxide extract on the quality and shelf life of Eastern European curd cheese. J. Dairy Sci. 2021, 104, 1504–1517. [Google Scholar] [CrossRef]
- Vasiliauskaite, A.; Mileriene, J.; Songisepp, E.; Rud, I.; Muizniece-Brasava, S.; Ciprovica, I.; Axelsson, L.; Lutter, L.; Aleksandrovas, E.; Tammsaar, E.; et al. Application of edible coating based on liquid acid whey protein concentrate with indigenous Lactobacillus helveticus for acid-curd cheese quality improvement. Foods 2022, 11, 3353. [Google Scholar] [CrossRef]
- Vasiliauskaite, A.; Mileriene, J.; Kasparaviciene, B.; Aleksandrovas, E.; Songisepp, E.; Rud, I.; Axelsson, L.; Muizniece-Brasava, S.; Ciprovica, I.; Paskevicius, A.; et al. Screening for antifungal indigenous lactobacilli strains isolated from local fermented milk for developing bioprotective fermentates and coatings based on acid whey protein concentrate for fresh cheese quality maintenance. Microorganisms 2023, 11, 557. [Google Scholar] [CrossRef]
- IPQ. IPQ-Portuguese Institute of Quality (NP-1598)-Cheese: Definition, Classification, Packaging and Marking; IPQ: Lisbon, Portugal, 1983. [Google Scholar]
- NP 2105:1983; Queijos. Determinação do Teor de Matéria Gorda. Técnica de Van Gulick. Processo Corrente. Direção Geral da Qualidade: Lisbon, Portugal, 1983. (In Portuguese)
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists, 16th ed.; AOAC: Rockville, MD, USA, 1997; Volume II, p. 33. [Google Scholar]
- AOAC. AOAC 920.124. Acidity for cheese. Titrimetric method. In Official Methods of Analysis of Association of Official Analytical Chemists, 17th ed.; AOAC: Rockville, MD, USA, 2002. [Google Scholar]
- IDF 117:2003; Yoghurt-Enumeration of Characteristic Microorganisms-Colony-Count Technique at 37 °C. International Organization for Standardization: Geneva, Switzerland, 2003.
- IDF 94:2004; Milk and Milk Products-Enumeration of Colony-Forming Units of Yeasts and/or Moulds—Colony-Count Technique at 25 °C. International Organization for Standardization: Geneva, Switzerland, 2004.
- Gonzalez-Fandos, E.; Sanz, S.; Olarte, C. Microbiological, physicochemical and sensory characteristics of Cameros cheese packaged under modified atmospheres. Food Microbiol. 2000, 17, 407–414. [Google Scholar] [CrossRef]
- Garnier, L.; Valence, F.; Mounier, J. Diversity and control of spoilage fungi in dairy products: An update. Microorganisms 2017, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.P.; Piras, F.; Siddi, G.; Cabras, D.; Comassi, E.; Lai, R.; McAuliffe, O.; de Santis, E.P.L.; Scarano, C. Comparison of activity of commercial protective cultures and thermophilic lactic acid bacteria against Listeria monocytogenes: A new perspective to improve the safety of sardinian PDO cheeses. Foods 2023, 12, 1182. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.L.S.M.; Araújo, G.P.; de Oliveira Ribeiro, K.; Torres, I.M.S.; de Martinis, E.C.P.; Marreto, R.N.; Alves, V. Use of encapsulated lactic acid bacteria as bioprotective cultures in fresh Brazilian cheese. Braz. J. Microbiol. 2021, 52, 2247–2256. [Google Scholar] [CrossRef] [PubMed]
- Sanna, R.; Piras, F.; Siddi, G.; Meloni, M.P.; Demontis, M.; Spanu, V.; Nieddu, G.; Cuccu, M.; de Santis, E.P.L.; Scarano, C. Use of commercial protective cultures in portioned sheep milk cheeses to control Listeria monocytogenes. Ital. J. Food Saf. 2023, 12, 10484. [Google Scholar] [CrossRef] [PubMed]
- Leite Junior, B.R.D.C.; Tribst, A.A.L. Use of nisin and bioprotective lactic cultures to extend the shelf life of sheep and goat cheese whey. Food Biosci. 2022, 50, 102096. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martín-Belloso, O. Improving the shelf life of low-fat cut cheese using nanoemulsion-based edible coatings containing oregano essential oil and mandarin fiber. Food Control 2017, 76, 1–12. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.; Bin Song, K. Development of a chicken feet protein film containing essential oils. Food Hydrocoll. 2015, 46, 208e215. [Google Scholar] [CrossRef]
- Christaki, S.; Moschakis, T.; Kyriakoudi, A.; Biliaderis, C.G.; Mourtzinos, I. Recent advances in plant essential oils and extracts: Delivery systems and potential uses as preservatives and antioxidants in cheese. Trends Food Sci. Technol. 2021, 116, 264–278. [Google Scholar] [CrossRef]
- Tarhan, Ö.; Sen, R. Heat-denatured and alcalase-hydrolyzed protein films/coatings containing marjoram essential oil and thyme extract. Food Biosci. 2022, 45, 101466. [Google Scholar] [CrossRef]
- Kheder, M.R.B.; Khedher, S.B.; Chaieb, I.; Tounsi, S.; Hammami, M. Chemical composition and biological activities of Salvia officinalis essential oil from Tunisia. EXCLI J. 2017, 16, 160–173. [Google Scholar] [CrossRef]
- Ture, H.; Eroglu, E.; Ozen, B.; Soyer, F. Effect of biopolymers containing natamycin against Aspergillus niger and Penicillium roquefortii on fresh kashar cheese. Int. J. Food Sci. Technol. 2011, 46, 154–160. [Google Scholar] [CrossRef]
- Cano Embuena, A.I.; Cháfer Nácher, M.; Chiralt Boix, A.; Molina Pons, M.P.; Borrás Llopis, M.; Beltran Martínez, M.C.; González Martínez, C. Quality of goat′ s milk cheese as affected by coating with edible chitosan-essential oil films. Int. J. Dairy Technol. 2017, 70, 68–76. [Google Scholar] [CrossRef]
- Fajardo, P.; Martins, J.T.; Fuciños, C.; Pastrana, L.; Teixeira, J.A.; Vicente, A.A. Evaluation of a chitosan-based edible film as carrier of natamycin to improve the storability of Saloio cheese. J. Food Eng. 2010, 101, 349–356. [Google Scholar] [CrossRef]
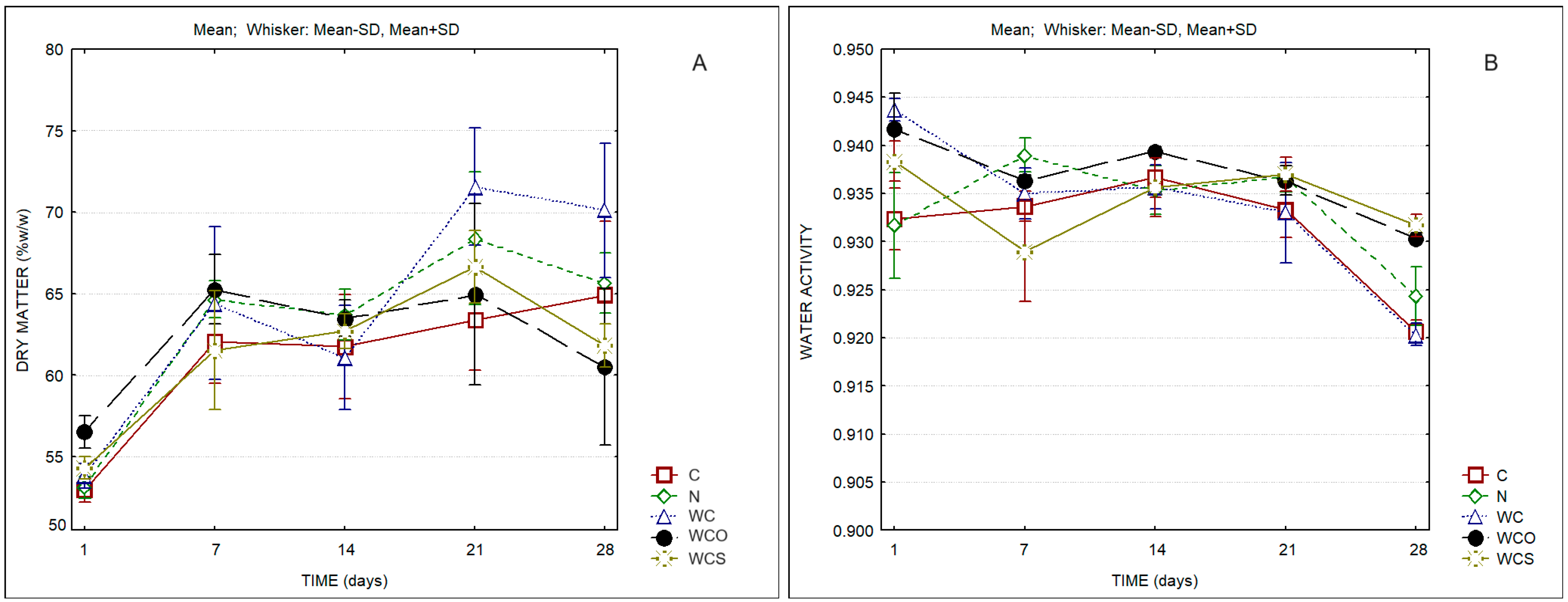
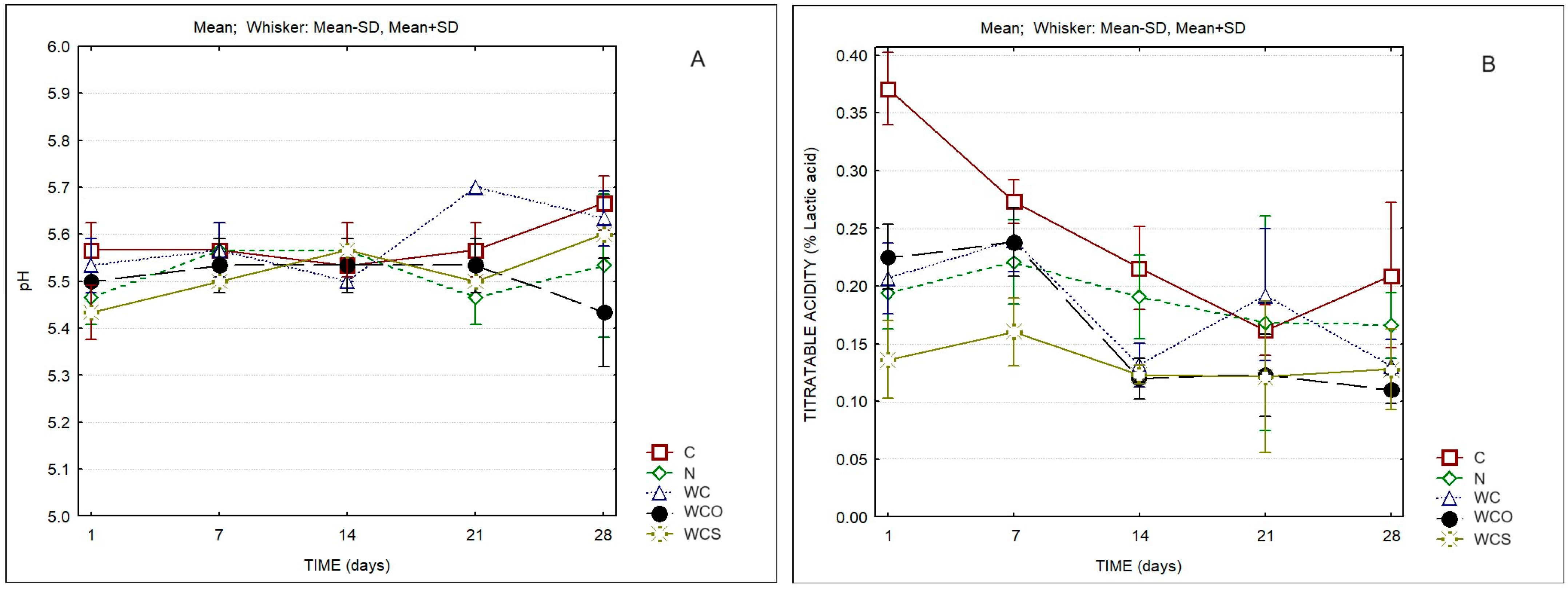
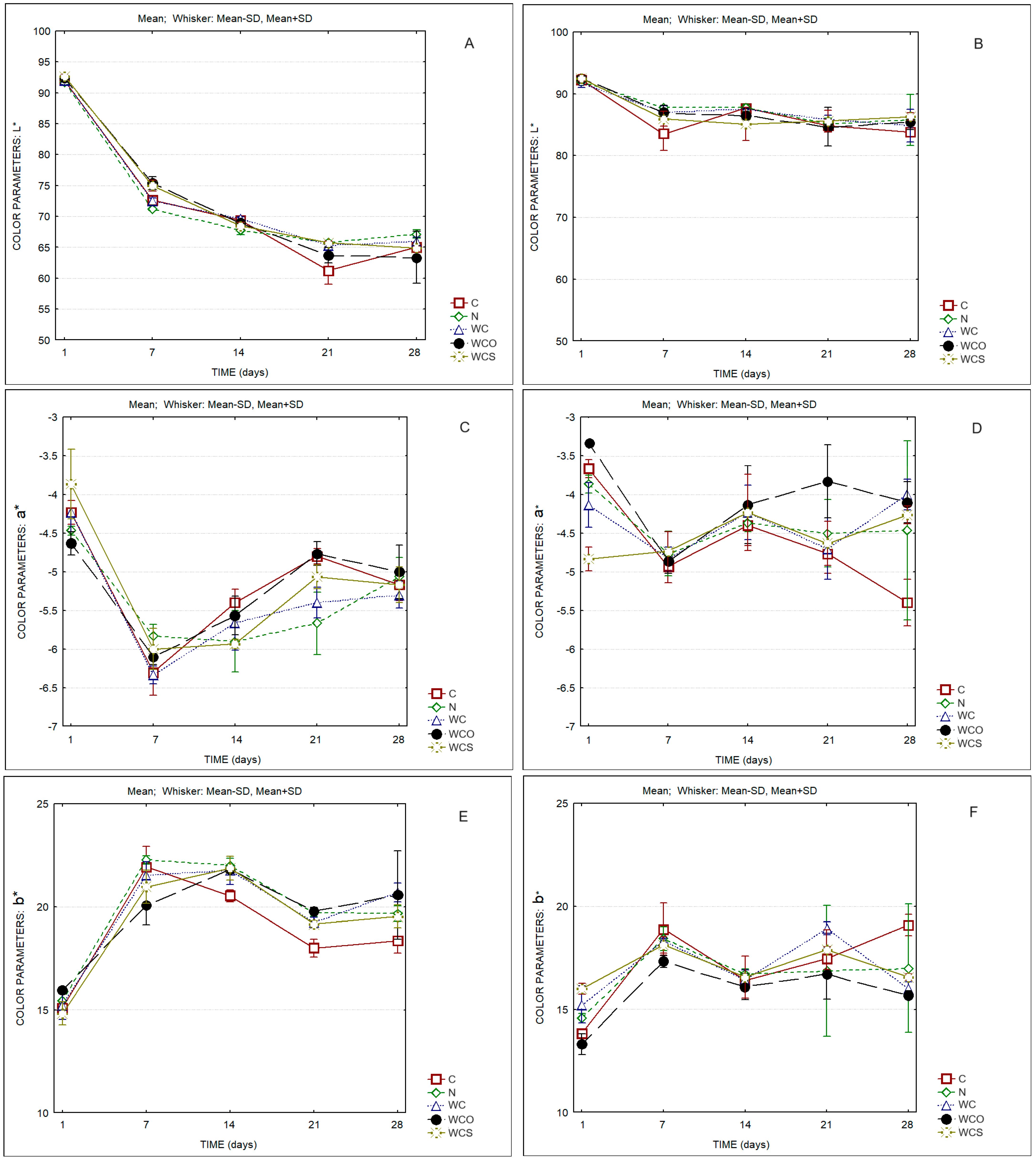

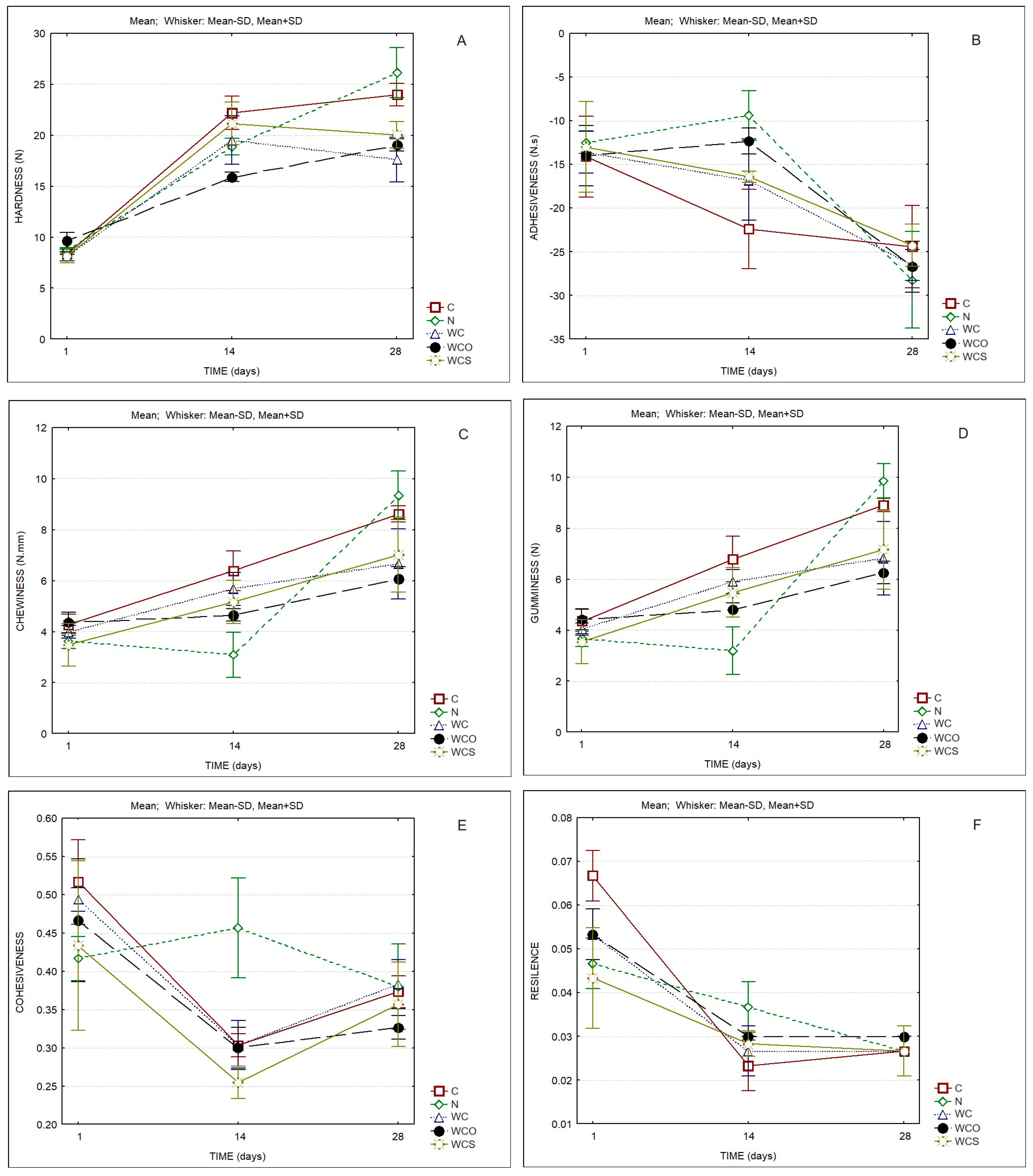
| Cheese Sample | Protein in Dry Matter (%w/w) | Fat in Dry Matter (%w/w) | Moisture in Defatted Cheese (%w/w) |
|---|---|---|---|
| C | 31.05 ± 1.08 a | 46.02 ± 1.43 a | 61.27 ± 1.40 a |
| N | 28.92 ± 1.30 a | 48.00 ± 2.16 a | 62.86 ± 0.91 a |
| WC | 30.08 ± 1.17 a | 48.07 ± 1.79 a | 62.28 ± 1.26 a |
| WCO | 31.89 ± 3.06 a | 49.26 ± 0.89 a | 60.73 ± 0.78 a |
| WCS | 29.61 ± 0.85 a | 49.07 ± 2.06 a | 62.79 ±0.57 a |
| Physicochemical Analysis | Two-Way ANOVA | ||
|---|---|---|---|
| F | p Value | ||
| Dry matter | Time | 41.42 | 0.000 |
| Product | 2.92 | 0.030 | |
| Interaction | 1.89 | 0.045 | |
| aw | Time | 48.83 | 0.000 |
| Product | 7.90 | 0.000 | |
| Interaction | 5.98 | 0.000 | |
| pH | Time | 2.91 | 0.031 |
| Product | 5.68 | 0.000 | |
| Interaction | 2.82 | 0.003 | |
| Titratable acidity | Time | 16.68 | 0.000 |
| Product | 17.35 | 0.000 | |
| Interaction | 2.67 | 0.004 | |
| ΔEab* values between products at different ripening days | |||||
| Ripening time (days) | 1 | 7 | 14 | 21 | 28 |
| Cheese samples | RIND | ||||
| N vs. C | 0.4 ± 0.7 | 1.6 ± 0.8 | 2.7 ± 1.1 | 17.9 ± 10.3 | 4.2 ± 4.9 |
| WC vs. C | 0.7 ± 0.5 | 0.3 ± 0.4 | 1.2 ± 0.9 | 19.2 ± 13.0 | 5.4 ± 5.3 |
| WCO vs. C | 0.6 ± 0.2 | 7.1 ± 6.5 | 1.0 ± 0.5 | 8.5 ± 10.2 | 15.0 ± 12.2 |
| WCS vs. C | 0.7 ± 0.7 | 3.5 ± 2.0 | 1.7 ± 1.4 | 12.7 ± 11.3 | 2.4 ± 2.4 |
| PASTE | |||||
| N vs. C | 0.3 ± 0.1 | 12.2 ± 11.7 | 0.4 ± 0.4 | 3.9 ± 4.7 | 14.6 ± 9.3 |
| WC vs. C | 1.7 ± 1.1 | 8.0 ± 8.5 | 0.2 ± 0.2 | 2.2 ± 2.9 | 9.3 ± 3.6 |
| WCO vs. C | 0.4 ± 0.4 | 11.4 ± 16.6 | 1.8 ± 2.7 | 6.5 ± 4.7 | 8.9 ± 0.6 |
| WCS vs. C | 3.2 ± 0.6 | 4.5 ± 4.4 | 5.6 ± 7.9 | 1.0 ± 1.5 | 7.4 ± 1.4 |
| ΔEab* values for the same product at different ripening days | |||||
| Cheese Sample | C | N | WC | WCO | WCS |
| Ripening time (days) | RIND | ||||
| 7 vs. 1 | 213.5 ± 15.6 | 234.5 ± 9.2 | 210.0 ± 21.1 | 155.3 ± 23.4 | 179.4 ± 26.1 |
| 14 vs. 7 | 7.5 ± 0.8 | 6.3 ± 3.0 | 4.9 ± 1.5 | 22.4 ± 5.7 | 21.6 ± 4.8 |
| 21 vs. 14 | 39.7 ± 19.1 | 16.2 ± 20.1 | 37.1 ± 24.9 | 16.7 ± 4.7 | 8.2 ± 2.5 |
| 28 vs. 21 | 7.7 ± 2.5 | 7.2 ± 9.5 | 18.9 ± 15.2 | 9.7 ± 7.4 | 0.6 ± 0.2 |
| PASTE | |||||
| 7 vs. 1 | 55.4 ± 32.0 | 16.9 ± 3.4 | 17.2 ± 4.5 | 26.0 ± 11.4 | 24.3 ± 5.3 |
| 14 vs. 7 | 14.3 ± 13.7 | 1.8 ± 0.9 | 2.7 ± 0.8 | 2.4 ± 1.3 | 3.0 ± 2.4 |
| 21 vs. 14 | 5.2 ± 2.6 | 7.3 ± 3.5 | 4.8 ± 1.6 | 3.7 ± 2.6 | 2.5 ± 1.9 |
| 28 vs. 21 | 2.8 ± 1.2 | 9.3 ± 2.3 | 8.6 ± 2.2 | 5.6 ± 5.1 | 2.2 ± 0.2 |
| Color Parameters | Two-Way ANOVA | ||
|---|---|---|---|
| Rind | F | p Value | |
| L* | Time | 1478.07 | 0.000 |
| Product | 2.71 | 0.040 | |
| Interaction | 4.74 | 0.000 | |
| a* | Time | 114.13 | 0.000 |
| Product | 2.56 | 0.050 | |
| Interaction | 3.49 | 0.000 | |
| b* | Time | 100.44 | 0.000 |
| Product | 3.07 | 0.025 | |
| Interaction | 3.63 | 0.000 | |
| Paste | |||
| L* | Time | 51.68 | 0.000 |
| Product | 1.35 | 0.264 | |
| Interaction | 1.31 | 0.227 | |
| a* | Time | 11.06 | 0.000 |
| Product | 5.44 | 0.001 | |
| Interaction | 3.09 | 0.001 | |
| b* | Time | 25.26 | 0.000 |
| Product | 3.73 | 0.010 | |
| Interaction | 1.80 | 0.059 | |
| Texture Parameters | Two-Way ANOVA | ||
|---|---|---|---|
| F | p Value | ||
| Hardness | Time | 267.79 | 0.000 |
| Product | 9.94 | 0.000 | |
| Interaction | 25.80 | 0.000 | |
| Adhesiveness | Time | 47.96 | 0.000 |
| Product | 1.28 | 0.300 | |
| Interaction | 2.45 | 0.038 | |
| Chewiness | Time | 84.76 | 0.000 |
| Product | 4.40 | 0.007 | |
| Interaction | 6.72 | 0.000 | |
| Gumminess | Time | 90.62 | 0.000 |
| Product | 4.98 | 0.004 | |
| Interaction | 7.72 | 0.000 | |
| Cohesiveness | Time | 30.28 | 0.000 |
| Product | 2.54 | 0.061 | |
| Interaction | 3.40 | 0.007 | |
| Resilience | Time | 88.87 | 0.000 |
| Product | 1.46 | 0.239 | |
| Interaction | 3.99 | 0.003 | |
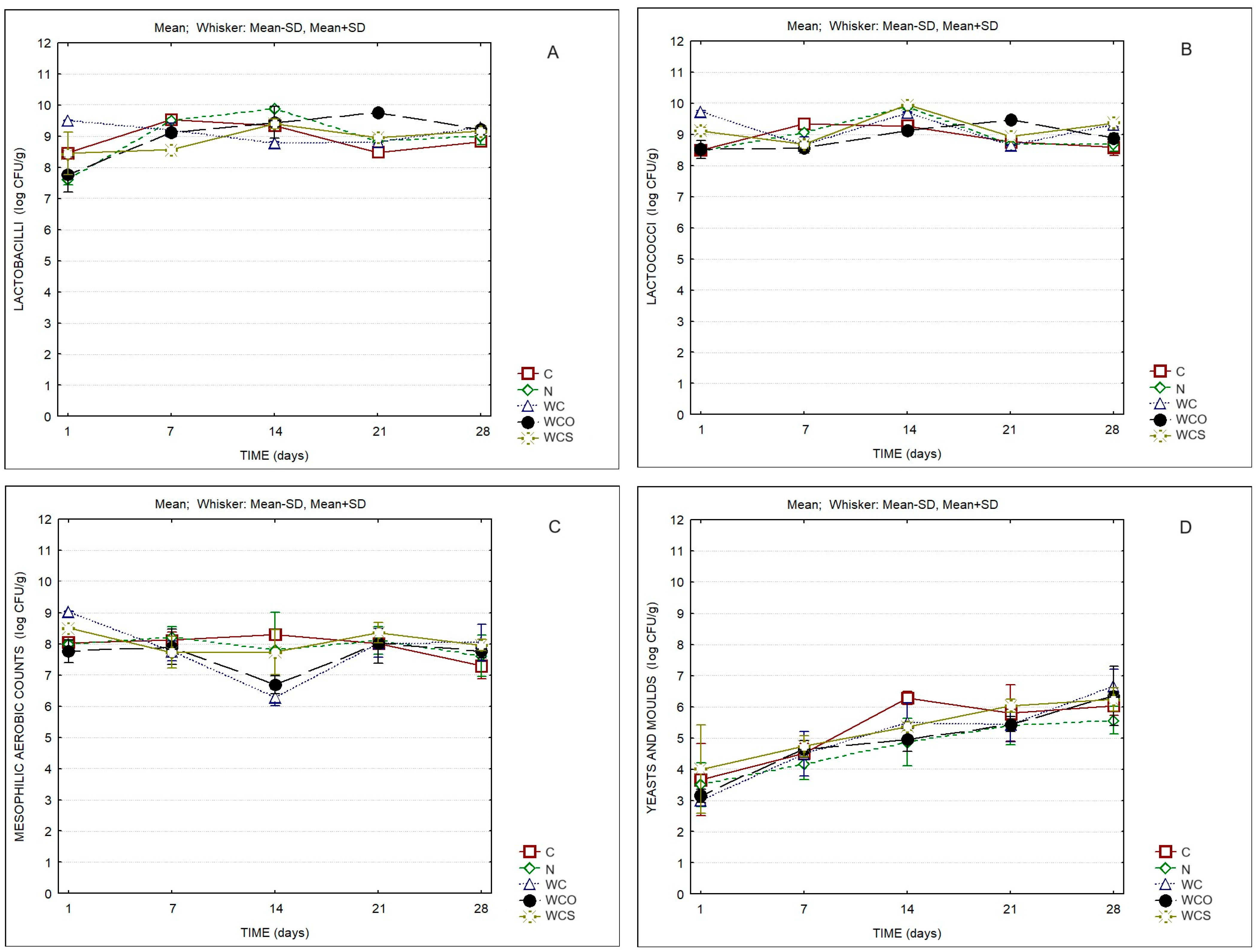
| Microbiological Analysis | Two-Way ANOVA | ||
|---|---|---|---|
| F | p Value | ||
| Lactococci | Time | 35.66 | 0.000 |
| Product | 9.58 | 0.000 | |
| Interaction | 12.63 | 0.000 | |
| Lactobacilli | Time | 22.64 | 0.000 |
| Product | 1.17 | 0.343 | |
| Interaction | 8.65 | 0.000 | |
| Mesophilic Aerobic Bacteria | Time | 9.37 | 0.000 |
| Product | 2.27 | 0.070 | |
| Interaction | 3.48 | 0.000 | |
| Yeasts and Molds | Time | 52.60 | 0.000 |
| Product | 3.13 | 0.019 | |
| Interaction | 1.29 | 0.225 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, A.; Pietruszka, H.; Bożek, A.; Szkolnicka, K.; Gomes, D.; Díaz, O.; Cobos, A.; Pereira, C. Sheep’s Second Cheese Whey Edible Coatings with Oregano and Clary Sage Essential Oils Used as Sustainable Packaging Material in Cheese. Foods 2024, 13, 674. https://doi.org/10.3390/foods13050674
Pires A, Pietruszka H, Bożek A, Szkolnicka K, Gomes D, Díaz O, Cobos A, Pereira C. Sheep’s Second Cheese Whey Edible Coatings with Oregano and Clary Sage Essential Oils Used as Sustainable Packaging Material in Cheese. Foods. 2024; 13(5):674. https://doi.org/10.3390/foods13050674
Chicago/Turabian StylePires, Arona, Hubert Pietruszka, Agata Bożek, Katarzyna Szkolnicka, David Gomes, Olga Díaz, Angel Cobos, and Carlos Pereira. 2024. "Sheep’s Second Cheese Whey Edible Coatings with Oregano and Clary Sage Essential Oils Used as Sustainable Packaging Material in Cheese" Foods 13, no. 5: 674. https://doi.org/10.3390/foods13050674
APA StylePires, A., Pietruszka, H., Bożek, A., Szkolnicka, K., Gomes, D., Díaz, O., Cobos, A., & Pereira, C. (2024). Sheep’s Second Cheese Whey Edible Coatings with Oregano and Clary Sage Essential Oils Used as Sustainable Packaging Material in Cheese. Foods, 13(5), 674. https://doi.org/10.3390/foods13050674







