Competition for Nitrogen Resources: An Explanation of the Effects of a Bioprotective Strain Metschnikowia pulcherrima on the Growth of Hanseniaspora Genus in Oenology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Media and Growth Condition
2.3. Growth Analysis
2.4. Nitrogen Analysis
2.5. Oxygen Requirement
2.6. UHPLC-Q-ToF-MS/MS Untargeted Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Interaction on Growth Kinetics
3.1.1. Interaction between Metschnikowia pulcherrima MCR24 and Hanseniaspora uvarum 3137
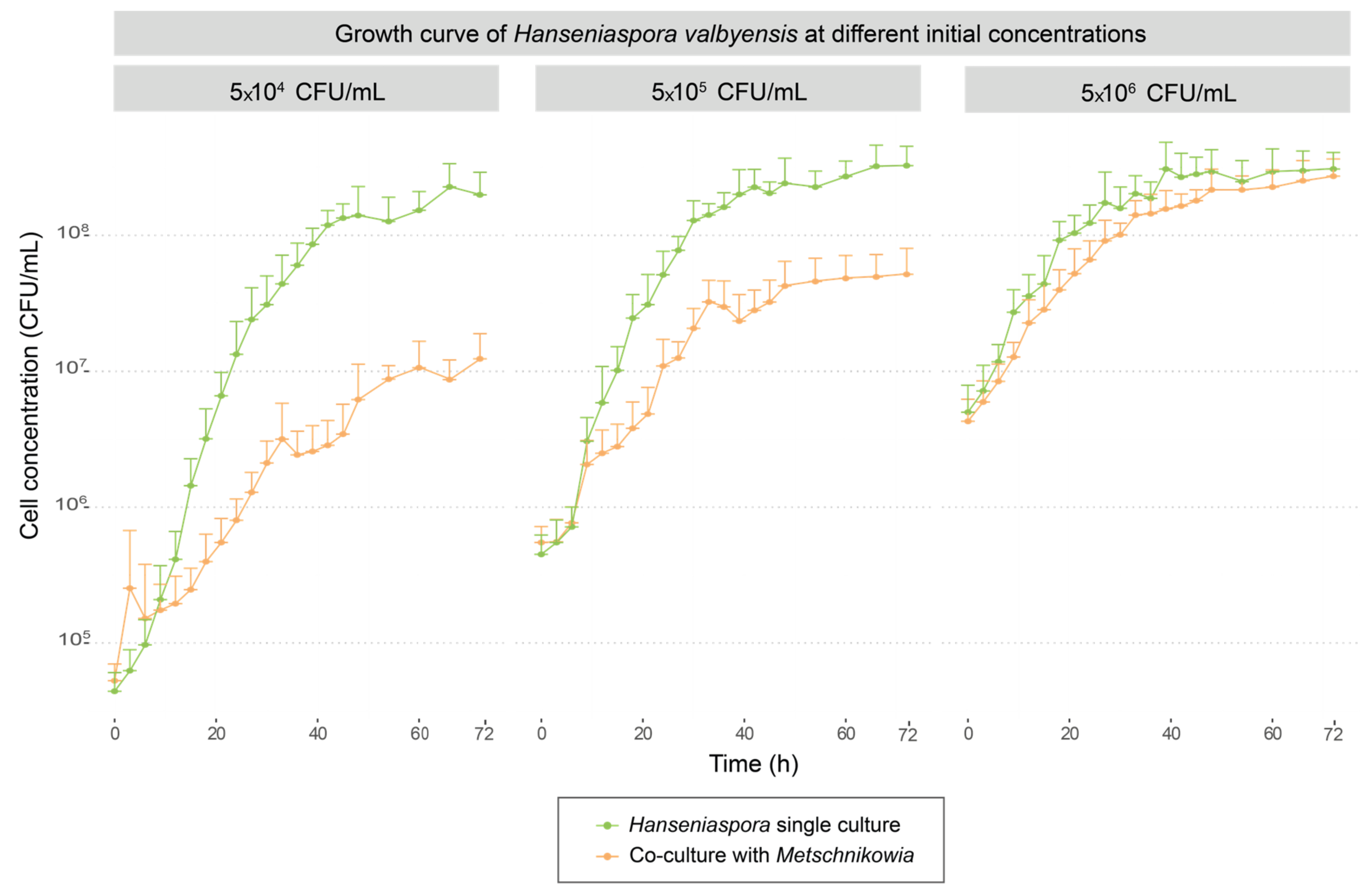
3.1.2. Interaction between Metschnikowia pulcherrima MCR24 and Hanseniaspora valbyensis ScS
3.2. Nitrogen Requirements
3.3. Oxygen Requirements
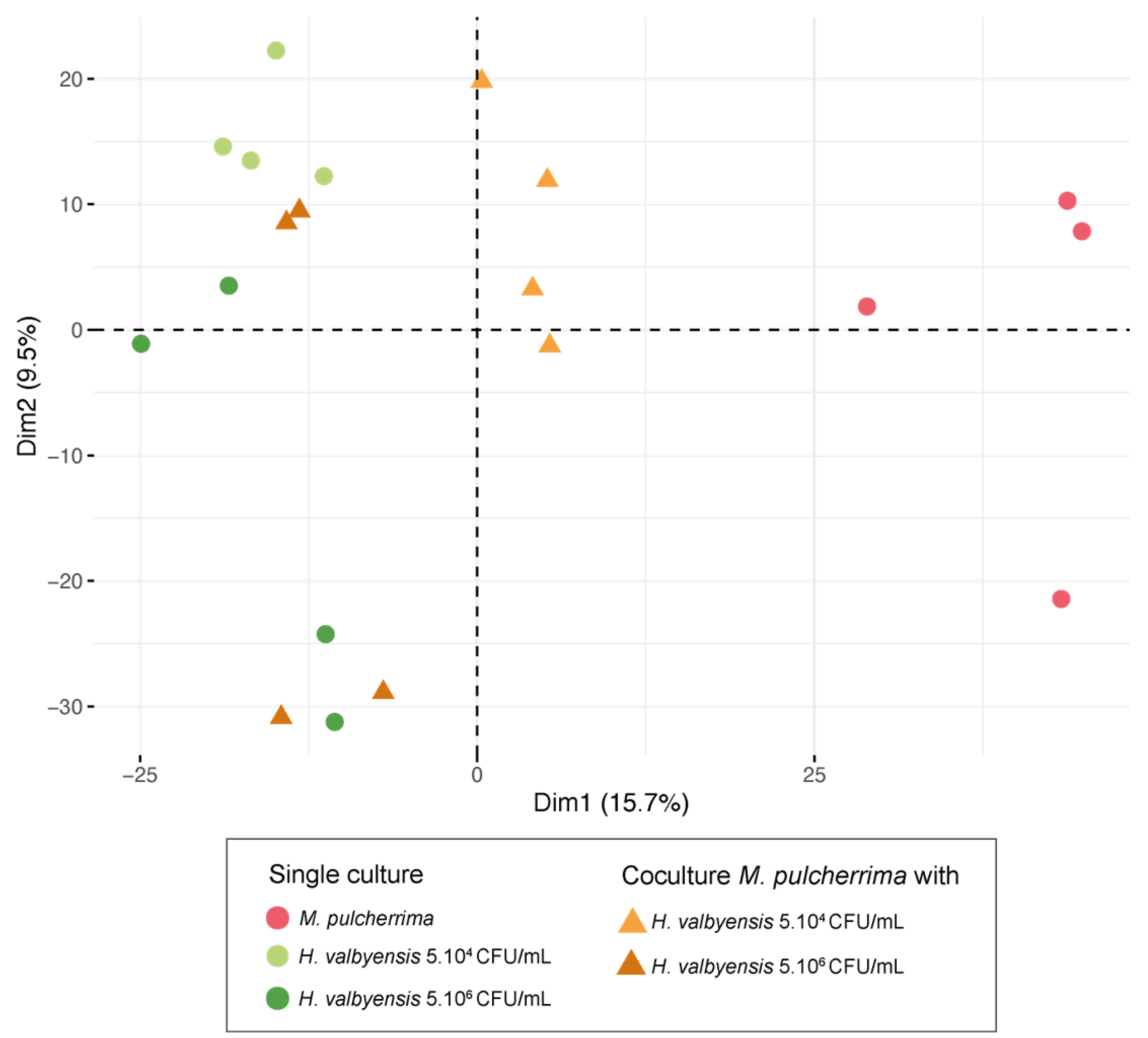
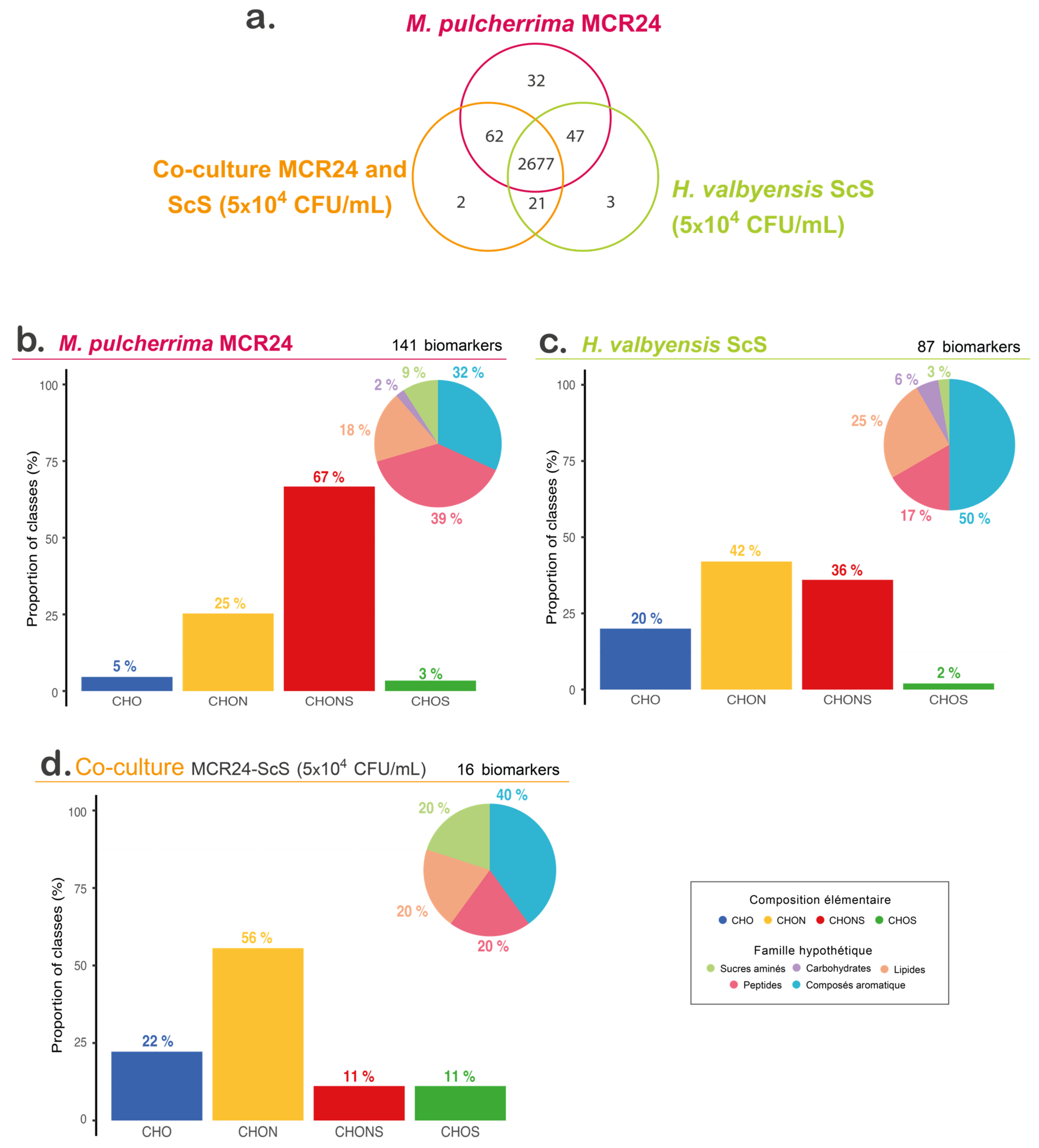
3.4. Untargeted Metabolomic Analyses of Non-Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nemcová, K.; Breierová, E.; Vadkertiová, R.; Molnárová, J. The Diversity of Yeasts Associated with Grapes and Musts of the Strekov Winegrowing Region, Slovakia. Folia Microbiol. 2015, 60, 103–109. [Google Scholar] [CrossRef]
- Albergaria, H.; Arneborg, N. Dominance of Saccharomyces cerevisiae in Alcoholic Fermentation Processes: Role of Physiological Fitness and Microbial Interactions. Appl. Microbiol. Biotechnol. 2016, 100, 2035–2046. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Q.; Zhu, S.; Du, F.; Mao, R.; Liu, L.; Tian, B.; Zhu, Y. Biodiversity of Non-Saccharomyces Yeasts Associated with Spontaneous Fermentation of Cabernet Sauvignon Wines from Shangri-La Wine Region, China. Sci. Rep. 2021, 11, 5150. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G.; Comi, G.; Zironi, R. Higher Alcohol and Acetic Acid Production by Apiculate Wine Yeasts. J. Appl. Bacteriol. 1992, 73, 126–130. [Google Scholar] [CrossRef]
- Ciani, M.; Beco, L.; Comitini, F. Fermentation Behaviour and Metabolic Interactions of Multistarter Wine Yeast Fermentations. Int. J. Food Microbiol. 2006, 108, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Jin, G.-J.; Xu, Y.-H.; Tao, Y.-S. Wine Aroma Response to Different Participation of Selected Hanseniaspora uvarum in Mixed Fermentation with Saccharomyces cerevisiae. Food Res. Int. 2018, 108, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. The Role of Sulfur Dioxide in Wine; Principles and Practices of Winemaking; Springer: Boston, MA, USA, 1999. [Google Scholar]
- Roudil, L.; Russo, P.; Berbegal, C.; Albertin, W.; Spano, G.; Capozzi, V. Non-Saccharomyces Commercial Starter Cultures: Scientific Trends, Recent Patents and Innovation in the Wine Sector. Recent Pat. Food Nutr. Agric. 2020, 11, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Simonin, S.; Alexandre, H.; Nikolantonaki, M.; Coelho, C.; Tourdot-Maréchal, R. Inoculation of Torulaspora delbrueckii as a Bio-Protection Agent in Winemaking. Food Res. Int. 2018, 107, 451–461. [Google Scholar] [CrossRef]
- Simonin, S.; Honoré-Chedozeau, C.; Monnin, L.; David-Vaizant, V.; Bach, B.; Alexandre, H.; Chatelet, B.; Tourdot-Marechal, R. Bioprotection on Chardonnay Grape: Limits and Impacts of Settling Parameters. Aust. J. Grape Wine Res. 2022, 2022, 1489094. [Google Scholar] [CrossRef]
- Windholtz, S.; Dutilh, L.; Lucas, M.; Maupeu, J.; Vallet-Courbin, A.; Farris, L.; Coulon, J.; Masneuf-Pomarède, I. Population Dynamics and Yeast Diversity in Early Winemaking Stages without Sulfites Revealed by Three Complementary Approaches. Appl. Sci. 2021, 11, 2494. [Google Scholar] [CrossRef]
- Windholtz, S.; Vinsonneau, E.; Farris, L.; Thibon, C.; Masneuf-Pomarède, I. Yeast and Filamentous Fungi Microbial Communities in Organic Red Grape Juice: Effect of Vintage, Maturity Stage, SO2, and Bioprotection. Front. Microbiol. 2021, 12, 748416. [Google Scholar] [CrossRef]
- Windholtz, S.; Redon, P.; Lacampagne, S.; Farris, L.; Lytra, G.; Cameleyre, M.; Barbe, J.-C.; Coulon, J.; Thibon, C.; Masneuf-Pomarède, I. Non-Saccharomyces Yeasts as Bioprotection in the Composition of Red Wine and in the Reduction of Sulfur Dioxide. LWT 2021, 149, 111781. [Google Scholar] [CrossRef]
- Agarbati, A.; Canonico, L.; Ciani, M.; Comitini, F. Metschnikowia pulcherrima in Cold Clarification: Biocontrol Activity and Aroma Enhancement in Verdicchio Wine. Fermentation 2023, 9, 302. [Google Scholar] [CrossRef]
- Bordet, F.; Joran, A.; Klein, G.; Roullier-Gall, C.; Alexandre, H. Yeast–Yeast Interactions: Mechanisms, Methodologies and Impact on Composition. Microorganisms 2020, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Zilelidou, E.A.; Nisiotou, A. Understanding Wine through Yeast Interactions. Microorganisms 2021, 9, 1620. [Google Scholar] [CrossRef]
- Velázquez, R.; Zamora, E.; Álvarez, M.L.; Hernández, L.M.; Ramírez, M. Effects of New Torulaspora delbrueckii Killer Yeasts on the Must Fermentation Kinetics and Aroma Compounds of White Table Wine. Front. Microbiol. 2015, 6, 1222. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.L.; Susana Sáez, J.; del Monaco, S.; Lopes, C.A.; Sangorrín, M.P. TdKT, a New Killer Toxin Produced by Torulaspora Delbrueckii Effective against Wine Spoilage Yeasts. Int. J. Food Microbiol. 2016, 217, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, M.B.; Ganga, M.A.; Sangorrín, M.P. Production of a Novel Killer Toxin from Saccharomyces eubayanus Using Agro-Industrial Waste and Its Application against Wine Spoilage Yeasts. Antonie Van Leeuwenhoek 2019, 112, 965–973. [Google Scholar] [CrossRef]
- Büyüksırıt Bedir, T.; Kuleaşan, H. A Natural Approach, the Use of Killer Toxin Produced by Metschnikowia pulcherrima in Fresh Ground Beef Patties for Shelf Life Extention. Int. J. Food Microbiol. 2021, 345, 109154. [Google Scholar] [CrossRef]
- Büyüksırıt-Bedir, T.; Kuleaşan, H. Purification and Characterization of a Metschnikowia pulcherrima Killer Toxin with Antagonistic Activity against Pathogenic Microorganisms. Arch. Microbiol. 2022, 204, 337. [Google Scholar] [CrossRef]
- Sipiczki, M. Metschnikowia Strains Isolated from Botrytized Grapes Antagonize Fungal and Bacterial Growth by Iron Depletion. Appl. Environ. Microbiol. 2006, 72, 6716–6724. [Google Scholar] [CrossRef] [PubMed]
- Oro, L.; Ciani, M.; Comitini, F. Antimicrobial Activity of Metschnikowia pulcherrima on Wine Yeasts. J. Appl. Microbiol. 2014, 116, 1209–1217. [Google Scholar] [CrossRef]
- Türkel, S.; Korukluoğlu, M.; Yavuz, M. Biocontrol Activity of the Local Strain of Metschnikowia pulcherrima on Different Postharvest Pathogens. Biotechnol. Res. Int. 2014, 2014, 397167. [Google Scholar] [CrossRef]
- Hicks, R.H.; Moreno-Beltrán, M.; Gore-Lloyd, D.; Chuck, C.J.; Henk, D.A. The Oleaginous Yeast Metschnikowia pulcherrima Displays Killer Activity against Avian-Derived Pathogenic Bacteria. Biology 2021, 10, 1227. [Google Scholar] [CrossRef]
- Kregiel, D.; Nowacka, M.; Rygala, A.; Vadkertiová, R. Biological Activity of Pulcherrimin from the Meschnikowia pulcherrima Clade. Molecules 2022, 27, 1855. [Google Scholar] [CrossRef]
- Kluyver, A.J.; van der Walt, J.P.; van Triet, A.J. Pulcherrimin, The Pigment of Candida pulcherrima. Proc. Natl. Acad. Sci. USA 1953, 39, 583–593. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J. Biosynthesis of Pulcherriminic Acid. Biochem. J. 1965, 96, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol Ability and Action Mechanism of Food-Isolated Yeast Strains against Botrytis cinerea Causing Post-Harvest Bunch Rot of Table Grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Oztekin, S.; Karbancioglu-Guler, F. Bioprospection of Metschnikowia sp. Isolates as Biocontrol Agents against Postharvest Fungal Decays on Lemons with Their Potential Modes of Action. Postharvest Biol. Technol. 2021, 181, 111634. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Ruan, C.; Yi, L.; Deng, L.; Zeng, K. Metschnikowia citriensis FL01 Antagonize Geotrichum Citri-Aurantii in Citrus Fruit through Key Action of Iron Depletion. Int. J. Food Microbiol. 2021, 357, 109384. [Google Scholar] [CrossRef]
- Chen, H.; Fink, G.R. Feedback Control of Morphogenesis in Fungi by Aromatic Alcohols. Genes Dev. 2006, 20, 1150–1161. [Google Scholar] [CrossRef]
- Hogan, D.A. Talking to Themselves: Autoregulation and Quorum Sensing in Fungi. Eukaryot. Cells 2006, 5, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Avbelj, M.; Zupan, J.; Kranjc, L.; Raspor, P. Quorum-Sensing Kinetics in Saccharomyces cerevisiae: A Symphony of ARO Genes and Aromatic Alcohols. J. Agric. Food Chem. 2015, 63, 8544–8550. [Google Scholar] [CrossRef] [PubMed]
- Avbelj, M.; Zupan, J.; Raspor, P. Quorum-Sensing in Yeast and Its Potential in Wine Making. Appl. Microbiol. Biotechnol. 2016, 100, 7841–7852. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.S.; Bedekar, A.A.; Rao, C.V. Quorum Sensing in Yeast. In ACS Symposium Series; Dhiman, S.S., Ed.; American Chemical Society: Washington, DC, USA, 2020; Volume 1374, pp. 235–250. ISBN 978-0-8412-9860-6. [Google Scholar]
- González, B.; Vázquez, J.; Morcillo-Parra, M.Á.; Mas, A.; Torija, M.J.; Beltran, G. The Production of Aromatic Alcohols in Non-Saccharomyces Wine Yeast Is Modulated by Nutrient Availability. Food Microbiol. 2018, 74, 64–74. [Google Scholar] [CrossRef]
- Prior, K.J.; Bauer, F.F.; Divol, B. The Utilisation of Nitrogenous Compounds by Commercial Non-Saccharomyces Yeasts Associated with Wine. Food Microbiol. 2019, 79, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Bely, M.; Sablayrolles, J.-M.; Barre, P. Automatic Detection of Assimilable Nitrogen Deficiencies during Alcoholic Fermentation in Oenological Conditions. J. Ferment. Bioeng. 1990, 70, 246–252. [Google Scholar] [CrossRef]
- Gobert, A.; Tourdot-Maréchal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of Nitrogen Status in Wine Alcoholic Fermentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Dellacassa, E.; Carrau, F. Growth of Non-Saccharomyces Yeasts Affects Nutrient Availability for Saccharomyces cerevisiae during Wine Fermentation. Int. J. Food Microbiol. 2012, 157, 245–250. [Google Scholar] [CrossRef]
- Vendramini, C.; Beltran, G.; Nadai, C.; Giacomini, A.; Mas, A.; Corich, V. The Role of Nitrogen Uptake on the Competition Ability of Three Vineyard Saccharomyces cerevisiae Strains. Int. J. Food Microbiol. 2017, 258, 1–11. [Google Scholar] [CrossRef]
- Roca-Mesa, H.; Sendra, S.; Mas, A.; Beltran, G.; Torija, M.-J. Nitrogen Preferences during Alcoholic Fermentation of Different Non-Saccharomyces Yeasts of Oenological Interest. Microorganisms 2020, 8, 157. [Google Scholar] [CrossRef]
- Gobert, A.; Tourdot-Maréchal, R.; Morge, C.; Sparrow, C.; Liu, Y.; Quintanilla-Casas, B.; Vichi, S.; Alexandre, H. Non-Saccharomyces Yeasts Nitrogen Source Preferences: Impact on Sequential Fermentation and Wine Volatile Compounds Profile. Front. Microbiol. 2017, 8, 2175. [Google Scholar] [CrossRef]
- Harlé, O.; Legrand, J.; Tesnière, C.; Pradal, M.; Mouret, J.-R.; Nidelet, T. Investigations of the Mechanisms of Interactions between Four Non-Conventional Species with Saccharomyces cerevisiae in Oenological Conditions. PLoS ONE 2020, 15, e0233285. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Seguinot, P.; Sanchez, I.; Ortiz-Julien, A.; Heras, J.M.; Querol, A.; Camarasa, C.; Guillamón, J.M. Nitrogen Sources Preferences of Non-Saccharomyces Yeasts to Sustain Growth and Fermentation under Winemaking Conditions. Food Microbiol. 2020, 85, 103287. [Google Scholar] [CrossRef] [PubMed]
- Rollero, S.; Bloem, A.; Ortiz-Julien, A.; Camarasa, C.; Divol, B. Altered Fermentation Performances, Growth, and Metabolic Footprints Reveal Competition for Nutrients between Yeast Species Inoculated in Synthetic Grape Juice-Like Medium. Front. Microbiol. 2018, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Puyo, M.; Mas, P.; Roullier-Gall, C.; Romanet, R.; Lebleux, M.; Klein, G.; Alexandre, H.; Tourdot-Maréchal, R. Bioprotection Efficiency of Metschnikowia Strains in Synthetic Must: Comparative Study and Metabolomic Investigation of the Mechanisms Involved. Foods 2023, 12, 3927. [Google Scholar] [CrossRef]
- Evers, M.S.; Roullier-Gall, C.; Morge, C.; Sparrow, C.; Gobert, A.; Vichi, S.; Alexandre, H. Thiamine and Biotin: Relevance in the Production of Volatile and Non-Volatile Compounds during Saccharomyces cerevisiae Alcoholic Fermentation in Synthetic Grape Must. Foods 2023, 12, 972. [Google Scholar] [CrossRef] [PubMed]
- Puyo, M.; Simonin, S.; Klein, G.; David-Vaizant, V.; Quijada-Morín, N.; Alexandre, H.; Tourdot-Maréchal, R. Use of Oenological Tannins to Protect the Colour of Rosé Wine in a Bioprotection Strategy with Metschnikowia pulcherrima. Foods 2023, 12, 735. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Liu, Y.; Bianchi, T.S.; Tolić, N.; Jansson, C.; Paša-Tolić, L. Moving beyond the van Krevelen Diagram: A New Stoichiometric Approach for Compound Classification in Organisms. Anal. Chem. 2018, 90, 6152–6160. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Chacon-Rodriguez, L.; Joseph, C.M.L.; Nazaris, B.; Coulon, J.; Richardson, S.; Dycus, D.A. Innovative Use of Non- Saccharomyces in Bio-Protection: T. delbrueckii and M. pulcherrima Applied to a Machine Harvester. Catal. Discov. Into Pract. 2020, 4, 82–90. [Google Scholar] [CrossRef]
- Lebleux, M.; Alexandre, H.; Romanet, R.; Ballester, J.; David-Vaizant, V.; Adrian, M.; Tourdot-Maréchal, R.; Rouiller-Gall, C. Must Protection, Sulfites versus Bioprotection: A Metabolomic Study. Food Res. Int. 2023, 173, 113383. [Google Scholar] [CrossRef] [PubMed]
- Albertin, W.; Setati, M.E.; Miot-Sertier, C.; Mostert, T.T.; Colonna-Ceccaldi, B.; Coulon, J.; Girard, P.; Moine, V.; Pillet, M.; Salin, F.; et al. Hanseniaspora uvarum from Winemaking Environments Show Spatial and Temporal Genetic Clustering. Front. Microbiol. 2016, 6, 1569. [Google Scholar] [CrossRef]
- Seguinot, P.; Ortiz-Julien, A.; Camarasa, C. Impact of Nutrient Availability on the Fermentation and Production of Aroma Compounds under Sequential Inoculation with M. pulcherrima and S. cerevisiae. Front. Microbiol. 2020, 11, 305. [Google Scholar] [CrossRef]
- Kemsawasd, V.; Viana, T.; Ardö, Y.; Arneborg, N. Influence of Nitrogen Sources on Growth and Fermentation Performance of Different Wine Yeast Species during Alcoholic Fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 10191–10207. [Google Scholar] [CrossRef]
- Bataillon, M.; Rico, A.; Sablayrolles, J.-M.; Salmon, J.-M.; Barre, P. Early Thiamin Assimilation by Yeasts under Enological Conditions: Impact on Alcoholic Fermentation Kinetics. J. Ferment. Bioeng. 1996, 82, 145–150. [Google Scholar] [CrossRef]
- Belviso, S.; Bardi, L.; Bartolini, A.B.; Marzona, M. Lipid Nutrition of Saccharomyces cerevisiae in Winemaking. Can. J. Microbiol. 2004, 50, 669–674. [Google Scholar] [CrossRef]
- Evers, M.S.; Ramousse, L.; Morge, C.; Sparrow, C.; Gobert, A.; Roullier-Gall, C.; Alexandre, H. To Be or Not to Be Required: Yeast Vitaminic Requirements in Winemaking. Food Microbiol. 2023, 115, 104330. [Google Scholar] [CrossRef]
- Tesnière, C.; Delobel, P.; Pradal, M.; Blondin, B. Impact of Nutrient Imbalance on Wine Alcoholic Fermentations: Nitrogen Excess Enhances Yeast Cell Death in Lipid-Limited Must. PLoS ONE 2013, 8, e61645. [Google Scholar] [CrossRef]
- Shekhawat, K.; Bauer, F.F.; Setati, M.E. Impact of Oxygenation on the Performance of Three Non-Saccharomyces Yeasts in Co-Fermentation with Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2016, 101, 2479–2491. [Google Scholar] [CrossRef]
- Holm Hansen, E.; Nissen, P.; Sommer, P.; Nielsen, J.C.; Arneborg, N. The Effect of Oxygen on the Survival of Non-Saccharomyces Yeasts during Mixed Culture Fermentations of Grape Juice with Saccharomyces cerevisiae. J. Appl. Microbiol. 2001, 91, 541–547. [Google Scholar] [CrossRef]
- Shekhawat, K.; Porter, T.J.; Bauer, F.F.; Setati, M.E. Employing Oxygen Pulses to Modulate Lachancea thermotolerans–Saccharomyces cerevisiae Chardonnay Fermentations. Ann. Microbiol. 2018, 68, 93–102. [Google Scholar] [CrossRef]
- Morales, P.; Rojas, V.; Quirós, M.; Gonzalez, R. The Impact of Oxygen on the Final Alcohol Content of Wine Fermented by a Mixed Starter Culture. Appl. Microbiol. Biotechnol. 2015, 99, 3993–4003. [Google Scholar] [CrossRef] [PubMed]
- Quirós, M.; Rojas, V.; Gonzalez, R.; Morales, P. Selection of Non-Saccharomyces Yeast Strains for Reducing Alcohol Levels in Wine by Sugar Respiration. Int. J. Food Microbiol. 2014, 181, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Visser, W.; Scheffers, W.A.; Batenburg-van der Vegte, W.H.; van Dijken, J.P. Oxygen Requirements of Yeasts. Appl. Environ. Microbiol. 1990, 56, 3785–3792. [Google Scholar] [CrossRef]
- Han, T.; Kim, G.B.; Lee, S.Y. Glutaric Acid Production by Systems Metabolic Engineering of an l-Lysine–Overproducing Corynebacterium glutamicum. Proc. Natl. Acad. Sci. USA 2020, 117, 30328–30334. [Google Scholar] [CrossRef]
- Prothstein, M.; Hart, J. Products of Lysine Metabolism in Yeast. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1964, 93, 439–441. [Google Scholar] [CrossRef]
- Bisson, L.F. Stuck and Sluggish Fermentations. Am. J. Enol. Vitic. 1999, 50, 107–119. [Google Scholar] [CrossRef]
- Martin, V.; Valera, M.; Medina, K.; Boido, E.; Carrau, F. Oenological Impact of the Hanseniaspora/Kloeckera Yeast Genus on Wines—A Review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef]
- Van Wyk, N.; Badura, J.; Von Wallbrunn, C.; Pretorius, I.S. Exploring Future Applications of the Apiculate Yeast Hanseniaspora. Crit. Rev. Biotechnol. 2024, 44, 100–119. [Google Scholar] [CrossRef]

| (a) Table of µmax (h−1) | ||||
| Hanseniaspora uvarum 3137 | Metschnikowia pulcherrima MCR24 | |||
| Initial Population of Hu (CFU/mL) | Single Culture | Co-Culture | Single Culture (at 5 × 105 CFU/mL) | Co-Culture |
| 5 × 104 | 0.394 ± 0.047 a | 0.324 ± 0.038 a | 0.249 ± 0.054 a | 0.230 ± 0.026 a |
| 5 × 105 | 0.283 ± 0.031 a | 0.294 ± 0.031 a | 0.249 ± 0.054 a | 0.196 ± 0.034 a |
| 5 × 106 | 0.277 ± 0.056 a | 0.238 ± 0.074 a | 0.249 ± 0.054 a | 0.182 ± 0.054 b |
| (b) Table of population maximal (CFU/mL) | ||||
| Hanseniaspora uvarum 3137 | Metschnikowia pulcherrima MCR24 | |||
| Initial Population of Hu (CFU/mL) | Single Culture | Co-Culture | Single Culture (at 5 × 105 CFU/mL) | Co-Culture |
| 5 × 104 | 2.27 × 107 ± 0.48 × 107 a | 1.68 × 107 ± 0.80×107 a | 4.58 × 107 ± 1.94 × 107 a | 9.36 × 106 ± 3.01 × 106 b |
| 5 × 105 | 3.56 × 107 ± 0.56 × 107 a | 3.90 × 107 ± 1.29×107 a | 4.58 × 107 ± 1.94 × 107 a | 5.54 × 106 ± 2.02 × 106 b |
| 5 × 106 | 4.79 × 107 ± 0.81 × 107 a | 4.68 × 107 ± 0.64×107 a | 4.58 × 107 ± 1.94 × 107 a | 2.91 × 106 ± 0.70 × 106 b |
| (a) Table of µmax (h−1) | ||||
| Hanseniaspora valbyensis ScS | Metschnikowia pulcherrima MCR24 | |||
| Initial Population of Hv (CFU/mL) | Single Culture | Co-Culture | Single Culture (at 5 × 105 CFU/mL) | Co-Culture |
| 5 × 104 | 0.378 ± 0.043 a | 0.171 ± 0.036 b | 0.249 ± 0.054 a | 0.270 ± 0.045 a |
| 5 × 105 | 0.325 ± 0.067 a | 0.213 ± 0.028 b | 0.249 ± 0.054 a | 0.237 ± 0.029 a |
| 5 × 106 | 0.209 ± 0.055 a | 0.177 ± 0.020 a | 0.249 ± 0.054 a | 0.213 ± 0.025 a |
| (b) Table of population maximal (CFU/mL) | ||||
| Hanseniaspora valbyensis ScS | Metschnikowia pulcherrima MCR24 | |||
| Initial Population of Hv (CFU/mL) | Single Culture | Co-Culture | Single Culture (at 5 × 105 CFU/mL) | Co-Culture |
| 5 × 104 | 1.55 × 108 ± 0.32 × 108 a | 9.06 × 106 ± 1.43 × 106 b | 4.58 × 107 ± 1.94 × 107 a | 3.34 × 107 ± 2.55 × 107 a |
| 5 × 105 | 2.72 × 108 ± 0.16 × 108 a | 4.70 × 107 ± 1.12 × 107 b | 4.58 × 107 ± 1.94 × 107 a | 2.63 × 107 ± 1.75 × 107 a |
| 5 × 106 | 2.89 × 108 ± 0.11 × 108 a | 2.33 × 108 ± 0.16 × 108 b | 4.58 × 107 ± 1.94 × 107 a | 1.33 × 107 ± 0.29 × 107 b |
| Hanseniaspora uvarum 3137 | Hanseniaspora valbyensis ScS | Metschnikowia pulcherrima | |||||
|---|---|---|---|---|---|---|---|
| 5 × 104 CFU/mL | 5 × 105 CFU/mL | 5 × 106 CFU/mL | 5 × 104 CFU/mL | 5 × 105 CFU/mL | 5 × 106 CFU/mL | 5 × 105 CFU/mL | |
 | |||||||
| H. uvarum | H. valbyensis | M. pulcherrima | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 × 104 CFU/mL | 5 × 105 CFU/mL | 5 × 106 CFU/mL | 5 × 104 CFU/mL | 5 × 105 CFU/mL | 5 × 106 CFU/mL | 5 × 105 CFU/mL | ||||||||||||||||||||||
| %24 h 1 | Lag 2 | Qs 3 | Rs 4 | %24 h | Lag | Qs | Rs | %24 h | Lag | Qs | Rs | %24 h | Lag | Qs | Rs | %24 h | Lag | Qs | Rs | 24%h | Lag | Qs | Rs | %24 h | Lag | Qs | Rs | |
| Ile | 45 | 7.00 | 0.088 | 0.107 | 74 | 4.00 | 0.059 | 0.942 | 81 | 0.50 | 0.040 | 0.872 | 6 | 16.50 | 0.299 | 0.061 | 18 | 9.75 | 0.059 | 0.141 | 36 | 6.25 | 0.007 | 0.451 | 24 | 7.00 | 0.037 | 0.405 |
| Leu | 41 | 7.00 | 0.143 | 0.399 | 68 | 3.50 | 0.094 | 1.304 | 76 | 0.75 | 0.059 | 1.291 | 6 | 17.50 | 0.589 | 0.097 | 18 | 10.25 | 0.050 | 0.117 | 36 | 5.50 | 0.015 | 0.599 | 28 | 5.50 | 0.061 | 0.419 |
| Lys | 90 | 7.00 | 0.039 | 0.379 | 93 | 0.75 | 0.039 | 0.376 | 90 | 0.00 | 0.042 | 0.968 | 30 | 10.75 | 0.377 | 0.059 | 70 | 8.00 | 0.023 | 0.127 | 87 | 3.00 | 0.011 | 0.259 | 50 | 3.25 | 0.039 | 0.181 |
| Trp | 42 | 5.00 | 0.769 | 1.031 | 57 | 1.00 | 0.463 | 4.339 | 70 | 0.50 | 0.225 | 5.536 | 19 | 13.00 | 5.359 | 1.547 | 20 | 7.75 | 0.666 | 1.448 | 38 | 1.25 | 0.134 | 2.684 | 39 | 2.75 | 0.760 | 4.617 |
| Species | Initial Concentration (CFU/mL) 1 | Maximal Speed (mg/L/h) 2 | T50 (h) 3 | Total Consumption (h) 4 |
|---|---|---|---|---|
| Hanseniaspora uvarum 3137 | 5 × 104 | 1.90 ± 0.30 A | 8.56 ± 0.25 A | 11.14 ± 1.18 A |
| 5 × 105 | 4.35 ± 0.98 B | 2.33 ± 0.11 B | 3.78 ± 0.54 B | |
| 5 × 106 | 7.41 ± 0.77 C | 1.06 ± 0.05 C | 2.24 ± 0.28 C | |
| Hanseniaspora valbyensis ScS | 5 × 104 | 2.31 ± 0.13 a | 9.50 ± 0.00 a | 11.00 ± 0.00 a |
| 5 × 105 | 2.90 ± 0.47 b | 6.33 ± 0.38 b | 8.03 ± 0.21 b | |
| 5 × 106 | 2.99 ± 0.30 b | 2.31 ± 0.24 c | 4.50 ± 1.00 c | |
| Metschnikowia pulcherrima MCR24 | 5 × 105 | 4.37 ± 0.64 B c | 1.93 ± 0.10 d D | 3.16 ± 0.19 d D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puyo, M.; Scalabrino, L.; Romanet, R.; Simonin, S.; Klein, G.; Alexandre, H.; Tourdot-Maréchal, R. Competition for Nitrogen Resources: An Explanation of the Effects of a Bioprotective Strain Metschnikowia pulcherrima on the Growth of Hanseniaspora Genus in Oenology. Foods 2024, 13, 724. https://doi.org/10.3390/foods13050724
Puyo M, Scalabrino L, Romanet R, Simonin S, Klein G, Alexandre H, Tourdot-Maréchal R. Competition for Nitrogen Resources: An Explanation of the Effects of a Bioprotective Strain Metschnikowia pulcherrima on the Growth of Hanseniaspora Genus in Oenology. Foods. 2024; 13(5):724. https://doi.org/10.3390/foods13050724
Chicago/Turabian StylePuyo, Maëlys, Léa Scalabrino, Rémy Romanet, Scott Simonin, Géraldine Klein, Hervé Alexandre, and Raphaëlle Tourdot-Maréchal. 2024. "Competition for Nitrogen Resources: An Explanation of the Effects of a Bioprotective Strain Metschnikowia pulcherrima on the Growth of Hanseniaspora Genus in Oenology" Foods 13, no. 5: 724. https://doi.org/10.3390/foods13050724






