Abstract
This study analysed the probabilistic risk to consumers associated with the presence of iAs, Cd, Cr, Hg, Pb, acrylamide (AA) and ochratoxin A (OTA) in instant coffee from Brazil, Colombia, Mexico and Peru. The results found iAs to be the metal with the highest concentrations (3.50 × 10−2 to 6.00 × 10−2 mg/kg), closely followed by Pb (1.70 × 10−2 to 2.70 × 10−2 mg/kg) and Cr (5.00 × 10−3 to 1.00 × 10−2 mg/kg), although these differences were not significant between countries. Cd and Hg were not detected. Focusing on AA, the concentrations ranged from 1.77 × 10−1 mg/kg (Peru) to 4.77 × 10−1 mg/kg (Brazil), while OTA ranged from 1.32 × 10−3 (Peru) to 1.77 × 10−3 mg/kg (Brazil) with significant differences between countries in both cases. As regards risk, the hazard quotient and hazard index were less than 1, meaning that the consumption of instant coffee represents a low level of concern for non-genotoxic effects. The results of the combination of margin of exposure and probability of exceedance indicated that the non-genotoxic effects of Pb, AA and OTA pose no threat. However, the probability values of suffering cancer from iAs and AA (between 1 × 10−6 and 1 × 10−4) indicated a moderate risk and that management measures should be taken.
1. Introduction
Coffee has now become the second most popular hot beverage, appreciated for its stimulating effect and organoleptic characteristics. The International Coffee Organization (ICO) estimated that world coffee consumption increased by 4.2% to 175.6 million 60 kg bags in 2021/22, representing € 165 billion per year [1]. Instant coffee accounts for the second form of coffee exports and represents 8.2% of the world’s market share of the total coffee consumed [2]. It is obtained by removing water from the coffee extract by techniques such as spray-drying or freeze-drying. The dry powder thus obtained has a moisture content of between 2% and 4% [3,4,5,6,7]. Instant coffee is widely consumed in Eastern Europe (45%), Asia/Pacific (53%), and Australia (79%), and has become established in countries where tea was traditionally consumed, such as the United Kingdom, where 90% of the coffee consumed is instant [8,9], a fact that has led many producing countries, including Brazil, Colombia, Mexico and Peru, to increase their production of instant coffee in recent years [10].
Different chemical hazards produced when cultivating, processing and storing coffee can jeopardize the consumer’s health. These compounds include heavy metal(oid)s, acrylamide or mycotoxins, whose presence and potential impact on instant coffee are of growing scientific interest, especially in countries whose economic development depends directly on coffee exports [11]. The heavy metals of natural or anthropomorphic origin present in the environment enter the food chain, causing a wide range of toxic and mutagenic effects in the organism [12,13,14]. The toxicity of arsenic is partly related to its form, valency, solubility, rate of absorption and elimination from the organism, so that organic arsenic is rapidly absorbed and excreted, while inorganic arsenic (iAs) is considered highly toxic and carcinogenic. A cadmium (Cd) exposure in food higher than 4 × 10−3 mg/kg/day can cause renal dysfunction, cardiovascular disorders, cancer and other illnesses [15,16,17,18]. Chromium (Cr), an essential nutrient for humans, is involved in synthesising nucleic acids and the proper functioning of the immune system [19]. However, clinical research has found that acute prolonged exposure to Cr can cause genotoxicity [20]. Mercury (Hg) is transformed into methylmercury by microbial action, its most toxic organic form since it is soluble and accumulates in the adipose tissue of animals and humans, mainly affecting the central nervous system and crossing the blood–brain barrier and the placenta, which can cause alterations in the neuronal development of the foetus and young children. Also, above the permissible limits, lead (Pb) increases the risk of high blood pressure, cardiovascular problems and kidney damage [17,21,22]. Previous studies have shown that mineral content is influenced by soil characteristics, biochemical plant parameters, variety, and processing stages [14,23,24,25,26,27]. Another aspect of interest is the optimisation of detection and quantification in the analytical method, improving the sensitivity and precision of the results [28,29].
Acrylamide (AA) is a by-product of the Maillard reaction of asparagine with reducing sugars, such as glucose, galactose and fructose, at temperatures above 120 °C and peaking at around 170 °C [30,31,32,33]. AA is a genotoxic and neurotoxic compound, classified as level 2A since 1994 (probable human carcinogen) by the International Agency for Research on Cancer [34,35,36]. Coffee is one of the main contributors to the intake of this hazard, as the roasting and spray-drying stages play an essential role in forming AA [33,37]. To ensure consumer protection on food safety, the European Commission has set a benchmark level of 8.50 × 10−4 mg per kg of AA in instant coffee [38].
Ochratoxin A (OTA) is a secondary metabolite produced by filamentous fungi, mainly of the genera Aspergillus and Penicillium [3]. The main origin of this metabolite in coffee is related to manufacturing conditions, which include processing and storage activities that can favour fungal growth, such as the inherent hygroscopicity of coffee beans, international transport time or the climatic and geographical conditions of the growing area [39]. OTA contamination is now a public health problem as it has toxic effects on the kidneys and liver and is also associated with possible adverse effects on the nervous, haematological and immune systems [40]. The International Agency for Research on Cancer has classified it as a class 2B carcinogen [41,42] and the European regulations have established a maximum OTA of 5 × 10−3 mg/kg in instant coffee [43].
The risk to the consumer can be determined by performing a risk assessment. This component of risk analysis is defined as a systematic process of identifying, analysing and characterising the risk to consumers’ health over a given period [44,45]. In this analysis, a probabilistic approach is essential to take into account both the variability of the input data (such as consumption, concentration of chemical contaminants, weight differences between population groups or digestibility) and also to reduce the uncertainty of the calculated risk [46,47,48,49]. In this approach, the input data are represented by a distribution, which must be combined to estimate the range and probability of a hazard, exposure or risk rather than a single-point estimate. This process is usually done by Monte Carlo simulation, resulting in a frequency distribution that provides not only the extreme values but also the most likely outcome [50]. On the other hand, humans are simultaneously exposed to many chemicals on a daily basis, so that their effect on health must be studied by considering their combined action, i.e., their additive, synergistic or antagonistic effect [51,52]. The aim of the present study was thus to assess the risk to consumers due to the presence of chemical contaminants generated in the field, such as metals (iAs, Cd, Cr, Hg and Pb) or due to storage and processing conditions (AA and OTA) in instant coffee from Brazil, Colombia, Mexico and Peru. The non-genotoxic effects were studied by calculating the hazard quotient and the hazard index (for the combined effect of Cd, Cr, Hg and AA) and the MOE-POE combination (of Pb, AA and OTA), and the genotoxic effects through calculating a MOE-POE combination (of iAs, AA and OTA) and the cancer risk (of iAs, Pb and AA), all under a probabilistic approach. This information could help manufacturers and health authorities to make informed risk decisions.
2. Materials and Methods
2.1. Study Material
A total of 180 instant coffee samples from Brazil (Sao Paulo and Belo Horizonte), Colombia (Bogota and Barranquilla), Mexico (Mexico City) and Peru (Lima and Chachapoyas) were purchased in retail outlets between July and September 2022. The samples were kept in the original containers (glass and bioriented polypropylene bags) and stored at room temperature until analysis. All the samples were analysed in triplicate.
2.2. Chemical Analysis
The chemical hazards studied in instant coffee, metals (iAs, Cd, Cr, Hg and Pb), AA and OTA were analysed at the Soil and Water Research Laboratory (LABISAG) of the Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas in Peru.
2.2.1. Heavy Metal(oid)s
The concentration of metals was determined following the method described by Leiva-Tafur et al. (2022) and Guadalupe et al. (2023) [24,53].
The acid digestion technique was used for sample preparation. One gram of the sample was weighed and placed in crucibles inside the muffle at a temperature of 450 °C for 6 h. The calcined sample was moistened with 3 drops of distilled water and 1 mL of concentrated HCl, leaving it to stand for 1 h. Subsequently, 1 mL of distilled water and 1 mL of concentrated HCl were added and left to stand for 1 h. Then, the solutions of the samples by filtration were transferred to a 25 mL flask and it was gauged with distilled water to take the volume to 25 mL for the analysis.
Total metals were determined by microwave plasma atomic emission plasma spectroscopy (ICP-AES) (Agilent 4100 MP-AES, Agilent Technologies, Santa Clara, CA, USA) equipped with a standard torch, an Inert One Neb nebulizer and a dual-pass glass cyclonic spray chamber (Agilent Technologies) [54].
Inorganic arsenic was determined by ion chromatography (IC) combined with inductively coupled plasma atomic emission spectroscopy [55]. Ultrapure water (4.1 mL) and concentrated HCl (18.4 mL) were added to the samples (0.5 g). The mixture was left overnight. The sample and extraction solvent were separated using a centrifuge and then the solution was filtered. HBr (2 mL) and 1.5% (w/v) hydrazine sulphate (1 mL) were added. The inorganic arsenic was extracted into 30 mL of chloroform, and then extracted into 20 mL of 1 M HCl. The solvent was removed by a rotary evaporator to about 1 mL. The evaporation flask was washed with ultrapure water. The solution was transferred to a 50 mL Falcon tube and the final volume was brought to 20 mL.
Detection (LOD) and quantification (LOQ) limits were calculated as the minimum detectable amount of analyte with a signal-to-noise ratio of 3:1 and 10:1, respectively [56], and are expressed as concentration (mg/kg). Detection wavelengths of 228.802 nm, 405.781 nm, 193.695 nm, 425.433 nm and 253.652 nm were selected to quantify iAs, Cd, Cr, Hg and Pb, respectively.
The equipment was calibrated using standard solutions of each element in different concentrations, prepared from a standard solution of 1.00 × 10 mg/kg. The analytical quality of detection and quantification of iAs, Cd, Cr, Hg and Pb was controlled by measuring blind and double samples. The parameters were validated from ten replicates, for iAs, Cd, Cr, Hg and Pb, the limit of quantification (LOQ) was 1.00 × 10−2, 1.50 × 10−2, 1.00 × 10−2, 1.50 × 10−2 and 1. 50 × 10−2 mg/kg, the limit of detection (LOD) was 3.00 × 10−3, 5.00 × 10−3, 3.00 × 10−3, 5.00 × 10−3 and 5.00 × 10−3 mg/kg, and the linearity (R2) was 9.96 × 10−1, 9.99 × 10−1, 9.99 × 10−1, 9.99 × 10−1, 9.98 × 10−1 and 9.99 × 10−1, respectively. The laboratory is accredited as conforming to the standard [57], granted by the National Institute of Quality (INACAL) of Peru.
2.2.2. Acrylamide
The AA was determined following the method described by Mesías and Morales (2016) [58]. The analysis was carried out on a high-performance liquid chromatograph (U-HPLC) (Agilent 1290 Infinity II, Santa Clara, CA, USA), coupled to an Agilent Triple Quadrupole MS detector (Agilent Technologies, Santa Clara, CA, USA) with positive electrospray ionization. The sample was separated on a Hypercarb column (50 × 2.1 mm, 5 µm; Thermo Scientific, Bremen, Germany) at 30 °C with formic acid in water eluent (2.00 × 10−1 mL/ 1.00 × 102 mL) at a flow rate of 4.00 × 10−1 mL/min. The linearity (R2) was 9.997 × 10−1, the LOD was set at 5.00 × 10−3 mg/Kg and the LOQ at 1.00 × 10−2 mg/Kg.
2.2.3. Ochratoxin A
OTA was quantified following the methodology described by Vecchio et al. (2012) [59]. Determinations were performed by ultra-high performance liquid chromatography (U-HPLC) (Agilent 1290 Infinity II, Santa Clara, CA, USA), equipped with a 20 μL loop and connected to a spectrofluorometer detector, Perkin Elmer Fluorescence Detector Series 200. The excitation and emission wavelengths were 330 and 460 nm. Chromatography was carried out isocratically using 4 mM sodium acetate/acetic acid (19:1): acetonitrile (60:40) as the mobile phase at a 1.0 mL/min flow rate. The working standard solution and sample volumes of 20 μL were injected in triplicate. The parameters were validated by six replicates. The LOD was 1.60 × 10−5 mg/kg, the LOQ was 4.80 × 10−5 mg/kg and the linearity coefficient (R2) was 9.997 × 10−1. The retention time was 9.09 ± 0.08 min.
2.3. Risk Characterisation
This section gives the equations and parameters involved in calculating the risk characterisation of the hazards studied, Table 1.
Risk characterisation was performed by a probabilistic approach; considering the random uncertainty of the input data and the AA and OTA concentrations of the samples studied, the consumption of instant coffee and the consumers’ weight were fitted to a probability density function (pdf) on @Risk version 8 software (Palisade, Newfield, NY, USA). The same program was also used to quantify risk by different metrics, by simulation with a standard Monte Carlo method and hypercube Latin sampling in 10 repetitions of 100,000 iterations, obtaining a pdf for each of the results. An HI of less than one was considered “acceptable” when interpreting the results, as the exposure to the hazard does not exceed the reference value, so that consumers are unlikely to suffer adverse health effects [60,61].
In interpreting the MOE, it is assumed that a value above 10,000 is of low concern from a public health point of view. For MOE values below this limit, the combination with the POE metric provides valuable information for making risk decisions. The POE is thus a measure of the probability that the change in the population’s response exceeds the predefined benchmark response. It could also be interpreted as the fraction of the total population exposed to increased risk. The POE ranges from zero to one, in which zero means that exposure is always lower than the reference value, and the risk can be considered negligible, while a POE value of one indicates that the reference value is always exceeded and must be considered as not tolerable. The POE metric can thus be used as a measure of the level of concern to complement the MOE [62]. With regard to the probability of developing cancer, values below 1 × 10−6 are not considered to be a health risk; between 1 × 10−6 and 1 × 10−4 the risk is acceptable, and above 1 × 10−4, the risk is high and indicates potential harm to consumers [63].

Table 1.
Parameters and equations of interest for risk characterisation of chemical hazards (x) and country (i).
Table 1.
Parameters and equations of interest for risk characterisation of chemical hazards (x) and country (i).
| Parameter | Description | Value | Units | Source |
|---|---|---|---|---|
| HQxi | Hazard quotient | EDIxi/RVx | ATSDR, 2022 | |
| HIi | Hazard index per country | ATSDR, 2022 | ||
| EDIxi | Estimated daily intake | Cxi · IR/Bw | mg/kgBw/day | EFSA, 2013 |
| Cxi | Concentration | Table 2 | mg/kg | This study |
| IR | Ingestion rate | Exponential (5%; 6.82 × 10−4; 95%; 1.2 × 10−2) | kg/day | EFSA, 2023 |
| Bw | Body weight | Gamma (2%; 54; 50%; 75; 98%; 110) | kgBw | CTCF, 2012 |
| RVx | Reference Value | RfD * (Cr): 3 × 10−4 | mg/kgBw/day | EPA, 2022 [64] |
| RfD (Cd): 1 × 10−3 | mg/kgBw/day | EPA, 2022 [64] | ||
| RfD (Hg): 1 × 10−4 | mg/kgBw/day | EPA, 2022 [64] | ||
| RfD (AA): 2 × 10−3 | mg/kgBw/day | EPA, 2022 [64] | ||
| MOExi | Margin of exposure | BMDL%X/EDIxi | EFSA, 2005 | |
| BMDL%X | Benchmark dose | BMDL01(iAs): Uniform (3 × 10−4; 8 × 10−3) (Carcinogenic) | mg/kgBw/day | EFSA, 2021 |
| BMDL01 (Pb): 1.5 × 10−3 (Cardiovascular) | mg/kgBw/day | EFSA, 2010 [65] | ||
| BMDL10 (Pb): 6.3 × 10−4 (Nephrotoxic) | mg/kgBw/day | EFSA, 2010 [65] | ||
| BMDL10 (AA): 1.7 × 10−1 (Carcinogenic) | mg/kgBw/day | EFSA, 2015 [66] | ||
| BMDL10 (AA): 4.3 × 10−1 (Neurotoxic) | mg/kgBw/day | EFSA, 2015 [66] | ||
| BMDL10 (OTA): 1.45 × 10−2 (Carcinogenic) | mg/kgBw/day | EFSA, 2020 | ||
| BMDL10 (OTA): 4.73 × 10−3 (Nephrotoxic) | mg/kgBw/day | EFSA, 2020 | ||
| POExi | Probability of exceedance | Domenech & Martorell, 2021 [62] | ||
| CRxi | Cancer risk | EDIxi · SFx | ATSDR, 2022 | |
| SFx | Slope factor | SF (iAs): 1.5 | (mg/kgBw/day)−1 | EPA, 2022 [64] |
| SF(Pb): 8.5 × 10−3 | (mg/kgBw/day)−1 | EPA, 2022 [64] | ||
| SF(AA): 5 × 10−1 | (mg/kgBw/day)−1 | EPA, 2022 [64] |
* The reference value used was the reference dose (RfD).
2.4. Statistical Analysis
Multivariate ANOVA and Tukey’s mean comparison test were performed on SPSS V.25 statistical software to find any differences between the groups. A p-value of less than 0.05 was considered statistically significant, while the F-ratio (variability due to the considered effect divided by the residual variance) was obtained to analyse the factor’s effect on a variable.
3. Results and Discussion
3.1. Hazard Concentration
The results showed that Cd and Hg values were below the LOD, while the concentrations of iAs, Cr, Pb, AA and OTA were above it in all the countries studied. Table 2 gives the mean, standard deviation, minimum and maximum concentration of quantified hazards, the multifactor analysis of variance (ANOVA), F-ratio and significant differences for the factor: country of origin. Figure S1 shows the concentrations of iAs, Cd, Cr, Pb, AA and OTA found by other authors and those obtained in the present study.

Table 2.
Metals above LOD, AA and OTA concentrations (mean, standard deviation (SD), minimum and maximum) in instant coffee from Brazil, Colombia, Mexico and Peru. ANOVA F-ratio per country.
The mean levels of iAs in the four countries studied ranged from 4.68 × 10−2 mg/kg in Colombia to 5.16 × 10−2 mg/kg in Peru. These values are higher than those obtained in a study carried out in Denmark by Pedersen et al. (1994) [67], in which the values ranged from 7.00 × 10−4 to 7.00 × 10−3 mg/kg. Values below the LOD (<1.00 × 10−2 mg/kg) were obtained by Dos Santos and De Oliveira in instant coffee from Brazil [25]. These different iAs concentrations in instant coffee can be related to very different variables such as the crop production area, the manufacturing process, the technique used to obtain instant coffee, the system of analysis, etc. [24,27,68].
The mean Cr values were of the order of 6.00 × 10−3 mg/kg in all the countries, being slightly higher in Peru (8.00 × 10−3 mg/kg). These values were higher by one order of magnitude than those obtained by Suseela et al. (2001) [69] in India (from 7.00 × 10−4 to 8.00 × 10−4 mg/kg). However, they were lower by two orders of magnitude to those found in the work by Dos Santos and De Oliveira [70] in samples from Brazil (5.20 × 10−1 mg/kg), Grembecka et al., (2007) [71] and Szymczycha-Madeja et al., (2015) [72] in instant coffee marketed in Poland (3.00 × 10−1 and 4.87 × 10−1 mg/kg, respectively), Pedersen et al., (1994) [67] in Denmark (2.30 × 10−1 mg/kg), Zaidi et al., (2005) [73] in Pakistan (1.07 × 10−1 mg/kg) and Oliveira et al., (2012) [74] in Portugal (5.00 × 10−2 mg/kg).
Our results on Pb contamination of instant coffee ranged from a minimum value of 1.70 × 10−2 mg/kg, obtained in Colombia, to a maximum of 2.70 × 10−2 mg/kg in Brazil. These values are in agreement with previous studies conducted in Poland by Winiarska-Mieczan et al. (2022) (8.26 × 10−2 mg/kg) [75] and in India by Suseela et al. (2001) (2.10 × 10−2 to 2.30 × 10−1 mg/kg) [69]. Concentrations notably lower or below the LOD were reported by Pedersen et al. (1994) (LOD—6.00 × 10−4 mg/kg) [67], Dos Santos and De Oliveira (2001) (<LOD: 1 mg/kg) [70], Grembecka et al. (2007) (<LOD: 1.00 × 10−1 mg/kg) [71], Szymczycha-Madeja et al. (2015) (<LOD: 2.70 × 10−1 mg/kg) [72]. The differences we found in iAs, Cr and Pb in the individual countries were not significant.
We also found a Cd of <LOD, in agreement with that obtained by Dos Santos and De Oliveira (2001) [70] in Brazil and slightly lower than those obtained by Pedersen et al. (1993) [67] in Denmark (2.50 × 10−3 mg/kg), Suseela et al. (2001) [69] in India (1.00 × 10−3 to 2.90 × 10−2 mg/kg) and Winiarska-Mieczan et al. (2022) (9.50 × 10−2 mg/kg) [75] and Grembecka et al. (2007) (1 × 10−1 mg/kg) [71] in Poland.
The findings for AA showed that the mean values ranged from 1.77 × 10−1 mg/kg in Peru to 4.77 × 10−1 mg/kg in Brazil. In relation to the maximum value, Brazil obtained the highest value, with 6.2 × 10−1 mg/kg. This result indicates that all the samples were below the limit of 8.5 × 10−1 mg/kg established by the EU (Regulation (EU) 2017/2158) [38]. On the other hand, the differences in AA content between the countries were significant (p < 0.05). Previous studies found that these differences can be attributed to many factors such as the type of coffee bean (Robusta and Arabica), the manufacturing process used (elimination of asparagine or its conversion into aspartic acid, which slows down the Maillard reaction), or the storage conditions (which can favour the formation of acrylamide in coffee) [30,76]. These results agree with numerous authors such as Granby and Fagt (2004) [77], Şenyuva and Gökmen (2005) [78], Baron Cortes et al. (2021) [79], Andrzejewski et al. (2004) [80], Loaëc et al. (2014) [81], Arisseto et al. (2007) [82], Surma et al. (2017) [83], Gonzalez et al. (2022) [84], and Lee et al. (2020) [85], whose values were from 1.07 × 10−1 to 7.1 × 10−1 mg/kg. Values in the order of 1 × 10−2 mg/kg were obtained in studies by Claeys et al. (2016) [86] and Health Canada (2012) [87]. A monitoring study carried out by EFSA (2015) showed that the mean AA values tested in instant European coffee were 7.16 × 10−1 mg/kg and 1.12 mg/kg at 95th percentile [66]. Karami et al. (2022) [88] found that the AA values ranged from 5.00 × 10−4 to 4.41 × 10−1 mg/kg. The study published by El-Zakhem et al. (2018) [89] is in line with the lowest value obtained (<5.00 × 10−4 mg/kg).
The OTA contamination in the samples analysed was 85%, with Peru and Mexico being the countries with the highest number of positive samples in instant coffee (100% in both). The high OTA percentages in instant coffee indicate that a greater effort must be made to control the presence of OTA to effectively safeguard human health. These results are lower than the 95% obtained by Vecchio et al. (2012) [59] and Yazdanfar et al. (2022) [90], and 100% by Aoyama et al. (2010) [91], Casal et al. (2014) [92], Galarce-Bustos et al. (2014) [93], T. P. Lee et al. (2012) [94] and Liu et al. (2008) [95]. In contrast, the values obtained in the present study are higher than the works of García-Moraleja et al. (2015) (33%) [96], Hajok et al. (2019) [97], Pokrzywa et al. (2022) [98] (43 and 63%, respectively) and Nielsen et al. (2015) (65%) [99].
The OTA concentrations we obtained were in the order of 1 × 10−3 mg/kg. Mexico and Brazil were the countries with the highest concentrations (1.71 × 10−3 mg/kg and 1.77 × 10−3 mg/kg, respectively). The ANOVA results indicated significant differences between the countries. It is noteworthy that the values obtained were below the limit allowed in the European Union (5.00 × 10−3 mg/kg) laid down in Commission Regulation (UE) 2023/915 [43]. These results are in agreement with those obtained by Galarce-Bustos et al. (2014) (1.80 × 10−3 mg/kg) [93], García-Moraleja et al. (2015) (4.93 × 10−3 mg/kg) [96], Hajok et al. (2019) (1.5 × 10−3 mg/kg) [97], Lee et al. (2012) (5.71 × 10−3 mg/kg) [94], Liu et al. (2008) (4.70 × 10−3 mg/kg) [95], Pokrzywa et al. (2022) (1.63 × 10−3 mg/kg) [98], Skarkova et al. (2013) (1.04 × 10−3 mg/kg) [100], Vecchio et al. (2012) (1.27 × 10−3 mg/kg) [59]. The studies by Aoyama et al. (2010), Casal et al. (2014) and Nielsen et al. (2015) stand out [91,92,99] with slightly lower values, in the order of 1E-04 mg/kg. Finally, values higher than 1 × 10−2 mg/kg and, therefore, above those permitted in the EU were obtained by Lindenmeier et al. (2011) [101] and Yazdanfar et al. (2022) [79]. According to a study by Gopinandhan et al. (2007) [102], high levels of OTA in instant coffee may be a consequence of adulteration with coffee husk, especially cherry husk. It is also known that when preparing instant coffee from blends with the robusta variety can directly lead to high levels of this mycotoxin [103,104,105]. Previous studies also concluded that OTA reduction by roasting ranges from zero to 100% [106,107]. Other studies focused on the stability of the toxin at processing temperatures and concluded that it is very stable [97,108]. The amount of OTA also depends on the season and storage conditions and time [97].
Table 3 shows the pdf-adjusted concentration of the hazards by country obtained using @Risk 8 software (Palisade, Newfield). For Cd and Hg, which were not detected, the concentration considered to characterise the risk was LOD/2 in both cases, i.e., 0.0025 mg/kg.

Table 3.
Distribution of the hazards studied in instant coffee by country of origin (mg/kg) (see Figure S2).
3.2. Risk Characterisation
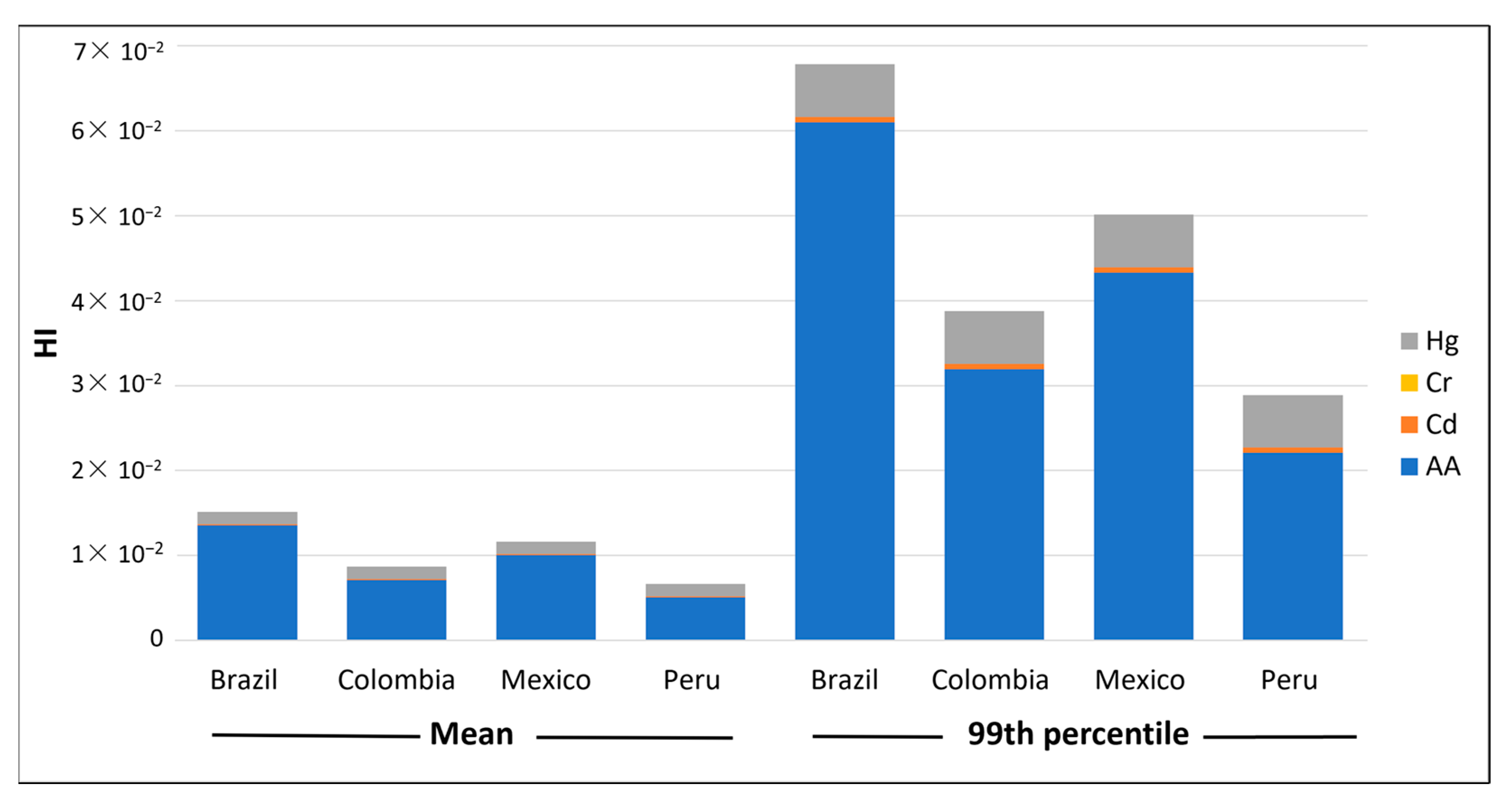
To assess both the non-genotoxic and genotoxic effects, we used metrics to characterise the risk from different countries (see Table 4). The HQ (for Cd, Cr, Hg and AA) and the HI values (summary of HQs) were used to estimate the partial and total chronic toxicity risk per country of Brazil, Colombia, Mexico and Peru, respectively (see Figure 1). As can be seen, all the findings were less than 1 in all cases, which indicates that the consumption of instant coffee, in relation to the non-genotoxic hazards studied, does not represent a level of concern.

Table 4.
Risk characterisation per country using HQ, HI, MOE and CR metrics considering non-genotoxic and genotoxic effects.

Figure 1.
Mean and 99th percentile values of the HQ contribution of each chemical hazard to HI in different countries.
The results indicated that AA (5.05 × 10−3 to 1.00 × 10−2) was the hazard with the highest HQ and, therefore, the major contributor to HI. The lowest HI value was found in Peru (6.63 × 10−3). Of the few previous works that characterised the risk in this product, Karami et al. (2022) [88] concluded that the HQ for AA determined in instant coffee purchased from different supermarkets in Tehran (Iran) was 2 × 10−4, indicating that the AA in instant coffee did not pose a health risk. Values lower than 1 were also found by Dybing et al. (2003) and Oroian et al. (2015) [109,110]. In the same vein, the calculated HQ for OTA in instant coffee was found to be below 1 [90,97], which indicated a safe level with no risk to human health.
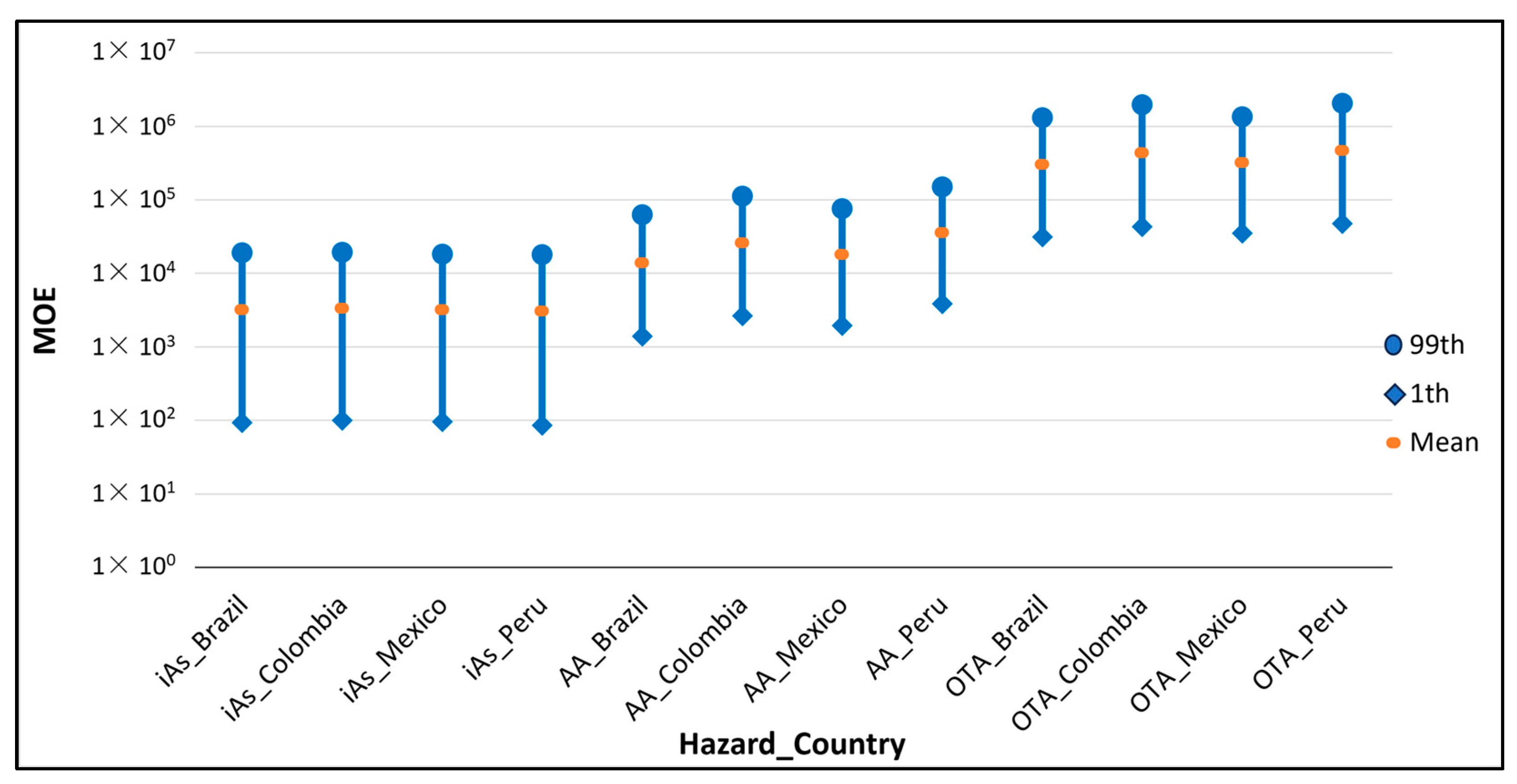
Figure 2 shows the MOE distributions per country obtained for iAs, AA and OTA, considering the BMDL established for the genotoxic effects of these hazards. The results showed that the highest MOE was obtained in OTA, for which almost all the values of the country distributions were above the threshold of 10,000, making the level of concern acceptable, while all the distributions obtained for iAs were below the threshold (see Table 4), indicating that the risk should be considered of high concern. However, the Committee on Carcinogenicity of Chemicals in Food, Consumer Products and the Environment proposed that an MOE of 10 or higher, associated with a 0.5% increased risk of lung cancer in humans, for iAs could be considered of low concern [111]. According to the Swedish National Food Agency, a value between 1–10 should be considered a high to moderate risk, between 10–100 moderate to low, and higher values no risk [112]. Along the same lines, the results obtained from the POE metric, equal to zero, indicated that the exposure did not exceed the BMDL in any case and that the risk could be considered acceptable.

Figure 2.
MOE for each country and hazard at the 1st, mean and 99th percentile when the BMDL considered was associated with a genotoxic effect (see Table 1).
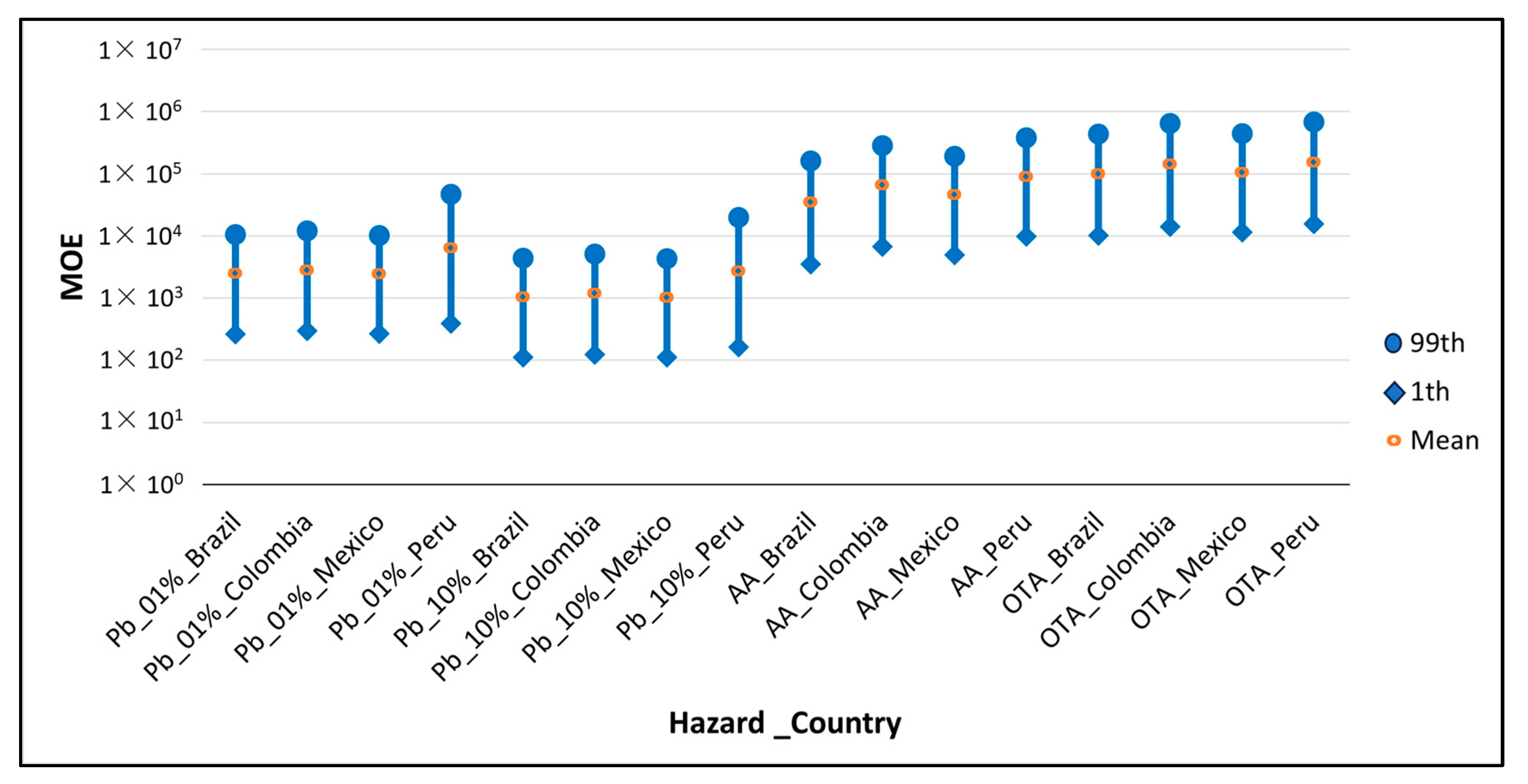
Figure 3 shows the MOE distributions obtained in the different countries for the non-genotoxic Pb effects (cardiovascular and nephrotoxic), AA and OTA. The findings show that AA ranged from 1 × 103 to 1 × 105 and OTA from 1 × 104 to 1 × 106, Table 4. Similar results were found by Karami et al., 2022, who obtained an MOE for the neurological effect of AA of 5 × 105 and Yazdanfar et al., 2022, for the non-neoplastic effect of OTA of 2.78 × 103 [88,90]. Pb had the smallest values, all being below the threshold of 10,000. However, the values were above 100, so taking into account the EFSA’s scientific opinion on lead in food, it was concluded that only when the MOE is <1 the possibility of effects on health cannot be excluded [65] and that the POE results were zero in all cases, which means that the exposure did not exceed the BMDL, indicating that the presence of all three hazards in instant coffee from the countries studied can be considered of low concern.

Figure 3.
MOE for each country and hazard in the 1st, mean and 99th percentile, in which the BMDL considered was associated with cardiovascular, nephrotoxic and neurotoxic effects (see Table 1).
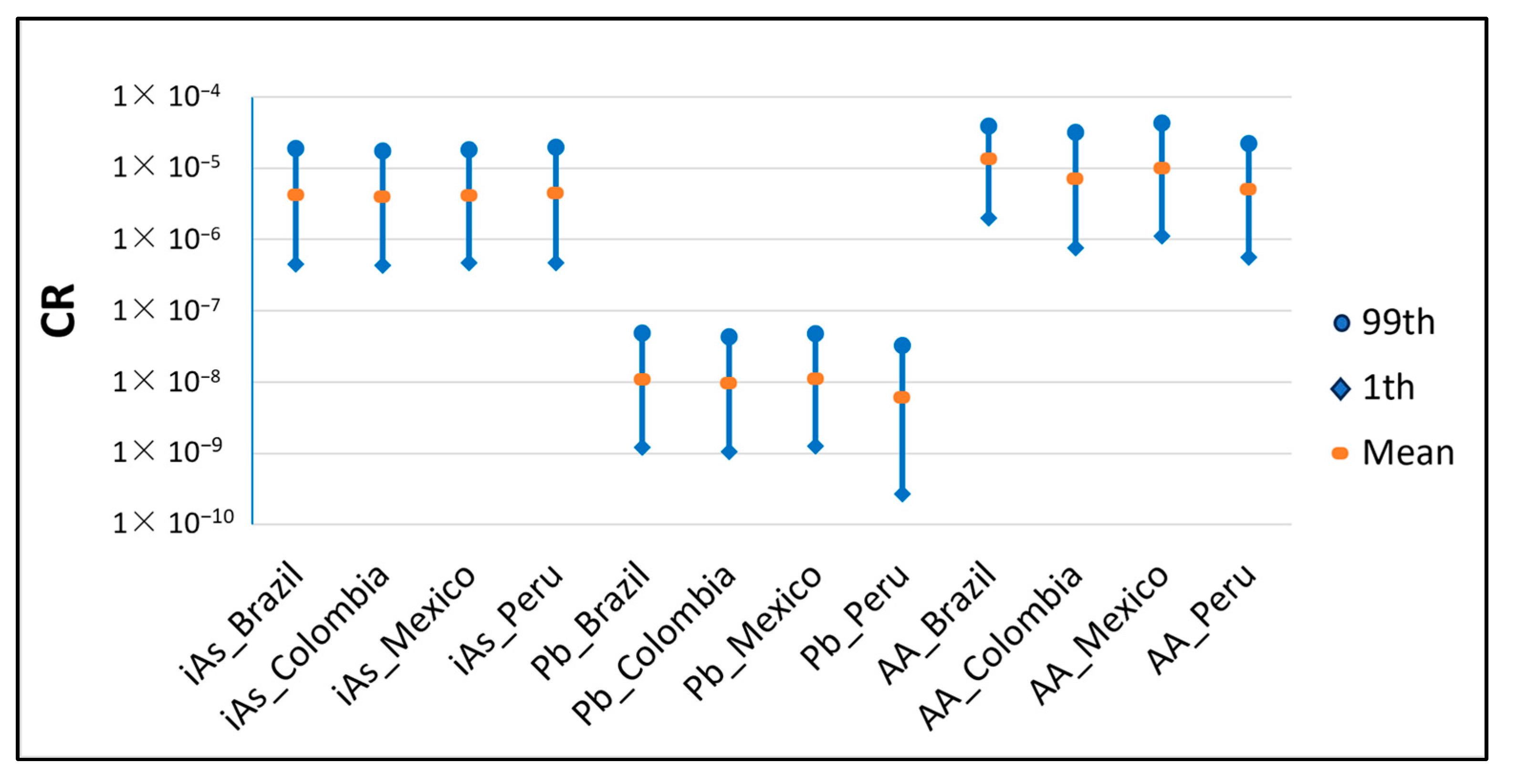
The CR or probability of an individual developing cancer over a lifetime due to the consumption of instant coffee was calculated for iAs, Pb and AA. Figure 4 represents the 1st percentile, mean and 99th percentile of the distribution obtained for each country and hazard. As can be seen, the results for Pb were lower than 1078 × 10−6, so this hazard in instant coffee does not result in any adverse health effects and the risk is considered low. However, almost the whole distribution is between 1.0 × 10−4 and 1.0 × 10−6 for the rest of the hazards, which is generally interpreted as a moderate risk.

Figure 4.
CR for each country and hazard at the 1st, mean and 99th percentile.
4. Conclusions
The present study reports on the levels of iAs, Cd, Cr, Hg, Pb, AA and OTA in instant coffee manufactured in Brazil, Colombia, Mexico and Peru. The results showed that AA reached the highest concentrations and the differences per country were significant. In relation to risk characterisation, the non-genotoxic effect studied for Cd, Cr, Hg and AA based on HQ and HI metrics revealed that the consumers are within a safe range of risk. The MOE-POE combination calculated for the non-genotoxic effect of Pb, AA and OTA also indicated a low level of concern. Focusing on the genotoxic effect, iAs and AA obtained the lowest MOEs and the highest probabilities of cancer risk, indicating that these hazards involve a moderate risk. The results highlight the need to focus on the pre-production stages of the finished product in order to find appropriate and practical solutions to develop strategies that will mitigate the chemical risks in instant coffee. Attention should focus on issues such as the selection of the growing areas, fermentation conditions, enzymatic treatment of the raw material, alternative vacuum or steam roasting technologies, etc. Only in this way, and following the principle of the ALARA concept (as low as reasonably achievable) at each stage of the process, will it be possible to guarantee a high level of human health protection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods13050726/s1; Figure S1: Comparison of the metal concentrations (iAs, Cd, Cr and Pb), AA and OTA in instant coffee found by other authors and those obtained in this study; Figure S2: Distributions for each hazard and country.
Author Contributions
Conceptualization, G.A.G. and E.D.; methodology, G.A.G. and E.D.; validation, G.A.G. and D.E.G.-Y.; formal analysis, E.A.; investigation, G.A.G. and E.A.; resources, G.A.G. and D.E.G.-Y.; writing—original draft preparation, G.A.G. and E.D.; writing—review and editing, G.A.G. and E.A.; visualization, G.A.G., E.A. and D.E.G.-Y.; supervision, E.D.; funding acquisition, G.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was financed by the Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Material, further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors wish to express their gratitude to the Peruvian National Public Investment System Project—SNIP N° 352439 “Creation of Coffee Research Services, Innovation and Technology Transfer Centre—CEINCAFE”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- International Coffee Organization (ICO) Coffee Market Report. October 2023. Available online: https://www.icocoffee.org/documents/cy2022-23/cmr-0523-c.pdf (accessed on 25 October 2023).
- International Coffee Organization (ICO) Monthly Coffee Market Report; The International Coffee Organization: London, UK, 2019; Volume 9.
- Vanesa, D.; Ana, P. Occurrence of Ochratoxin A in Coffee Beans, Ground Roasted Coffee and Soluble Coffee and Method Validation. Food Control 2013, 30, 675–678. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Anandharamakrishnan, C. Spray-Freeze-Drying Approach for Soluble Coffee Processing and Its Effect on Quality Characteristics. J. Food Eng. 2015, 149, 171–180. [Google Scholar] [CrossRef]
- Cunha, S.C.; Senra, L.; Cruz, R.; Casal, S.; Fernandes, J.O. 4-Methylimidazole in Soluble Coffee and Coffee Substitutes. Food Control 2016, 63, 15–20. [Google Scholar] [CrossRef]
- Deotale, S.M.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Influence of Drying Techniques on Sensory Profile and Chlorogenic Acid Content of Instant Coffee Powders. Meas. Food 2022, 6, 100030. [Google Scholar] [CrossRef]
- Massulo Souza, R.; Moreira, C.Q.; Vieira, R.P.; Coltro, L.; Alves, R.M.V. Alternative Flexible Plastic Packaging for Instant Coffees. Food Res. Int. 2023, 17, 1131652. [Google Scholar] [CrossRef] [PubMed]
- Gosalvitr, P.; Cuéllar-Franca, R.M.; Smith, R.; Azapagic, A. An Environmental and Economic Sustainability Assessment of Coffee Production in the UK. Chem. Eng. J. 2023, 465, 142793. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant Activity, Polyphenols, Caffeine and Melanoidins in Soluble Coffee: The Influence of Processing Conditions and Raw Material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- Mendes, K.; Luchine, A. Non-Tariff Barriers Removal in the Brazilian Coffee Industry. J. Int. Trade Law Policy 2020, 19, 139–157. [Google Scholar] [CrossRef]
- Chavez, S.G.; Mendoza, M.M.; Caetano, A.C. Antioxidants, Phenols, Caffeine Content and Volatile Compounds in Coffee Beverages Obtained by Different Methods. Food Sci. Technol. 2022, 42, 1–8. [Google Scholar] [CrossRef]
- Hseu, Z.Y.; Su, S.W.; Lai, H.Y.; Guo, H.Y.; Chen, T.C.; Chen, Z.S. Remediation Techniques and Heavy Metal Uptake by Different Rice Varieties in Metal-Contaminated Soils of Taiwan: New Aspects for Food Safety Regulation and Sustainable Agriculture. Soil Sci. Plant Nutr. 2010, 56, 31–52. [Google Scholar] [CrossRef]
- Pigozzi, M.T.; Passos, F.R.; Mendes, F.Q. Quality of Commercial Coffees: Heavy Metal and Ash Contents. J. Food Qual. 2018, 2018, 5908463. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kwiatkowska, K.; Kwiecień, M.; Zaricka, E. Assessment of the Risk of Exposure to Cadmium and Lead as a Result of the Consumption of Coffee Infusions. Biol. Trace Elem. Res. 2021, 199, 2420–2428. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chen, F.A.; Hsu, M.J. Threat of Heavy Metal Pollution in Halophytic and Mangrove Plants of Tamil Nadu, India. Environ. Pollut. 2008, 155, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Dghaim, R.; Al Khatib, S.; Rasool, H.; Ali Khan, M. Determination of Heavy Metals Concentration in Tradi. J. Environ. Public Health 2015, 2015, 973878. [Google Scholar] [CrossRef] [PubMed]
- Fathabad, A.E.; Shariatifar, N.; Moazzen, M.; Nazmara, S.; Fakhri, Y.; Alimohammadi, M.; Azari, A.; Mousavi Khaneghah, A. Determination of Heavy Metal Content of Processed Fruit Products from Tehran’s Market Using ICP- OES: A Risk Assessment Study. Food Chem. Toxicol. 2018, 115, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Barone, G.; Dambrosio, A.; Storelli, A.; Garofalo, R.; Busco, V.; Pietro; Storelli, M.M. Estimated Dietary Intake of Trace Metals from Swordfish Consumption: A Human Health Problem. Toxics 2018, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. New Evidence against Chromium as an Essential Trace Element. J. Nutr. 2017, 147, 2212–2219. [Google Scholar] [CrossRef]
- Monga, A.; Fulke, A.B.; Dasgupta, D. Recent Developments in Essentiality of Trivalent Chromium and Toxicity of Hexavalent Chromium: Implications on Human Health and Remediation Strategies. J. Hazard. Mater. Adv. 2022, 7, 100113. [Google Scholar] [CrossRef]
- Paz, S.; Rubio, C.; Frías, I.; Gutiérrez, Á.J.; González-Weller, D.; Martín, V.; Revert, C.; Hardisson, A. Toxic Metals (Al, Cd, Pb and Hg) in the Most Consumed Edible Seaweeds in Europe. Chemosphere 2019, 218, 879–884. [Google Scholar] [CrossRef]
- Hsu, M.J.; Selvaraj, K.; Agoramoorthy, G. Taiwan’s Industrial Heavy Metal Pollution Threatens Terrestrial Biota. Environ. Pollut. 2006, 143, 327–334. [Google Scholar] [CrossRef]
- Adler, G.; Nędzarek, A.; Tórz, A. Concentrations of Selected Metals (NA, K, CA, MG, FE, CU, ZN, AL, NI, PB, CD) in Coffee. Slov. J. Public Health 2019, 58, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe, G.A.; Chavez, S.G.; Arellanos, E.; Doménech, E. Probabilistic Risk Characterization of Heavy Metals in Peruvian Coffee: Implications of Variety, Region and Processing. Foods 2023, 12, 3254. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, É.J.; De Oliveira, E. Evaluation of Arsenic and Selenium in Brazilian Soluble Coffee by Inductively Coupled Plasma Atomic Emission Spectrometry with Hydride Generation. Braz. Arch. Biol. Technol. 2001, 44, 233–238. [Google Scholar] [CrossRef]
- Santos, E.E.; Lauria, D.C.; Porto Da Silveira, C.L. Assessment of Daily Intake of Trace Elements Due to Consumption of Foodstuffs by Adult Inhabitants of Rio de Janeiro City. Sci. Total Environ. 2004, 327, 69–79. [Google Scholar] [CrossRef]
- Vezzulli, F.; Fontanella, M.C.; Lambri, M.; Beone, G.M. Specialty and High-Quality Coffee: Discrimination through Elemental Characterization via ICP-OES, ICP-MS, and ICP-MS/MS of Origin, Species, and Variety. J. Sci. Food Agric. 2023, 103, 4303–4316. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, H.; Bibi, R.; Balal Arain, M.; Safi, F.; Ullah, S.; Castro-Muñoz, R.; Boczkaj, G. Deep Eutectic Solvent (DES) with Silver Nanoparticles (Ag-NPs) Based Assay for Analysis of Lead (II) in Edible Oils. Food Chem. 2022, 379, 132085. [Google Scholar] [CrossRef] [PubMed]
- Elahi, F.; Arain, M.B.; Ali Khan, W.; Ul Haq, H.; Khan, A.; Jan, F.; Castro-Muñoz, R.; Boczkaj, G. Ultrasound-Assisted Deep Eutectic Solvent-Based Liquid–Liquid Microextraction for Simultaneous Determination of Ni (II) and Zn (II) in Food Samples. Food Chem. 2022, 393, 133384. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide Is Formed in the Maillard Reaction. Nature 2002, 76, 448–449. [Google Scholar] [CrossRef]
- Robert, F.; Vuataz, G.; Pollien, P.; Saucy, F.; Alonso, M.I.; Bauwens, I.; Blank, I. Acrylamide Formation from Asparagine under Low-Moisture Maillard Reaction Conditions. 1. Physical and Chemical Aspects in Crystalline Model Systems. J. Agric. Food Chem. 2004, 52, 6837–6842. [Google Scholar] [CrossRef]
- Boyaci Gunduz, C.P. Formulation and Processing Strategies to Reduce Acrylamide in Thermally Processed Cereal-Based Foods. Int. J. Environ. Res. Public Health 2023, 20, 6272. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Deng, P.; He, Z.; Qin, F.; Chen, Q.; Wang, Z.; Pan, H.; Chen, J.; Zeng, M. Research Progress on Generation, Detection and Inhibition of Multiple Hazards—Acrylamide, 5-Hydroxymethylfurfural, Advanced Glycation End Products, Methylimidazole—In Baked Goods. Food Chem. 2024, 431, 137152. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Xu, X.; Lang, W.; Wang, W.; Wang, X.; Xin, A.; Zhou, F.; Ding, Z.; Ye, X.; Zhu, B. Toxicity, Formation, Contamination, Determination and Mitigation of Acrylamide in Thermally Processed Plant-Based Foods and Herbal Medicines: A Review. Ecotoxicol. Environ. Saf. 2023, 260, 115059. [Google Scholar] [CrossRef] [PubMed]
- Acquaticci, L.; Angeloni, S.; Cela, N.; Galgano, F.; Vittori, S.; Caprioli, G.; Condelli, N. Impact of Coffee Species, Post-Harvesting Treatments and Roasting Conditions on Coffee Quality and Safety Related Compounds. Food Control 2023, 149, 109714. [Google Scholar] [CrossRef]
- IARC. Mycotoxin Exposure and Human Cancer Risk: A Systematic Review of Epidemiological Studies. Available online: https://www.iarc.who.int/news-events/mycotoxin-exposure-and-human-cancer-risk-a-systematic-review-of-epidemiological-studies/ (accessed on 17 September 2023).
- Pedreschi, F.; Mariotti, S.; Granby, K.; Risum, J. Acrylamide Reduction in Potato Chips by Using Commercial Asparaginase in Combination with Conventional Blanching. LWT 2011, 44, 1473–1476. [Google Scholar] [CrossRef]
- European Union (UE). Commission Regulation (EU) 2017/2158 of 20 November 2017 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food. Off. J. Eur. Union 2017, 2017, 24–44. [Google Scholar]
- Pakshir, K.; Dehghani, A.; Nouraei, H.; Zareshahrabadi, Z.; Zomorodian, K. Evaluation of Fungal Contamination and Ochratoxin A Detection in Different Types of Coffee by HPLC-Based Method. J. Clin. Lab. Anal. 2021, 35, e24001. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Chang, C.W.; Chen, C.Y. Measuring Ochratoxin a Concentrations in Coffee Beverages with Immunoaffinity Columns and Ultra-Performance Liquid Chromatography/Tandem Mass Spectrometry. J. AOAC Int. 2016, 99, 469–474. [Google Scholar] [CrossRef]
- Li, R.; Zhu, L.; Yang, M.; Liu, A.; Xu, W.; He, P. Silver Nanocluster-Based Aptasensor for the Label-Free and Enzyme-Free Detection of Ochratoxin A. Food Chem. 2024, 431, 137126. [Google Scholar] [CrossRef]
- IARC. Experimental and Pan-Cancer Genome Analyses Reveal Widespread Contribution of Acrylamide Exposure to Carcinogenesis in Humans. Available online: https://www.iarc.who.int/news-events/experimental-and-pan-cancer-genome-analyses-reveal-widespread-contribution-of-acrylamide-exposure-to-carcinogenesis-in-humans/ (accessed on 20 September 2023).
- European Commission. Commission Regulation (EC) No 2023/915 of 25 April 2023 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union L 2023, 119, 103–157. [Google Scholar]
- Roychowdhury, T.; Tokunaga, H.; Ando, M. Survey of Arsenic and Other Heavy Metals in Food Composites and Drinking Water and Estimation of Dietary Intake by the Villagers from an Arsenic-Affected Area of West Bengal, India. Sci. Total Environ. 2003, 308, 15–35. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand. Food Standards Australia New Zealand–24th Australian Total Diet Study—1778-FSANZ_AustDietStudy-Web.Pdf; Food Standards Australia New Zealand: Wellington, New Zealand, 2014; ISBN 9780642345837.
- WHO/IPCS. Guidance Document on Evaluating and Expressing Uncertenty in Hazard Characterization; World Health Organization: Geneva, Switzerland; International Labour Organization: Geneva, Switzerland; United Nations Environment Programme: Nairobi, Kenya, 2014; ISBN 9789241513548. [Google Scholar]
- Doménech, E.; Martorell, S. Assessment of Safety Margins of Exposure to Non-Genotoxic Chemical Substances in Food. Food Control 2017, 79, 1–9. [Google Scholar] [CrossRef]
- Guo, G.; Zhang, D.; Wang, Y. Probabilistic Human Health Risk Assessment of Heavy Metal Intake via Vegetable Consumption around Pb/Zn Smelters in Southwest China. Int. J. Environ. Res. Public Health 2019, 16, 3267. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.M.C.; Faria, M.A.; Cunha, S.C.; Miladinovic, B.; Ferreira, I.M. Transport of Mycotoxins across Human Gastric NCI–N87 and Intestinal Caco-2 cell Models. Food Chem. Toxicol. 2019, 131, 110595. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.; Products, P.; Ppr, R. Guidance on the Use of Probabilistic Methodology for Modelling Dietary Exposure to Pesticide Residues. EFSA J. 2012, 10, 2839. [Google Scholar] [CrossRef]
- Maertens, A.; Golden, E.; Luechtefeld, T.H.; Hoffmann, S.; Tsaioun, K.; Hartung, T. Probabilistic Risk Assessment—The Keystone for the Future of Toxicology. ALTEX 2022, 39, 3–29. [Google Scholar] [CrossRef] [PubMed]
- IGHRC. Chemical Mixtures: A Framework for Assessing Risk to Human Health (CR14); Institute of Environment and Health: Bedfordshire, UK, 2008; ISBN 9781899110445. [Google Scholar]
- Leiva-Tafur, D.; Goñas, M.; Culqui, L.; Santa Cruz, C.; Rascón, J.; Oliva-Cruz, M. Spatiotemporal Distribution of Physicochemical Parameters and Toxic Elements in Lake Pomacochas, Amazonas, Peru. Front. Environ. Sci. 2022, 10, 885591. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater. 2017, p. 1496. Available online: https://secure.apha.org/imis/ItemDetail?iProductCode=978-087553-2998&CATEGORY=BK (accessed on 3 April 2022).
- Cui, S.; Na, J.S.; Kim, N.Y.; Lee, Y.; Nam, S.H. An Investigation on Inorganic Arsenic in Seaweed by Ion Chromatography Combined with Inductively Coupled Plasma-Atomic Emission Spectrometry. Bull. Korean Chem. Soc. 2013, 34, 3206–3210. [Google Scholar] [CrossRef][Green Version]
- Oliveira, M.; Ramos, S.; Delerue-Matos, C.; Morais, S. Espresso Beverages of Pure Origin Coffee: Mineral Characterization, Contribution for Mineral Intake and Geographical Discrimination. Food Chem. 2015, 177, 330–338. [Google Scholar] [CrossRef]
- NTP-ISO/IEC 17025; General Requirements for the Competence of Testing an Calibration Laboratories. INACAL: Lima, Perú, 2017.
- Mesías, M.; Morales, F.J. Acrylamide in Coffee: Estimation of Exposure from Vending Machines. J. Food Compos. Anal. 2016, 48, 8–12. [Google Scholar] [CrossRef]
- Vecchio, A.; Mineo, V.; Planeta, D. Ochratoxin A in Instant Coffee in Italy. Food Control 2012, 28, 220–223. [Google Scholar] [CrossRef]
- Evans, R.M.; Scholze, M.; Kortenkamp, A. Examining the Feasibility of Mixture Risk Assessment: A Case Study Using a Tiered Approach with Data of 67 Pesticides from the Joint FAO/WHO Meeting on Pesticide Residues (JMPR). Food Chem. Toxicol. 2015, 84, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Liu, Q.; Liu, J.; Wang, Q.; Wang, Y. Concentrations of Organophosphorus Pesticides in Fresh Vegetables and Related Human Health Risk Assessment in Changchun, Northeast China. Food Control 2016, 60, 353–360. [Google Scholar] [CrossRef]
- Doménech, E.; Martorell, S. Formulation and Application of the Probability of Exceedance Metric for Risk Characterization of Non-Threshold Chemical Hazards in Food. Food Control 2021, 124, 107910. [Google Scholar] [CrossRef]
- Kowalska, G. The Safety Assessment of Toxic Metals in Commonly Used Herbs, Spices, Tea, and Coffee in Poland. Int. J. Environ. Res. Public Health 2021, 18, 5779. [Google Scholar] [CrossRef] [PubMed]
- EPA Environmental Protection Agency. 2022. Available online: https://iris.epa.gov/AdvancedSearch/ (accessed on 1 April 2022).
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Lead in Food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Acrylamide in Food. EFSA J. 2015, 13, e4104. [Google Scholar] [CrossRef]
- Pedersen, G.A.; Larsen, E.H.; Mortensen, G.K. Beverages as a Source of Toxic Trace Element Intake. Food Addit. Contam. 1994, 11, 351–363. [Google Scholar] [CrossRef]
- Santato, A.; Bertoldi, D.; Perini, M.; Camin, F.; Larcher, R. Using Elemental Profiles and Stable Isotopes to Trace the Origin of Green Coffee Beans on the Global Market. J. Mass Spectrom. 2012, 47, 1132–1140. [Google Scholar] [CrossRef]
- Suseela, B.; Bhalke, S.; Kumar, A.V.; Tripathi, R.M.; Sastry, V.N. Daily Intake of Trace Metals through Coffee Consumption in India. Food Addit. Contam. 2001, 18, 115–120. [Google Scholar] [CrossRef]
- Dos Santos, É.J.; De Oliveira, E. Determination of Mineral Nutrients and Toxic Elements in Brazilian Soluble Coffee by ICP-AES. J. Food Compos. Anal. 2001, 14, 523–531. [Google Scholar] [CrossRef]
- Grembecka, M.; Malinowska, E.; Szefer, P. Differentiation of Market Coffee and Its Infusions in View of Their Mineral Composition. Sci. Total Environ. 2007, 383, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Szymczycha-Madeja, A.; Welna, M.; Pohl, P. Simplified Multi-Element Analysis of Ground and Instant Coffees by ICP-OES and FAAS. Food Addit. Contam.—Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, J.H.; Fatima, I.; Arif, M.; Qureshi, I.H. Determination of Trace Elements in Coffee Beans and Instant Coffee of Various Origins by INAA. J. Radioanal. Nucl. Chem. 2005, 267, 109–112. [Google Scholar] [CrossRef]
- Oliveira, M.; Casal, S.; Morais, S.; Alves, C.; Dias, F.; Ramos, S.; Mendes, E.; Delerue-Matos, C.; Beatriz, M. Intra- and Interspecific Mineral Composition Variability of Commercial Instant Coffees and Coffee Substitutes: Contribution to Mineral Intake. Food Chem. 2012, 130, 702–709. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Jachimowicz, K.; Kislova, S.; Kwiecień, M.; Zasadna, Z.; Yanovych, D. Cadmium and Lead Concentration in Drinking Instant Coffee, Instant Coffee Drinks and Coffee Substitutes: Safety and Health Risk Assessment. Biol. Trace Elem. Res. 2022, 201, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Akillioglu, H.G.; Gökmen, V. Mitigation of Acrylamide and Hydroxymethyl Furfural in Instant Coffee by Yeast Fermentation. Food Res. Int. 2014, 61, 252–256. [Google Scholar] [CrossRef]
- Granby, K.; Fagt, S. Analysis of Acrylamide in Coffee and Dietary Exposure to Acrylamide from Coffee. Anal. Chim. Acta 2004, 520, 177–182. [Google Scholar] [CrossRef]
- Şenyuva, H.Z.; Gökmen, V. Study of Acrylamide in Coffee Using an Improved Liquid Chromatography Mass Spectrometry Method: Investigation of Colour Changes and Acrylamide Formation in Coffee during Roasting. Food Addit. Contam. 2005, 22, 214–220. [Google Scholar] [CrossRef]
- Barón Cortés, W.R.; Vásquez Mejía, S.M.; Suárez Mahecha, H. Consumption Study and Margin of Exposure of Acrylamide in Food Consumed by the Bogotá Population in Colombia. J. Food Compos. Anal. 2021, 100, 103934. [Google Scholar] [CrossRef]
- Andrzejewski, D.; Roach, J.A.G.; Gay, M.L.; Musser, S.M. Analysis of Coffee for the Presence of Acrylamide by LC-MS/MS. J. Agric. Food Chem. 2004, 52, 1996–2002. [Google Scholar] [CrossRef]
- Loaëc, G.; Jacolot, P.; Helou, C.; Niquet-Léridon, C.; Tessier, F.J. Acrylamide, 5-Hydroxymethylfurfural and Nε-Carboxymethyl-Lysine in Coffee Substitutes and Instant Coffees. Food Addit. Contam.—Part A 2014, 31, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Arisseto, A.P.; Toledo, M.C.; Govaert, Y.; Van Loco, J.; Fraselle, S.; Weverbergh, E.; Degroodt, J.M. Determination of Acrylamide Levels in Selected Foods in Brazil. Food Addit. Contam. 2007, 24, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Surma, M.; Sadowska-Rociek, A.; Cieślik, E.; Sznajder-Katarzyńska, K. Optimization of QuEChERS Sample Preparation Method for Acrylamide Level Determination in Coffee and Coffee Substitutes. Microchem. J. 2017, 131, 98–102. [Google Scholar] [CrossRef]
- González, N.; Marquès, M.; Calderón, J.; Collantes, R.; Corraliza, L.; Timoner, I.; Bosch, J.; Castell, V.; Domingo, J.L.; Nadal, M. Occurrence and Dietary Intake of Food Processing Contaminants (FPCs) in Catalonia, Spain. J. Food Compos. Anal. 2022, 106, 104272. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.J. Dietary Exposure to Acrylamide and Associated Health Risks for the Korean Population. Int. J. Environ. Res. Public Health 2020, 17, 7619. [Google Scholar] [CrossRef] [PubMed]
- Claeys, W.; De Meulenaer, B.; Huyghebaert, A.; Scippo, M.L.; Hoet, P.; Matthys, C. Reassessment of the Acrylamide Risk: Belgium as a Case-Study. Food Control 2016, 59, 628–635. [Google Scholar] [CrossRef]
- Health Canada Health Canada Bureau of Chemical Safety. Health Canada Revised Exposure Assessment of Acrylamide in Food; Health Canada Bureau of Chemical Safety, 2012; Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/pdf/securit/chem-chim/food-aliment/acrylamide/rev-eval-exposure-exposition-eng.pdf (accessed on 23 April 2023).
- Karami, M.; Akbari-Adergani, B.; Jahed Khaniki, G.; Shariatifar, N.; Sadighara, P. Determination and Health Risk Assessment of Acrylamide Levels in Instant Coffee Products Available in Tehran Markets by GC-MS. Int. J. Environ. Anal. Chem. 2022, 14, 1–10. [Google Scholar] [CrossRef]
- El-Zakhem Naous, G.; Merhi, A.; Abboud, M.I.; Mroueh, M.; Taleb, R.I. Carcinogenic and Neurotoxic Risks of Acrylamide Consumed through Caffeinated Beverages among the Lebanese Population. Chemosphere 2018, 208, 352–357. [Google Scholar] [CrossRef]
- Yazdanfar, N.; Mahmudiono, T.; Fakhri, Y.; Mahvi, A.H.; Sadighara, P.; Mohammadi, A.A.; Yousefi, M. Concentration of Ochratoxin A in Coffee Products and Probabilistic Health Risk Assessment. Arab. J. Chem. 2022, 15, 104376. [Google Scholar] [CrossRef]
- Aoyama, K.; Nakajima, M.; Tabata, S.; Eiichi, I.; Tanaka, T.; Norizuki, H.; Itoh, Y.; Fujita, K.; Kai, S.; Tsutsumi, T.; et al. Four-Year Surveillance for Ochratoxin a and Fumonisins in Retail Foods in Japan. J. Food Prot. 2010, 73, 344–352. [Google Scholar] [CrossRef]
- Casal, S.; Vieira, T.; Cruz, R.; Cunha, S.C. Ochratoxin A in Commercial Soluble Coffee and Coffee Substitutes. Food Res. Int. 2014, 61, 56–60. [Google Scholar] [CrossRef]
- Galarce-Bustos, O.; Alvarado, M.; Vega, M.; Aranda, M. Occurrence of Ochratoxin A in Roasted and Instant Coffees in Chilean Market. Food Control 2014, 46, 102–107. [Google Scholar] [CrossRef]
- Lee, T.P.; Saad, B.; Ng, E.P.; Salleh, B. Zeolite Linde Type L as Micro-Solid Phase Extraction Sorbent for the High Performance Liquid Chromatography Determination of Ochratoxin A in Coffee and Cereal. J. Chromatogr. A 2012, 1237, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Tsao, Z.J.; Wang, J.J.; Yu, F.Y. Development of a Monoclonal Antibody against Ochratoxin A and Its Application in Enzyme-Linked Immunosorbent Assay and Gold Nanoparticle Immunochromatographic Strip. Anal. Chem. 2008, 80, 7029–7035. [Google Scholar] [CrossRef]
- García-Moraleja, A.; Font, G.; Mañes, J.; Ferrer, E. Analysis of Mycotoxins in Coffee and Risk Assessment in Spanish Adolescents and Adults. Food Chem. Toxicol. 2015, 86, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Hajok, I.; Kowalska, A.; Piekut, A.; Ćwieląg-Drabek, M. A Risk Assessment of Dietary Exposure to Ochratoxin A for the Polish Population. Food Chem. 2019, 284, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Pokrzywa, P.; Surma, M.; Szarek, S. Coffee and Wine with Ochratoxin A—Exposure Risk Assessment Resulting from Its Consumption. Med. Ogólna Nauk. Zdrowiu 2022, 28, 190–195. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Ngemela, A.F.; Jensen, L.B.; De Medeiros, L.S.; Rasmussen, P.H. UHPLC-MS/MS Determination of Ochratoxin a and Fumonisins in Coffee Using QuEChERS Extraction Combined with Mixed-Mode SPE Purification. J. Agric. Food Chem. 2015, 63, 1029–1034. [Google Scholar] [CrossRef]
- Skarkova, J.; Ostry, V.; Malir, F.; Roubal, T. Determination of Ochratoxin A in Food by High Performance Liquid Chromatography. Anal. Lett. 2013, 46, 1495–1504. [Google Scholar] [CrossRef]
- Lindenmeier, M.; Schieberle, P.; Rychlik, M. Determination of Ochratoxin A in Food: Comparison of a Stable Isotope Dilution Assay, Liquid Chromatography-Fluorescence Detection and an Enzyme-Linked Immunosorbent Assay. Mycotoxin Res. 2011, 27, 115–121. [Google Scholar] [CrossRef]
- Gopinandhan, T.; Velmourougane, K.; Panneerselvam, P.; Keshamma, E.; Raghuramulu, Y. Occurrence of Ochratoxin-A (OT-A) in Green and Commercial Coffee Samples. J. Food Sci. Technol. 2007, 44, 247–249. [Google Scholar]
- Culliao, A.G.L.; Barcelo, J.M. Fungal and Mycotoxin Contamination of Coffee Beans in Benguet Province, Philippines. Food Addit. Contam.—Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Duris, D.; Mburu, J.K.; Durand, N.; Clarke, R.; Frank, J.M.; Guyot, B. Ochratoxin a Contamination of Coffee Batches from Kenya in Relation to Cultivation Methods and Post-Harvest Processing Treatments. Food Addit. Contam.—Part A 2010, 27, 836–841. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khaneghah, A.M.; Fakhri, Y.; Abdi, L.; Coppa, C.F.S.C.; Franco, L.T.; de Oliveira, C.A.F. The Concentration and Prevalence of Ochratoxin A in Coffee and Coffee-Based Products: A Global Systematic Review, Meta-Analysis and Meta-Regression. Fungal Biol. 2019, 123, 611–617. [Google Scholar] [CrossRef]
- Tozlovanu, M.; Pfohl-Leszkowicz, A. Ochratoxin A in Roasted Coffee from French Supermarkets and Transfer in Coffee Beverages: Comparison of Analysis Methods. Toxins 2010, 2, 1928–1942. [Google Scholar] [CrossRef] [PubMed]
- Vatinno, R.; Aresta, A.; Zambonin, C.G.; Palmisano, F. Determination of Ochratoxin A in Green Coffee Beans by Solid-Phase Microextraction and Liquid Chromatography with Fluorescence Detection. J. Chromatogr. A 2008, 1187, 145–150. [Google Scholar] [CrossRef]
- Lobeau, M.; De Saeger, S.; Sibanda, L.; Barna-Vetró, I.; Van Peteghem, C. Development of a New Clean-up Tandem Assay Column for the Detection of Ochratoxin A in Roasted Coffee. Anal. Chim. Acta 2005, 538, 57–61. [Google Scholar] [CrossRef]
- Dybing, E.; Sanner, T. Risk Assessment of Acrylamide in Foods. Toxicol. Sci. 2003, 75, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Amariei, S.; Gutt, G. Acrylamide in Romanian Food Using HPLC-UV and a Health Risk Assessment. Food Addit. Contam. Part B Surveill. 2015, 8, 136–141. [Google Scholar] [CrossRef]
- Central Oklahoma Telephone Co. On Toxicity of Chemicals in Food, Consumer Products and the Environment. Statement on Potential Risks from Arsenic in the Diet of Infants Aged 0 to 12 Months and Children Aged 1 to 5 Years. 2016; pp. 1–47. Available online: https://cot.food.gov.uk/sites/default/files/finalstatementonarsenic_0.pdf, (accessed on 23 April 2023).
- Kollander, B.; Sundström, B.; Öhrvik, V.; Abramsson, L. Inorganic Arsenic in Rice and Rice Products on the Swedish Market 2015 Part 2: Survey. In Proceedings of the Arsenic Research and Global Sustainability Proceedings of the Sixth International Congress on Arsenic in the Environment (As2016), Stockholm, Sweden, 19–23 June 2016; pp. 277–278. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).