Probabilistic Risk Assessment of Metals, Acrylamide and Ochratoxin A in Instant Coffee from Brazil, Colombia, Mexico and Peru
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Material
2.2. Chemical Analysis
2.2.1. Heavy Metal(oid)s
2.2.2. Acrylamide
2.2.3. Ochratoxin A
2.3. Risk Characterisation
| Parameter | Description | Value | Units | Source |
|---|---|---|---|---|
| HQxi | Hazard quotient | EDIxi/RVx | ATSDR, 2022 | |
| HIi | Hazard index per country | ATSDR, 2022 | ||
| EDIxi | Estimated daily intake | Cxi · IR/Bw | mg/kgBw/day | EFSA, 2013 |
| Cxi | Concentration | Table 2 | mg/kg | This study |
| IR | Ingestion rate | Exponential (5%; 6.82 × 10−4; 95%; 1.2 × 10−2) | kg/day | EFSA, 2023 |
| Bw | Body weight | Gamma (2%; 54; 50%; 75; 98%; 110) | kgBw | CTCF, 2012 |
| RVx | Reference Value | RfD * (Cr): 3 × 10−4 | mg/kgBw/day | EPA, 2022 [64] |
| RfD (Cd): 1 × 10−3 | mg/kgBw/day | EPA, 2022 [64] | ||
| RfD (Hg): 1 × 10−4 | mg/kgBw/day | EPA, 2022 [64] | ||
| RfD (AA): 2 × 10−3 | mg/kgBw/day | EPA, 2022 [64] | ||
| MOExi | Margin of exposure | BMDL%X/EDIxi | EFSA, 2005 | |
| BMDL%X | Benchmark dose | BMDL01(iAs): Uniform (3 × 10−4; 8 × 10−3) (Carcinogenic) | mg/kgBw/day | EFSA, 2021 |
| BMDL01 (Pb): 1.5 × 10−3 (Cardiovascular) | mg/kgBw/day | EFSA, 2010 [65] | ||
| BMDL10 (Pb): 6.3 × 10−4 (Nephrotoxic) | mg/kgBw/day | EFSA, 2010 [65] | ||
| BMDL10 (AA): 1.7 × 10−1 (Carcinogenic) | mg/kgBw/day | EFSA, 2015 [66] | ||
| BMDL10 (AA): 4.3 × 10−1 (Neurotoxic) | mg/kgBw/day | EFSA, 2015 [66] | ||
| BMDL10 (OTA): 1.45 × 10−2 (Carcinogenic) | mg/kgBw/day | EFSA, 2020 | ||
| BMDL10 (OTA): 4.73 × 10−3 (Nephrotoxic) | mg/kgBw/day | EFSA, 2020 | ||
| POExi | Probability of exceedance | Domenech & Martorell, 2021 [62] | ||
| CRxi | Cancer risk | EDIxi · SFx | ATSDR, 2022 | |
| SFx | Slope factor | SF (iAs): 1.5 | (mg/kgBw/day)−1 | EPA, 2022 [64] |
| SF(Pb): 8.5 × 10−3 | (mg/kgBw/day)−1 | EPA, 2022 [64] | ||
| SF(AA): 5 × 10−1 | (mg/kgBw/day)−1 | EPA, 2022 [64] |
2.4. Statistical Analysis
3. Results and Discussion
3.1. Hazard Concentration
3.2. Risk Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Coffee Organization (ICO) Coffee Market Report. October 2023. Available online: https://www.icocoffee.org/documents/cy2022-23/cmr-0523-c.pdf (accessed on 25 October 2023).
- International Coffee Organization (ICO) Monthly Coffee Market Report; The International Coffee Organization: London, UK, 2019; Volume 9.
- Vanesa, D.; Ana, P. Occurrence of Ochratoxin A in Coffee Beans, Ground Roasted Coffee and Soluble Coffee and Method Validation. Food Control 2013, 30, 675–678. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Anandharamakrishnan, C. Spray-Freeze-Drying Approach for Soluble Coffee Processing and Its Effect on Quality Characteristics. J. Food Eng. 2015, 149, 171–180. [Google Scholar] [CrossRef]
- Cunha, S.C.; Senra, L.; Cruz, R.; Casal, S.; Fernandes, J.O. 4-Methylimidazole in Soluble Coffee and Coffee Substitutes. Food Control 2016, 63, 15–20. [Google Scholar] [CrossRef]
- Deotale, S.M.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Influence of Drying Techniques on Sensory Profile and Chlorogenic Acid Content of Instant Coffee Powders. Meas. Food 2022, 6, 100030. [Google Scholar] [CrossRef]
- Massulo Souza, R.; Moreira, C.Q.; Vieira, R.P.; Coltro, L.; Alves, R.M.V. Alternative Flexible Plastic Packaging for Instant Coffees. Food Res. Int. 2023, 17, 1131652. [Google Scholar] [CrossRef] [PubMed]
- Gosalvitr, P.; Cuéllar-Franca, R.M.; Smith, R.; Azapagic, A. An Environmental and Economic Sustainability Assessment of Coffee Production in the UK. Chem. Eng. J. 2023, 465, 142793. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant Activity, Polyphenols, Caffeine and Melanoidins in Soluble Coffee: The Influence of Processing Conditions and Raw Material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- Mendes, K.; Luchine, A. Non-Tariff Barriers Removal in the Brazilian Coffee Industry. J. Int. Trade Law Policy 2020, 19, 139–157. [Google Scholar] [CrossRef]
- Chavez, S.G.; Mendoza, M.M.; Caetano, A.C. Antioxidants, Phenols, Caffeine Content and Volatile Compounds in Coffee Beverages Obtained by Different Methods. Food Sci. Technol. 2022, 42, 1–8. [Google Scholar] [CrossRef]
- Hseu, Z.Y.; Su, S.W.; Lai, H.Y.; Guo, H.Y.; Chen, T.C.; Chen, Z.S. Remediation Techniques and Heavy Metal Uptake by Different Rice Varieties in Metal-Contaminated Soils of Taiwan: New Aspects for Food Safety Regulation and Sustainable Agriculture. Soil Sci. Plant Nutr. 2010, 56, 31–52. [Google Scholar] [CrossRef]
- Pigozzi, M.T.; Passos, F.R.; Mendes, F.Q. Quality of Commercial Coffees: Heavy Metal and Ash Contents. J. Food Qual. 2018, 2018, 5908463. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kwiatkowska, K.; Kwiecień, M.; Zaricka, E. Assessment of the Risk of Exposure to Cadmium and Lead as a Result of the Consumption of Coffee Infusions. Biol. Trace Elem. Res. 2021, 199, 2420–2428. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chen, F.A.; Hsu, M.J. Threat of Heavy Metal Pollution in Halophytic and Mangrove Plants of Tamil Nadu, India. Environ. Pollut. 2008, 155, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Dghaim, R.; Al Khatib, S.; Rasool, H.; Ali Khan, M. Determination of Heavy Metals Concentration in Tradi. J. Environ. Public Health 2015, 2015, 973878. [Google Scholar] [CrossRef] [PubMed]
- Fathabad, A.E.; Shariatifar, N.; Moazzen, M.; Nazmara, S.; Fakhri, Y.; Alimohammadi, M.; Azari, A.; Mousavi Khaneghah, A. Determination of Heavy Metal Content of Processed Fruit Products from Tehran’s Market Using ICP- OES: A Risk Assessment Study. Food Chem. Toxicol. 2018, 115, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Barone, G.; Dambrosio, A.; Storelli, A.; Garofalo, R.; Busco, V.; Pietro; Storelli, M.M. Estimated Dietary Intake of Trace Metals from Swordfish Consumption: A Human Health Problem. Toxics 2018, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. New Evidence against Chromium as an Essential Trace Element. J. Nutr. 2017, 147, 2212–2219. [Google Scholar] [CrossRef]
- Monga, A.; Fulke, A.B.; Dasgupta, D. Recent Developments in Essentiality of Trivalent Chromium and Toxicity of Hexavalent Chromium: Implications on Human Health and Remediation Strategies. J. Hazard. Mater. Adv. 2022, 7, 100113. [Google Scholar] [CrossRef]
- Paz, S.; Rubio, C.; Frías, I.; Gutiérrez, Á.J.; González-Weller, D.; Martín, V.; Revert, C.; Hardisson, A. Toxic Metals (Al, Cd, Pb and Hg) in the Most Consumed Edible Seaweeds in Europe. Chemosphere 2019, 218, 879–884. [Google Scholar] [CrossRef]
- Hsu, M.J.; Selvaraj, K.; Agoramoorthy, G. Taiwan’s Industrial Heavy Metal Pollution Threatens Terrestrial Biota. Environ. Pollut. 2006, 143, 327–334. [Google Scholar] [CrossRef]
- Adler, G.; Nędzarek, A.; Tórz, A. Concentrations of Selected Metals (NA, K, CA, MG, FE, CU, ZN, AL, NI, PB, CD) in Coffee. Slov. J. Public Health 2019, 58, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe, G.A.; Chavez, S.G.; Arellanos, E.; Doménech, E. Probabilistic Risk Characterization of Heavy Metals in Peruvian Coffee: Implications of Variety, Region and Processing. Foods 2023, 12, 3254. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, É.J.; De Oliveira, E. Evaluation of Arsenic and Selenium in Brazilian Soluble Coffee by Inductively Coupled Plasma Atomic Emission Spectrometry with Hydride Generation. Braz. Arch. Biol. Technol. 2001, 44, 233–238. [Google Scholar] [CrossRef]
- Santos, E.E.; Lauria, D.C.; Porto Da Silveira, C.L. Assessment of Daily Intake of Trace Elements Due to Consumption of Foodstuffs by Adult Inhabitants of Rio de Janeiro City. Sci. Total Environ. 2004, 327, 69–79. [Google Scholar] [CrossRef]
- Vezzulli, F.; Fontanella, M.C.; Lambri, M.; Beone, G.M. Specialty and High-Quality Coffee: Discrimination through Elemental Characterization via ICP-OES, ICP-MS, and ICP-MS/MS of Origin, Species, and Variety. J. Sci. Food Agric. 2023, 103, 4303–4316. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, H.; Bibi, R.; Balal Arain, M.; Safi, F.; Ullah, S.; Castro-Muñoz, R.; Boczkaj, G. Deep Eutectic Solvent (DES) with Silver Nanoparticles (Ag-NPs) Based Assay for Analysis of Lead (II) in Edible Oils. Food Chem. 2022, 379, 132085. [Google Scholar] [CrossRef] [PubMed]
- Elahi, F.; Arain, M.B.; Ali Khan, W.; Ul Haq, H.; Khan, A.; Jan, F.; Castro-Muñoz, R.; Boczkaj, G. Ultrasound-Assisted Deep Eutectic Solvent-Based Liquid–Liquid Microextraction for Simultaneous Determination of Ni (II) and Zn (II) in Food Samples. Food Chem. 2022, 393, 133384. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide Is Formed in the Maillard Reaction. Nature 2002, 76, 448–449. [Google Scholar] [CrossRef]
- Robert, F.; Vuataz, G.; Pollien, P.; Saucy, F.; Alonso, M.I.; Bauwens, I.; Blank, I. Acrylamide Formation from Asparagine under Low-Moisture Maillard Reaction Conditions. 1. Physical and Chemical Aspects in Crystalline Model Systems. J. Agric. Food Chem. 2004, 52, 6837–6842. [Google Scholar] [CrossRef]
- Boyaci Gunduz, C.P. Formulation and Processing Strategies to Reduce Acrylamide in Thermally Processed Cereal-Based Foods. Int. J. Environ. Res. Public Health 2023, 20, 6272. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Deng, P.; He, Z.; Qin, F.; Chen, Q.; Wang, Z.; Pan, H.; Chen, J.; Zeng, M. Research Progress on Generation, Detection and Inhibition of Multiple Hazards—Acrylamide, 5-Hydroxymethylfurfural, Advanced Glycation End Products, Methylimidazole—In Baked Goods. Food Chem. 2024, 431, 137152. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Xu, X.; Lang, W.; Wang, W.; Wang, X.; Xin, A.; Zhou, F.; Ding, Z.; Ye, X.; Zhu, B. Toxicity, Formation, Contamination, Determination and Mitigation of Acrylamide in Thermally Processed Plant-Based Foods and Herbal Medicines: A Review. Ecotoxicol. Environ. Saf. 2023, 260, 115059. [Google Scholar] [CrossRef] [PubMed]
- Acquaticci, L.; Angeloni, S.; Cela, N.; Galgano, F.; Vittori, S.; Caprioli, G.; Condelli, N. Impact of Coffee Species, Post-Harvesting Treatments and Roasting Conditions on Coffee Quality and Safety Related Compounds. Food Control 2023, 149, 109714. [Google Scholar] [CrossRef]
- IARC. Mycotoxin Exposure and Human Cancer Risk: A Systematic Review of Epidemiological Studies. Available online: https://www.iarc.who.int/news-events/mycotoxin-exposure-and-human-cancer-risk-a-systematic-review-of-epidemiological-studies/ (accessed on 17 September 2023).
- Pedreschi, F.; Mariotti, S.; Granby, K.; Risum, J. Acrylamide Reduction in Potato Chips by Using Commercial Asparaginase in Combination with Conventional Blanching. LWT 2011, 44, 1473–1476. [Google Scholar] [CrossRef]
- European Union (UE). Commission Regulation (EU) 2017/2158 of 20 November 2017 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food. Off. J. Eur. Union 2017, 2017, 24–44. [Google Scholar]
- Pakshir, K.; Dehghani, A.; Nouraei, H.; Zareshahrabadi, Z.; Zomorodian, K. Evaluation of Fungal Contamination and Ochratoxin A Detection in Different Types of Coffee by HPLC-Based Method. J. Clin. Lab. Anal. 2021, 35, e24001. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Chang, C.W.; Chen, C.Y. Measuring Ochratoxin a Concentrations in Coffee Beverages with Immunoaffinity Columns and Ultra-Performance Liquid Chromatography/Tandem Mass Spectrometry. J. AOAC Int. 2016, 99, 469–474. [Google Scholar] [CrossRef]
- Li, R.; Zhu, L.; Yang, M.; Liu, A.; Xu, W.; He, P. Silver Nanocluster-Based Aptasensor for the Label-Free and Enzyme-Free Detection of Ochratoxin A. Food Chem. 2024, 431, 137126. [Google Scholar] [CrossRef]
- IARC. Experimental and Pan-Cancer Genome Analyses Reveal Widespread Contribution of Acrylamide Exposure to Carcinogenesis in Humans. Available online: https://www.iarc.who.int/news-events/experimental-and-pan-cancer-genome-analyses-reveal-widespread-contribution-of-acrylamide-exposure-to-carcinogenesis-in-humans/ (accessed on 20 September 2023).
- European Commission. Commission Regulation (EC) No 2023/915 of 25 April 2023 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union L 2023, 119, 103–157. [Google Scholar]
- Roychowdhury, T.; Tokunaga, H.; Ando, M. Survey of Arsenic and Other Heavy Metals in Food Composites and Drinking Water and Estimation of Dietary Intake by the Villagers from an Arsenic-Affected Area of West Bengal, India. Sci. Total Environ. 2003, 308, 15–35. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand. Food Standards Australia New Zealand–24th Australian Total Diet Study—1778-FSANZ_AustDietStudy-Web.Pdf; Food Standards Australia New Zealand: Wellington, New Zealand, 2014; ISBN 9780642345837.
- WHO/IPCS. Guidance Document on Evaluating and Expressing Uncertenty in Hazard Characterization; World Health Organization: Geneva, Switzerland; International Labour Organization: Geneva, Switzerland; United Nations Environment Programme: Nairobi, Kenya, 2014; ISBN 9789241513548. [Google Scholar]
- Doménech, E.; Martorell, S. Assessment of Safety Margins of Exposure to Non-Genotoxic Chemical Substances in Food. Food Control 2017, 79, 1–9. [Google Scholar] [CrossRef]
- Guo, G.; Zhang, D.; Wang, Y. Probabilistic Human Health Risk Assessment of Heavy Metal Intake via Vegetable Consumption around Pb/Zn Smelters in Southwest China. Int. J. Environ. Res. Public Health 2019, 16, 3267. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.M.C.; Faria, M.A.; Cunha, S.C.; Miladinovic, B.; Ferreira, I.M. Transport of Mycotoxins across Human Gastric NCI–N87 and Intestinal Caco-2 cell Models. Food Chem. Toxicol. 2019, 131, 110595. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.; Products, P.; Ppr, R. Guidance on the Use of Probabilistic Methodology for Modelling Dietary Exposure to Pesticide Residues. EFSA J. 2012, 10, 2839. [Google Scholar] [CrossRef]
- Maertens, A.; Golden, E.; Luechtefeld, T.H.; Hoffmann, S.; Tsaioun, K.; Hartung, T. Probabilistic Risk Assessment—The Keystone for the Future of Toxicology. ALTEX 2022, 39, 3–29. [Google Scholar] [CrossRef] [PubMed]
- IGHRC. Chemical Mixtures: A Framework for Assessing Risk to Human Health (CR14); Institute of Environment and Health: Bedfordshire, UK, 2008; ISBN 9781899110445. [Google Scholar]
- Leiva-Tafur, D.; Goñas, M.; Culqui, L.; Santa Cruz, C.; Rascón, J.; Oliva-Cruz, M. Spatiotemporal Distribution of Physicochemical Parameters and Toxic Elements in Lake Pomacochas, Amazonas, Peru. Front. Environ. Sci. 2022, 10, 885591. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater. 2017, p. 1496. Available online: https://secure.apha.org/imis/ItemDetail?iProductCode=978-087553-2998&CATEGORY=BK (accessed on 3 April 2022).
- Cui, S.; Na, J.S.; Kim, N.Y.; Lee, Y.; Nam, S.H. An Investigation on Inorganic Arsenic in Seaweed by Ion Chromatography Combined with Inductively Coupled Plasma-Atomic Emission Spectrometry. Bull. Korean Chem. Soc. 2013, 34, 3206–3210. [Google Scholar] [CrossRef]
- Oliveira, M.; Ramos, S.; Delerue-Matos, C.; Morais, S. Espresso Beverages of Pure Origin Coffee: Mineral Characterization, Contribution for Mineral Intake and Geographical Discrimination. Food Chem. 2015, 177, 330–338. [Google Scholar] [CrossRef]
- NTP-ISO/IEC 17025; General Requirements for the Competence of Testing an Calibration Laboratories. INACAL: Lima, Perú, 2017.
- Mesías, M.; Morales, F.J. Acrylamide in Coffee: Estimation of Exposure from Vending Machines. J. Food Compos. Anal. 2016, 48, 8–12. [Google Scholar] [CrossRef]
- Vecchio, A.; Mineo, V.; Planeta, D. Ochratoxin A in Instant Coffee in Italy. Food Control 2012, 28, 220–223. [Google Scholar] [CrossRef]
- Evans, R.M.; Scholze, M.; Kortenkamp, A. Examining the Feasibility of Mixture Risk Assessment: A Case Study Using a Tiered Approach with Data of 67 Pesticides from the Joint FAO/WHO Meeting on Pesticide Residues (JMPR). Food Chem. Toxicol. 2015, 84, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Liu, Q.; Liu, J.; Wang, Q.; Wang, Y. Concentrations of Organophosphorus Pesticides in Fresh Vegetables and Related Human Health Risk Assessment in Changchun, Northeast China. Food Control 2016, 60, 353–360. [Google Scholar] [CrossRef]
- Doménech, E.; Martorell, S. Formulation and Application of the Probability of Exceedance Metric for Risk Characterization of Non-Threshold Chemical Hazards in Food. Food Control 2021, 124, 107910. [Google Scholar] [CrossRef]
- Kowalska, G. The Safety Assessment of Toxic Metals in Commonly Used Herbs, Spices, Tea, and Coffee in Poland. Int. J. Environ. Res. Public Health 2021, 18, 5779. [Google Scholar] [CrossRef] [PubMed]
- EPA Environmental Protection Agency. 2022. Available online: https://iris.epa.gov/AdvancedSearch/ (accessed on 1 April 2022).
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Lead in Food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Acrylamide in Food. EFSA J. 2015, 13, e4104. [Google Scholar] [CrossRef]
- Pedersen, G.A.; Larsen, E.H.; Mortensen, G.K. Beverages as a Source of Toxic Trace Element Intake. Food Addit. Contam. 1994, 11, 351–363. [Google Scholar] [CrossRef]
- Santato, A.; Bertoldi, D.; Perini, M.; Camin, F.; Larcher, R. Using Elemental Profiles and Stable Isotopes to Trace the Origin of Green Coffee Beans on the Global Market. J. Mass Spectrom. 2012, 47, 1132–1140. [Google Scholar] [CrossRef]
- Suseela, B.; Bhalke, S.; Kumar, A.V.; Tripathi, R.M.; Sastry, V.N. Daily Intake of Trace Metals through Coffee Consumption in India. Food Addit. Contam. 2001, 18, 115–120. [Google Scholar] [CrossRef]
- Dos Santos, É.J.; De Oliveira, E. Determination of Mineral Nutrients and Toxic Elements in Brazilian Soluble Coffee by ICP-AES. J. Food Compos. Anal. 2001, 14, 523–531. [Google Scholar] [CrossRef]
- Grembecka, M.; Malinowska, E.; Szefer, P. Differentiation of Market Coffee and Its Infusions in View of Their Mineral Composition. Sci. Total Environ. 2007, 383, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Szymczycha-Madeja, A.; Welna, M.; Pohl, P. Simplified Multi-Element Analysis of Ground and Instant Coffees by ICP-OES and FAAS. Food Addit. Contam.—Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, J.H.; Fatima, I.; Arif, M.; Qureshi, I.H. Determination of Trace Elements in Coffee Beans and Instant Coffee of Various Origins by INAA. J. Radioanal. Nucl. Chem. 2005, 267, 109–112. [Google Scholar] [CrossRef]
- Oliveira, M.; Casal, S.; Morais, S.; Alves, C.; Dias, F.; Ramos, S.; Mendes, E.; Delerue-Matos, C.; Beatriz, M. Intra- and Interspecific Mineral Composition Variability of Commercial Instant Coffees and Coffee Substitutes: Contribution to Mineral Intake. Food Chem. 2012, 130, 702–709. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Jachimowicz, K.; Kislova, S.; Kwiecień, M.; Zasadna, Z.; Yanovych, D. Cadmium and Lead Concentration in Drinking Instant Coffee, Instant Coffee Drinks and Coffee Substitutes: Safety and Health Risk Assessment. Biol. Trace Elem. Res. 2022, 201, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Akillioglu, H.G.; Gökmen, V. Mitigation of Acrylamide and Hydroxymethyl Furfural in Instant Coffee by Yeast Fermentation. Food Res. Int. 2014, 61, 252–256. [Google Scholar] [CrossRef]
- Granby, K.; Fagt, S. Analysis of Acrylamide in Coffee and Dietary Exposure to Acrylamide from Coffee. Anal. Chim. Acta 2004, 520, 177–182. [Google Scholar] [CrossRef]
- Şenyuva, H.Z.; Gökmen, V. Study of Acrylamide in Coffee Using an Improved Liquid Chromatography Mass Spectrometry Method: Investigation of Colour Changes and Acrylamide Formation in Coffee during Roasting. Food Addit. Contam. 2005, 22, 214–220. [Google Scholar] [CrossRef]
- Barón Cortés, W.R.; Vásquez Mejía, S.M.; Suárez Mahecha, H. Consumption Study and Margin of Exposure of Acrylamide in Food Consumed by the Bogotá Population in Colombia. J. Food Compos. Anal. 2021, 100, 103934. [Google Scholar] [CrossRef]
- Andrzejewski, D.; Roach, J.A.G.; Gay, M.L.; Musser, S.M. Analysis of Coffee for the Presence of Acrylamide by LC-MS/MS. J. Agric. Food Chem. 2004, 52, 1996–2002. [Google Scholar] [CrossRef]
- Loaëc, G.; Jacolot, P.; Helou, C.; Niquet-Léridon, C.; Tessier, F.J. Acrylamide, 5-Hydroxymethylfurfural and Nε-Carboxymethyl-Lysine in Coffee Substitutes and Instant Coffees. Food Addit. Contam.—Part A 2014, 31, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Arisseto, A.P.; Toledo, M.C.; Govaert, Y.; Van Loco, J.; Fraselle, S.; Weverbergh, E.; Degroodt, J.M. Determination of Acrylamide Levels in Selected Foods in Brazil. Food Addit. Contam. 2007, 24, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Surma, M.; Sadowska-Rociek, A.; Cieślik, E.; Sznajder-Katarzyńska, K. Optimization of QuEChERS Sample Preparation Method for Acrylamide Level Determination in Coffee and Coffee Substitutes. Microchem. J. 2017, 131, 98–102. [Google Scholar] [CrossRef]
- González, N.; Marquès, M.; Calderón, J.; Collantes, R.; Corraliza, L.; Timoner, I.; Bosch, J.; Castell, V.; Domingo, J.L.; Nadal, M. Occurrence and Dietary Intake of Food Processing Contaminants (FPCs) in Catalonia, Spain. J. Food Compos. Anal. 2022, 106, 104272. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.J. Dietary Exposure to Acrylamide and Associated Health Risks for the Korean Population. Int. J. Environ. Res. Public Health 2020, 17, 7619. [Google Scholar] [CrossRef] [PubMed]
- Claeys, W.; De Meulenaer, B.; Huyghebaert, A.; Scippo, M.L.; Hoet, P.; Matthys, C. Reassessment of the Acrylamide Risk: Belgium as a Case-Study. Food Control 2016, 59, 628–635. [Google Scholar] [CrossRef]
- Health Canada Health Canada Bureau of Chemical Safety. Health Canada Revised Exposure Assessment of Acrylamide in Food; Health Canada Bureau of Chemical Safety, 2012; Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/pdf/securit/chem-chim/food-aliment/acrylamide/rev-eval-exposure-exposition-eng.pdf (accessed on 23 April 2023).
- Karami, M.; Akbari-Adergani, B.; Jahed Khaniki, G.; Shariatifar, N.; Sadighara, P. Determination and Health Risk Assessment of Acrylamide Levels in Instant Coffee Products Available in Tehran Markets by GC-MS. Int. J. Environ. Anal. Chem. 2022, 14, 1–10. [Google Scholar] [CrossRef]
- El-Zakhem Naous, G.; Merhi, A.; Abboud, M.I.; Mroueh, M.; Taleb, R.I. Carcinogenic and Neurotoxic Risks of Acrylamide Consumed through Caffeinated Beverages among the Lebanese Population. Chemosphere 2018, 208, 352–357. [Google Scholar] [CrossRef]
- Yazdanfar, N.; Mahmudiono, T.; Fakhri, Y.; Mahvi, A.H.; Sadighara, P.; Mohammadi, A.A.; Yousefi, M. Concentration of Ochratoxin A in Coffee Products and Probabilistic Health Risk Assessment. Arab. J. Chem. 2022, 15, 104376. [Google Scholar] [CrossRef]
- Aoyama, K.; Nakajima, M.; Tabata, S.; Eiichi, I.; Tanaka, T.; Norizuki, H.; Itoh, Y.; Fujita, K.; Kai, S.; Tsutsumi, T.; et al. Four-Year Surveillance for Ochratoxin a and Fumonisins in Retail Foods in Japan. J. Food Prot. 2010, 73, 344–352. [Google Scholar] [CrossRef]
- Casal, S.; Vieira, T.; Cruz, R.; Cunha, S.C. Ochratoxin A in Commercial Soluble Coffee and Coffee Substitutes. Food Res. Int. 2014, 61, 56–60. [Google Scholar] [CrossRef]
- Galarce-Bustos, O.; Alvarado, M.; Vega, M.; Aranda, M. Occurrence of Ochratoxin A in Roasted and Instant Coffees in Chilean Market. Food Control 2014, 46, 102–107. [Google Scholar] [CrossRef]
- Lee, T.P.; Saad, B.; Ng, E.P.; Salleh, B. Zeolite Linde Type L as Micro-Solid Phase Extraction Sorbent for the High Performance Liquid Chromatography Determination of Ochratoxin A in Coffee and Cereal. J. Chromatogr. A 2012, 1237, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Tsao, Z.J.; Wang, J.J.; Yu, F.Y. Development of a Monoclonal Antibody against Ochratoxin A and Its Application in Enzyme-Linked Immunosorbent Assay and Gold Nanoparticle Immunochromatographic Strip. Anal. Chem. 2008, 80, 7029–7035. [Google Scholar] [CrossRef]
- García-Moraleja, A.; Font, G.; Mañes, J.; Ferrer, E. Analysis of Mycotoxins in Coffee and Risk Assessment in Spanish Adolescents and Adults. Food Chem. Toxicol. 2015, 86, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Hajok, I.; Kowalska, A.; Piekut, A.; Ćwieląg-Drabek, M. A Risk Assessment of Dietary Exposure to Ochratoxin A for the Polish Population. Food Chem. 2019, 284, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Pokrzywa, P.; Surma, M.; Szarek, S. Coffee and Wine with Ochratoxin A—Exposure Risk Assessment Resulting from Its Consumption. Med. Ogólna Nauk. Zdrowiu 2022, 28, 190–195. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Ngemela, A.F.; Jensen, L.B.; De Medeiros, L.S.; Rasmussen, P.H. UHPLC-MS/MS Determination of Ochratoxin a and Fumonisins in Coffee Using QuEChERS Extraction Combined with Mixed-Mode SPE Purification. J. Agric. Food Chem. 2015, 63, 1029–1034. [Google Scholar] [CrossRef]
- Skarkova, J.; Ostry, V.; Malir, F.; Roubal, T. Determination of Ochratoxin A in Food by High Performance Liquid Chromatography. Anal. Lett. 2013, 46, 1495–1504. [Google Scholar] [CrossRef]
- Lindenmeier, M.; Schieberle, P.; Rychlik, M. Determination of Ochratoxin A in Food: Comparison of a Stable Isotope Dilution Assay, Liquid Chromatography-Fluorescence Detection and an Enzyme-Linked Immunosorbent Assay. Mycotoxin Res. 2011, 27, 115–121. [Google Scholar] [CrossRef]
- Gopinandhan, T.; Velmourougane, K.; Panneerselvam, P.; Keshamma, E.; Raghuramulu, Y. Occurrence of Ochratoxin-A (OT-A) in Green and Commercial Coffee Samples. J. Food Sci. Technol. 2007, 44, 247–249. [Google Scholar]
- Culliao, A.G.L.; Barcelo, J.M. Fungal and Mycotoxin Contamination of Coffee Beans in Benguet Province, Philippines. Food Addit. Contam.—Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Duris, D.; Mburu, J.K.; Durand, N.; Clarke, R.; Frank, J.M.; Guyot, B. Ochratoxin a Contamination of Coffee Batches from Kenya in Relation to Cultivation Methods and Post-Harvest Processing Treatments. Food Addit. Contam.—Part A 2010, 27, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Khaneghah, A.M.; Fakhri, Y.; Abdi, L.; Coppa, C.F.S.C.; Franco, L.T.; de Oliveira, C.A.F. The Concentration and Prevalence of Ochratoxin A in Coffee and Coffee-Based Products: A Global Systematic Review, Meta-Analysis and Meta-Regression. Fungal Biol. 2019, 123, 611–617. [Google Scholar] [CrossRef]
- Tozlovanu, M.; Pfohl-Leszkowicz, A. Ochratoxin A in Roasted Coffee from French Supermarkets and Transfer in Coffee Beverages: Comparison of Analysis Methods. Toxins 2010, 2, 1928–1942. [Google Scholar] [CrossRef] [PubMed]
- Vatinno, R.; Aresta, A.; Zambonin, C.G.; Palmisano, F. Determination of Ochratoxin A in Green Coffee Beans by Solid-Phase Microextraction and Liquid Chromatography with Fluorescence Detection. J. Chromatogr. A 2008, 1187, 145–150. [Google Scholar] [CrossRef]
- Lobeau, M.; De Saeger, S.; Sibanda, L.; Barna-Vetró, I.; Van Peteghem, C. Development of a New Clean-up Tandem Assay Column for the Detection of Ochratoxin A in Roasted Coffee. Anal. Chim. Acta 2005, 538, 57–61. [Google Scholar] [CrossRef]
- Dybing, E.; Sanner, T. Risk Assessment of Acrylamide in Foods. Toxicol. Sci. 2003, 75, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Amariei, S.; Gutt, G. Acrylamide in Romanian Food Using HPLC-UV and a Health Risk Assessment. Food Addit. Contam. Part B Surveill. 2015, 8, 136–141. [Google Scholar] [CrossRef]
- Central Oklahoma Telephone Co. On Toxicity of Chemicals in Food, Consumer Products and the Environment. Statement on Potential Risks from Arsenic in the Diet of Infants Aged 0 to 12 Months and Children Aged 1 to 5 Years. 2016; pp. 1–47. Available online: https://cot.food.gov.uk/sites/default/files/finalstatementonarsenic_0.pdf, (accessed on 23 April 2023).
- Kollander, B.; Sundström, B.; Öhrvik, V.; Abramsson, L. Inorganic Arsenic in Rice and Rice Products on the Swedish Market 2015 Part 2: Survey. In Proceedings of the Arsenic Research and Global Sustainability Proceedings of the Sixth International Congress on Arsenic in the Environment (As2016), Stockholm, Sweden, 19–23 June 2016; pp. 277–278. [Google Scholar] [CrossRef]
| Country of Origin | Concentration (mg/kg) | |||||
|---|---|---|---|---|---|---|
| iAs | Cr | Pb | AA | OTA | ||
| Brazil (n = 72) | Mean | 4.86 × 10−2 a | 6.88 × 10−3 a | 2.25 × 10−2 a | 4.77 × 10−1 c | 1.77 × 10−3 b |
| SD | 5.85 × 10−3 | 2.47 × 10−3 | 2.11 × 10−3 | 9.58 × 10−2 | 2.80 × 10−4 | |
| Min | 3.50 × 10−2 | 5.00 × 10−3 | 1.90 × 10−2 | 3.20 × 10−1 | 9.80 × 10−4 | |
| Max | 6.00 × 10−2 | 1.00 × 10−2 | 2.70 × 10−2 | 6.18 × 10−1 | 2.21 × 10−3 | |
| Colombia (n = 81) | Mean | 4.68 × 10−2 a | 6.67 × 10−3 a | 2.00 × 10−2 a | 2.50 × 10−1 a,b | 1.26 × 10−3 a |
| SD | 6.22 × 10−3 | 2.40 × 10−3 | 2.90 × 10−3 | 3.60 × 10−2 | 2.80 × 10−4 | |
| Min | 3.50 × 10−2 | 5.00 × 10−3 | 1.70 × 10−2 | 1.87 × 10−1 | 3.20 × 10−4 | |
| Max | 5.40 × 10−2 | 1.00 × 10−2 | 2.50 × 10−2 | 3.05 × 10−1 | 1.64 × 10−3 | |
| Mexico (n = 12) | Mean | 5.03 × 10−2 a | 6.25 × 10−3 a | 2.25 × 10−2 a | 3.31 × 10−1 b | 1.71 × 10−3 b |
| SD | 4.86 × 10−3 | 2.50 × 10−3 | 1.29 × 10−3 | 4.51 × 10−2 | 1.73 × 10−4 | |
| Min | 4.30 × 10−2 | 5.00 × 10−3 | 2.10 × 10−2 | 2.98 × 10−1 | 1.46 × 10−3 | |
| Max | 5.30 × 10−2 | 1.00 × 10−2 | 2.40 × 10−2 | 3.98 × 10−1 | 1.85 × 10−3 | |
| Peru (n = 15) | Mean | 5.16 × 10−2 a | 8.00 × 10−3 a | 2.12 × 10−2 a | 1.77 × 10−1 a | 1.23 × 10−3 a |
| SD | 6.19 × 10−3 | 2.74 × 10−3 | 1.79 × 10−3 | 1.07 × 10−2 | 4.49 × 10−4 | |
| Min | 4.30 × 10−2 | 5.00 × 10−3 | 1.90 × 10−2 | 1.63 × 10−1 | 9.80 × 10−4 | |
| Max | 6.00 × 10−2 | 1.00 × 10−2 | 2.30 × 10−2 | 1.87 × 10−1 | 2.03 × 10−3 | |
| ANOVA F-ratio | 1.209 n.s. | 0.493 n.s. | 4.74 n.s. | 59.22 *** | 12.91 *** | |
| Country | iAs | Cr | Pb | AA | OTA |
|---|---|---|---|---|---|
| Brazil | Logistic (0.039; 0.049; 0.058) | Exponential (0.005; 0.006; 0.009) | Extravalue (0.019; 0.022; 0.0265) | Uniform (0.307; 0.631) | Logitic (0.001; 0.002; 0.002) |
| Colombia | Triangular (0.036; 0.047; 0.053) | Exponential (0.005; 0.006; 0.010) | Exponential (0.017; 0.0189; 0.0258) | Uniform (0.183; 0.309) | Logistic (0.001; 0.001; 0.002) |
| Mexico | Uniform (0.043; 0.053) | Uniform (0.005; 0.01) | Uniform (0.021; 0.024) | Uniform (0.298; 0.398) | Uniform (0.001; 0.002) |
| Peru | Uniform (0.039; 0.064) | Uniform (0.004; 0.011) | Uniform (0.002; 0.023) | Uniform (0.157; 0.193) | Exponential (0.001; 0.001; 0.002) |
| Metrics | Hazard | Country of Origin | Mean | 1st | 5th | 50th | 95th | 99th |
|---|---|---|---|---|---|---|---|---|
| HQ | Cd | All countries | 1.44 × 10−7 | 1.62 × 10−5 | 2.24 × 10−5 | 1.04 × 10−4 | 4.00 × 10−4 | 6.22 × 10−4 |
| Cr | Brazil | 2.61 × 10−7 | 2.58 × 10−8 | 3.78 × 10−8 | 1.80 × 10−7 | 7.60 × 10−7 | 1.24 × 10−6 | |
| Colombia | 2.54 × 10−7 | 2.59 × 10−8 | 3.71 × 10−8 | 1.76 × 10−7 | 7.31 × 10−7 | 1.19 × 10−6 | ||
| Mexico | 2.88 × 10−7 | 2.90 × 10−8 | 4.29 × 10−8 | 2.02 × 10−7 | 8.24 × 10−7 | 1.29 × 10−6 | ||
| Peru | 2.89 × 10−7 | 2.48 × 10−8 | 3.89 × 10−8 | 1.96 × 10−7 | 8.47 × 10−7 | 1.34 × 10−6 | ||
| Hg | All countries | 1.44 × 10−7 | 1.62 × 10−4 | 2.24 × 10−4 | 1.04 × 10−3 | 4.00 × 10−3 | 6.22 × 10−3 | |
| AA | Brazil | 1.35 × 10−2 | 1.34 × 10−3 | 2.01 × 10−3 | 9.44 × 10−3 | 3.92 × 10−2 | 6.10 × 10−2 | |
| Colombia | 7.08 × 10−3 | 7.60 × 10−4 | 1.07 × 10−3 | 5.01 × 10−3 | 2.00 × 10−2 | 3.20 × 10−2 | ||
| Mexico | 1.00 × 10−2 | 1.11 × 10−3 | 1.55 × 10−3 | 7.14 × 10−3 | 2.81 × 10−2 | 4.33 × 10−2 | ||
| Peru | 5.05 × 10−3 | 5.67 × 10−4 | 7.84 × 10−4 | 3.61 × 10−3 | 1.41 × 10−2 | 2.21 × 10−2 | ||
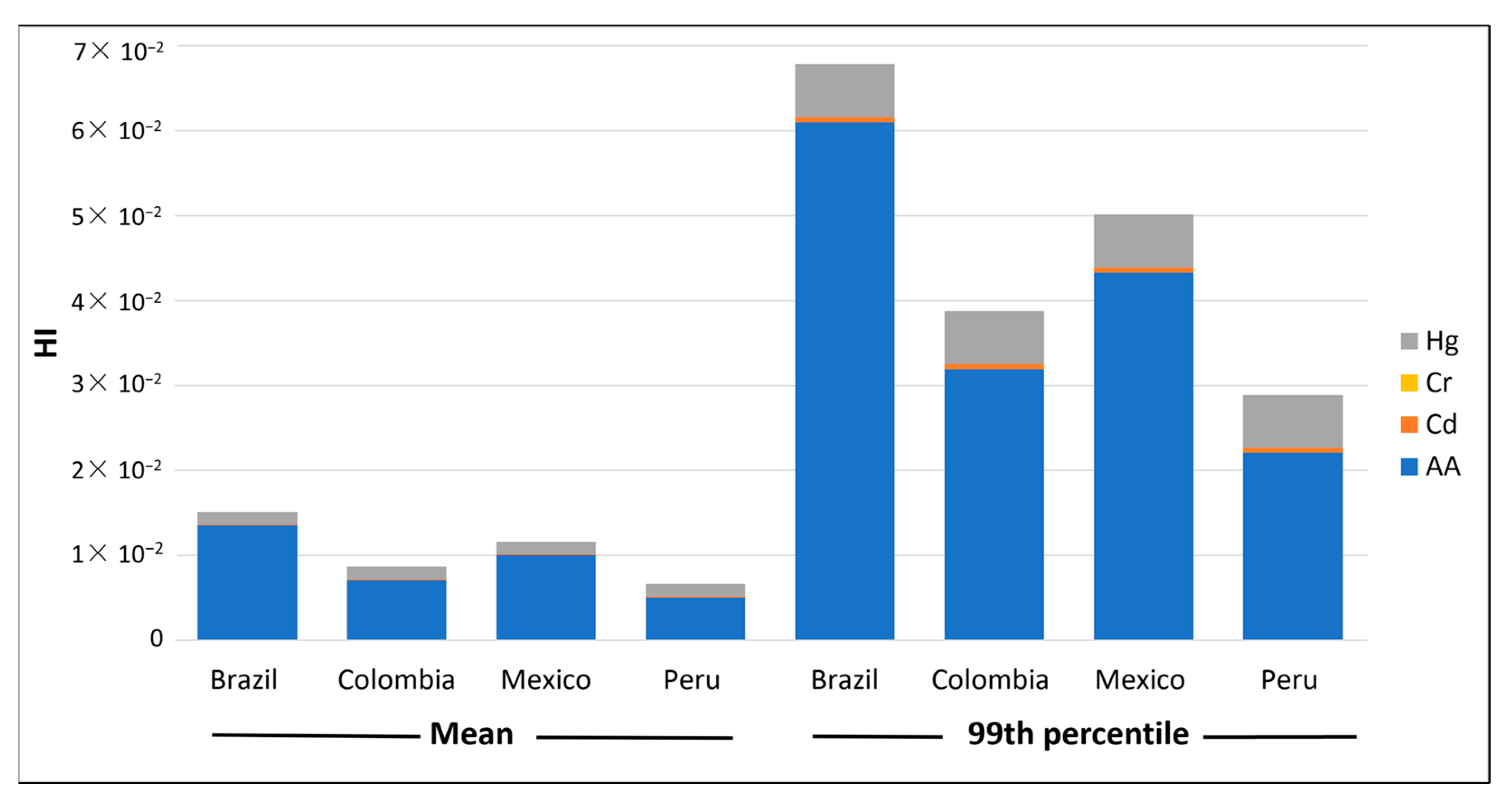
| HI | ∑HQ | Brazil | 1.51 × 10−2 | 1.52 × 10−3 | 2.24 × 10−3 | 1.05 × 10−2 | 4.30 × 10−2 | 7.07 × 10−2 |
| ∑HQ | Colombia | 8.67 × 10−3 | 9.10 × 10−4 | 1.32 × 10−3 | 6.13 × 10−3 | 2.48 × 10−2 | 3.76 × 10−2 | |
| ∑HQ | Mexico | 1.16 × 10−2 | 1.27 × 10−3 | 1.78 × 10−3 | 8.27 × 10−3 | 3.27 × 10−2 | 5.10 × 10−2 | |
| ∑HQ | Peru | 6.63 × 10−3 | 7.45 × 10−4 | 1.03 × 10−3 | 4.73 × 10−3 | 1.88 × 10−2 | 2.87 × 10−2 | |
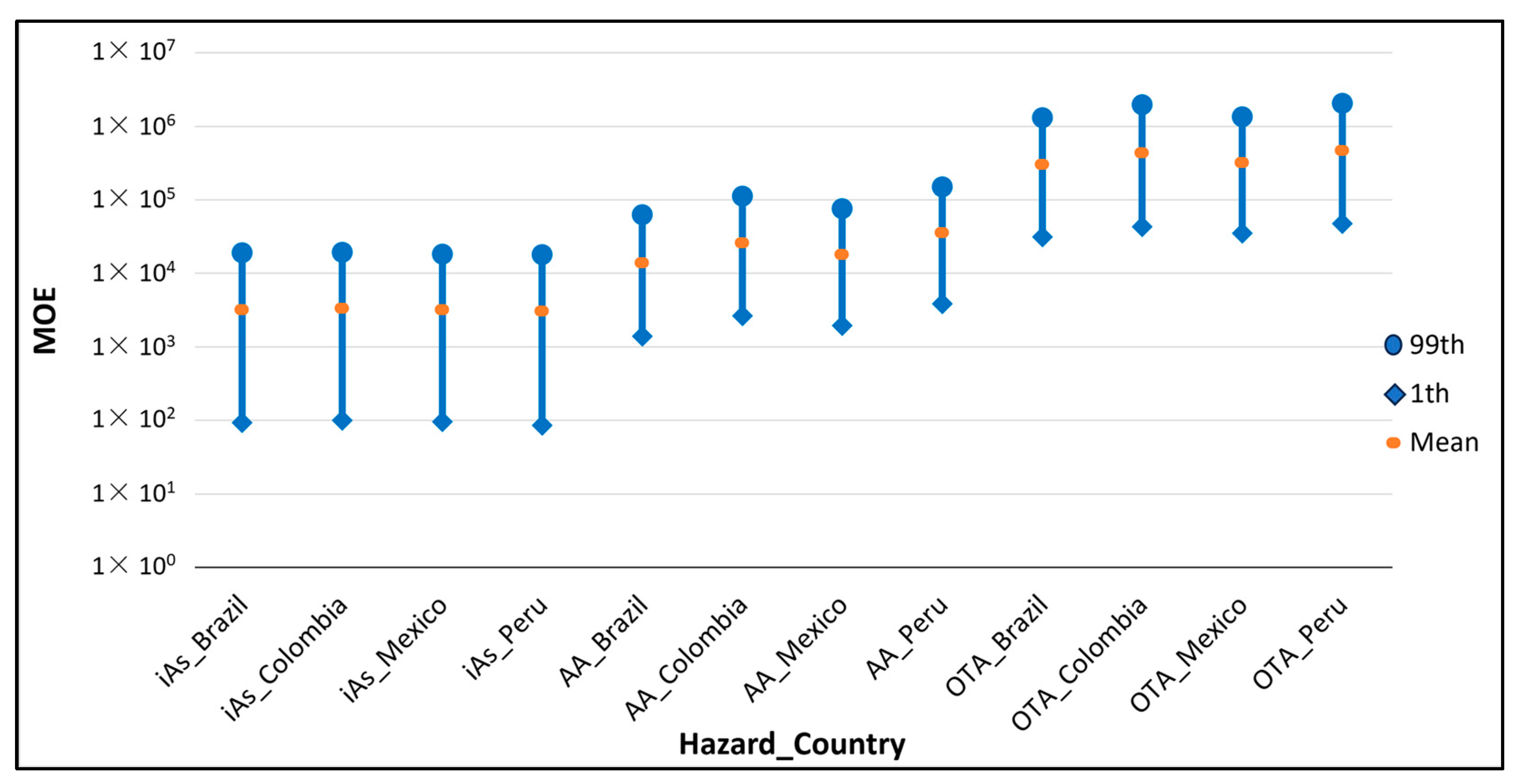
| MOE | iAs | Brazil | 3.18 × 103 | 9.34 × 10 | 2.24 × 102 | 1.78 × 103 | 1.13 × 104 | 1.91 × 104 |
| Colombia | 3.36 × 103 | 1.00 × 102 | 2.31 × 102 | 1.90 × 103 | 1.19 × 104 | 1.94 × 104 | ||
| Mexico | 3.20 × 103 | 9.66 × 10 | 2.24 × 102 | 1.81 × 103 | 1.13 × 104 | 1.82 × 104 | ||
| Peru | 3.04 × 103 | 8.55 × 10 | 2.10 × 102 | 1.71 × 103 | 1.08 × 104 | 1.81 × 104 | ||
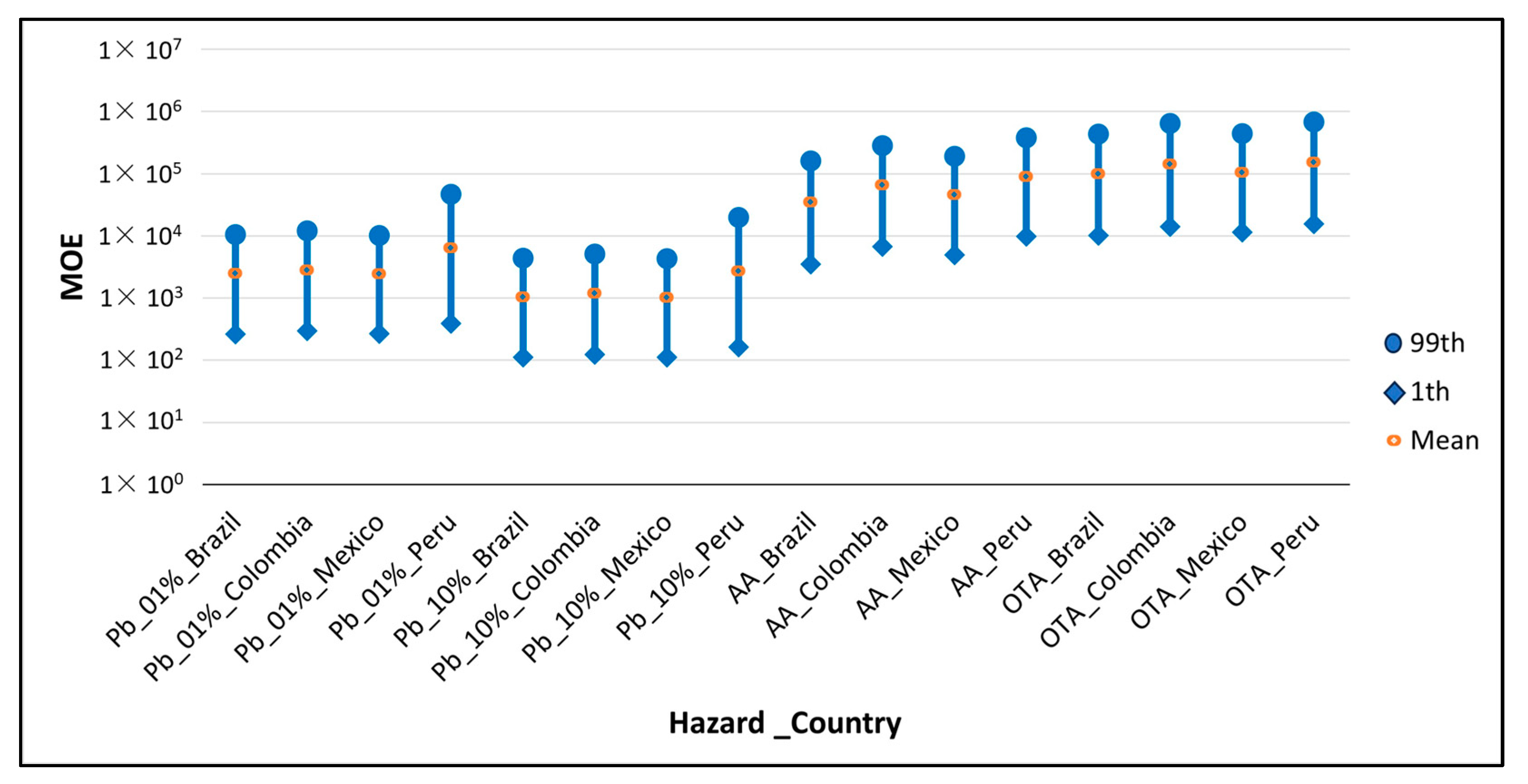
| Pb * | Brazil | 2.47 × 103 | 2.63 × 102 | 4.11 × 102 | 1.62 × 103 | 7.51 × 103 | 1.05 × 104 | |
| Colombia | 2.82 × 103 | 2.94 × 102 | 4.65 × 102 | 1.85 × 103 | 8.64 × 103 | 1.21 × 104 | ||
| Mexico | 2.45 × 103 | 2.67 × 102 | 4.14 × 102 | 1.61 × 103 | 7.43 × 103 | 1.02 × 104 | ||
| Peru | 6.41 × 103 | 3.89 × 102 | 6.52 × 102 | 3.38 × 103 | 2.22 × 104 | 4.71 × 104 | ||
| Pb ** | Brazil | 1.04 × 103 | 1.11 × 102 | 1.73 × 102 | 6.81 × 102 | 3.16 × 103 | 4.41 × 103 | |
| Colombia | 1.19 × 103 | 1.24 × 102 | 1.95 × 102 | 7.78 × 102 | 3.63 × 103 | 5.09 × 103 | ||
| Mexico | 1.03 × 103 | 1.12 × 102 | 1.74 × 102 | 6.78 × 102 | 3.12 × 103 | 4.27 × 103 | ||
| Peru | 2.69 × 103 | 1.63 × 102 | 2.74 × 102 | 1.42 × 103 | 9.33 × 103 | 1.98 × 104 | ||
| AA | Brazil | 1.38 × 104 | 1.39 × 103 | 2.17 × 103 | 9.00 × 103 | 4.21 × 104 | 6.31 × 104 | |
| Colombia | 2.59 × 104 | 2.66 × 103 | 4.25 × 103 | 1.69 × 104 | 7.91 × 104 | 1.12 × 105 | ||
| Mexico | 1.80 × 104 | 1.96 × 103 | 3.03 × 103 | 1.19 × 104 | 5.49 × 104 | 7.60 × 104 | ||
| Peru | 3.57 × 104 | 3.85 × 103 | 5.99 × 103 | 2.36 × 104 | 1.08 × 105 | 1.50 × 105 | ||
| OTA | Brazil | 3.03 × 105 | 3.13 × 104 | 4.96 × 104 | 1.97 × 105 | 9.17 × 105 | 1.32 × 106 | |
| Colombia | 4.34 × 105 | 4.31 × 104 | 6.83 × 104 | 2.80 × 105 | 1.32 × 106 | 1.97 × 106 | ||
| Mexico | 3.23 × 105 | 3.52 × 104 | 5.38 × 104 | 2.12 × 105 | 9.79 × 105 | 1.36 × 106 | ||
| Peru | 4.66 × 105 | 4.78 × 104 | 7.51 × 104 | 3.01 × 105 | 1.43 × 106 | 2.06 × 106 | ||
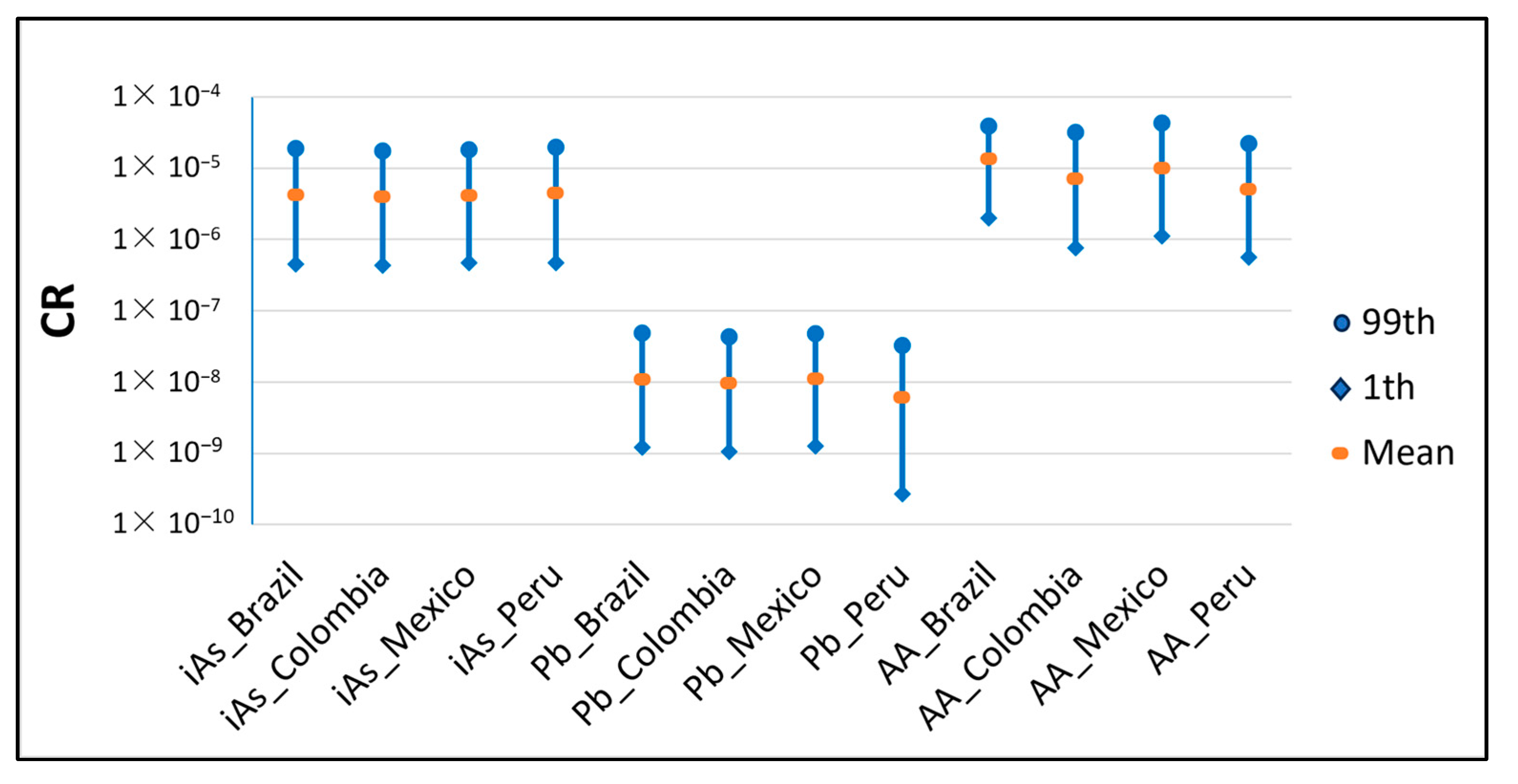
| CR | iAs | Brazil | 4.24 × 10−6 | 4.49 × 10−7 | 6.46 × 10−7 | 3.04 × 10−6 | 1.19 × 10−5 | 1.87 × 10−5 |
| Colombia | 4.00 × 10−6 | 4.31 × 10−7 | 6.13 × 10−7 | 2.87 × 10−6 | 1.12 × 10−5 | 1.75 × 10−5 | ||
| Mexico | 4.15 × 10−6 | 4.72 × 10−7 | 6.42 × 10−7 | 2.96 × 10−6 | 1.16 × 10−5 | 1.81 × 10−5 | ||
| Peru | 4.46 × 10−6 | 4.68 × 10−7 | 6.73 × 10−7 | 3.14 × 10−6 | 1.27 × 10−5 | 1.97 × 10−5 | ||
| Pb | Brazil | 1.10 × 10−8 | 1.21 × 10−9 | 1.70 × 10−9 | 7.87 × 10−9 | 3.10 × 10−8 | 4.83 × 10−8 | |
| Colombia | 9.74 × 10−9 | 1.05 × 10−9 | 1.47 × 10−9 | 6.88 × 10−9 | 2.74 × 10−8 | 4.33 × 10−8 | ||
| Mexico | 1.10 × 10−8 | 1.25 × 10−9 | 1.71 × 10−9 | 7.90 × 10−9 | 3.08 × 10−8 | 4.76 × 10−8 | ||
| Peru | 6.08 × 10−9 | 2.71 × 10−10 | 5.73 × 10−10 | 3.77 × 10−9 | 1.95 × 10−8 | 3.27 × 10−8 | ||
| AA | Brazil | 1.35 × 10−5 | 1.34 × 10−6 | 2.01 × 10−6 | 9.44 × 10−6 | 3.92 × 10−5 | 6.10 × 10−5 | |
| Colombia | 7.08 × 10−6 | 7.60 × 10−7 | 1.07 × 10−6 | 5.01 × 10−6 | 2.00 × 10−5 | 3.20 × 10−5 | ||
| Mexico | 1.00 × 10−5 | 1.11 × 10−6 | 1.55 × 10−6 | 7.14 × 10−6 | 2.81 × 10−5 | 4.33 × 10−5 | ||
| Peru | 5.05 × 10−6 | 5.67 × 10−7 | 7.84 × 10−7 | 3.61 × 10−6 | 1.41 × 10−5 | 2.21 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guadalupe, G.A.; Grandez-Yoplac, D.E.; Arellanos, E.; Doménech, E. Probabilistic Risk Assessment of Metals, Acrylamide and Ochratoxin A in Instant Coffee from Brazil, Colombia, Mexico and Peru. Foods 2024, 13, 726. https://doi.org/10.3390/foods13050726
Guadalupe GA, Grandez-Yoplac DE, Arellanos E, Doménech E. Probabilistic Risk Assessment of Metals, Acrylamide and Ochratoxin A in Instant Coffee from Brazil, Colombia, Mexico and Peru. Foods. 2024; 13(5):726. https://doi.org/10.3390/foods13050726
Chicago/Turabian StyleGuadalupe, Grobert A., Dorila E. Grandez-Yoplac, Erick Arellanos, and Eva Doménech. 2024. "Probabilistic Risk Assessment of Metals, Acrylamide and Ochratoxin A in Instant Coffee from Brazil, Colombia, Mexico and Peru" Foods 13, no. 5: 726. https://doi.org/10.3390/foods13050726
APA StyleGuadalupe, G. A., Grandez-Yoplac, D. E., Arellanos, E., & Doménech, E. (2024). Probabilistic Risk Assessment of Metals, Acrylamide and Ochratoxin A in Instant Coffee from Brazil, Colombia, Mexico and Peru. Foods, 13(5), 726. https://doi.org/10.3390/foods13050726








