Protective Effect of Black Rice Cyanidin-3-Glucoside on Testicular Damage in STZ-Induced Type 1 Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. C3G Preparation
2.2. Animals and Diet
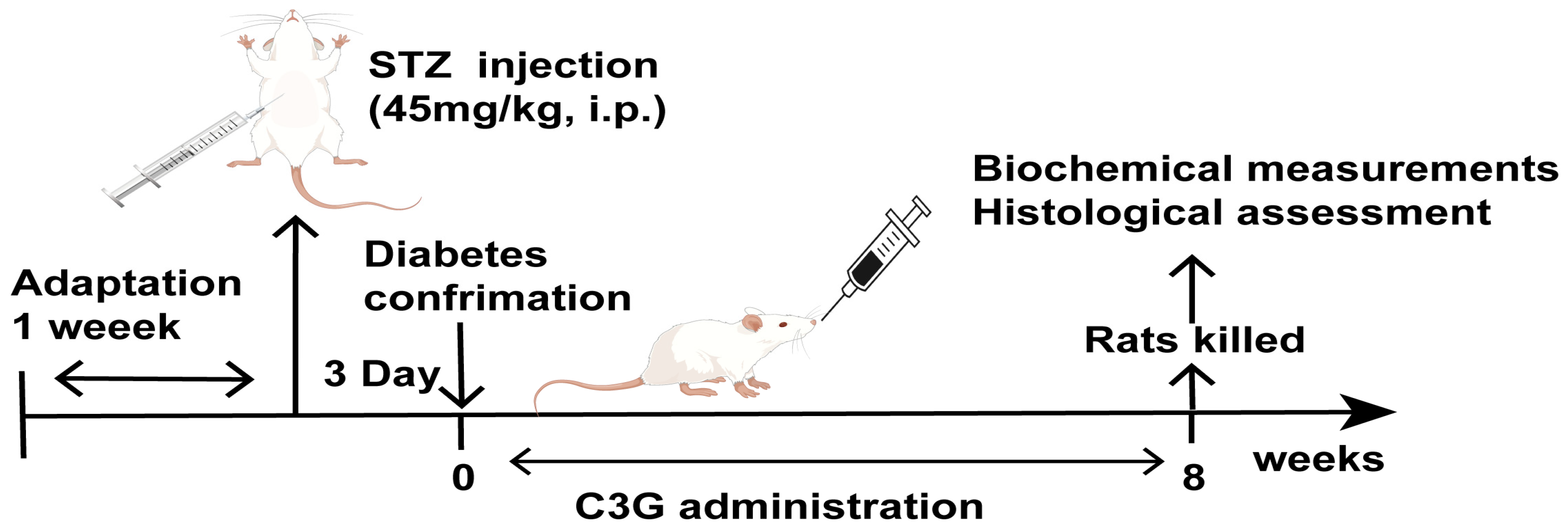
2.3. Type 1 Diabetes Animal Model Induction
2.4. Animal Grouping and C3G Treatment
2.5. Sperm Count, Motility and Viability
2.6. Sperm Abnormality Assessment
2.7. Biochemical Assay in Serum and Testis Homogenate Samples
2.8. Histology Evaluation of Testicles
2.9. Masson Staining for Testicular Interstitial Fibrosis Evaluation
2.10. PAS Staining to Observe Testicular Seminiferous Tubules Basement Membrane
2.11. Ultrastructure Observation of Testicular Tissue
2.12. Immunohistochemical Analysis
2.13. Statistical Analysis
3. Results
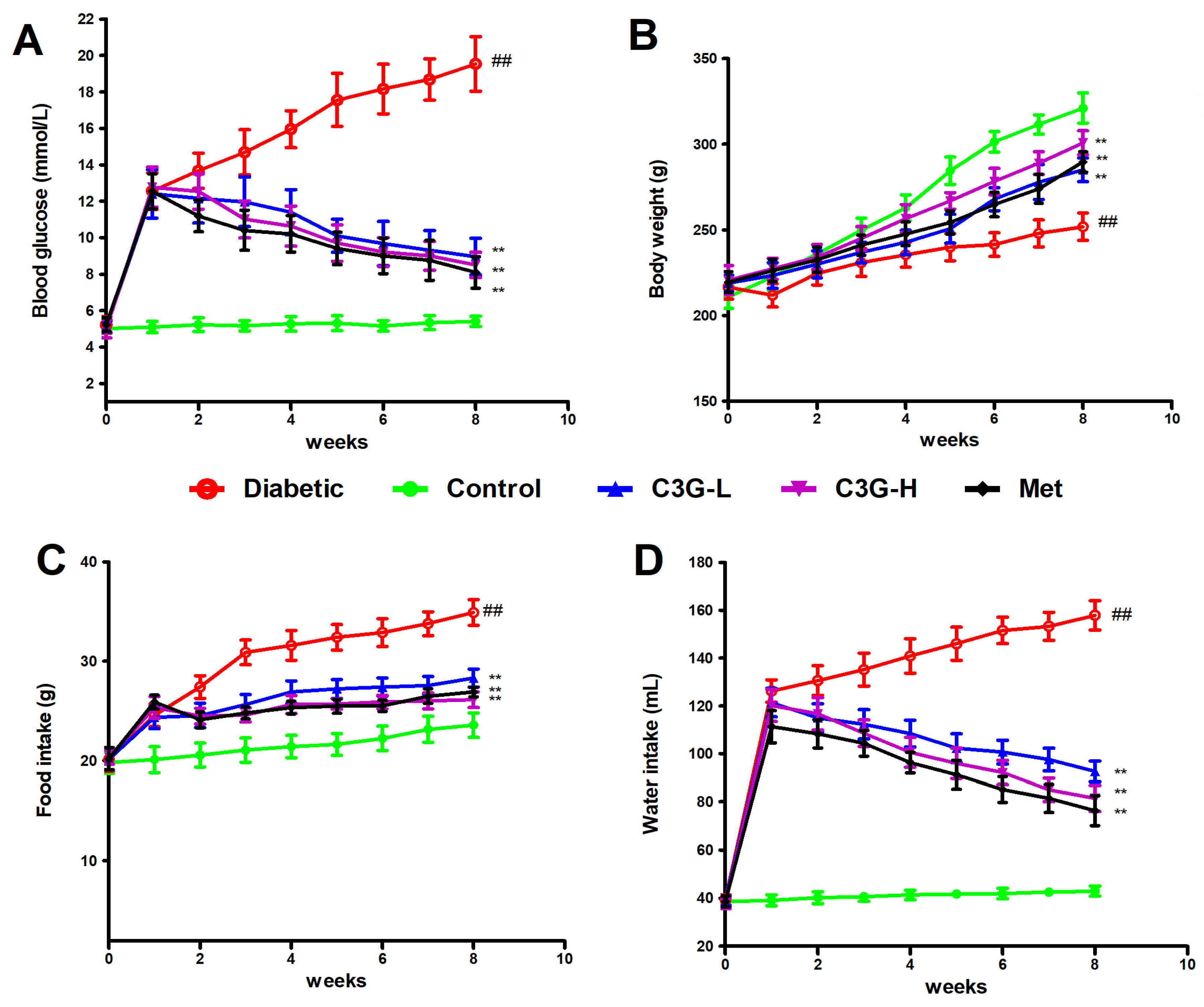
3.1. C3G Lowered Blood Glucose, Water and Food Intake, Increased Weight of Diabetic Rats
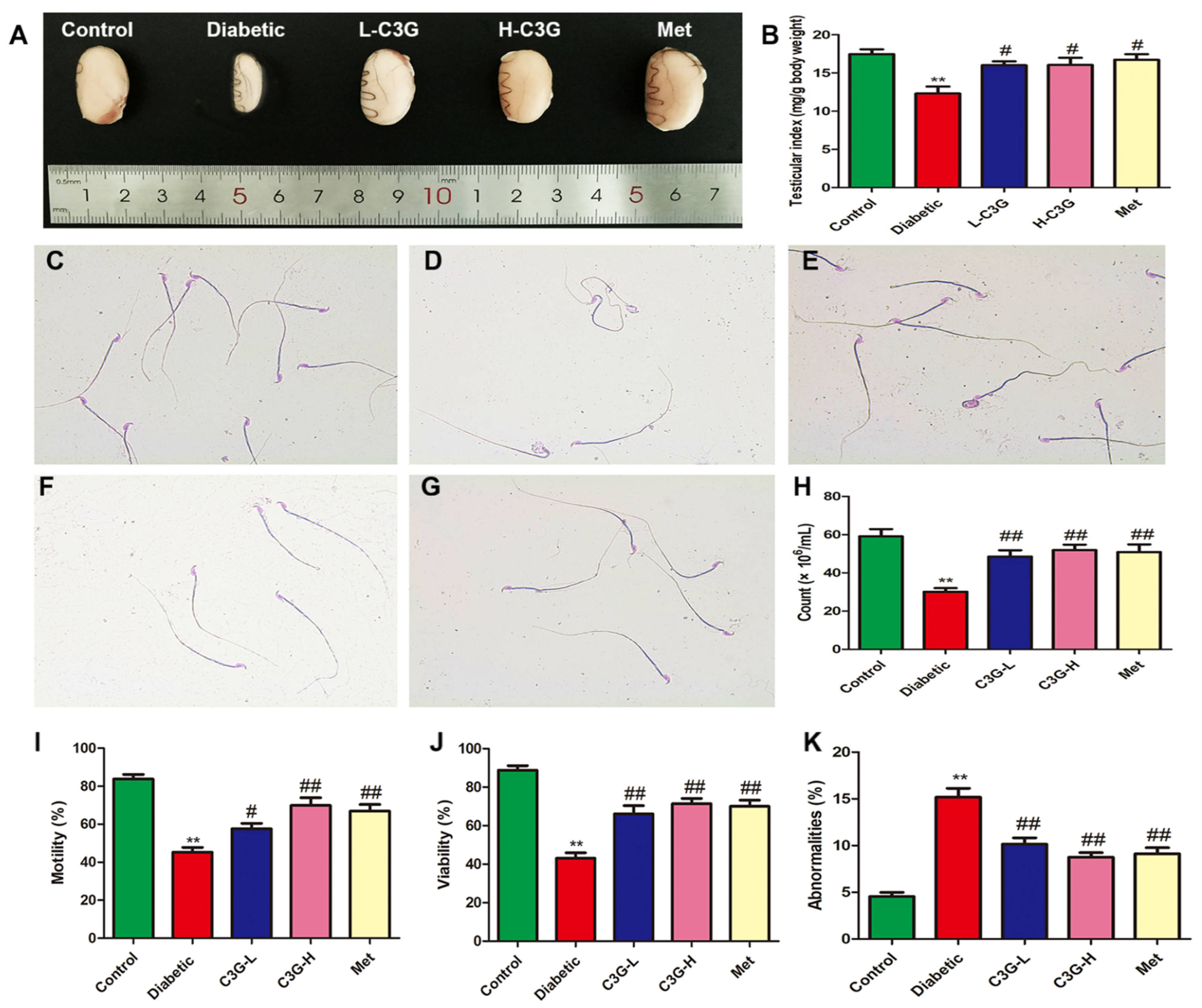
3.2. C3G Increased the Testicular Index and Repaired the Sperm Quality of Diabetic Rats
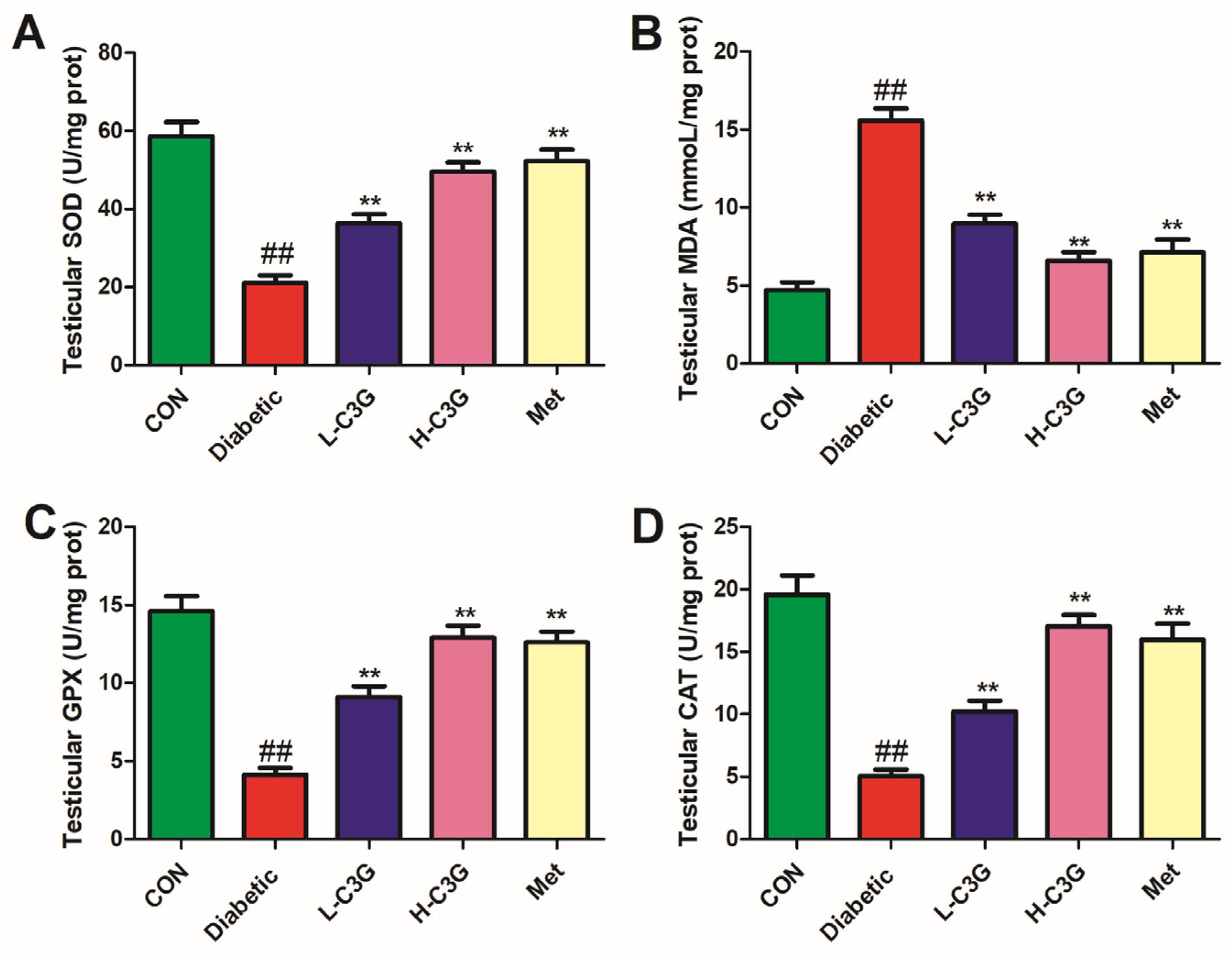
3.3. C3G Supplementation Relieved Testicular SOD, GPX, MDA, and CAT of Diabetic Rats
3.4. C3G Up-Regulated the Testosterone, LH, and FSH Content of Serum in Diabetic Rats
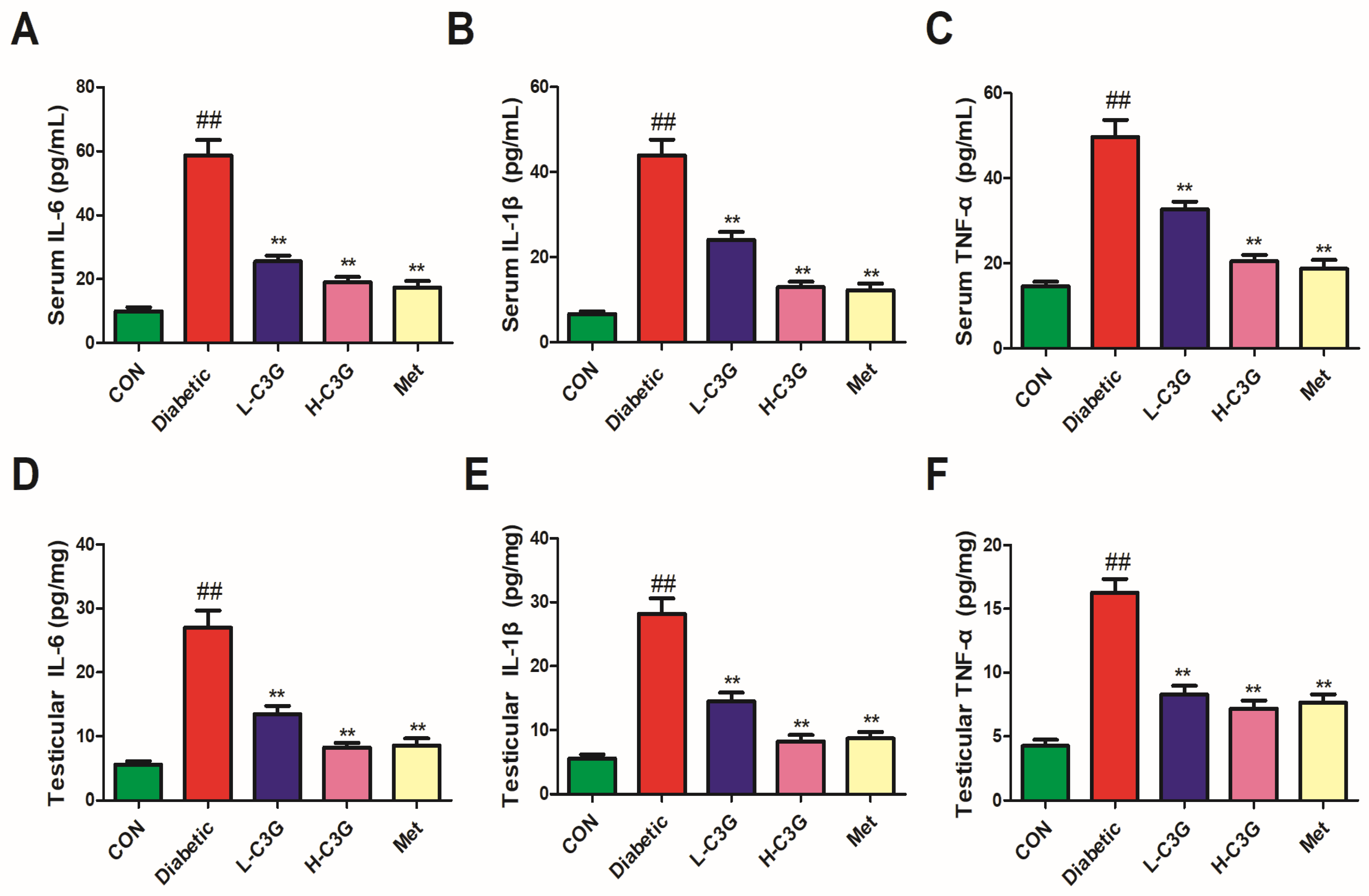
3.5. C3G Reduced Inflammatory Cytokines in Serum and Testicular Tissue of Diabetic Rats
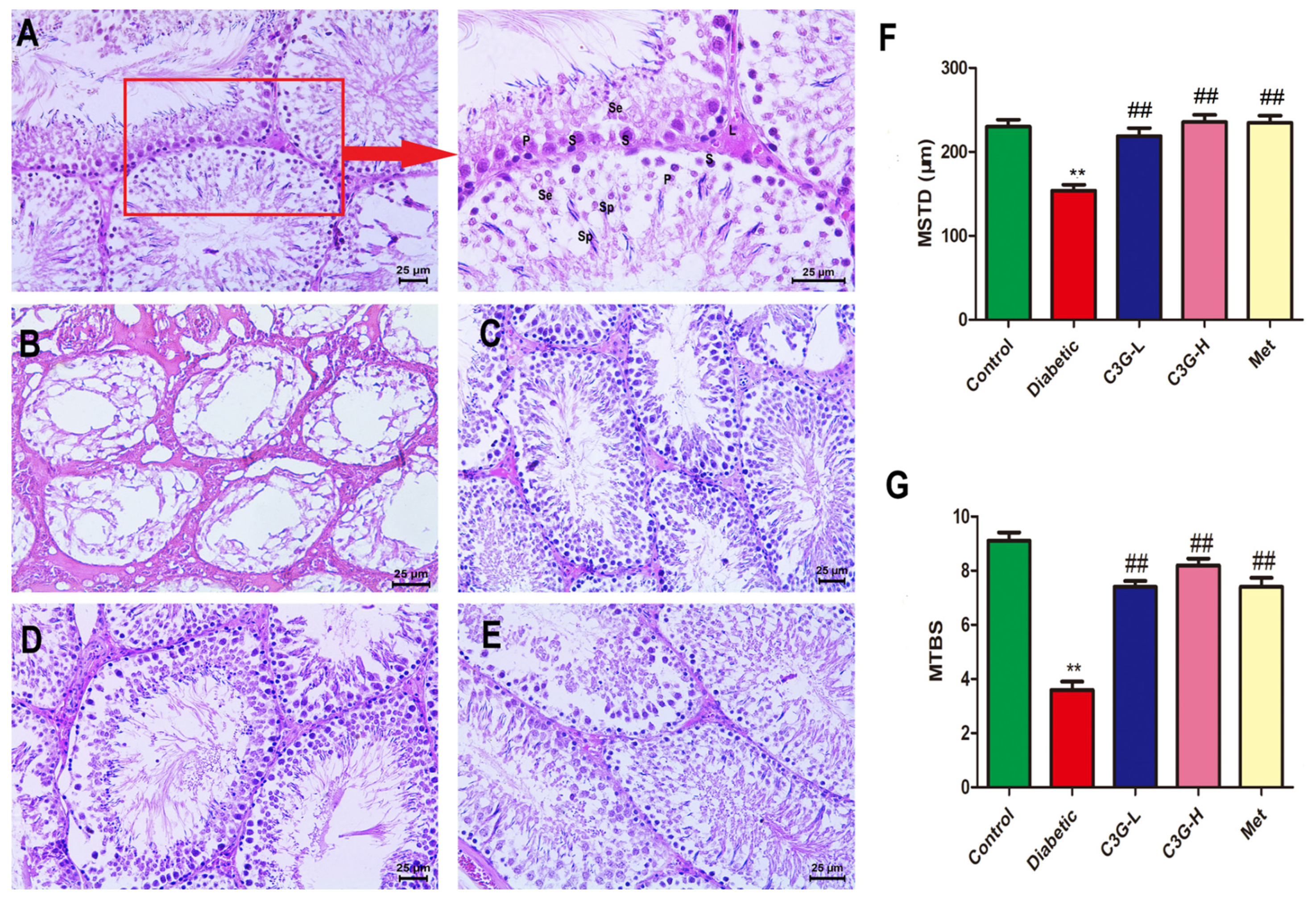
3.6. C3G Repaired Pathological Structure of Testicular Tissue of Diabetic Rats
3.7. C3G Inhibited the Testicular Interstitial Fibrosis of Diabetic Rats
3.8. C3G-Repaired Morphology of the Seminiferous Tubule Basement Membrane of Diabetic Rats
3.9. C3G-Repaired Ultrastructure of Testis of Diabetic Rats
3.10. C3G Regulated Cell-Apoptosis-Related Proteins in the Testis of Diabetic Rats
3.11. C3G Down-Regulated TGF-β1/Smads Pathway in the Testis of Diabetic Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sarmah, S.; Roy, A.S. A review on prevention of glycation of proteins: Potential therapeutic substances to mitigate the severity of diabetes complications. Int. J. Biol. Macromol. 2022, 195, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Letourneau, L.R.; Greeley, S.A.W. Congenital diabetes: Comprehensive genetic testing allows for improved diagnosis and treatment of diabetes and other associated features. Curr. Diab. Rep. 2018, 18, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Maresch, C.C.; Stute, D.C.; Alves, M.G.; Oliveira, P.F.; de Kretser, D.M.; Linn, T. Diabetes-induced hyperglycemia impairs male reproductive function: A systematic review. Hum. Reprod. Update 2018, 24, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Vignera, S.; Condorelli, R.A.; Di Mauro, M.; Lo Presti, D.; Mongioì, L.M.; Russo, G.; Calogero, A.E. Reproductive function in male patients with type 1 diabetes mellitus. Andrology 2015, 3, 1082–1087. [Google Scholar] [CrossRef]
- He, Z.; Yin, G.; Li, Q.Q.; Zeng, Q.; Duan, J. Diabetes mellitus causes male reproductive dysfunction: A review of the evidence and mechanisms. Vivo 2021, 35, 2503–2511. [Google Scholar] [CrossRef]
- Pavlinkova, G.; Margaryan, H.; Zatecka, E.; Valaskova, E.; Elzeinova, F.; Kubatova, A.; Bohuslavova, R.; Peknicova, J. Transgenerational inheritance of susceptibility to diabetes-induced male subfertility. Sci. Rep. 2017, 7, 4940. [Google Scholar] [CrossRef]
- Facondo, P.; Lodovico, E.; Delbarba, A.; Anelli, V.; Pezzaioli, L.C.; Filippini, E.; Cappelli, C.; Corona, G.; Ferlin, A. The impact of diabetes mellitus type 1 on male fertility: Systematic review and meta-analysis. Andrology 2022, 10, 426–440. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.; Vicari, E.; Agata, R.; Calogero, A.E. Diabetes mellitus and sperm parameters. J. Androl. 2012, 33, 145–153. [Google Scholar] [CrossRef]
- Ding, G.L.; Liu, Y.; Liu, M.E.; Pan, J.X.; Guo, M.X.; Sheng, J.Z.; Huang, H.F. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J. Androl. 2015, 17, 948–953. [Google Scholar]
- Shi, G.J.; Li, Z.M.; Zheng, J.; Chen, J.; Han, X.X.; Wu, J.; Li, G.Y.; Chang, Q.; Li, Y.X.; Yu, J.Q. Diabetes associated with male reproductive system damages: Onset of presentation, pathophysiological mechanisms and drug intervention. Biomed. Pharmacother. 2017, 90, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tan, Y.; Dai, J.; Li, B.; Guo, L.; Cui, J.; Wang, G.; Shi, X.; Zhang, X.; Mellen, N.; et al. Exacerbation of diabetes-induced testicular apoptosis by zinc deficiency is most likely associated with oxidative stress, p38 MAPK activation, and p53 activation in mice. Toxicol. Lett. 2011, 200, 100–106. [Google Scholar] [CrossRef]
- Amaral, S.; Oliveira, P.J.; Ramalho-Santos, J. Diabetes and the impairment of reproductive function: Possible role of mitochondria and reactive oxygen species. Curr. Diabetes Rev. 2008, 4, 46–54. [Google Scholar] [PubMed]
- Rashid, K.; Sil, P.C. Curcumin ameliorates testicular damage in diabetic rats by suppressing cellular stress-mediated mitochondria and endoplasmic reticulum-dependent apoptotic death. Biochim. Biophys. Acta 2015, 1852, 70–82. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Mongioì, L.M.; Alamo, A.; Calogero, A.E. Diabetes mellitus and infertility: Different pathophysiological effects in type 1 and type 2 on sperm function. Front. Endocrinol. 2018, 9, 268–282. [Google Scholar] [CrossRef]
- Nna, V.U.; Abu Bakar, A.B.; Ahmad, A.; Eleazu, C.O.; Mohamed, M. Oxidative stress, NF-κB-mediated inflammation and apoptosis in the testes of streptozotocin-induced diabetic rats: Combined protective effects of malaysian propolis and metformin. Antioxidants 2019, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, Z.; Sedaghat, R.; Baluchnejadmojarad, T.; Roghani, M. Diosgenin ameliorates testicular damage in streptozotocin-diabetic rats through attenuation of apoptosis, oxidative stress, and inflammation. Int. Immunopharmacol. 2019, 70, 37–46. [Google Scholar] [CrossRef]
- Orman, D.; Vardi, N.; Ates, B.; Taslidere, E.; Elbe, H. Aminoguanidine mitigates apoptosis, testicular seminiferous tubules damage, and oxidative stress in streptozotocin-induced diabetic rats. Tissue Cell 2015, 47, 284–290. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Feng, Y.-L.; Wang, Y.-H.; Kong, L.-J.; Zhou, M.-S.; Wu, M.-M.; Liu, C.-Y.; Weng, H.-C.; Wang, H.-W. Islet transplantation ameliorates diabetes-induced testicular interstitial fibrosis and is associated with inhibition of TGF-β1/Smad2 pathway in a rat model of type 1 diabetes. Mol. Med. Rep. 2021, 23, 376–385. [Google Scholar] [CrossRef]
- Shiraishi, K.; Takihara, H.; Naito, K.; Aktuelle, U. Quantitative analysis of testicular interstitial fibrosis after vasectomy in humans. Aktuel. Urol. 2003, 34, 262–264. [Google Scholar]
- Li, J.; Kang, S.W.; Kim, J.L.; Sung, H.Y.; Kwun, I.S.; Kang, Y.H. Isoliquiritigenin entails blockade of TGF-β1-smad signaling for retarding high glucose-induced mesangial matrix accumulation. J. Agric. Food Chem. 2010, 58, 3205–3212. [Google Scholar] [CrossRef]
- Ma, B.; Qiao, H.; Guo, Y.; Wei, J.; Yang, Q.; Feng, X.; Li, Z. TGF-β1 induced activations of Smad2 and miRNAs inhibit SF-1- and LRH-1-dependent CYP19 expression in rat Leydig cells. Biol. Reprod. 2023, 58, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Cai, F.L.; Wen, C.G.; Li, Z.Y.; Qin, G.W.; Qi, S.S.; Jiang, H.; Li, X.S.; Liu, M.H.; Yan, F.; et al. Anthocyanins are converted into anthocyanidins and phenolic acids and effectively absorbed in the jejunum and ileum. J. Agric. Food Chem. 2021, 69, 992–1002. [Google Scholar] [CrossRef]
- Tancharoen, S.; Shakya, P.; Napkpinit, S.; Dararat, P.; Kikuchi, K. Anthocyanins extracted from oryza sativa L. prevent fluorouracil-induced nuclear factor-κβ activation in oral mucositis: In vitro and in vivo studies. Int. J. Mol. Sci. 2018, 19, 2981. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, D.; Ji, Y.; Liu, Y.; Xu, L.; Guo, Y. Dietary supplementation of black rice anthocyanin extract regulates cholesterol metabolism and improves gut microbiota dysbiosis in c57BL/6J mice fed a high-fat and cholesterol diet. Mol. Nutr. Food Res. 2020, 64, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Guo, X.Q.; Zhang, M.; Yang, L.; Liu, R.; Yin, J. Anthocyanins in black rice, soybean and purple corn increase fecal butyric acid and prevent liver inflammation in high fat diet-induced obese mice. Food Funct. 2017, 8, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Guo, H.; Shen, T.; Tang, X.; Yang, Y.; Ling, W. Cyanidin-3-O-β-glucoside purified from black rice protects mice against hepatic fibrosis induced by carbon tetrachloride via inhibiting hepatic stellate cell activation. J. Agric. Food Chem. 2015, 63, 6221–6230. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhou, Y.H.; Wu, T.; Hao, L. Ameliorative effect of black rice anthocyanin on senescent mice induced by D-galactose. Food Funct. 2014, 5, 2892–2907. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.X.; Qi, S.S.; He, J.; Hu, C.Y.; Han, H.; Jiang, H.; Li, X.S. Cyanidin-3-glucoside from black rice ameliorates diabetic nephropathy via reducing blood glucose, suppressing oxidative stress and inflammation, and regulating transforming growth factor β1/Smad expression. J. Agric. Food Chem. 2020, 68, 4399–4410. [Google Scholar] [CrossRef]
- Qi, S.S.; He, J.; Dong, L.C.; Yuan, L.P.; Wu, J.L.; Zu, Y.X.; Zheng, H.X. Cyanidin-3-glucoside from black rice prevents renal dysfunction and renal fibrosis in streptozotocin-diabetic rats. J. Funct. Foods 2020, 72, 1040–1062. [Google Scholar] [CrossRef]
- Qi, S.S.; He, J.; Han, H.; Zheng, H.X.; Jiang, H.; Ching, Y.H.; Zhang, Z.J.; Li, X.S. Anthocyanin-rich extract from black rice (Oryza sativa L. Japonica) ameliorates diabetic osteoporosis in rats. Food Funct. 2019, 10, 5350–5360. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. 2021, 4, e78. [Google Scholar] [CrossRef]
- Johnsen, S.G. Testicular biopsy score count-a method for registration of sperma togenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1970, 1, 2–25. [Google Scholar] [PubMed]
- Zheng, H.; Hu, Y.; Shao, M.; Chen, S.; Qi, S. Chromium picolinate protects against testicular damage in STZ-induced diabetic rats via anti-Inflammation, anti-oxidation, inhibiting apoptosis, and regulating the TGF-β1/Smad pathway. Molecules 2023, 28, 7669. [Google Scholar] [CrossRef] [PubMed]
- Rabadiya, S.; Bhadada, S.; Dudhrejiya, A.; Vaishnav, D.; Patel, B. Magnesium valproate ameliorates type 1 diabetes and cardiomyopathy in diabetic rats through estrogen receptors. Biomed. Pharmacother. 2018, 97, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Alblihd, M.A.; Alsharif, K.F.; Hamad, A.A. Okra Abelmoschus esculentus (L.) Moench] improved blood glucose and restored histopathological alterations in splenic tissues in a rat model with streptozotocin-induced type 1 diabetes through CD8+ T cells and NF-kβ expression. Front. Vet. Sci. 2023, 10, 1268968. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, E.S.; Dallie, S.K.; Withyachumnarnkul, B. Reproductive system of the obese male zucker rat. Reproductive capacity, artificial insemination and plasma testosterone levels. Biol. Reprod. 1982, 27, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Vendramini, V.; Cedenho, A.P.; Miraglia, S.M.; Spaine, D.M. Reproductive function of the male obese Zucker rats: Alteration in sperm production and sperm DNA damage. Reprod. Sci. 2014, 21, 221–229. [Google Scholar] [CrossRef]
- Chen, Y.; Jiao, N.; Jiang, M.; Liu, L.; Zhu, Y.; Wu, H.; Chen, J.; Fu, Y.; Du, Q.; Xu, H.; et al. Loganin alleviates testicular damage and germ cell apoptosis induced by AGEs upon diabetes mellitus by suppressing the RAGE/p38MAPK/NF-κB pathway. J. Cell. Mol. Med. 2020, 24, 6083–6095. [Google Scholar] [CrossRef]
- Oladipo, G.O.; Nlekerem, C.M.; Ibukun, E.O.; Kolawole, A.O. Quail (Coturnix japonica) egg yolk bioactive components attenuate streptozotocin-induced testicular damage and oxidative stress in diabetic rats. Eur. J. Nutr. 2018, 57, 2857–2867. [Google Scholar] [CrossRef]
- Nna, V.U.; Bakar, A.; Ahmad, A.; Mohamed, M. Down-regulation of steroidogenesis-related genes and its accompanying fertility decline in streptozotocin-induced diabetic male rats: Ameliorative effect of metformin. Andrology 2019, 7, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Shoorei, H.; Khaki, A.; Khaki, A.A.; Hemmati, A.A.; Moghimian, M.; Shokoohi, M. The ameliorative effect of carvacrol on oxidative stress and germ cell apoptosis in testicular tissue of adult diabetic rats. Biomed. Pharmacother. 2019, 111, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; De Iuliis, G.N.; Finnie, J.M.; Hedges, A.; McLachlan, R.I. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: Development of diagnostic criteria. Hum. Reprod. 2010, 25, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T. Role of oxidative stress in male infertility: An updated review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Laleethambika, N.; Anila, V.; Manojkumar, C.; Muruganandam, I.; Giridharan, B.; Ravimanickam, T. Diabetes and sperm DNA damage: Efficacy of antioxidants. SN Compr. Clin. Med. 2019, 1, 49–59. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Zrieq, R.; Al-Quraishy, S.A.; Moneim, A.E. Selenium nanoparticles attenuate oxidative stress and testicular damage in streptozotocin-induced diabetic rats. Molecules 2016, 21, 1517. [Google Scholar] [CrossRef]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian, K.M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: A review. J. Clin. Diagn. Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef]
- Guazzone, V.A.; Jacobo, P.; Theas, M.S.; Lustig, L. Cytokines and chemokines in testicular inflammation: A brief review. Microsc. Res. Tech. 2009, 72, 620–628. [Google Scholar] [CrossRef]
- Dogan, Y.; Akarsu, S.; Ustundag, B.; Yilmaz, E.; Gurgoze, M.K. Serum IL-1beta, IL-2, and IL-6 in insulin-dependent diabetic children. Mediat. Inflamm. 2006, 1, 592–596. [Google Scholar]
- Mohamad, N.V.; Wong, S.K.; Wan Hasan, W.N.; Jolly, J.J.; Nur-Farhana, M.F.; Ima-Nirwana, S.; Chin, K.Y. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 2019, 22, 129–140. [Google Scholar] [CrossRef]
- Nasiri, K.; Akbari, A.; Nimrouzi, M.; Ruyvaran, M.; Mohamadian, A. Safflower seed oil improves steroidogenesis and spermatogenesis in rats with type II diabetes mellitus by modulating the genes expression involved in steroidogenesis, inflammation and oxidative stress. J. Ethnopharmacol. 2021, 275, 114–139. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.M.; Elshaer, S.L.; Gangaraju, R.; Abdelaziz, R.R.; Nader, M.A. CysLTR1 antagonism by montelukast can ameliorate diabetes-induced aortic and testicular inflammation. Int. Immunopharmacol. 2023, 125, 111127. [Google Scholar] [CrossRef] [PubMed]
- Nna, V.U.; Bakar, A.B.A.; Ahmad, A.; Mohamed, M. Diabetes-induced testicular oxidative stress, inflammation, and caspase-dependent apoptosis: The protective role of metformin. Arch. Physiol. Biochem. 2020, 126, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Bai, Y.; Zhang, Z.; Xin, Y.; Cai, L. Protection by sulforaphane from type 1 diabetes-induced testicular apoptosis is associated with the up-regulation of Nrf2 expression and function. Toxicol. Appl. Pharmacol. 2014, 279, 198–210. [Google Scholar] [CrossRef]
- Hu, L.; Wei, S.; Wu, Y.; Li, S.; Zhu, P.; Wang, X. MicroRNA regulation of the proliferation and apoptosis of Leydig cells in diabetes. Mol. Med. 2021, 27, 104–112. [Google Scholar] [CrossRef]
- Aly, H. Mitochondria-mediated apoptosis induced testicular dysfunction in diabetic rats: Ameliorative effect of resveratrol. Endocrinology 2021, 162, 18–25. [Google Scholar] [CrossRef]
- Long, L.; Wang, J.; Lu, X.; Xu, Y.; Zheng, S.; Luo, C.; Li, Y. Protective effects of scutellarin on type II diabetes mellitus-induced testicular damages related to reactive oxygen species/Bcl-2/Bax and reactive oxygen species microcirculation staving pathway in diabetic rat. J. Diabetes Res. 2015, 2015, 252530. [Google Scholar] [CrossRef]
- Cayan, S.; Dusmez, D.; Bozlu, M.; Gorur, S.; Akbay, E. Human testicular mast cells and fibrosis in infertile men. Fertil. Steril. 2001, 76, S258. [Google Scholar] [CrossRef]
- Bhanmeechao, C.; Srisuwatanasagul, S.; Ponglowhapan, S. Age-related changes in interstitial fibrosis and germ cell degeneration of the canine testis. Reprod. Domest. Anim. 2018, 53, 37–43. [Google Scholar] [CrossRef]
- Yang, D.; Li, L.; Qian, S.; Liu, L. Evodiamine ameliorates liver fibrosis in rats via TGF-β1/smad signaling pathway. J. Nat. Med. 2018, 72, 145–154. [Google Scholar] [CrossRef]
- Gonzalez, C.R.; Matzkin, M.E.; Frungieri, M.B.; Terradas, C.; Ponzio, R.; Puigdomenech, E.; Levalle, O.; Calandra, R.S.; Gonzalez-Calvar, S.I. Expression of the TGF-beta1 system in human testicular pathologies. Reprod. Biol. Endocrinol. 2010, 8, 148. [Google Scholar] [CrossRef] [PubMed]





| Score | Description |
|---|---|
| 1 | No cells |
| 2 | Sertoli cells without germ cells |
| 3 | Only spermatogonia |
| 4 | Only a few spermatocytes |
| 5 | Many spermatocytes |
| 6 | Only a few early spermatids |
| 7 | Many early spermatids without differentiation |
| 8 | Few late spermatids |
| 9 | Many late spermatids |
| 10 | Full spermatogenesis |
| Groups | LH (IU/L) | FSH (IU/L) | Testosterone (ng/mL) |
|---|---|---|---|
| Control | 1.36 ± 0.21 | 16.75 ± 1.46 | 1.29 ± 0.13 |
| Diabetic | 0.74 ± 0.14 ** | 8.36 ± 1.21 ** | 0.36 ± 0.09 ** |
| L-C3G | 1.15 ± 0.18 # | 13.28 ± 1.31 ## | 0.96 ± 0.14 ## |
| H-C3G | 1.25 ± 0.22 ## | 14.22 ± 1.90 ## | 1.26 ± 0.10 ## |
| Met | 1.36 ± 0.19 ## | 13.44 ± 1.57 ## | 1.22 ± 0.13 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Hu, Y.; Zhou, J.; Zhou, B.; Qi, S. Protective Effect of Black Rice Cyanidin-3-Glucoside on Testicular Damage in STZ-Induced Type 1 Diabetic Rats. Foods 2024, 13, 727. https://doi.org/10.3390/foods13050727
Zheng H, Hu Y, Zhou J, Zhou B, Qi S. Protective Effect of Black Rice Cyanidin-3-Glucoside on Testicular Damage in STZ-Induced Type 1 Diabetic Rats. Foods. 2024; 13(5):727. https://doi.org/10.3390/foods13050727
Chicago/Turabian StyleZheng, Hongxing, Yingjun Hu, Jia Zhou, Baolong Zhou, and Shanshan Qi. 2024. "Protective Effect of Black Rice Cyanidin-3-Glucoside on Testicular Damage in STZ-Induced Type 1 Diabetic Rats" Foods 13, no. 5: 727. https://doi.org/10.3390/foods13050727







