Metabolomic Profiling Reveals the Quality Variations in Citri Reticulatae Pericarpium (Citrus reticulata Blanco cv. Chachiensis) with Different Storage Ages in Response to “Candidatus Liberibacter Asiaticus” Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of PCRC Samples
2.2. DNA Extraction and CLas Detection
2.3. Untargeted Metabolomics Analysis
2.4. Data Processing and Chemical Metabolite Identification
2.5. Determination of Total Flavonoids (TFs) Content, Total Polyphenols (TPs) Content, and Total Antioxidant Capacity (TAC) of PCRC
2.6. Statistical Analysis
3. Results
3.1. CLas Detection and Phenotypic Characteristics of PCRC Samples
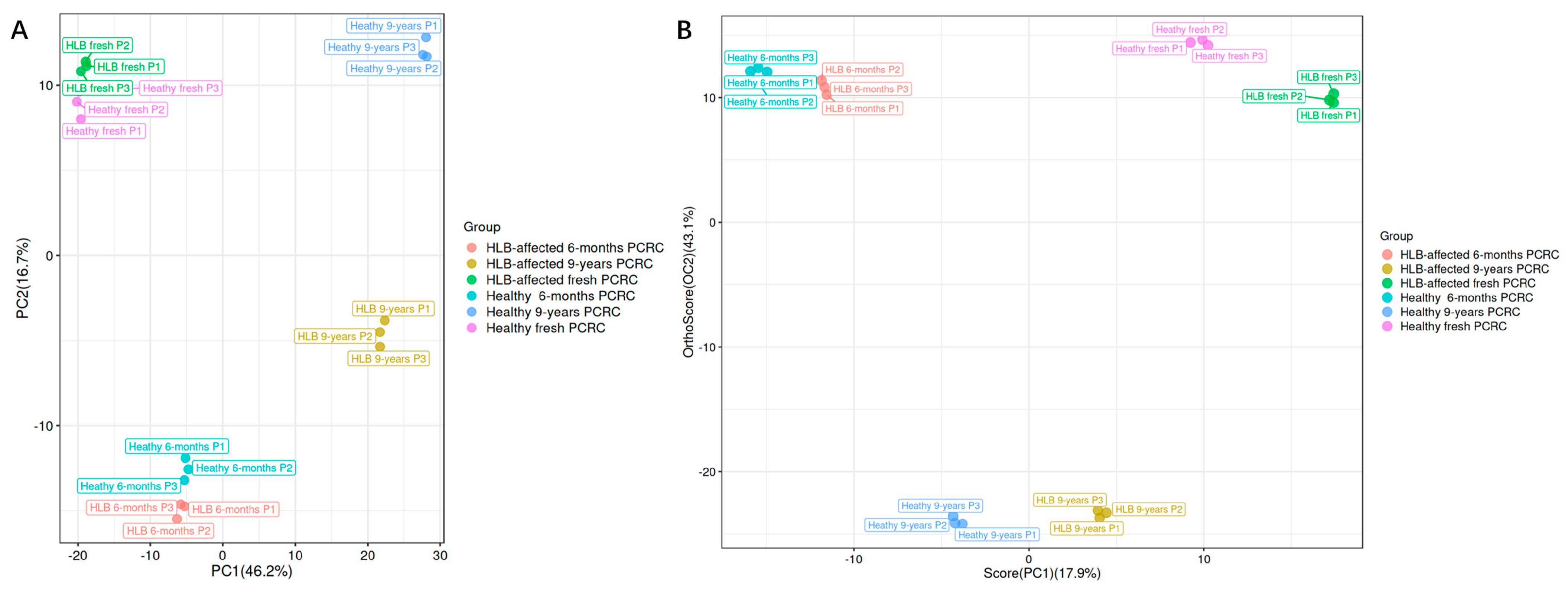
3.2. Multivariate Analyses of Metabolites in Healthy and HLB-Affected PCRC
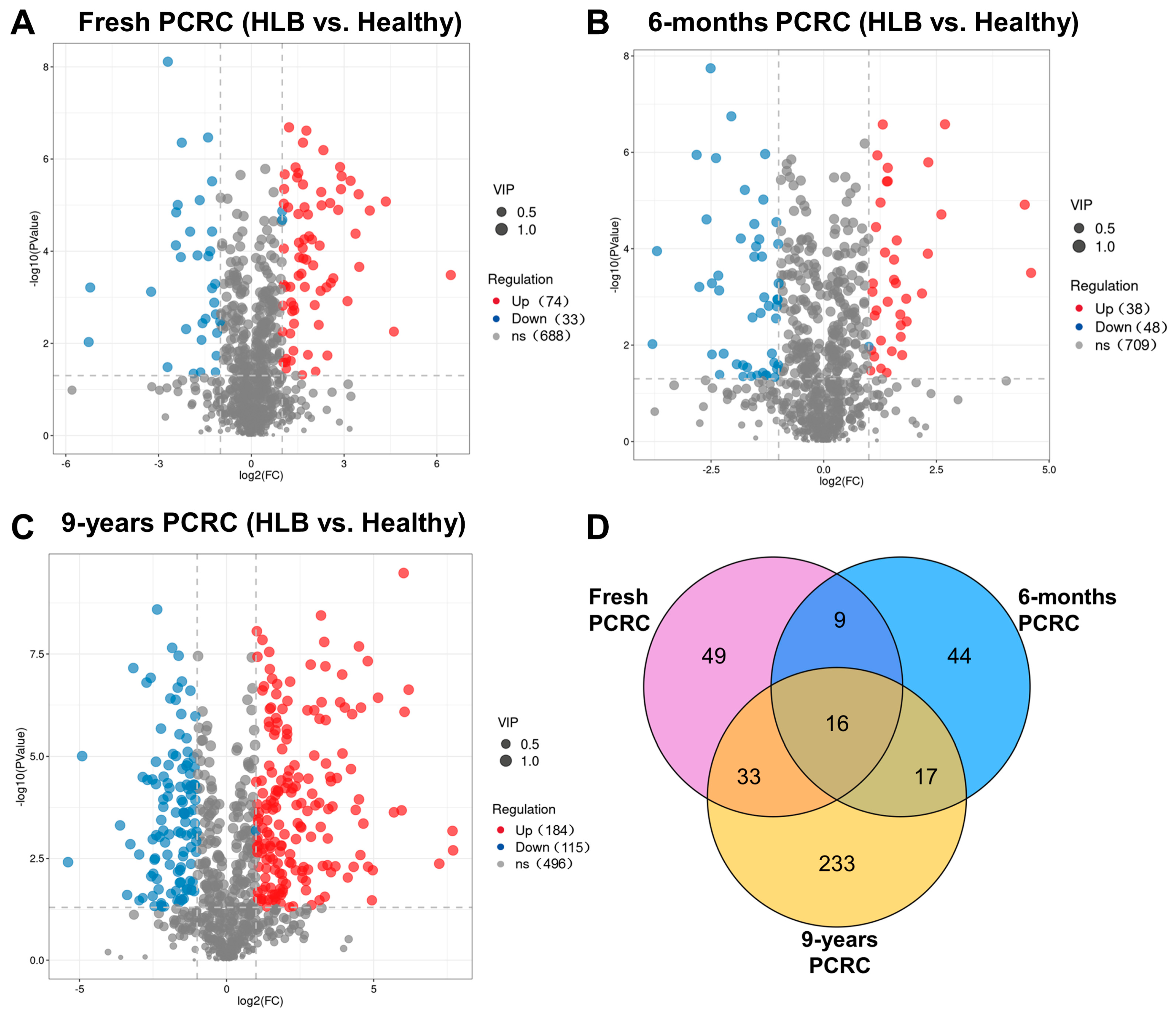
3.3. Comparative Metabolic Analysis of Healthy and HLB-Affected PCRC
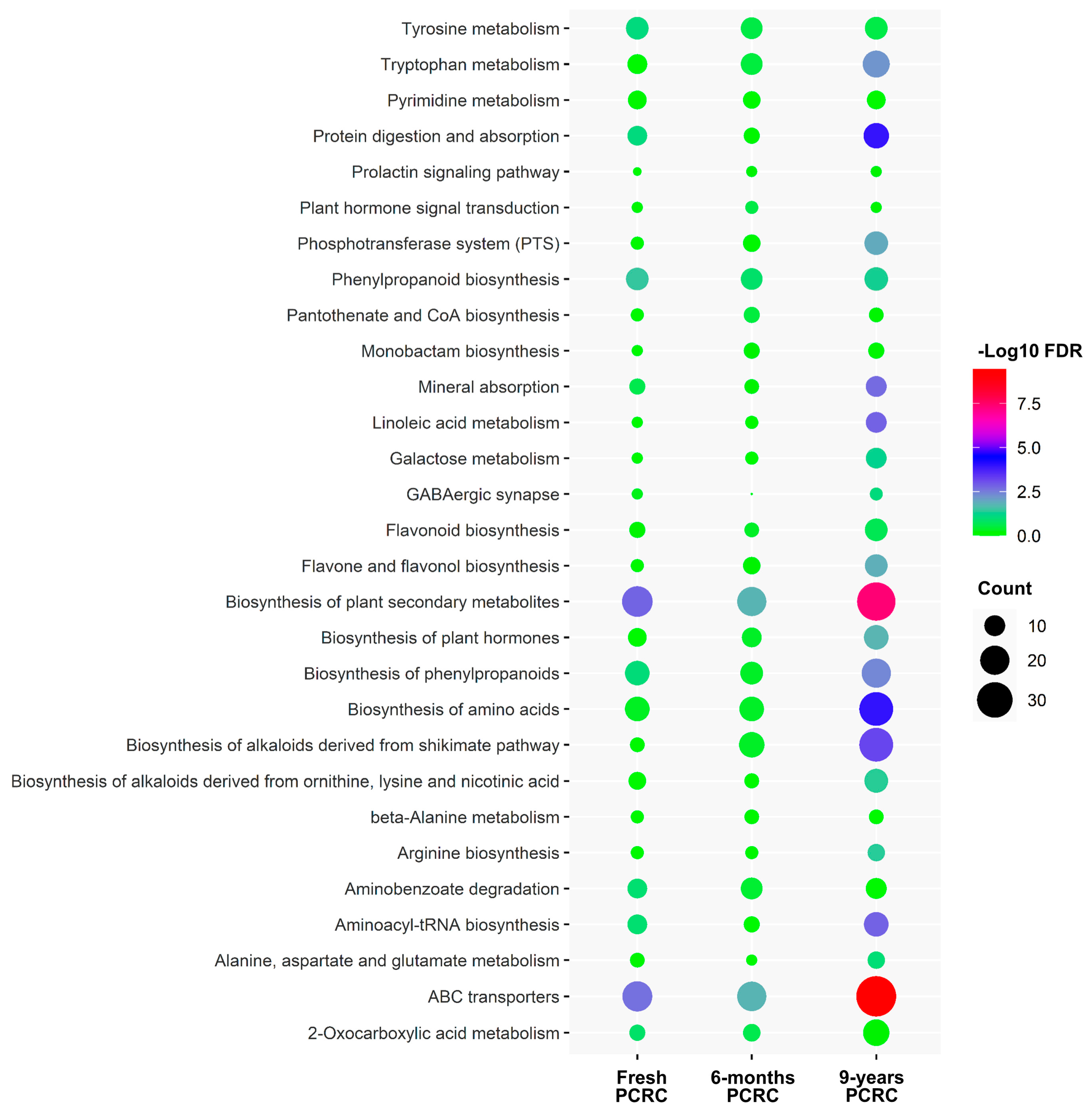
3.4. KEGG Pathway Analysis
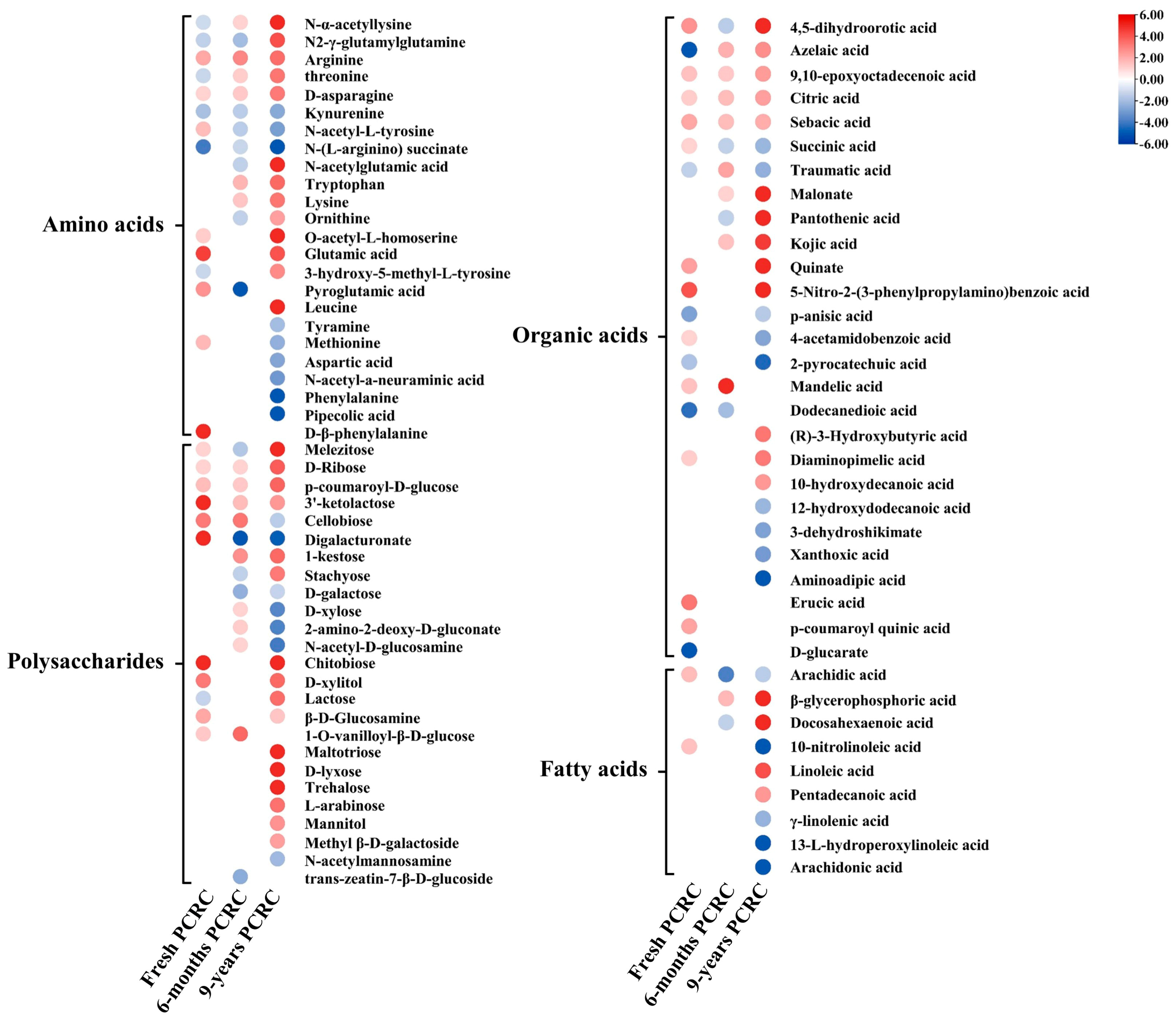
3.5. Alteration of Secondary Metabolites in PCRC by HLB
3.6. Changes in Primary Metabolites of PCRC Affected by HLB
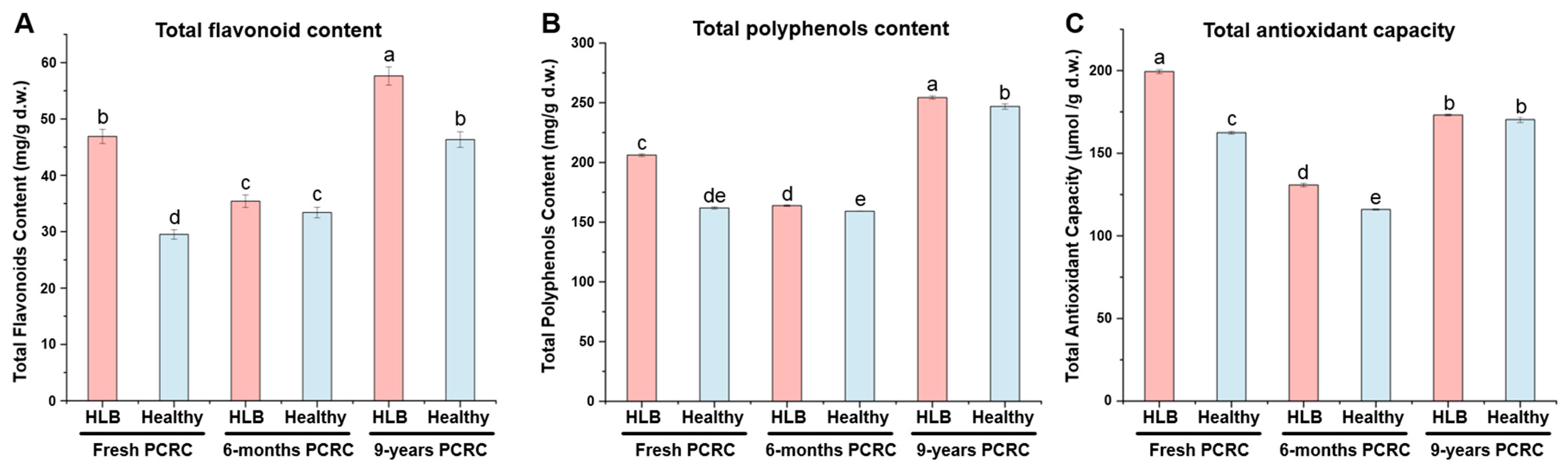
3.7. TFs Content, TPs Content and TAC of HLB-Affected and Healthy PCRC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, X.; Sun, S.; Guo, Y.; Liu, Y.; Yang, D.; Li, G.; Lü, S. Citri Reticulatae Pericarpium (Chenpi): Botany, ethnopharmacology, phytochemistry, and pharmacology of a frequently used traditional Chinese medicine. J. Ethnopharmacol. 2018, 220, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, X.; Qiu, J.; Zhang, L.; Zhang, Y.; Qiu, X.; Huang, Z.; Xu, W. Comprehensive comparison on the chemical profile of guang chen pi at different ripeness stages using untargeted and pseudotargeted metabolomics. J. Agric. Food Chem. 2020, 68, 8483–8495. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, J.; Gu, S.; Liu, Z.; Zhang, Y.; Zhang, X. Simultaneous determination of flavonoids in different parts of Citrus reticulata ‘Chachi’ fruit by high performance liquid chromatography-photodiode array detection. Molecules 2010, 15, 5378–5388. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Xu, Y.; Chen, Y.; Wu, J.; Yu, Y.; Zou, B.; An, K.; Xiao, G. Evaluation of bioactive flavonoids and antioxidant activity in Pericarpium Citri Reticulatae (Citrus reticulata ‘Chachi’) during storage. Food Chem. 2017, 230, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lai, C.S.; Ho, C.T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Xie, X.; Cai, J.; Zhao, Y.; Miao, W.; Chen, X.; Xiao, Y.; Li, N.; Wu, J.L. Dynamic changes of phenolic acids and antioxidant activity of Citri Reticulatae Pericarpium during aging processes. Food Chem. 2022, 373 Pt A, 131399. [Google Scholar] [CrossRef]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Folimonova, S.Y.; Robertson, C.J.; Garnsey, S.M.; Gowda, S.; Dawson, W.O. Examination of the responses of different genotypes of citrus to huanglongbing (citrus greening) under different conditions. Phytopathology 2009, 1346–1354. [Google Scholar] [CrossRef]
- Shokrollah, H.; Abdullah, T.L.; Sijam, K.; Abdullah, S.N. Potential use of selected citrus rootstocks and interstocks against HLB disease in Malaysia. Crop Prot. 2011, 30, 521–525. [Google Scholar] [CrossRef]
- Lin, K.H. Observations on yellow shoot of citrus. Etiological studies of yellow shoot of Citrus. Acta Phytopathol. Sinca 1956, 2, 1–11. [Google Scholar]
- Zheng, Z.; Chen, J.; Deng, X. Historical perspectives, management, and current research of citrus HLB in Guangdong province of china, where the disease has been endemic for over a hundred years. Phytopathology 2018, 108, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Bassanezi, R.B.; Montesino, L.H.; Stuchi, E.S. Effects of huanglongbing on fruit quality of sweet orange cultivars in Brazil. Eur. J. Plant Pathol. 2009, 125, 565–572. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Plotto, A.; Bai, J.; Manthey, J.A.; Baldwin, E.A.; Ferrarezi, R.S.; Gloria, M.B.A. Effect of Huanglongbing or greening disease on orange juice quality, a review. Front. Plant Sci. 2019, 9, 1976. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, M.; Bao, M.; Wu, F.; Chen, J.; Deng, X. Unusual five copies and dual forms of nrdB in “Candidatus Liberibacter asiaticus”: Biological implications and PCR detection application. Sci. Rep. 2016, 6, 39020. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Zheng, Z.; Sun, X.; Chen, J.; Deng, X. Enhancing PCR capacity to detect ‘Candidatus Liberibacter asiaticus’ utilizing whole genome sequence information. Plant Dis. 2020, 104, 527–532. [Google Scholar] [CrossRef]
- Yan, J.; Yuan, F.; Long, G.; Qin, L.; Deng, Z. Selection of reference genes for quantitative real-time RT-PCR analysis in citrus. Mol. Biol. Rep. 2012, 39, 1831–1838. [Google Scholar] [CrossRef]
- Smith, C.A.; Wan, T.E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Gagnebin, Y.; Tonoli, D.; Lescuyer, P.; Ponte, B.; de Seigneux, S.; Martin, P.Y.; Schappler, J.; Boccard, J.; Rudaz, S. Metabolomic analysis of urine samples by UHPLC-QTOF-MS: Impact of normalization strategies. Anal. Chim. Acta 2017, 955, 27–35. [Google Scholar] [CrossRef]
- Hung, W.L.; Wang, Y. A targeted mass spectrometry-based metabolomics approach toward the understanding of host responses to huanglongbing disease. J. Agric. Food Chem. 2018, 66, 10651–10661. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S. Bioactivity of naringin and related mechanisms. Pharmazie 2021, 76, 359–363. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-inflammatory and active biological properties of the plant-derived bioactive compounds luteolin and luteolin 7-glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef]
- Wang, K.L.; Yu, Y.C.; Hsia, S.M. Perspectives on the Role of Isoliquiritigenin in Cancer. Cancers 2021, 13, 115. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika, S.R.; Dwi, A.A.; Yasir, B.; Rahma, R.N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Li, J.; Zou, S.; Yang, W.; Peng, M.; Chen, B.; Deng, J.; Wei, M.; Zheng, G. Identification of volatile and nonvolatile compounds in Citri Reticulatae Pericarpium Viride using GC-MS, UPLC-Q-Exactive Orbitrap-MS, and HPLC-PDA. Food Sci. Nutr. 2023, 11, 1415–1425. [Google Scholar] [CrossRef]
- Gualdani, R.; Cavalluzzi, M.M.; Lentini, G.; Habtemariam, S. The chemistry and pharmacology of citrus limonoids. Molecules 2016, 21, 1530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Zhang, R.Q.; Rahman, K.; Cao, Z.X.; Zhang, H.; Peng, C. Diverse pharmacological activities and potential medicinal benefits of geniposide. Evid. Based Complement. Alternat. Med. 2019, 2019, 4925682. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Wang, J.; Ye, W.; Lu, J.; Li, C.; Zhang, D.; Ye, W.; Xu, S.; Chen, C.; Liu, P.; et al. Citri Reticulatae Pericarpium (Chenpi): A multi-efficacy pericarp in treating cardiovascular diseases. Biomed. Pharmacother. 2022, 154, 113626. [Google Scholar] [CrossRef]
- El-Kalyoubi, S.A.; Fayed, E.A.; Abdel-Razek, A.S. One pot synthesis, antimicrobial and antioxidant activities of fused uracils: Pyrimidodiazepines, lumazines, triazolouracil and xanthines. Chem. Cent. J. 2017, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Cao, N.; Wang, C. A review on β-carboline alkaloids and their distribution in foodstuffs: A class of potential functional components or not? Food Chem. 2021, 348, 129067. [Google Scholar] [CrossRef] [PubMed]
- Bradner, W.T. Mitomycin C: A clinical update. Cancer Treat. Rev. 2001, 27, 35–50. [Google Scholar] [CrossRef]
- Ginnan, N.A.; Dang, T.; Bodaghi, S.; Ruegger, P.M.; Englang, G.; Vidalakis, G.; Borneman, J.; Rolshausen, P.E.; Roper, M.E. Disease-induced microbial shifts in citrus indicate microbiome-derived responses to huanglongbing across the disease severity spectrum. Phytobiomes J. 2020, 4, 375–387. [Google Scholar] [CrossRef]
- Balaha, M.; Ahmed, N.; Geddawy, A.; Kandeel, S. Fraxetin prevented sodium fluoride-induced chronic pancreatitis in rats: Role of anti-inflammatory, antioxidant, antifibrotic and anti-apoptotic activities. Int. Immunopharmacol. 2021, 93, 107372. [Google Scholar] [CrossRef]
- Hui, Y.; Wang, X.; Yu, Z.; Fan, X.; Cui, B.; Zhao, T.; Mao, L.; Feng, H.; Lin, L.; Yu, Q.; et al. Scoparone as a therapeutic drug in liver diseases: Pharmacology, pharmacokinetics and molecular mechanisms of action. Pharmacol. Res. 2020, 160, 105170. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Moselhy, S.S.; Razvi, S.S.; ALshibili, F.A.; Kuerban, A.; Hasan, M.N.; Balamash, K.S.; Huwait, E.A.; Abdulaal, W.H.; Al-Ghamdi, M.A.; Kumosani, T.A.; et al. m-Coumaric acid attenuates non-catalytic protein glycosylation in the retinas of diabetic rats. J. Pestic. Sci. 2018, 43, 180–185. [Google Scholar] [CrossRef]
- Adaszek, Ł.; Gadomska, D.; Mazurek, Ł.; Łyp, P.; Madany, J.; Winiarczyk, S. Properties of capsaicin and its utility in veterinary and human medicine. Res. Vet. Sci. 2019, 123, 14–19. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, Z.; Wen, M.; Wu, Z.; Zha, M.; Xu, W.; Zhang, L. Chemical variation of chenpi (citrus peels) and corresponding correlated bioactive compounds by LC-MS metabolomics and multibioassay analysis. Front. Nutr. 2022, 9, 825381. [Google Scholar] [CrossRef] [PubMed]
- Killiny, N.; Nehela, Y. Metabolomic Response to Huanglongbing: Role of Carboxylic Compounds in Citrus sinensis Response to ‘Candidatus Liberibacter asiaticus’ and Its Vector, Diaphorina citri. Mol. Plant Microbe Interact. 2017, 30, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Jiang, Y.; Wen, L.; Yang, B. Characterization of polysaccharide structure in Citrus reticulate ‘Chachi’ peel during storage and their bioactivity. Carbohydr. Res. 2021, 508, 108398. [Google Scholar] [CrossRef]
- Chen, A.S.; Taguchi, T.; Sakai, K.; Kikuchi, K.; Wang, M.W.; Miwa, I. Antioxidant activities of chitobiose and chitotriose. Biol. Pharm. Bull. 2003, 26, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Min, A.; Luo, S.; He, J.; Wu, R.; Lin, X.; Wang, Y.; He, W.; Zhang, Y.; Lin, Y.; et al. Metabolomic Analysis Revealed Distinct Physiological Responses of Leaves and Roots to Huanglongbing in a Citrus Rootstock. Int. J. Mol. Sci. 2022, 23, 9242. [Google Scholar] [CrossRef] [PubMed]
- Prag, H.A.; Aksentijevic, D.; Dannhorn, A.; Giles, A.V.; Mulvey, J.F.; Sauchanka, O.; Du, L.; Bates, G.; Reinhold, J.; Kula-Alwar, D.; et al. Ischemia-Selective Cardioprotection by Malonate for Ischemia/Reperfusion Injury. Circ. Res. 2022, 131, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W.; Rucker, R.B. Pantothenic acid. In Present Knowledge in Nutrition, 11th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Xi, Y.; Li, J.; Xu, S.; Zheng, Y.; Xu, M.; Zheng, Z.; Deng, X. Metabolomic Profiling Reveals the Quality Variations in Citri Reticulatae Pericarpium (Citrus reticulata Blanco cv. Chachiensis) with Different Storage Ages in Response to “Candidatus Liberibacter Asiaticus” Infection. Foods 2024, 13, 827. https://doi.org/10.3390/foods13060827
Liang J, Xi Y, Li J, Xu S, Zheng Y, Xu M, Zheng Z, Deng X. Metabolomic Profiling Reveals the Quality Variations in Citri Reticulatae Pericarpium (Citrus reticulata Blanco cv. Chachiensis) with Different Storage Ages in Response to “Candidatus Liberibacter Asiaticus” Infection. Foods. 2024; 13(6):827. https://doi.org/10.3390/foods13060827
Chicago/Turabian StyleLiang, Jiayin, Yuqing Xi, Jiaming Li, Shugui Xu, Yongqin Zheng, Meirong Xu, Zheng Zheng, and Xiaoling Deng. 2024. "Metabolomic Profiling Reveals the Quality Variations in Citri Reticulatae Pericarpium (Citrus reticulata Blanco cv. Chachiensis) with Different Storage Ages in Response to “Candidatus Liberibacter Asiaticus” Infection" Foods 13, no. 6: 827. https://doi.org/10.3390/foods13060827
APA StyleLiang, J., Xi, Y., Li, J., Xu, S., Zheng, Y., Xu, M., Zheng, Z., & Deng, X. (2024). Metabolomic Profiling Reveals the Quality Variations in Citri Reticulatae Pericarpium (Citrus reticulata Blanco cv. Chachiensis) with Different Storage Ages in Response to “Candidatus Liberibacter Asiaticus” Infection. Foods, 13(6), 827. https://doi.org/10.3390/foods13060827





