Impact of Technological Processes on the Formation of Furosine, Acrylamide and Furan in Traditional Venezuelan Cocoa
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Furosine Assay
2.4. Acrylamide Assay
2.5. Furan Assay
2.6. Other Determinations
2.7. Statistical Analysis
3. Results and Discussion
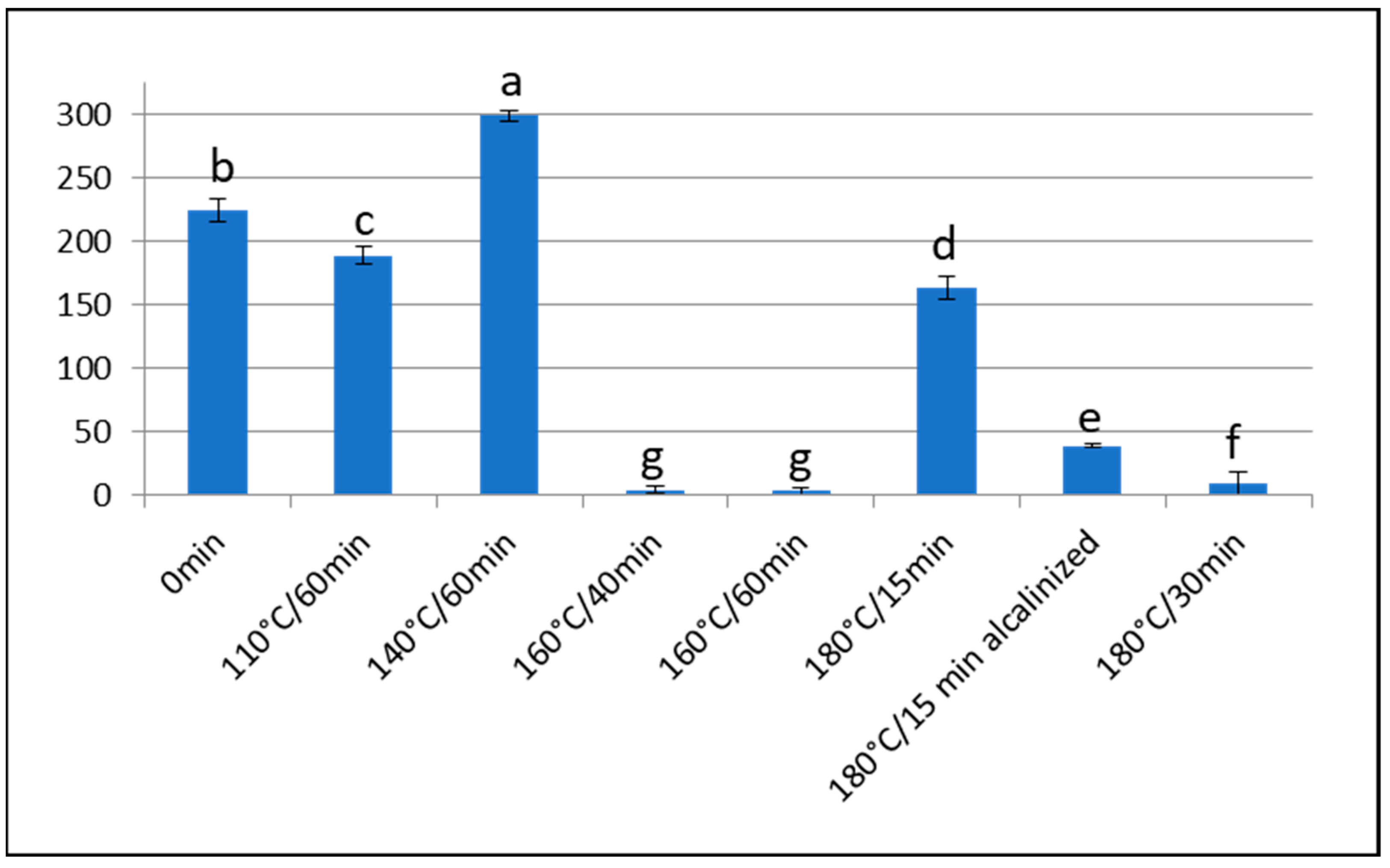
3.1. Furosine
Effect of Over-Roasting on Furosine Content
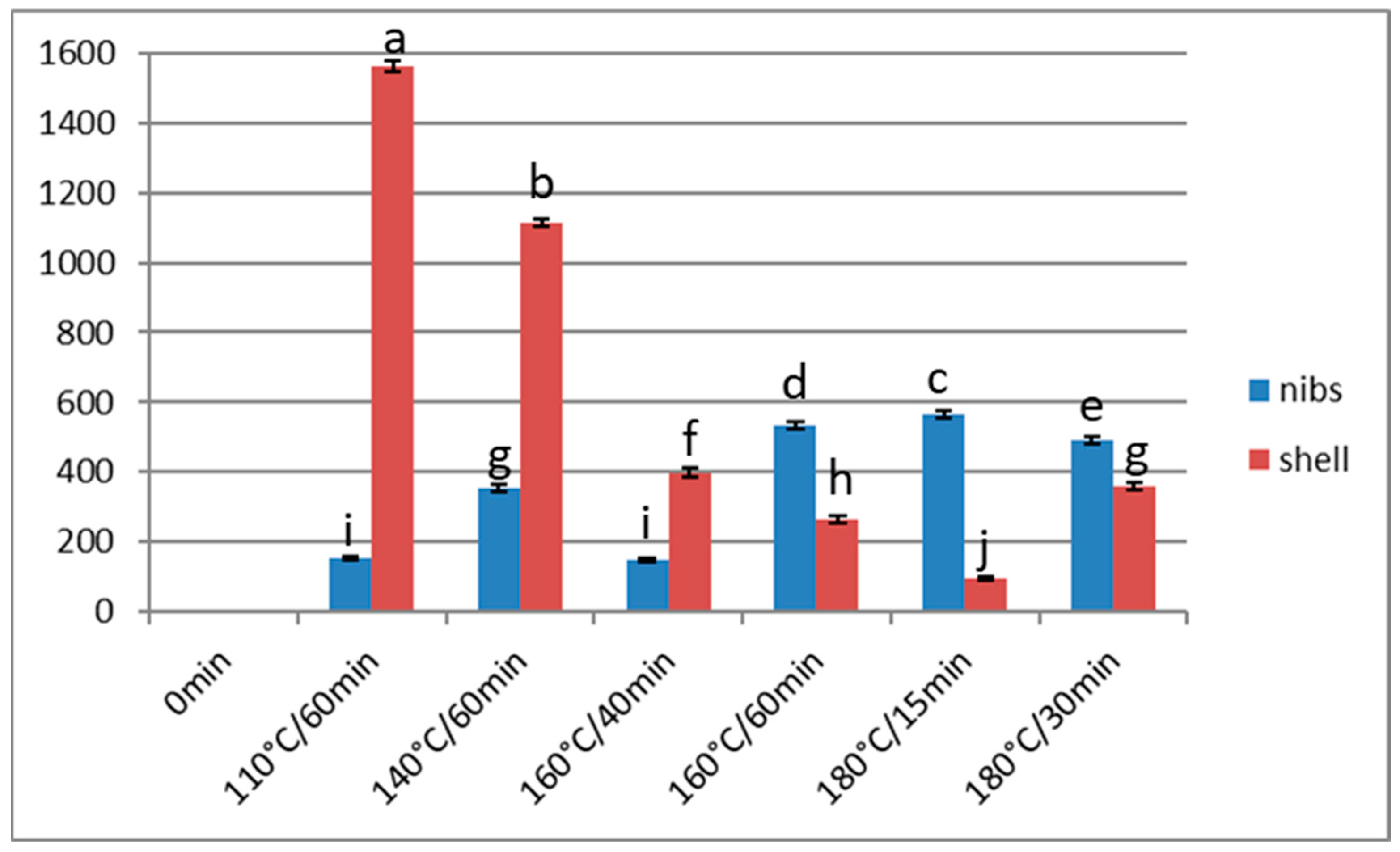
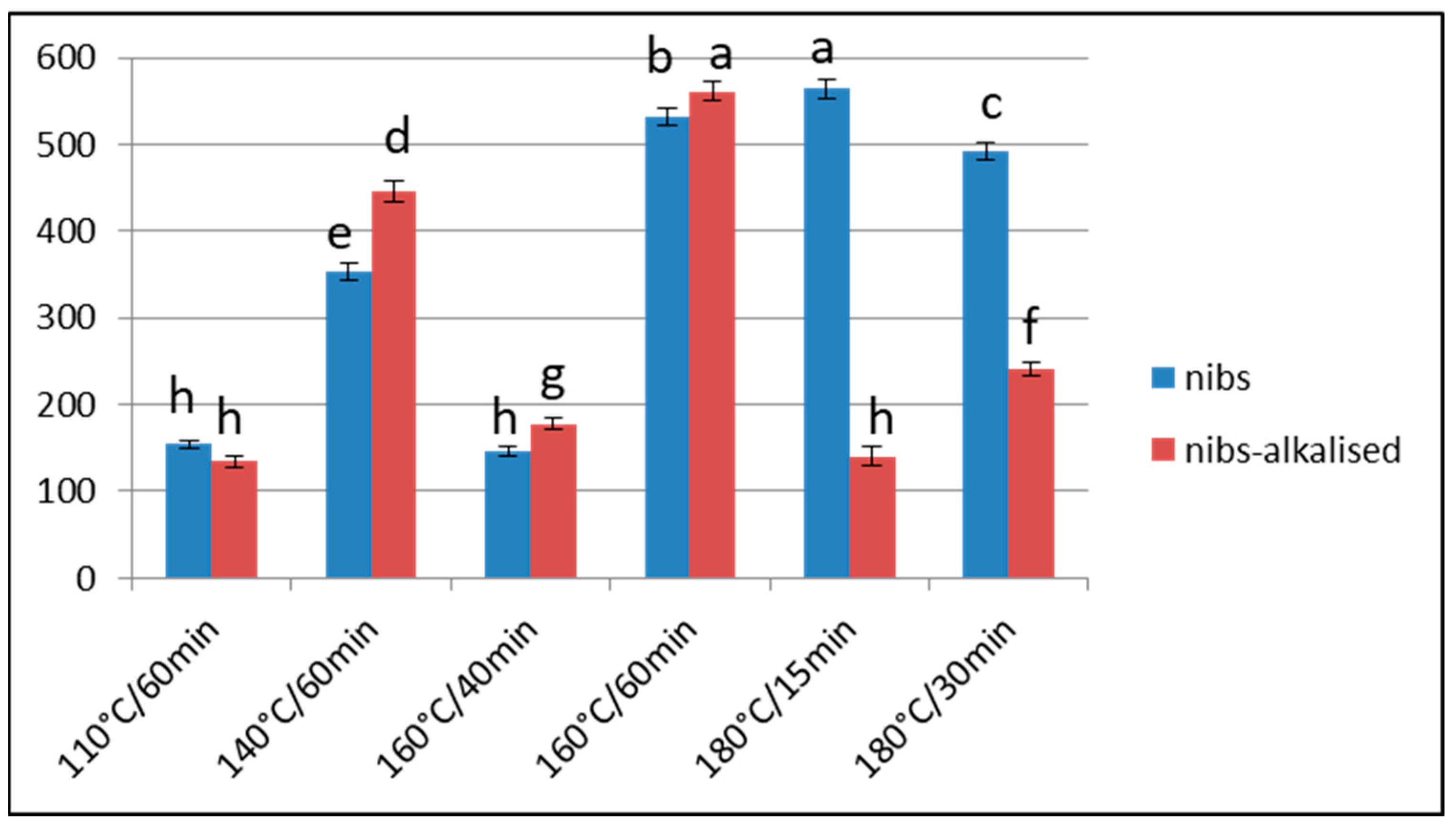
3.2. Acrylamide
Effect of Over-Roasting on Acrylamide Content
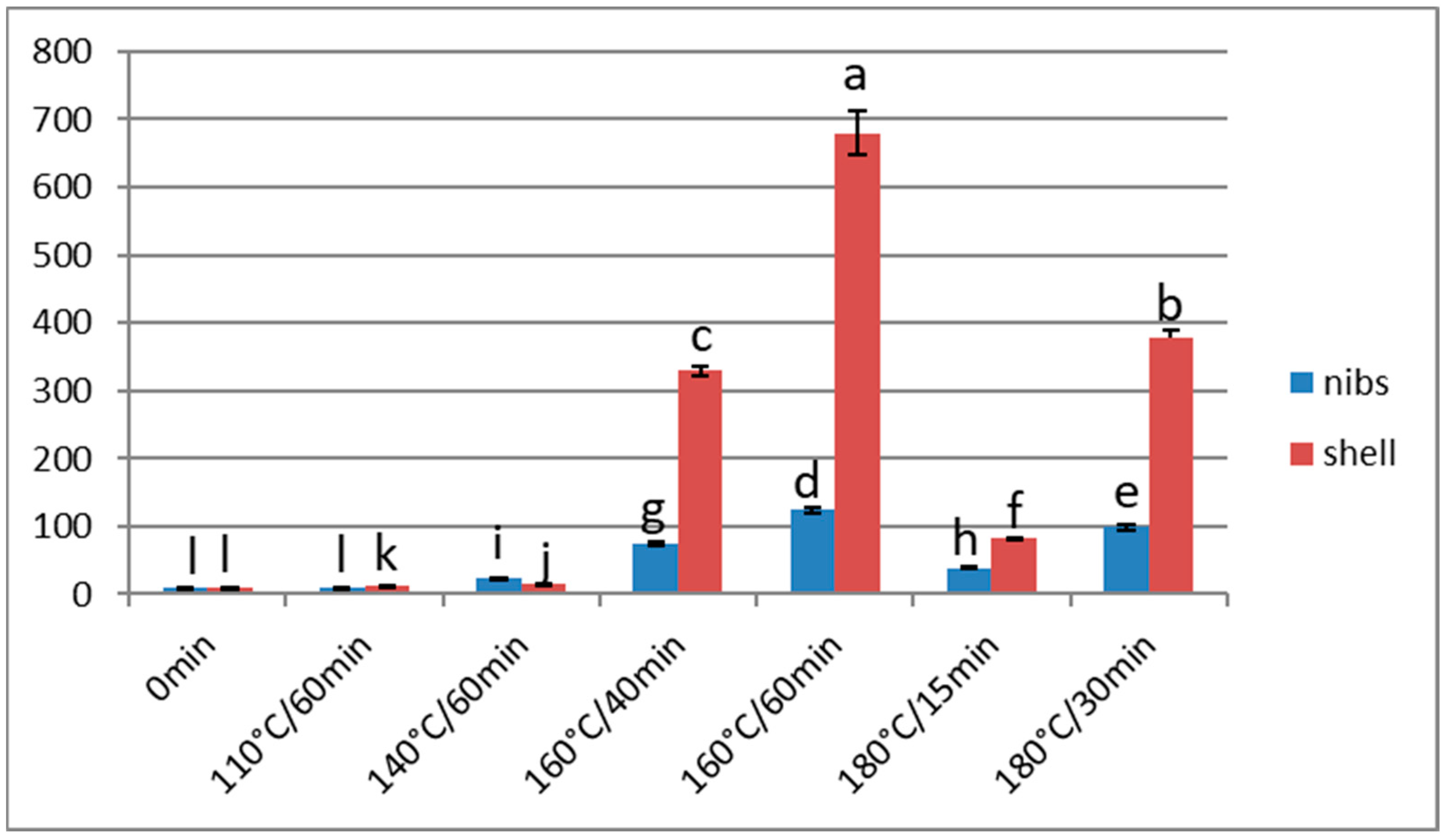
3.3. Furan
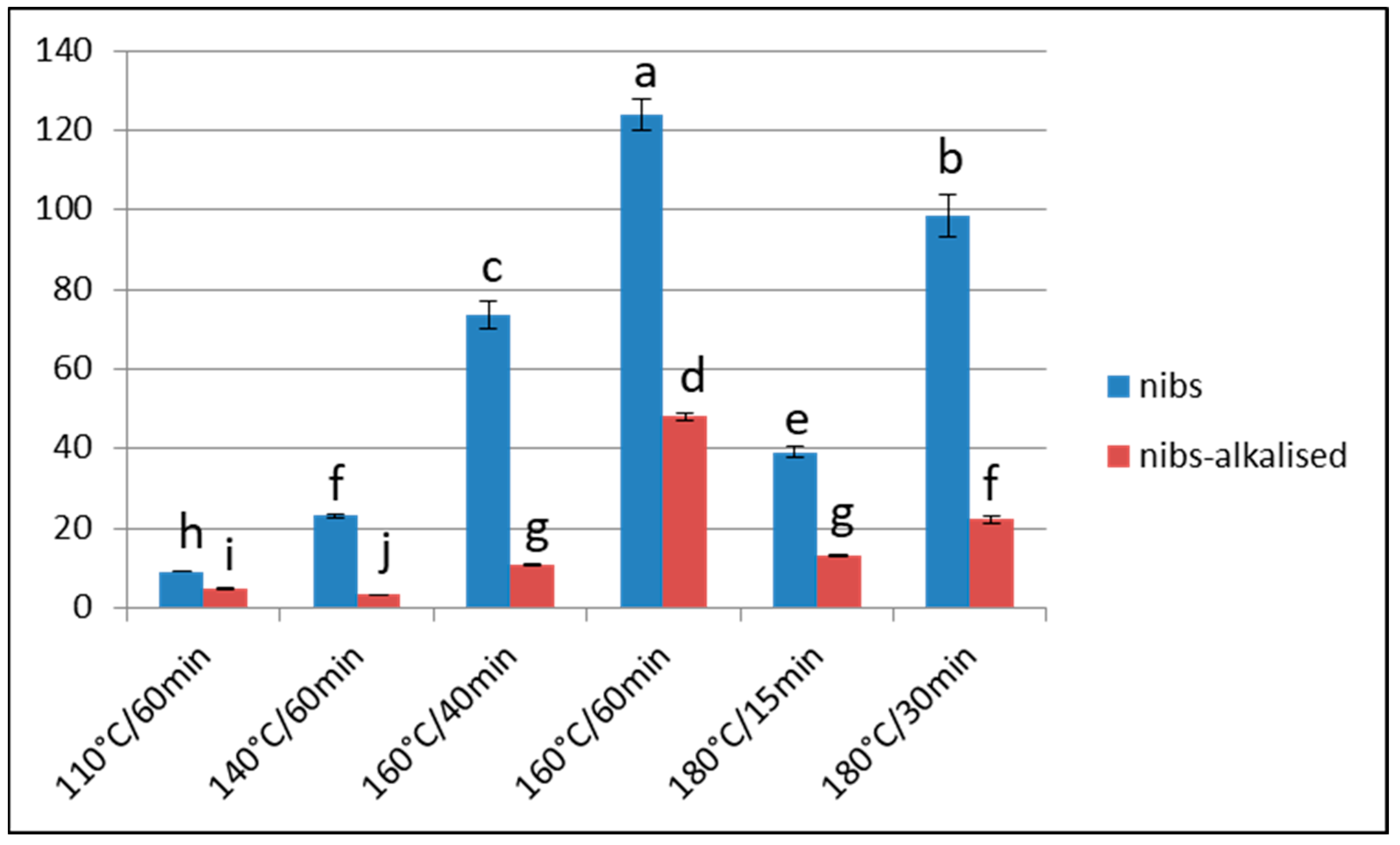
Effect of Over-Roasting on Furan Content
3.4. Correlations between Furosine, Furan and Acrylamide
3.4.1. Correlations between Acrylamide and Furan
3.4.2. Correlations between Furosine and Acrylamide/Furan
3.4.3. Other Correlations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beckett, S.T. Industrial Chocolate Manufacture and Use, 4th ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2009; pp. 10–47, 121–141. [Google Scholar]
- Rodriguez, P.; Pérez, E.; Guzman, R. Effect of the types and concentrations of alkali on the color of cocoa liquor. J. Sci. Food Agric. 2009, 89, 1186–1194. [Google Scholar] [CrossRef]
- Santos Rocha, I.; Radomille de Santana, L.R.; Soares, S.E.; da Silva Bispo, E. Effect of the roasting temperature and time of cocoa beans on the sensory characteristics and acceptability of chocolate. Food Sci. Technol. 2017, 37, 522–530. [Google Scholar] [CrossRef]
- Hinneh, M.; Abotsi, E.E.; Van de Walle, D.; Tzompa-Sosa, D.A.; Da Winne, A.; Simonis, J.; Messens, K.; Van Durme, J.; Afoakwa, E.O.; De Cooman, L.; et al. Pod storage with roasting: A tool to diversifying the flavor profiles of dark chocolates produced from ‘bulk’ cocoa beans? (part I: Aroma profiling of chocolates). Food Res. Int. 2019, 119, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Quelal-Vásconez, M.A.; Lerma-García, M.J.; Pérez-Esteve, E.; Talens, P.; Barat, J.M. Roadmap of cocoa quality and authenticity control in the industry: A review of conventional and alternative methods. Compr. Rev. Food Saf. 2020, 19, 448–478. [Google Scholar] [CrossRef]
- Martín, M.A.; Ramos, S. Health beneficial effects of cocoa phenolic compounds: A mini-review. Curr. Opin. Food Sci. 2017, 14, 20–25. [Google Scholar] [CrossRef]
- Kruszewski, B.; Obiedziński, M.W. Impact of raw materials and production processes on furan and acrylamide contents in dark chocolate. J. Agri. Food Chem. 2020, 68, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Ciecierska, M. Cocoa beans of different origins and varieties and their derived products contamination with polycyclic aromatic hydrocarbons. Food Chem. 2020, 317, 126408. [Google Scholar] [CrossRef]
- Ofosu, I.W.; Ankar-Brewoo, G.M.; Lutterodt, H.E.; Benefo, E.O.; Menyah, C.A. Estimated daily intake and risk of prevailing acrylamide content of alkalized roasted cocoa beans. Sci. Afr. 2019, 6, e00176. [Google Scholar] [CrossRef]
- Mesías, M.; Guerra-Hernández, E.; García-Villanova, B. Furan content in Spanish baby foods and its relation with potential precursors. CyTA-J. Food 2013, 11, 1–6. [Google Scholar] [CrossRef]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- FAO/WHO (Food and Agriculture Organisation/World Health Organisation). Summary and conclusions of the sixty-fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). JECFA 2005, 64/SC, 1–17. [Google Scholar]
- Granvogl, M.; Schieberle, P. Quantification of 3-aminopropionamide in cocoa, coffee and cereal products. Eur. Food Res. Technol. 2007, 225, 857–863. [Google Scholar] [CrossRef]
- Pardo, O.; Yusa, V.; Coscollà, C.; León, N.; Pastor, A. Determination of acrylamide in coffee and chocolate by pressurised fluid extraction and liquid chromatography-tandem mass spectrometry. Food Addit. Contam. 2007, 24, 663–672. [Google Scholar] [CrossRef]
- Arisseto, A.P.; de Figueiredo Toledo, M.C.; Govaert, Y.; van Loco, J.; Fraselle, S.; Degroodt, J.-M. A modified sample preparation for acrylamide determination in cocoa and coffee products. Food Anal. Methods 2008, 1, 49–55. [Google Scholar] [CrossRef]
- Köppen, R.; Rasenko, T.; Koch, M. Overview of the acrylamide content of cocoa and chocolate. Dtsch. Lebensmitt. Rundsch. 2015, 111, 261–267. [Google Scholar]
- EFSA. Scientific opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar] [CrossRef]
- Raters, M.; Matissek, R. Acrylamide in cocoa: A survey of acrylamide levels in cocoa and cocoa products sourced from the German market. Eur. Food Res.Technol. 2018, 244, 1381–1388. [Google Scholar] [CrossRef]
- Farah, D.M.H.; Zaibunnisa, A.H.; Misnawi, J.; Zainal, S. Effect of roasting process on the concentration of acrylamide and pyrizines in roasted cocoa beans from different origins. APCBEE Procedia 2012, 4, 204–208. [Google Scholar] [CrossRef]
- Gil, M.; Ruiz, P.; Quijano, J.; Londono-Londono, J.; Jaramillo, Y.; Gallego, V.; Tessier, F.; Notario, R. Effect of temperature on the formation of acrylamide in cocoa beans during drying treatment: An experimental and computational study. Heliyon 2020, 6, e03321. [Google Scholar] [CrossRef]
- FDA, U.S. Food and Drug Administration (FDA). Exploratory Data on Furan in Food. Individual Food Products. 2010. Available online: https://www.fda.gov/Food/FoodborneIllnessContaminants/ChemicalContaminants/ucm078439.htm#table4/ (accessed on 4 November 2023).
- EFSA. European Food Safety Authority. Results on acrylamide levels in food from monitoring years 2007–2009: An exposure assessment. EFSA J. 2011, 9, 2133. [Google Scholar] [CrossRef]
- FSA (Food Standards Agency). Acrylamide and Furan in a Range of UK Retail Foodstuffs. 2014. Available online: http://www.food.gov.uk/science/research/surveillance/food-surveys/food-survey-information-sheets-2014/acrylamide-and-furan (accessed on 24 October 2023).
- Taş, N.G.; Gökmen, V. Effect of alkalization on the Maillard reaction products formed in cocoa during roasting. Food Res. Int. 2016, 89, 930–936. [Google Scholar] [CrossRef]
- Wissgott, U. Process of Alkalization of Cocoa in Aqueous Phase. U.S. Patent 4784866, 15 November 1988. [Google Scholar]
- Resmini, P.; Pellegrino, L.; Battelli, G. Accurate quantification of furosine in milk and dairy products by a direct HPLC method. Ital. J. Food Sci. 1990, 2, 173–184. [Google Scholar]
- Sánchez-Oliver, A.J.; Contreras-Calderón, J.; Puya-Braza, J.M.; Guerra-Hernández, E. Quality analysis of commercial protein powder supplements and relation to characteristics declared by manufacturer. LWT-Food Sci. Technol. 2018, 97, 100–108. [Google Scholar] [CrossRef]
- Kocadağlı, T.; Palazoğlu, T.; Gökmen, V. Mitigation of acrylamide formation in cookies by using Maillard reaction products as recipe modifier in a combined partial conventional baking and radio frequency post-baking process. Eur. Food Res. Technol. 2012, 235, 711–717. [Google Scholar] [CrossRef]
- FDA, U.S. Food and Drug Administration (FDA). Determination of Furan in Food. 2004. Available online: https://www.fda.gov/Food/FoodborneIllnessContaminants/ChemicalContaminants/ucm078400.htm (accessed on 4 November 2023).
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 16th ed.; AOAC International: Arlington, VA, USA, 1990. [Google Scholar]
- Cros, E.; Jeanjean, N. Cocoa quality: Effect of fermentation and drying. Plant. Rech. Développement 1995, 124, 25–27. [Google Scholar]
- Biehl, B.; Brunner, E.; Passern, D.; Quesnel, V.C.; Adomako, D. Acidification, proteolysis and flavour potential in fermenting cocoa beans. J. Sci. Food Agric. 1985, 36, 583–598. [Google Scholar] [CrossRef]
- Fang, Y.; Li, R.; Chu, Z.; Zhu, K.; Gu, F.; Zhang, Y. Chemical and flavour profile changes of cocoa beans (Theobroma cacao L.) during primary fermentation. Food Sci. Nutr. 2020, 8, 4121–4133. [Google Scholar] [CrossRef]
- Agus, B.A.P.; Mohamad, N.N.; Hussain, N. Composition of unfermented, unroasted, roasted cocoa beans and cocoa shells from Peninsular Malaysia. Food Meas. 2018, 12, 2581–2589. [Google Scholar] [CrossRef]
- Adeyeye, E.I.; Akinyeye, R.O.; Ogunlade, I.; Olaofe, O.; Boluwade, J.O. Effect of farm and industrial processing on the amino acid profile of cocoa beans. Food Chem. 2010, 118, 357–363. [Google Scholar] [CrossRef]
- Barišić, V.; Flanjak, I.; Tot, A.; Budeč, M.; Benšić, M.; Jozinović, A.; Babić, J.; Šubarić, D.; Miličević, B.; Ačkar, A. 5-Hydroxymethylfurfural and acrylamide content of cocoa shell treated with high voltage electrical discharge. Food Control 2020, 110, 107043. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Oracz, J.; Krysiak, W.; Budryn, G.; Nebesny, E. Effects of various roasting conditions on acrylamide, acrolein, and polycyclic aromatic hydrocarbons content in cocoa bean and the derived chocolates. Dry. Technol. 2017, 35, 363–374. [Google Scholar] [CrossRef]
- Desmarchelier, A.; Bebius, A.; Reding, F.; Griffin, A.; Ahijado, M.; Beasley, J.; Clauzier, E.; Delatour, T. Towards a consensus LC-MS/MS method for the determination of acrylamide in food that prevents overestimation due to interferences. Food Addit. Contam. Part A 2022, 39, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Gallego, V.; Jaramillo, Y.; Gil, M.; Uribe, D. Advances on acrylamide in cocoa and its derivates: A challenge to control from postharvest to the industrialization. Curr. Opin. Food Sci. 2022, 47, 100903. [Google Scholar] [CrossRef]
- The European Commission. Commission recommendation (EU) 2019/1888 of 7 November 2019 on the monitoring of the presence of acrylamide in certain foods. Off. J. Eur. Union 2019, 62, L290/31. [Google Scholar]
- The European Commission. Commission regulation (EU) 2017/2158 of 20 November 2017 Establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. Off. J. Eur. Union 2017, 60, L304/24. [Google Scholar]
- Huang, Y.; Barringer, S.A. Monitoring of cocoa volatiles produced during roasting by selected ion flow tube-mass spectrometry (SIFT-MS). J. Food Sci. 2011, 76, C279–C286. [Google Scholar] [CrossRef]
- Taeymans, D.; Andersson, A.; Ashby, P.; Blank, I.; Gondé, P.; van Eijck, P.; Faivre, V.; Lalljie, S.P.; Lingnert, H.; Lindblom, M.; et al. Acrylamide: Update on selected research activities conducted by the European food and drink industry. J. AOAC Int. 2005, 88, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Huang, J.; Hu, J.; Zhang, Y.; Wang, S.; Chang, L.; Marcone, M.; Xie, M. Effect of pH, temperature and heating time on the formation of furan from typical carbohydrates and ascorbic acid. J. Food Agric. Environ. 2013, 11, 121–125. [Google Scholar]
- Soares, T.F.; Oliveira, M.B.P.P. Cocoa By-Products: Characterization of Bioactive Compounds and Beneficial Health Effects. Molecules 2022, 27, 1625. [Google Scholar] [CrossRef]
| Code | Sample | Code | Sample |
|---|---|---|---|
| NC0 | Nibs Chuao control | NB0 | Nibs Barlovento control |
| SC0 | Shell Chuao control | SB0 | Shell Barlovento control |
| NC110 | Nibs Chuao roasted 110 °C (60 min) | NB110 | Nibs Barlovento roasted 110 °C (60 min) |
| SC110 | Shell Chuao roasted 110 °C (60 min) | SB110 | Shell Barlovento roasted 110 °C (60 min) |
| NC140 | Nibs Chuao roasted 140 °C (60 min) | NB140 | Nibs Barlovento roasted 140 °C (60 min) |
| SC140 | Shell Chuao roasted 140 °C (60 min) | SB140 | Shell Barlovento roasted 140 °C (60 min) |
| NC110Na10 | Nibs Chuao roasted 110 °C (60 min) NaHCO3 10 g kg−1 | NB110Na10 | Nibs Barlovento roasted 110 °C (60 min) NaHCO3 10 g kg−1 |
| NC110Na15 | Nibs Chuao roasted 110 °C (60 min) NaHCO3 15 g kg−1 | NB110Na15 | Nibs Barlovento roasted 110 °C (60 min) NaHCO3 15 g kg−1 |
| NC110Na20 | Nibs Chuao roasted 110 °C (60 min) NaHCO3 20 g kg−1 | NB110Na20 | Nibs Barlovento roasted 110 °C (60 min) NaHCO3 20 g kg−1 |
| NC110Na25 | Nibs Chuao roasted 110 °C (60 min) NaHCO3 25 g kg−1 | NB110Na25 | Nibs Barlovento roasted 110 °C (60 min) NaHCO3 25 g kg−1 |
| NC110K10 | Nibs Chuao roasted 110 °C (60 min) K2CO3 10 g kg−1 | NB110K10 | Nibs Barlovento roasted 110 °C (60 min) K2CO3 10 g kg−1 |
| NC110K15 | Nibs Chuao roasted 110 °C (60 min) K2CO3 15 g kg−1 | NB110K15 | Nibs Barlovento roasted 110 °C (60 min) K2CO3 15 g kg−1 |
| NC110K20 | Nibs Chuao roasted 110 °C (60 min) K2CO3 20 g kg−1 | NB110K20 | Nibs Barlovento roasted 110 °C (60 min) K2CO3 20 g kg−1 |
| NC110K25 | Nibs Chuao roasted 110 °C (60 min) K2CO3 25 g kg−1 | NB110K25 | Nibs Barlovento roasted 110 °C (60 min) K2CO3 25 g kg−1 |
| NC140Na10 | Nibs Chuao roasted 140 °C (60 min) NaHCO3 10 g kg−1 | NB140Na10 | Nibs Barlovento roasted 140 °C (60 min) NaHCO3 10 g kg−1 |
| NC140Na15 | Nibs Chuao roasted 140 °C (60 min) NaHCO3 15 g kg−1 | NB140Na15 | Nibs Barlovento roasted 140 °C (60 min) NaHCO3 15 g kg−1 |
| NC140Na20 | Nibs Chuao roasted 140 °C (60 min) NaHCO3 20 g kg−1 | NB140Na20 | Nibs Barlovento roasted 140 °C (60 min) NaHCO3 20 g kg−1 |
| NC140Na25 | Nibs Chuao roasted 140 °C (60 min) NaHCO3 25 g kg−1 | NB140Na25 | Nibs Barlovento roasted 140 °C (60 min) NaHCO3 25 g kg−1 |
| NC140K10 | Nibs Chuao roasted 140 °C (60 min) K2CO3 10 g kg−1 | NB140K10 | Nibs Barlovento roasted 140 °C (60 min) K2CO3 10 g kg−1 |
| NC140K15 | Nibs Chuao roasted 140 °C (60 min) K2CO3 15 g kg−1 | NB140K15 | Nibs Barlovento roasted 140 °C (60 min) K2CO3 15 g kg−1 |
| NC140K20 | Nibs Chuao roasted 140 °C (60 min) K2CO3 20 g kg−1 | NB140K20 | Nibs Barlovento roasted 140 °C (60 min) K2CO3 20 g kg−1 |
| NC140K25 | Nibs Chuao roasted 140 °C (60 min) K2CO3 25 g kg−1 | NB140K25 | Nibs Barlovento roasted 140 °C (60 min) K2CO3 25 g kg−1 |
| NB16040 | Nibs Barlovento roasted 160 °C (40 min) | SB16040 | Shell Barlovento roasted 160 °C (40 min) |
| NB16060 | Nibs Barlovento roasted 160 °C (60 min) | SB16060 | Shell Barlovento roasted 160 °C (60 min) |
| NB18015 | Nibs Barlovento roasted 180 °C (15 min) | SB18015 | Shell Barlovento roasted 180 °C (15 min) |
| NB18030 | Nibs Barlovento roasted 180 °C (30 min) | SB18030 | Shell Barlovento roasted 180 °C (30 min) |
| NB16040K20 | Nibs Barlovento roasted 160 °C (40 min) K2CO3 20 g kg−1 | NB18015K20 | Nibs Barlovento roasted 180 °C (15 min) K2CO3 20 g kg−1 |
| NB16060K20 | Nibs Barlovento roasted 160 °C (60 min) K2CO3 20 g kg−1 | NB18030K20 | Nibs Barlovento roasted 180 °C (30 min) K2CO3 20 g kg−1 |
| Code | Furosine (mg/100 g of Protein) | Acrylamide (µg/kg) | Furan (µg/kg) | Moisture (%) | L | Browning Index (100-L•) | pH | Ash (%) |
|---|---|---|---|---|---|---|---|---|
| NC0 | 249 ± 1 a | nd j | 7.60 ± 0.99 k | - | 29.08 ± 0.02 b | 70.92 | 5.0 | - |
| SC0 | 151 ± 1 b | nd j | 4.18 ± 0.08 l | - | - | - | - | - |
| NC110 | 129 ±12 c | 222 ± 7 g | 17.9 ± 0.5 e,f | - | - | - | - | - |
| SC110 | 49.1 ± 3.9 d | 204 ± 10 g | 16.0 ± 0.4 f,g | - | - | - | - | - |
| NC140 | 129 ± 12 c | 627 ± 8 e | 46.2 ± 1.4 b | - | - | - | - | - |
| SC140 | 9.88 ± 0.91 g,h,i | 956 ± 12 b | 65.2 ± 1.9 a | - | - | - | - | - |
| NC110Na10 | 59.6 ± 5.4 d | 95 ± 8 h | 34.6 ± 1.5 c | 4.81 ± 0.21 i | 21.60 ± 0.02 j | 78.40 | 6.5 | 3.19 ± 0.09 g |
| NC110Na15 | 22.3 ± 1.9 e,f | - | 12.5 ± 0.3 h,i,j | 5.15 ± 0.26 h,i | 19.10 ± 0.03 n | 80.90 | 7.1 | 3.85 ± 0.02 d,e,f |
| NC110Na20 | 34.3 ± 1.9 e | - | 19.8 ± 0.6 e | 5.01 ± 0.01 i | 23.90 ± 0.02 f | 76.10 | 7.9 | 4.02 ± 0.03 c,d |
| NC110Na25 | 16.5 ± 1.5 f,g,h,i | 1253 ± 11 a | 13.0 ± 0.4 h,i | 7.05 ± 0.05 e,f | 17.20 ± 0.02 p | 82.80 | 8.0 | 4.51 ± 0.01 b,c |
| NC110K10 | 51.2 ± 3.9 d | 385 ± 12 f | 7.31 ± 0.30 k | 5.09 ± 0.15 h,i | 27.90 ± 0.02 c | 72.10 | - | 4.15 ± 0.38 b,c,d |
| NC110K15 | 25.9 ± 2.3 e,f | - | 14.7 ± 0.8 g,h | 7.58 ± 0.08 d | 20.50 ± 0.03 k | 79.50 | - | 4.04 ± 0.33 c,d |
| NC110K20 | 19.8 ± 1.3 f,g,h | - | 12.6 ± 0.1 h,i | 8.56 ± 0.37 c | 18.00 ± 0.05 o | 82.00 | - | 4.20 ± 0.36 b,c,d |
| NC110K25 | 15.6 ± 1.4 f,g,h,i | 25.0 ± 0.8 i | 14.6 ± 0.4 g,h | 4.81 ± 0.68 i | 16.90 ± 0.03 q | 83.10 | - | 3.23 ± 0.19 f,g |
| NC140Na10 | 22.8 ± 1.3 e,f | 25.0 ± 1.1 i | 17.5 ± 0.4 e,f | 3.35 ± 0.27 j | 25.20 ± 0.04 e | 74.80 | - | 3.16 ± 0.23 g |
| NC140Na15 | 12.9 ± 1.3 f,g,h,i | - | 18.8 ± 0.4 e | 4.44 ± 0.00 i | 29.20 ± 0.02 a | 70.80 | - | 2.50 ± 0.08 h |
| NC140Na20 | 11.9 ± 1.0 g,h,i | - | 14.0 ± 0.2 g,h | 4.38 ± 0.07 i | 21.90 ± 0.03 i | 78.10 | - | 3.35 ± 0.08 e,f,g |
| NC140Na25 | 8.30 ± 0.15 g,h,i | 85.0 ± 7.4 h | 23.0 ± 0.9 d | 4.59 ± 0.32 i | 23.10 ± 0.03 h | 76.90 | - | 4.20 ± 0.21 b,c,d |
| NC140K10 | 21.8 ± 1.0 e,f | 769 ± 12 c | 12.5 ± 0.5 h,i,j | 5.84 ± 0.04 g,h | 23.70 ± 0.03 g | 76.30 | - | 4.33 ± 0.04 b,c,d |
| NC140K15 | 18.1 ± 1.6 f,g,h,i | - | 19.2 ± 0.2 e | 6.35 ± 0.04 f,g | 26.10 ± 0.04 d | 73.90 | - | 3.89 ± 0.04 c,d,e |
| NC140K20 | 13.3 ± 0.7 f,g,h,i | - | 10.7 ± 0.3 i,j | 9.76 ± 0.07 b | 19.40 ± 0.01 m | 80.60 | - | 4.71 ± 0.25 a,b |
| NC140K25 | 6.13 ± 0.21 i | 697 ± 8 d | 10.2 ± 0.4 j | 11.62 ± 0.36 a | 19.90 ± 0.05 l | 80.10 | - | 5.25 ± 0.36 a |
| Code | Furosine (mg/100 g of Protein) | Acrylamide (µg/kg) | Furan (µg/kg) | Moisture (%) | L | Browning Index (100-L•) | pH | Ash (%) |
|---|---|---|---|---|---|---|---|---|
| NB0 | 225 ± 9 b | nd j | 9.30 ± 0.20 f,g | - | 30.03 ± 0.00 a | 69.97 | - | - |
| SB0 | 180 ± 8 c | nd j | 9.83 ± 0.11 e,f | - | - | - | - | - |
| NB110 | 189 ± 7 c | 154 ± 5 i | 9.16 ± 0.11 f,g | - | - | - | - | - |
| SB110 | 41.9 ± 4.0 h,i | 1562 ± 14 a | 11.6 ± 0.3 d | - | - | - | - | - |
| NB140 | 299 ± 5 a | 353 ± 11 e | 23.2 ± 0.5 a | - | - | - | - | - |
| SB140 | 52.0 ± 3.9 g,h,i | 1113 ± 12 b | 16.0 ± 0.5 b | - | - | - | - | - |
| NB110Na10 | 49.9 ± 0.4 g,h,i | 352 ± 7 e | 12.5 ± 0.4 c | 4.80 ± 0.49 f | 17.60 ± 0.03 o | 82.40 | - | 2.68 ± 0.04 g |
| NB110Na15 | 98.3 ± 1.7 e | - | 10.6 ± 0.3 e | 5.14 ± 0.27 e,f | 22.20 ± 0.03 k | 77.80 | - | 4.16 ± 0.10 e,f |
| NB110Na20 | 61.7 ± 5.7 f,g | - | 5.81 ± 0.08 j | 7.33 ± 0.12 c | 19.70 ± 0.02 n | 80.30 | - | 4.44 ± 0.19 c,d,e,f |
| NB110Na25 | 36.3 ± 3.6 i | 307 ± 9 f | 6.22 ± 0.12 j | 4.85 ± 0.05 e,f | 25.90 ± 0.03 f | 74.10 | - | 4.30 ± 0.19 d,e,f |
| NB110K10 | 3.49 ± 0.19 j | 370 ± 11 e | 7.91 ± 0.20 i | 7.08 ± 0.29 c | 26.30 ± 0.02 e | 73.70 | - | 3.82 ± 0.31 f |
| NB110K15 | 115 ± 2 e | - | 8.28 ± 0.23 h,i | 6.34 ± 0.19 c,d | 25.20 ± 0.09 g | 74.80 | - | 4.25 ± 0.36 d,e,f |
| NB110K20 | 55.2 ± 2.1 f,g,h | - | 4.86 ± 0.23 k | 9.12 ± 0.09 a,b | 20.00 ± 0.01m | 80.00 | - | 4.81 ± 0.41 b,c,d,e |
| NB110K25 | 2.03 ± 0.14 j | 134 ± 8 i | 1.77 ± 0 m | 7.05 ± 0.04 c | 19.50 ± 0.02 ñ | 80.50 | - | 4.82 ± 0.38 b,c,d,e |
| NB140Na10 | 142 ± 9 d | 212 ± 4 h | 11.8 ± 0.1 c,d | 4.71 ± 0.34 f | 27.20 ± 0.03 c | 72.80 | - | 4.27 ± 0.05 d,e,f |
| NB140Na15 | 61.2 ± 5.6 f,g | - | 7.91 ± 0.12 i | 5.08 ± 0.75 e,f | 22.50 ± 0.02 j | 77.50 | - | 4.01 ± 0.35 e,f |
| NB140Na20 | 112 ± 1 e | - | 8.90 ± 0.28 g,h | 5.64 ± 0.07 d,e,f | 20.90 ± 0.04 l | 79.10 | - | 5.19 ± 0.17 a,b,c,d |
| NB140Na25 | 139 ± 9 d | 250 ± 10 g | 11.7 ± 0.1 d | 5.24 ± 0.03 e,f | 20.90 ± 0.02 l | 79.10 | - | 5.28 ± 0.07 a,b,c |
| NB140K10 | 116 ± 6 e | 527 ± 11 c | 10.6 ± 0.2 e | 5.82 ± 0.04 d,e | 27.40 ± 0.20 b | 72.60 | 6.6 | 4.51 ± 0.04 c,d,e,f |
| NB140K15 | 147 ± 15 d | - | 4.94 ± 0.12 k | 7.10 ± 0.70 c | 26.60 ± 0.04 d | 73.40 | 7.2 | 4.52 ± 0.70 c,d,e,f |
| NB140K20 | 71.4 ± 2.7 f | - | 3.32 ± 0.11 l | 8.63 ± 0.30 b | 24.90 ± 0.02 h | 75.10 | 8.1 | 5.90 ± 0.40 a |
| NB140K25 | 72.8 ± 0.6 f | 446 ± 11 d | 3.40 ± 0.04 l | 9.96 ± 0.30 a | 23.40 ± 0.02 i | 76.60 | 8.7 | 5.48 ± 0.40 a,b |
| Code | Furosine (mg/100 g of Protein) | Acrylamide (µg/kg) | Furan (µg/kg) |
|---|---|---|---|
| NB16040 | 4.39 ± 0.43 c | 146 ± 5 h | 73.8 ± 3.5 g |
| SB16040 | - | 398 ± 12 d | 329 ± 8 c |
| NB16060 | 3.24 ± 0.17 c | 532 ± 11 b | 124 ± 4 d |
| SB16060 | - | 263 ± 11 f | 680 ± 32 a |
| NB18015 | 164 ± 9 a | 564 ± 11 a | 39.1 ± 1.4 i |
| SB18015 | - | 97.0 ± 4.9 i | 81.7 ± 1.8 f |
| NB18030 | 8.97 ± 0.81 c | 492 ± 10 c | 98.6 ± 5.2 e |
| SB18030 | - | 358 ± 11 e | 378 ± 10 b |
| NB16040K20 | - | 178 ± 6 g | 10.8 ± 0.3 k |
| NB16060K20 | - | 561 ± 11 a | 48.1 ± 0.8 h |
| NB18015K20 | 39.0 ± 1.8 b | 140 ± 11 h | 13.2 ± 0.3 k |
| NB18030K20 | - | 241 ± 7 f | 22.2 ± 1.0 j |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Trujillo, T.R.; Perez, E.; Verardo, V.; García-Villanova, B.; Guerra-Hernández, E.J. Impact of Technological Processes on the Formation of Furosine, Acrylamide and Furan in Traditional Venezuelan Cocoa. Foods 2024, 13, 829. https://doi.org/10.3390/foods13060829
Moreno-Trujillo TR, Perez E, Verardo V, García-Villanova B, Guerra-Hernández EJ. Impact of Technological Processes on the Formation of Furosine, Acrylamide and Furan in Traditional Venezuelan Cocoa. Foods. 2024; 13(6):829. https://doi.org/10.3390/foods13060829
Chicago/Turabian StyleMoreno-Trujillo, Thayra Rocio, Elevina Perez, Vito Verardo, Belén García-Villanova, and Eduardo Jesús Guerra-Hernández. 2024. "Impact of Technological Processes on the Formation of Furosine, Acrylamide and Furan in Traditional Venezuelan Cocoa" Foods 13, no. 6: 829. https://doi.org/10.3390/foods13060829
APA StyleMoreno-Trujillo, T. R., Perez, E., Verardo, V., García-Villanova, B., & Guerra-Hernández, E. J. (2024). Impact of Technological Processes on the Formation of Furosine, Acrylamide and Furan in Traditional Venezuelan Cocoa. Foods, 13(6), 829. https://doi.org/10.3390/foods13060829







