The Shelf Life of Milk—A Novel Concept for the Identification of Marker Peptides Using Multivariate Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Proteins
2.3. Sample Material
2.4. Sample Preparation and Extraction
2.4.1. Sample Preparation for the Model System
2.4.2. Sample Preparation ‘Milk’
2.4.3. Enzymatic Hydrolysis
2.5. Sensory Analysis
2.6. UPLC-IMS-QToF Analysis
2.7. Data Analysis and Statistics
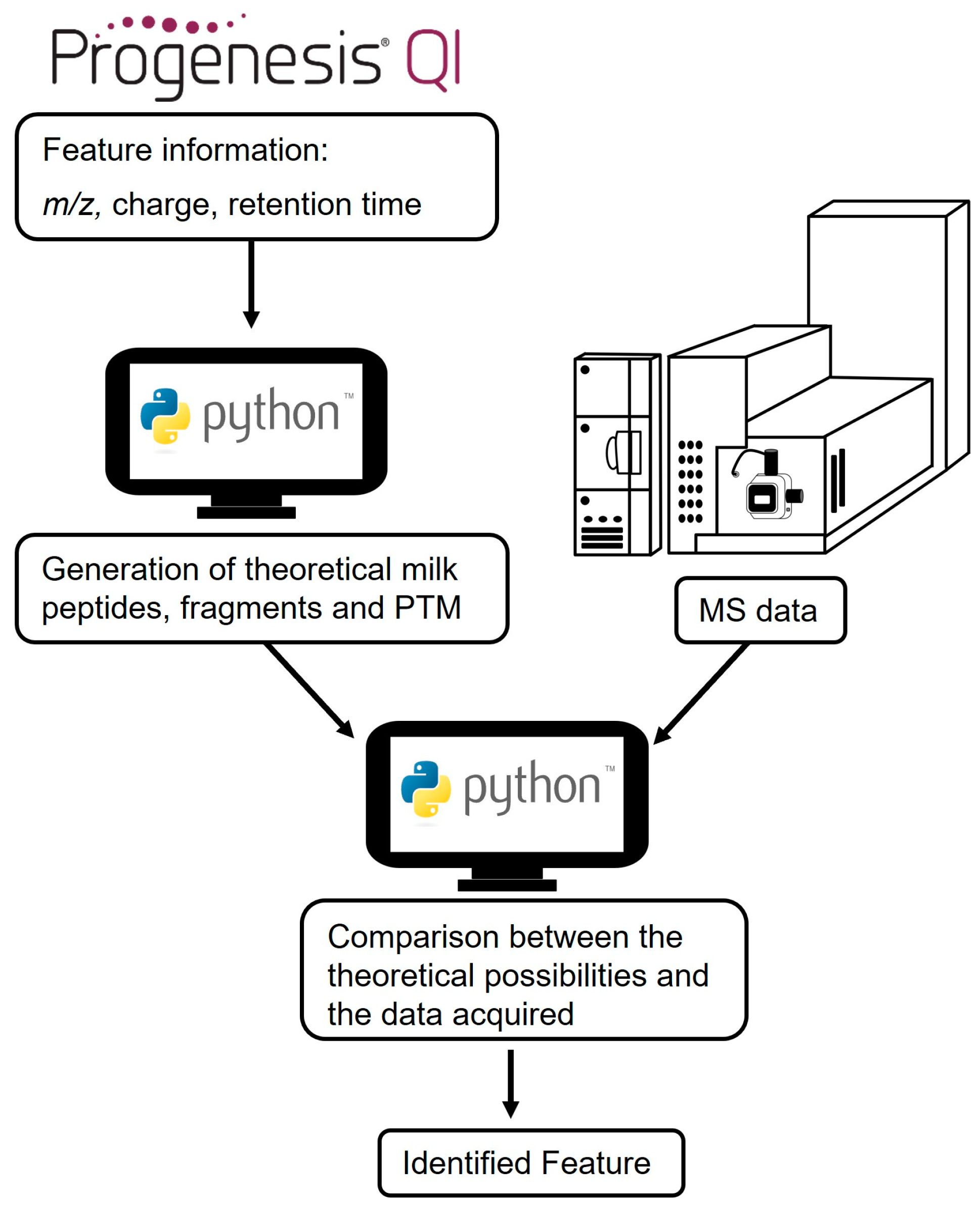
2.8. Feature Identification
3. Results and Discussion
3.1. Sensory Analysis
3.2. Data Processing and Statistical Analysis
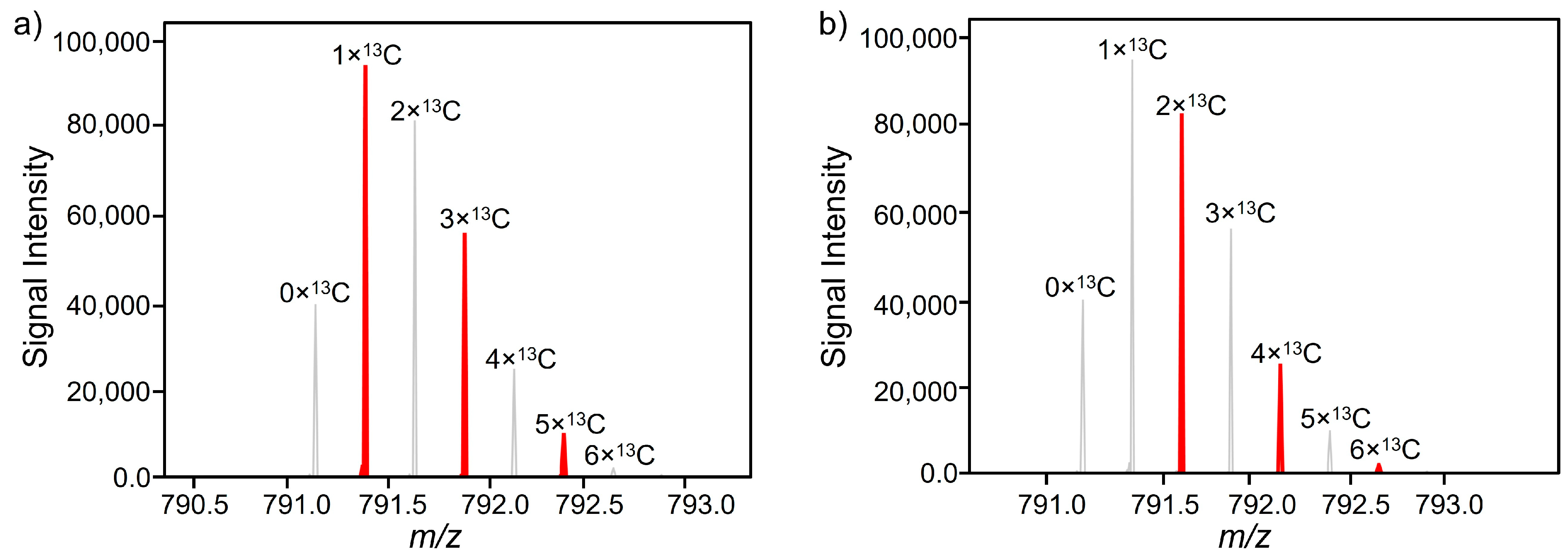
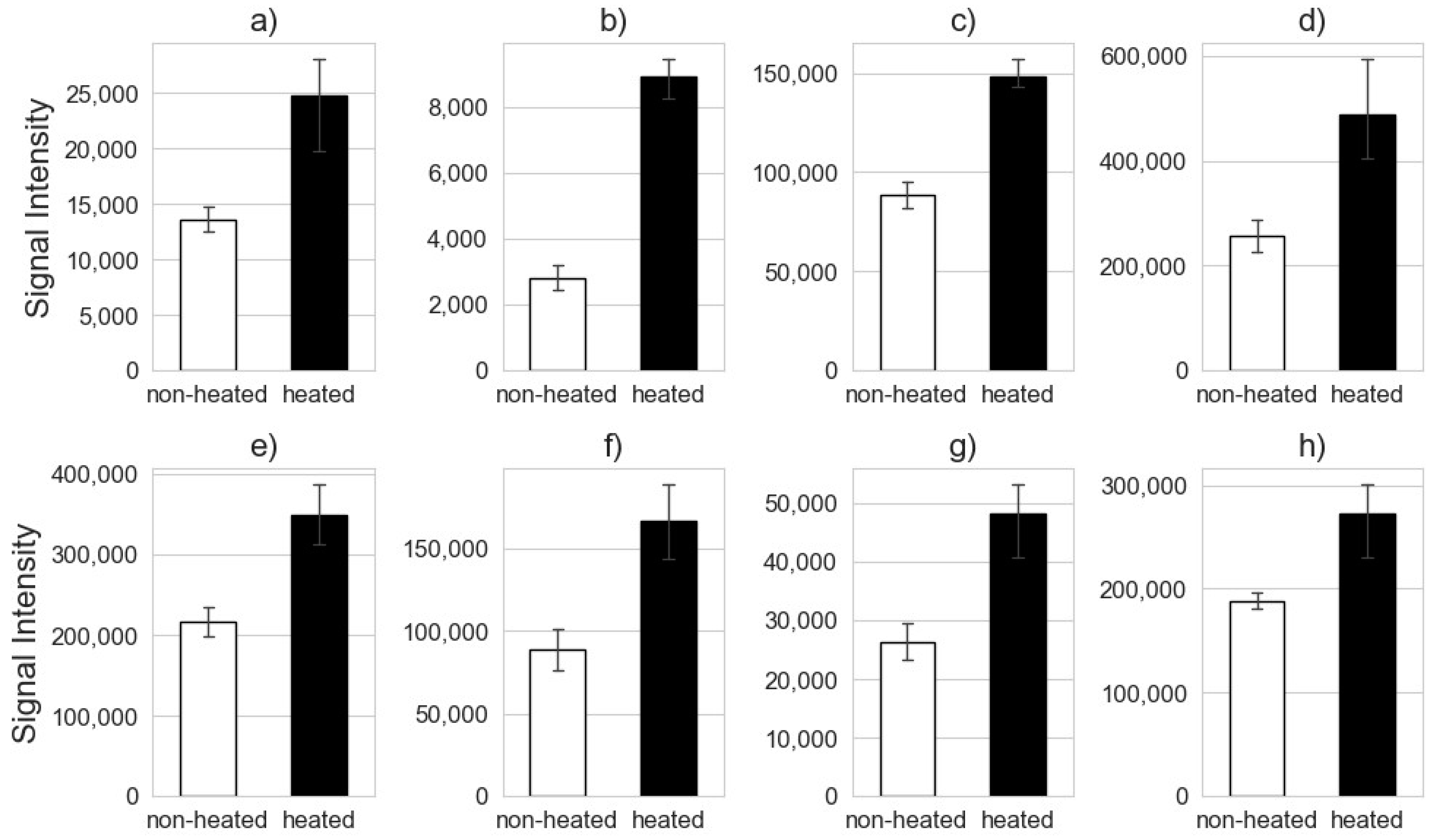
3.3. Feature Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grunert, K.G. Food quality and safety: Consumer perception and demand. Eur. Rev. Agric. Econ. 2005, 32, 369–391. [Google Scholar] [CrossRef]
- Ling, E.K. Integrity of food supply chain: Going beyond food safety and food quality Siti Norida Wahab. Int. J. Product. Qual. Manag. 2020, 29, 216–232. [Google Scholar] [CrossRef]
- Wang, J.; Yue, H. Food safety pre-warning system based on data mining for a sustainable food supply chain. Food Control 2017, 73, 223–229. [Google Scholar] [CrossRef]
- Toma, L.; Revoredo-Giha, C.; Costa-Font, M.; Thompson, B. Food Waste and Food Safety Linkages along the Supply Chain. EuroChoices 2020, 19, 24–29. [Google Scholar] [CrossRef]
- Corradini, M.G. Shelf Life of Food Products: From Open Labeling to Real-Time Measurements. Annu. Rev. Food Sci. Technol. 2018, 9, 251–269. [Google Scholar] [CrossRef]
- Scherhaufer, S.; Moates, G.; Hartikainen, H.; Waldron, K.; Obersteiner, G. Environmental impacts of food waste in Europe. Waste Manag. 2018, 77, 98–113. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Emanuelsson, A. The Methodology of the FAO Study: “Global Food Losses and Food Waste-Extent, Causes and Prevention”; Volume SIK Report; Swedish Institute for Food and Biotechnology (SIK): Göteborg, Sweden, 2013; ISBN 9789172903234. [Google Scholar]
- Deeth, H.C.; Datta, N. Heat Treatment of Milk: Ultra-High Temperature Treatment (UHT): Heating Systems. Encycl. Dairy Sci. Second Ed. 2011, 2, 699–707. [Google Scholar] [CrossRef]
- Linares, D.M.; Del Río, B.; Ladero, V.; Martínez, N.; Fernández, M.; Martín, M.C.; Álvarez, M.A. Factors influencing biogenic amines accumulation in dairy products. Front. Microbiol. 2012, 3, 180. [Google Scholar] [CrossRef]
- Dalabasmaz, S.; Dittrich, D.; Kellner, I.; Drewello, T.; Pischetsrieder, M. Identification of peptides reflecting the storage of UHT milk by MALDI-TOF-MS peptide profiling. J. Proteom. 2019, 207, 103444. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Lehrbuch der Lebensmittelchemie; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Class, L.; Kuhnen, G.; Rohn, S.; Kuballa, J. Diving Deep into the Data: A Review of Deep Learning Approaches and Potential Applications in Foodomics. Foods 2021, 10, 1803. [Google Scholar] [CrossRef]
- Martin, N.H.; Ranieri, M.L.; Murphy, S.C.; Ralyea, R.D.; Wiedmann, M.; Boor, K.J. Results from raw milk microbiological tests do not predict the shelf-life performance of commercially pasteurized fluid milk. J. Dairy Sci. 2011, 94, 1211–1222. [Google Scholar] [CrossRef]
- Beckmann, S.; Scheller, S. Bestimmung des Mindesthaltbarkeitsdatums. Der Leb.—Ernährung Aktuell 2011, 22, 3. [Google Scholar]
- Kretschmer, B.; Smith, C.; Watkins, E.; Allen, B.; Buckwell, A.; Desbarats, J.; Kieve, D. Technology Options for Feeding 10 Billion People—Recycling Agricultural, Forestry & Food Wastes and Residues for Sustainable Bioenergy and Biomaterials. Inst. Eur. Environ. Policy 2013. [Google Scholar]
- Newsome, R.; Balestrini, C.G.; Baum, M.D.; Corby, J.; Fisher, W.; Goodburn, K.; Labuza, T.P.; Prince, G.; Thesmar, H.S.; Yiannas, F. Applications and perceptions of date labeling of food. Compr. Rev. Food Sci. Food Saf. 2014, 13, 745–769. [Google Scholar] [CrossRef]
- Wansink, B.; Wright, A.O. “Best if used by...” How freshness dating influences food acceptance. J. Food Sci. 2006, 71, 354–357. [Google Scholar] [CrossRef]
- Wilson, N.L.W.; Rickard, B.J.; Saputo, R.; Ho, S.T. Food waste: The role of date labels, package size, and product category. Food Qual. Prefer. 2017, 55, 35–44. [Google Scholar] [CrossRef]
- Theotokis, A.; Pramatari, K.; Tsiros, M. Effects of Expiration Date-Based Pricing on Brand Image Perceptions. J. Retail. 2012, 88, 72–87. [Google Scholar] [CrossRef]
- Buttlar, B.; Löwenstein, L.; Geske, M.S.; Ahlmer, H.; Walther, E. Love food, hate waste? Ambivalence towards food Fosters people’s willingness to waste food. Sustainability 2021, 13, 3971. [Google Scholar] [CrossRef]
- Kavanaugh, M.; Quinlan, J.J. Consumer knowledge and behaviors regarding food date labels and food waste. Food Control 2020, 115, 107285. [Google Scholar] [CrossRef]
- Patra, D.; Feng, S.; Howard, J.W. Confusion of food-date label with food safety—implications for food waste. Curr. Opin. Food Sci. 2022, 48, 100917. [Google Scholar] [CrossRef]
- Madilo, F.K.; Owusu-Kwarteng, J.; Parry-Hanson Kunadu, A.; Tano-Debrah, K. Self-reported use and understanding of food label information among tertiary education students in Ghana. Food Control 2020, 108, 106841. [Google Scholar] [CrossRef]
- Toma, L.; Costa Font, M.; Thompson, B. Impact of consumers’ understanding of date labelling on food waste behaviour. Oper. Res. 2020, 20, 543–560. [Google Scholar] [CrossRef]
- Zielińska, D.; Bilska, B.; Marciniak-łukasiak, K.; Łepecka, A.; Trząskowska, M.; Neffe-skocińska, K.; Tomaszewska, M.; Szydłowska, A.; Kołożyn-krajewska, D. Consumer understanding of the date of minimum durability of food in association with quality evaluation of food products after expiration. Int. J. Environ. Res. Public Health 2020, 17, 1632. [Google Scholar] [CrossRef]
- Gašo-Sokač, D.; Kovač, S.; Josić, D. Use of proteomic methodology in optimization of processing and quality control of food of animal origin. Food Technol. Biotechnol. 2011, 49, 397–412. [Google Scholar]
- Johnson, M.E.; Lucey, J.A. Major technological advances and trends in cheese. J. Dairy Sci. 2006, 89, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Dambrouck, T.; Marchal, R.; Marchal-Delahaut, L.; Parmentier, M.; Maujean, A.; Jeandet, P. Immunodetection of proteins from grapes and yeast in a white wine. J. Agric. Food Chem. 2003, 51, 2727–2732. [Google Scholar] [CrossRef]
- Gagnaire, V.; Jardin, J.; Jan, G.; Lortal, S. Invited review: Proteomics of milk and bacteria used in fermented dairy products: From qualitative to quantitative advances. J. Dairy Sci. 2009, 92, 811–825. [Google Scholar] [CrossRef]
- Matissek, R.; Fischer, M. Lebensmittelanalytik; Springer: Berlin/Heidelberg, Germany, 2021; ISBN 978-3-662-63409-7. [Google Scholar]
- Meltretter, J.; Becker, C.M.; Pischetsrieder, M. Identification and site-specific relative quantification of β-lactoglobulin modifications in heated milk and dairy products. J. Agric. Food Chem. 2008, 56, 5165–5171. [Google Scholar] [CrossRef] [PubMed]
- Ebner, J.; Baum, F.; Pischetsrieder, M. Identification of sixteen peptides reflecting heat and/or storage induced processes by profiling of commercial milk samples. J. Proteom. 2016, 147, 66–75. [Google Scholar] [CrossRef]
- Liu, Y.; Pischetsrieder, M. Identification and Relative Quantification of Bioactive Peptides Sequentially Released during Simulated Gastrointestinal Digestion of Commercial Kefir. J. Agric. Food Chem. 2017, 65, 1865–1873. [Google Scholar] [CrossRef]
- von Oesen, T.; Treblin, M.; Clawin-Rädecker, I.; Martin, D.; Maul, R.; Hoffmann, W.; Schrader, K.; Wegner, B.; Bode, K.; Zink, R.; et al. Identification of Marker Peptides for the Whey Protein Quantification in Edam-Type Cheese. Foods 2023, 12, 2002. [Google Scholar] [CrossRef] [PubMed]
- Cristobal, A.; Marino, F.; Post, H.; Van Den Toorn, H.W.P.; Mohammed, S.; Heck, A.J.R. Toward an Optimized Workflow for Middle-Down Proteomics. Anal. Chem. 2017, 89, 3318–3325. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, P.; Tsiatsiani, L.; Low, T.Y.; Heck, A.J.R. Six alternative proteases for mass spectrometry-based proteomics beyond trypsin. Nat. Protoc. 2016, 11, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; de Araújo Calado, V.Ô.M.; Jarvis, B. Observations on the use of statistical methods in Food Science and Technology. Food Res. Int. 2014, 55, 137–149. [Google Scholar] [CrossRef]
- Morschheuser, L.; Mink, K.; Horst, R.; Kallinich, C.; Rohn, S. Immunological analysis of food proteins using high-performance thin-layer chromatography-immunostaining. J. Chromatogr. A 2017, 1526, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Post, A.E.; Arnold, B.; Weiss, J.; Hinrichs, J. Effect of temperature and pH on the solubility of caseins: Environmental influences on the dissociation of α S- and β-casein. J. Dairy Sci. 2012, 95, 1603–1616. [Google Scholar] [CrossRef]
- Pelegrine, D.H.G.; Gasparetto, C.A. Whey proteins solubility as function of temperature and pH. LWT 2005, 38, 77–80. [Google Scholar] [CrossRef]
- BVL. Amtliche Sammlung von Untersuchungsverfahren nach §64 LFGB ASU L00.90-6, 2015.
- DIN 10964:2014-11; DIN Deutsches Institut für Normung e., V. Sensory Analysis-Simple Descriptive Test. Beuth Verlag GmbH: Berlin, Germany, 2014.
- BVL. Amtliche Sammlung von Untersuchungsverfahren nach §64 LFGB ASU L00.90-7, 2021.
- DIN EN ISO 4120 2021-06; DIN Deutsches Institut für Normung e., V. Sensory Analysis-Triangle Test. Beuth Verlag GmbH: Berlin, Germany, 2021.
- Regueiro, J.; Negreira, N.; Berntssen, M.H.G. Ion-mobility-derived collision cross section as an additional identification point for multiresidue screening of pesticides in fish feed. Anal. Chem. 2016, 88, 11169–11177. [Google Scholar] [CrossRef]
- Bijlsma, L.; Berntssen, M.H.G.; Merel, S. A Refined Nontarget Workflow for the Investigation of Metabolites through the Prioritization by in Silico Prediction Tools. Anal. Chem. 2019, 91, 6321–6328. [Google Scholar] [CrossRef]
- Kim, N.; Kim, K.; Choi, B.Y.; Lee, D.; Shin, Y.S.; Bang, K.H.; Cha, S.W.; Lee, J.W.; Choi, H.K.; Jang, D.S.; et al. Metabolomic approach for age discrimination of Panax ginseng using UPLC-Q-Tof MS. J. Agric. Food Chem. 2011, 59, 10435–10441. [Google Scholar] [CrossRef]
- Sumner, L.W.; Mendes, P.; Dixon, R.A. Plant metabolomics: Large-scale phytochemistry in the functional genomics era. Phytochemistry 2003, 62, 817–836. [Google Scholar] [CrossRef]
- Röst, H.L.; Schmitt, U.; Aebersold, R.; Malmström, L. pyOpenMS: A Python-based interface to the OpenMS mass-spectrometry algorithm library. Proteomics 2014, 14, 74–77. [Google Scholar] [CrossRef]
- Chavan, R.S.; Chavan, S.R.; Khedkar, C.D.; Jana, A.H. UHT milk processing and effect of plasmin activity on shelf life: A review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 251–268. [Google Scholar] [CrossRef]
- Nielsen, B.R.; Stapelfeldt, H.; Skibsted, L.H. Early prediction of the shelf-life of medium-heat whole milk powders using stepwise multiple regression and principal component analysis. Int. Dairy J. 1997, 7, 341–348. [Google Scholar] [CrossRef]
- Coolbear, T.; Janin, N.; Traill, R.; Shingleton, R. Heat-induced changes in the sensory properties of milk. Int. Dairy J. 2022, 126, 105199. [Google Scholar] [CrossRef]
- Calvo, M.M.; de la Hoz, L. Flavour of heated milks. A review. Int. Dairy J. 1992, 2, 69–81. [Google Scholar] [CrossRef]
- van Boekel, M.A.J.S. Effect of heating on Maillard reactions in milk. Food Chem. 1998, 62, 403–414. [Google Scholar] [CrossRef]
- Jo, Y.; Benoist, D.M.; Barbano, D.M.; Drake, M.A. Flavor and flavor chemistry differences among milks processed by high-temperature, short-time pasteurization or ultra-pasteurization. J. Dairy Sci. 2018, 101, 3812–3828. [Google Scholar] [CrossRef] [PubMed]
- Kühn, J.; Considine, T.; Singh, H. Interactions of milk proteins and volatile flavor compounds: Implications in the development of protein foods. J. Food Sci. 2006, 71, R72–R82. [Google Scholar] [CrossRef]
- Hutton, J.T.; Patton, S. The Origin of Sulfhydryl Groups in Milk Proteins and their Contributions to “Cooked” Flavor. J. Dairy Sci. 1952, 35, 699–705. [Google Scholar] [CrossRef]
- Al-Attabi, Z.; D’Arcy, B.R.; Deeth, H.C. Volatile sulphur compounds in UHT milk. Crit. Rev. Food Sci. Nutr. 2009, 49, 28–47. [Google Scholar] [CrossRef]
- Mehta, R.S. Milk Processed at Ultra-High-Temperatures—A Review. J. Food Prot. 1980, 43, 212–225. [Google Scholar] [CrossRef]
- Mahoney, R.R. Galactosyl-oligosaccharide formation during lactose hydrolysis: A review. Food Chem. 1998, 63, 147–154. [Google Scholar] [CrossRef]
- Adhikari, K.; Dooley, L.M.; Chambers, I.V.E.; Bhumiratana, N. Sensory characteristics of commercial lactose-free milks manufactured in the United States. LWT 2010, 43, 113–118. [Google Scholar] [CrossRef]
- Aktağ, I.G.; Hamzalıoğlu, A.; Gökmen, V. Lactose hydrolysis and protein fortification pose an increased risk for the formation of Maillard reaction products in UHT treated milk products. J. Food Compos. Anal. 2019, 84, 103308. [Google Scholar] [CrossRef]
- Kong, Y.; Dong, Q.; Yu, Z.; Yan, H.; Liu, L.; Shen, Y. The effect of lactose and its isomerization product lactulose on functional and structural properties of glycated casein. Food Res. Int. 2023, 168, 112683. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, E.M.; Hudson, C.S. Relations between rotatory power and structure in the sugar group. XXIX. Synthesis of a new disaccharide ketose (Lactulose) from Lactose. J. Am. Chem. Soc. 1930, 52, 2519–2524. [Google Scholar] [CrossRef]
- Parrish, F.W.; Talley, F.B.; Ross, K.D.; Clark, J.; Phillips, J.G. Sweetness of Lactulose Relative To Sucrose. J. Food Sci. 1979, 44, 813–815. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kumari, S. Lactulose: Production, purification and potential applications. Biotechnol. Adv. 2011, 29, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, R.A.; Lindsay, R.C.; Libbey, L.M.; Day, E.A. Heat-Induced Volatile Compounds in Milk. J. Dairy Sci. 1968, 51, 1001–1007. [Google Scholar] [CrossRef]
- Colahan-Sederstrom, P.M.; Peterson, D.G. Inhibition of key aroma compound generated during ultrahigh-temperature processing of bovine milk via epicatechin addition. J. Agric. Food Chem. 2005, 53, 398–402. [Google Scholar] [CrossRef]
- Klockmann, S.; Reiner, E.; Bachmann, R.; Hackl, T.; Fischer, M. Food Fingerprinting: Metabolomic Approaches for Geographical Origin Discrimination of Hazelnuts (Corylus avellana) by UPLC-QTOF-MS. J. Agric. Food Chem. 2016, 64, 9253–9262. [Google Scholar] [CrossRef] [PubMed]
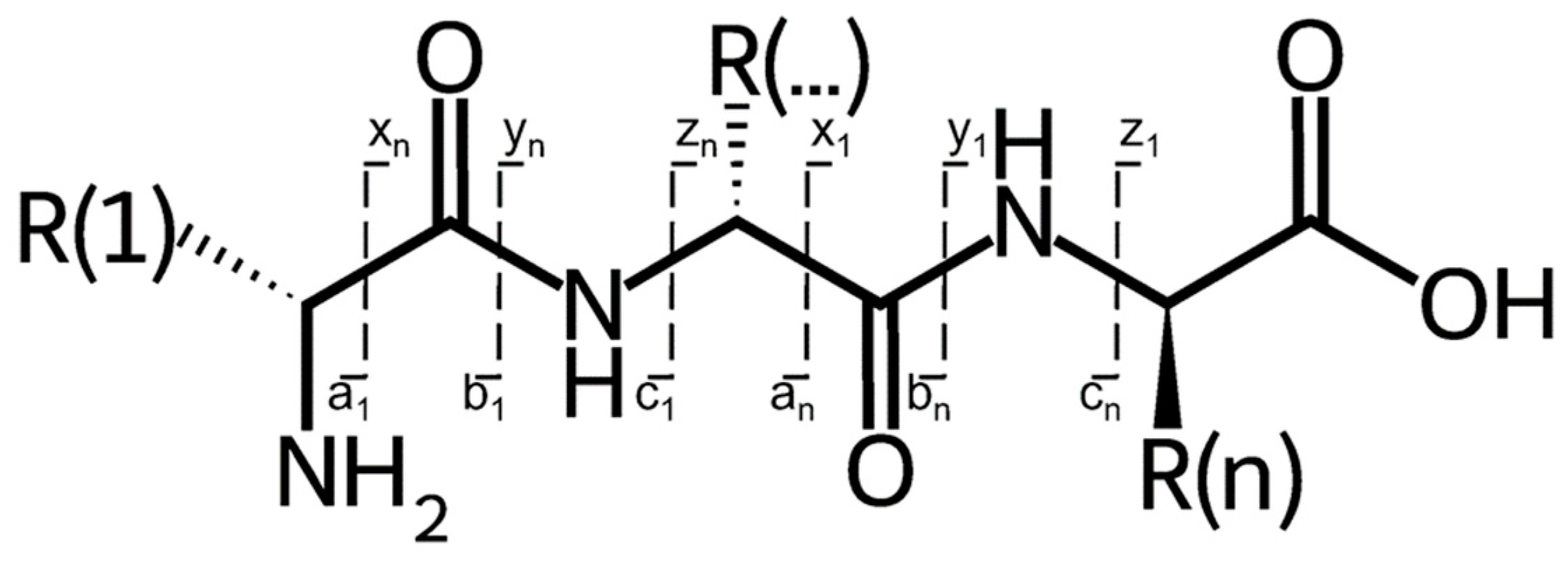
- Bensadek, D.; Monigatti, F.; Steen, J.A.J.; Steen, H. Why b, y’s? Sodiation-induced tryptic peptide-like fragmentation of non-tryptic peptides. Int. J. Mass Spectrom. 2007, 268, 181–189. [Google Scholar] [CrossRef]
- Steen, H.; Mann, M. The ABC’s (and XYZ’s) of peptide sequencing. Nat. Rev. Mol. Cell Biol. 2004, 5, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, J.; Klempt, M.; Clawin-Rädecker, I.; Lorenzen, P.C.; Meisel, H. Identification of a NFκB inhibitory peptide from tryptic β-casein hydrolysate. Food Chem. 2014, 165, 129–133. [Google Scholar] [CrossRef]
- Tu, M.; Feng, L.; Wang, Z.; Qiao, M.; Shahidi, F.; Lu, W.; Du, M. Sequence analysis and molecular docking of antithrombotic peptides from casein hydrolysate by trypsin digestion. J. Funct. Foods 2017, 32, 313–323. [Google Scholar] [CrossRef]
- Meltretter, J.; Schmidt, A.; Humeny, A.; Becker, C.M.; Pischetsrieder, M. Analysis of the peptide profile of milk and its changes during thermal treatment and storage. J. Agric. Food Chem. 2008, 56, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- von Oesen, T.; Treblin, M.; Staudacher, A.; Clawin-Rädecker, I.; Martin, D.; Hoffmann, W.; Schrader, K.; Bode, K.; Zink, R.; Rohn, S.; et al. Determination and evaluation of whey protein content in matured cheese via liquid chromatography. LWT 2023, 174, 114347. [Google Scholar] [CrossRef]
- Van Vlierberghe, K.; Gavage, M.; Dieu, M.; Renard, P.; Arnould, T.; Gillard, N.; Coudijzer, K.; De Loose, M.; Gevaert, K.; Van Poucke, C. Selecting Processing Robust Markers Using High-Resolution Mass Spectrometry for the Detection of Milk in Food Products. J. AOAC Int. 2022, 105, 463–475. [Google Scholar] [CrossRef]
- Dalabasmaz, S.; Ebner, J.; Pischetsrieder, M. Identification of the Peptide PyroQ-βCasein194-209 as a Highly Specific and Sensitive Marker to Differentiate between Ultrahigh-Temperature Processed (UHT) Milk and Mildly Heated Milk. J. Agric. Food Chem. 2017, 65, 10781–10791. [Google Scholar] [CrossRef]
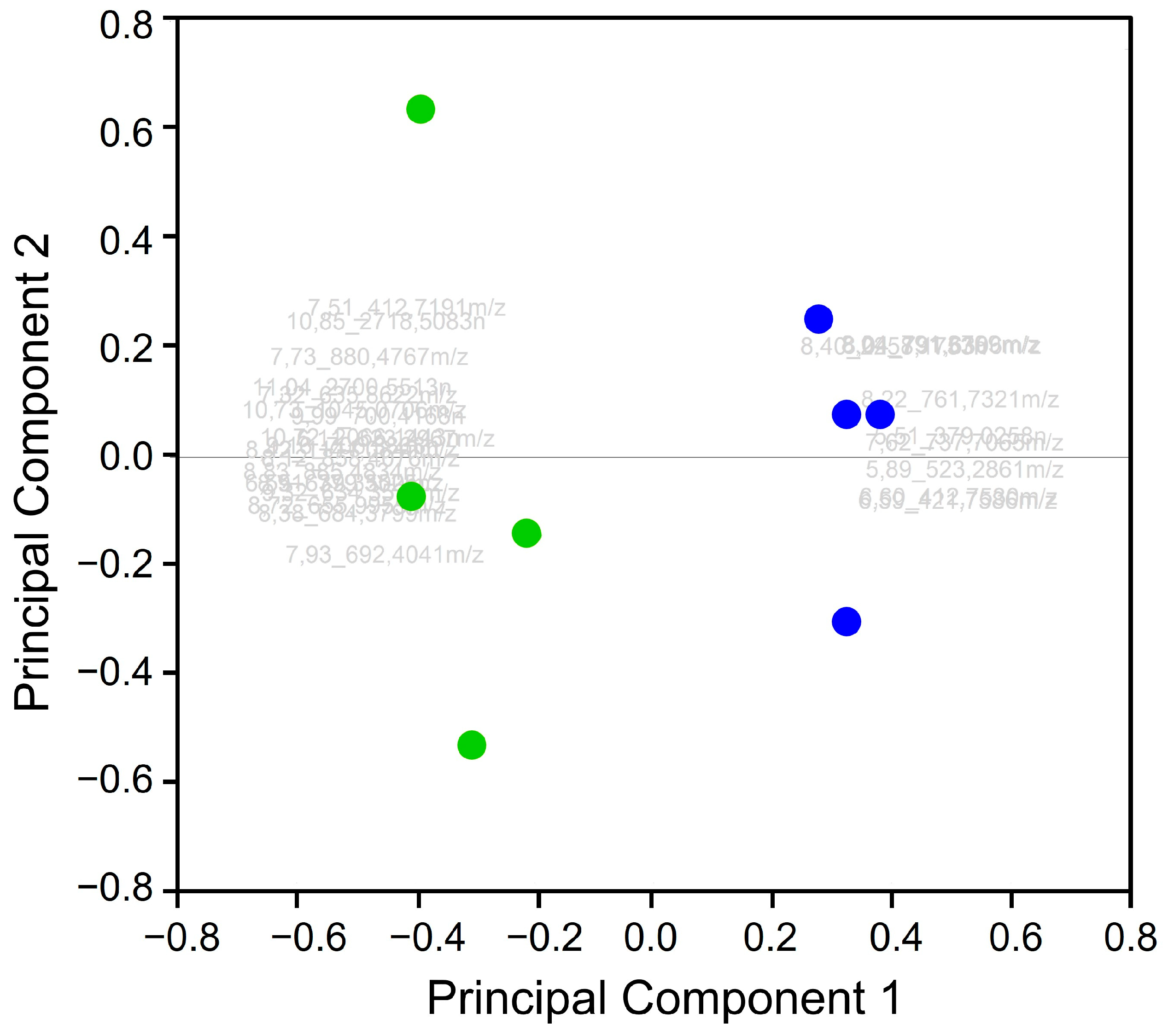


| Feature Name | m/z | Charge | Retention Time [min] | CCS [Å] | ANOVA (p) | q Value |
|---|---|---|---|---|---|---|
| FT 01 | 655.9955 | 3 | 8.72 | 592.71 | 7.98 × 10−5 | 4.79 × 10−3 |
| FT 02 | 885.4834 | 2 | 8.83 | 454.25 | 1.87 × 10−4 | 6.62 × 10−3 |
| FT 03 | 639.3500 | 3 | 6.55 | 526.31 | 1.89 × 10−4 | 6.62 × 10−3 |
| FT 04 | 635.8622 | 2 | 7.32 | 376.85 | 2.39 × 10−4 | 6.87 × 10−3 |
| FT 05 | 634.3570 | 2 | 9.32 | 391.93 | 5.33 × 10−4 | 8.19 × 10−3 |
| FT 06 | 821.3864 | 1 | 8.91 | 281.99 | 6.24 × 10−4 | 8.44 × 10−3 |
| FT 07 | 858.4076 | 2 | 8.12 | 438.96 | 1.15 × 10−3 | 1.11 × 10−2 |
| FT 08 | 697.0494 | 3 | 10.72 | 539.40 | 1.76 × 10−3 | 1.23 × 10−2 |
| FT 09 | 761.7321 | 3 | 8.22 | 559.93 | 2.01 × 10−3 | 1.26 × 10−2 |
| FT 10 | 443.0417 | 1 | 5.51 | 191.23 | 2.03 × 10−3 | 1.26 × 10−2 |
| FT 11 | 684.3799 | 3 | 8.38 | 539.73 | 2.56 × 10−3 | 1.32 × 10−2 |
| FT 12 | 412.7191 | 2 | 7.51 | 319.19 | 2.81 × 10−3 | 1.32 × 10−2 |
| FT 13 | 523.2861 | 2 | 5.89 | 349.85 | 3.26 × 10−3 | 1.36 × 10−2 |
| FT 14 | 737.7065 | 3 | 7.62 | 560.52 | 3.38 × 10−3 | 1.37 × 10−2 |
| FT 15 | 1045.0706 | 2 | 10.73 | 494.44 | 3.55 × 10−3 | 1.39 × 10−2 |
| FT 16 | 791.6306 | 2 | 8.04 | 393.91 | 4.55 × 10−3 | 1.47 × 10−2 |
| FT 17 | 748.3706 | 1 | 9.16 | 266.04 | 6.14 × 10−3 | 1.61 × 10−2 |
| FT 18 | 791.3799 | 2 | 8.04 | 388.88 | 6.48 × 10−3 | 1.65 × 10−2 |
| FT 19 | 742.4507 | 1 | 9.99 | 268.93 | 6.64 × 10−3 | 1.66 × 10−2 |
| FT 20 | 1141.0840 | 2 | 8.42 | 520.30 | 7.52 × 10−3 | 1.77 × 10−2 |
| FT 21 | 908.5183 | 3 | 11.04 | 601.58 | 9.02 × 10−3 | 1.93 × 10−2 |
| FT 22 | 412.7530 | 2 | 6.60 | 319.19 | 1.35 × 10−2 | 2.31 × 10−2 |
| FT 23 | 421.7586 | 2 | 6.59 | 323.67 | 1.45 × 10−2 | 2.41 × 10−2 |
| FT 24 | 623.2967 | 2 | 6.11 | 377.12 | 1.67 × 10−2 | 2.58 × 10−2 |
| FT 25 | 761.0597 | 3 | 8.40 | 567.39 | 2.82 × 10−2 | 3.46 × 10−2 |
| FT 26 | 880.4767 | 2 | 7.73 | 459.62 | 3.39 × 10−2 | 3.82 × 10−2 |
| FT 27 | 914.5040 | 3 | 10.85 | 601.45 | 3.84 × 10−2 | 4.08 × 10−2 |
| FT 28 | 692.4041 | 2 | 7.93 | 390.73 | 4.74 × 10−2 | 4.67 × 10−2 |
| Feature Name | Retentiontime [min] | m/z | Charge | Modification Type | Fragment | Tryptic Peptide | Protein |
|---|---|---|---|---|---|---|---|
| FT 02 | 8.83 | 885.4834 | 2 | - | y15++ | FPQYLQYLYQGPIVLNPWDQVK | α-s2-casein |
| FT 03 | 6.55 | 639.3500 | 3 | Lactulosyl-lysine | - | VLPVPQKAVPYPQR | β-casein |
| FT 04 | 7.32 | 635.8622 | 2 | - | y11++ | DMPIQAFLLYQEPVLGPVR | β-casein |
| FT 05 | 9.32 | 634.3570 | 2 | - | - | YLGYLEQLLR | α-s1-casein |
| FT 12 | 7.51 | 412.7191 | 2 | - | y6++ | EPMIGVNQELAYFYPELFR | α-s1-casein |
| FT 24 | 6.11 | 623.2967 | 2 | - | - | TPEVDDEALEK | β-lactoglobulin |
| FT 26 | 7.73 | 880.4767 | 2 | - | - | HQGLPQEVLNENLLR | α-s1-casein |
| FT 28 | 7.93 | 692.4041 | 2 | - | y12++ | DMPIQAFLLYQEPVLGPVR | β-casein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Class, L.-C.; Kuhnen, G.; Hanisch, K.L.; Badekow, S.; Rohn, S.; Kuballa, J. The Shelf Life of Milk—A Novel Concept for the Identification of Marker Peptides Using Multivariate Analysis. Foods 2024, 13, 831. https://doi.org/10.3390/foods13060831
Class L-C, Kuhnen G, Hanisch KL, Badekow S, Rohn S, Kuballa J. The Shelf Life of Milk—A Novel Concept for the Identification of Marker Peptides Using Multivariate Analysis. Foods. 2024; 13(6):831. https://doi.org/10.3390/foods13060831
Chicago/Turabian StyleClass, Lisa-Carina, Gesine Kuhnen, Kim Lara Hanisch, Svenja Badekow, Sascha Rohn, and Jürgen Kuballa. 2024. "The Shelf Life of Milk—A Novel Concept for the Identification of Marker Peptides Using Multivariate Analysis" Foods 13, no. 6: 831. https://doi.org/10.3390/foods13060831








