Application of L-Cysteine Hydrochloride Delays the Ripening of Harvested Tomato Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Fruit Ripening Characteristics
2.3. H2O2 Content and Activities of Antioxidant Enzymes
2.4. RNA-Seq Analysis
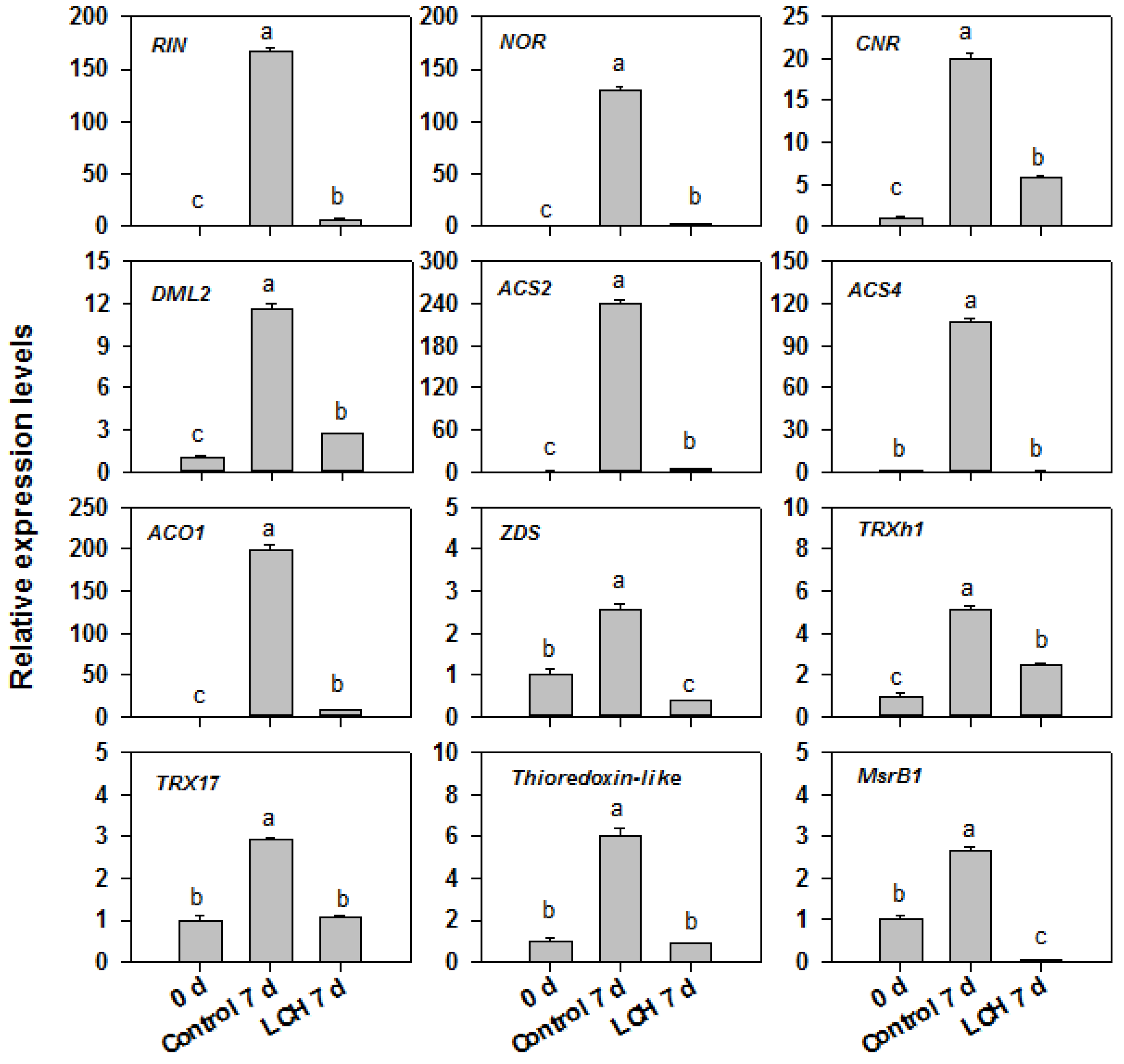
2.5. Real-Time Quantitative PCR (RT-qPCR) Analysis
2.6. Statistical Analysis
3. Results
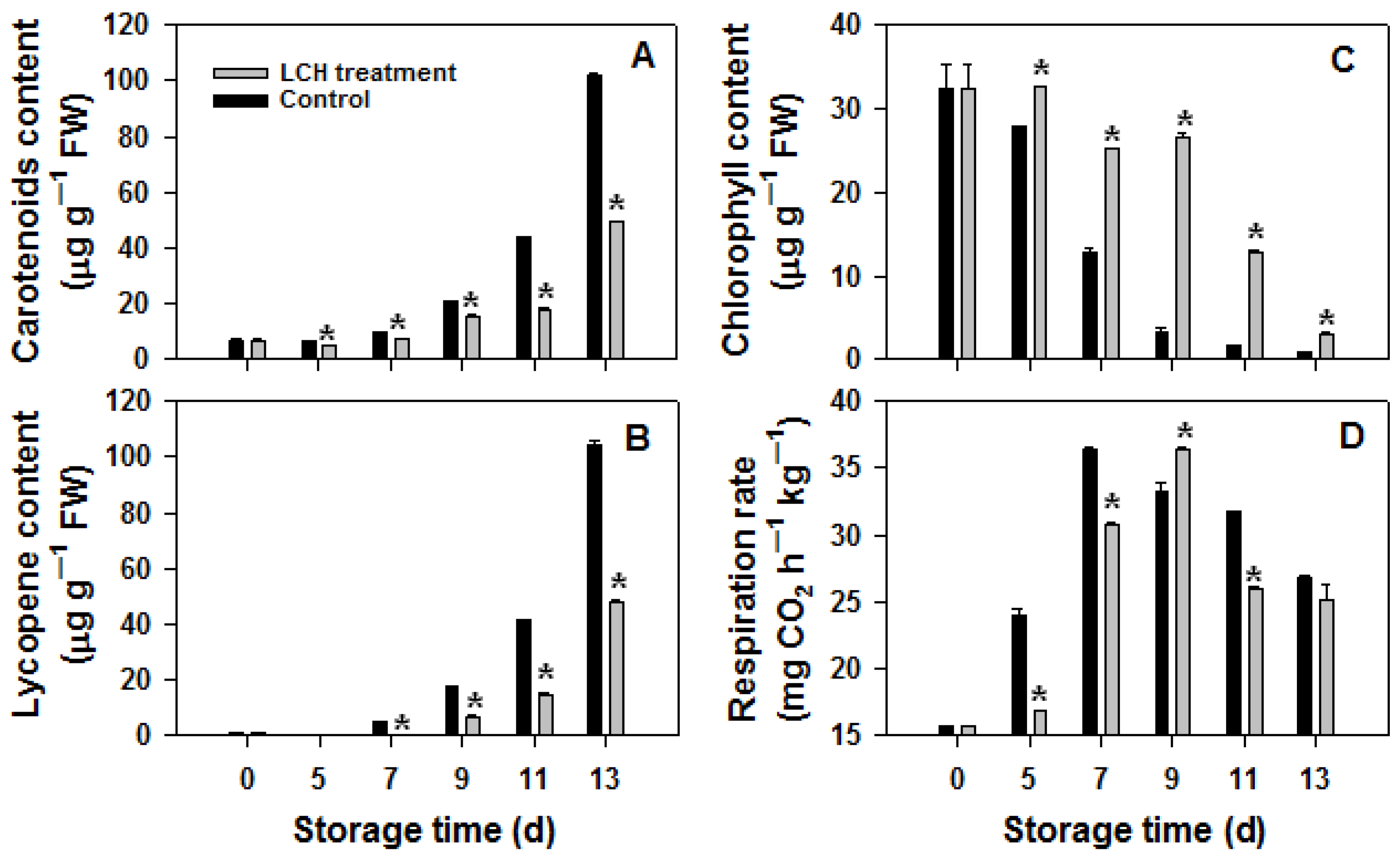
3.1. Effects of LCH Treatment on Ripening and Pigment Metabolism of Tomato Fruit
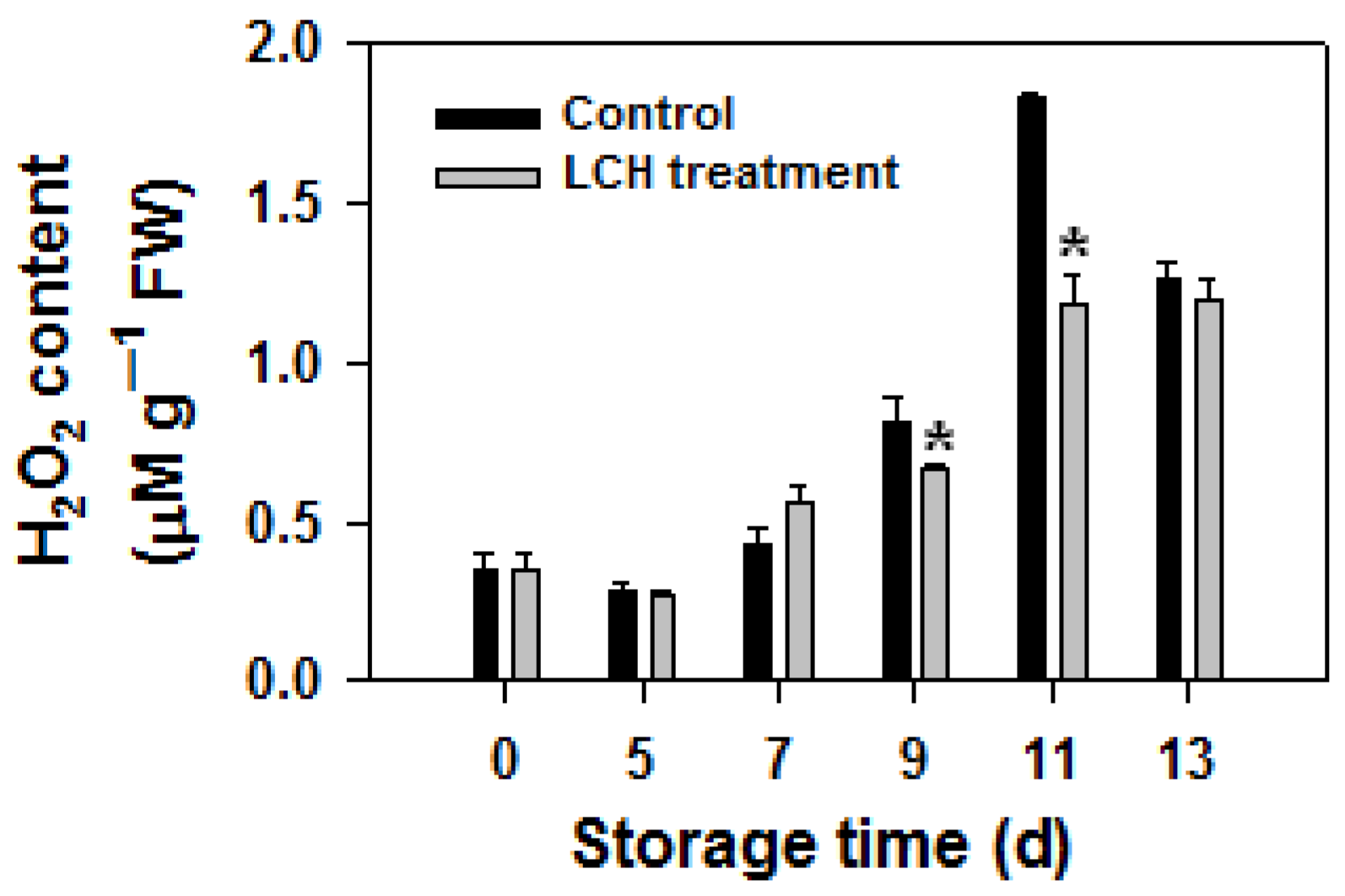
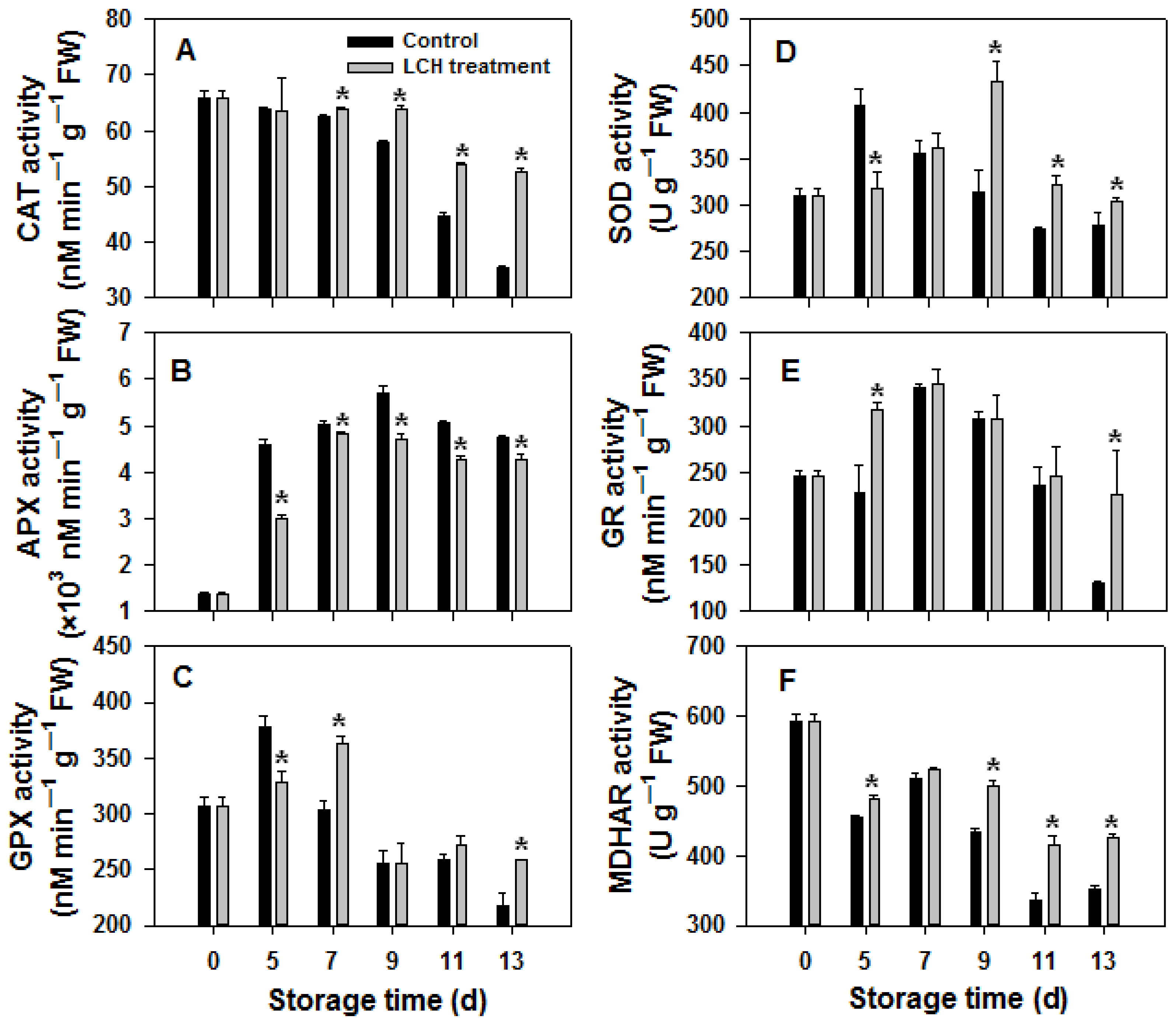
3.2. H2O2 Content and Activities of Antioxidant Enzymes
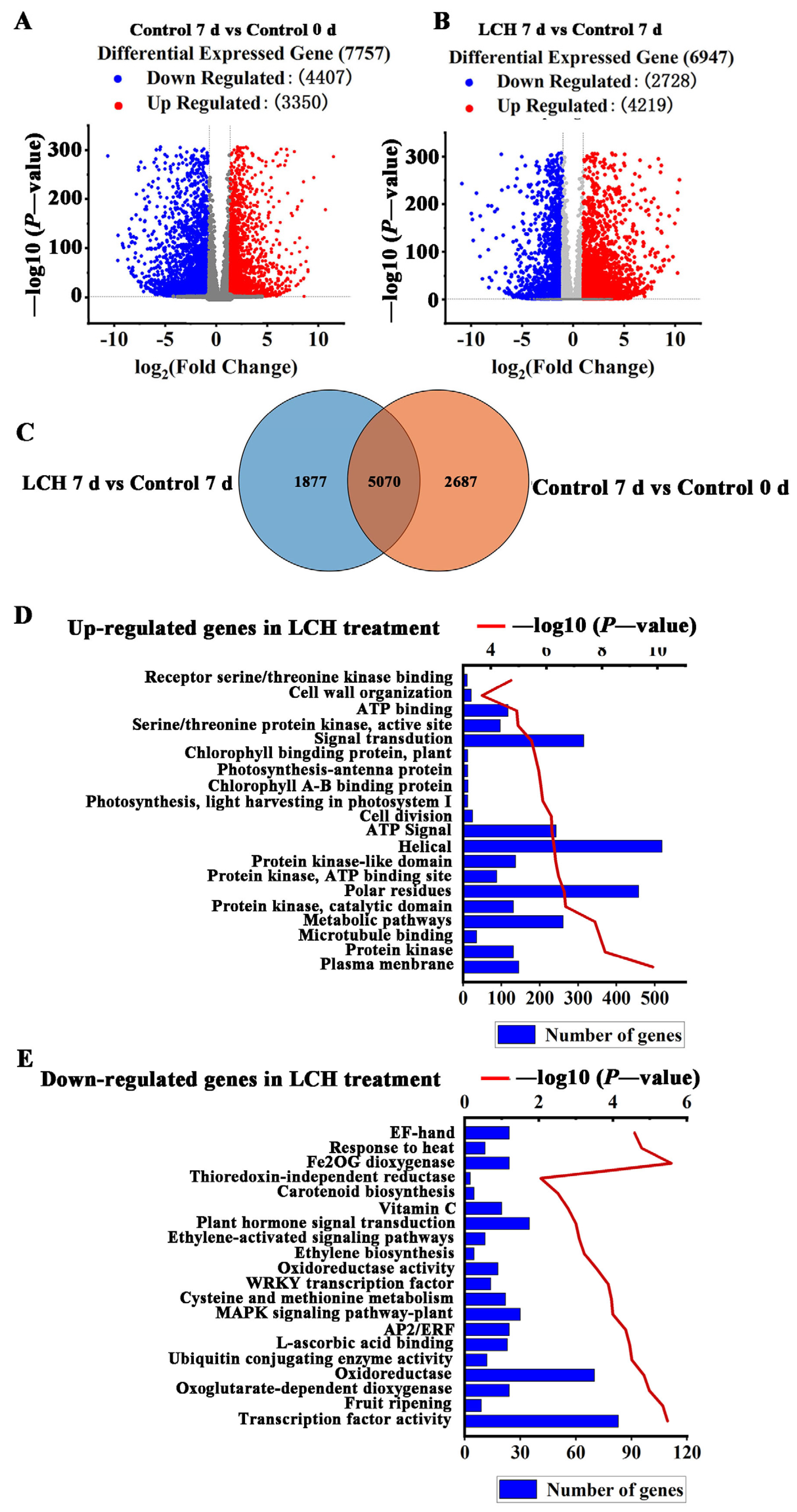
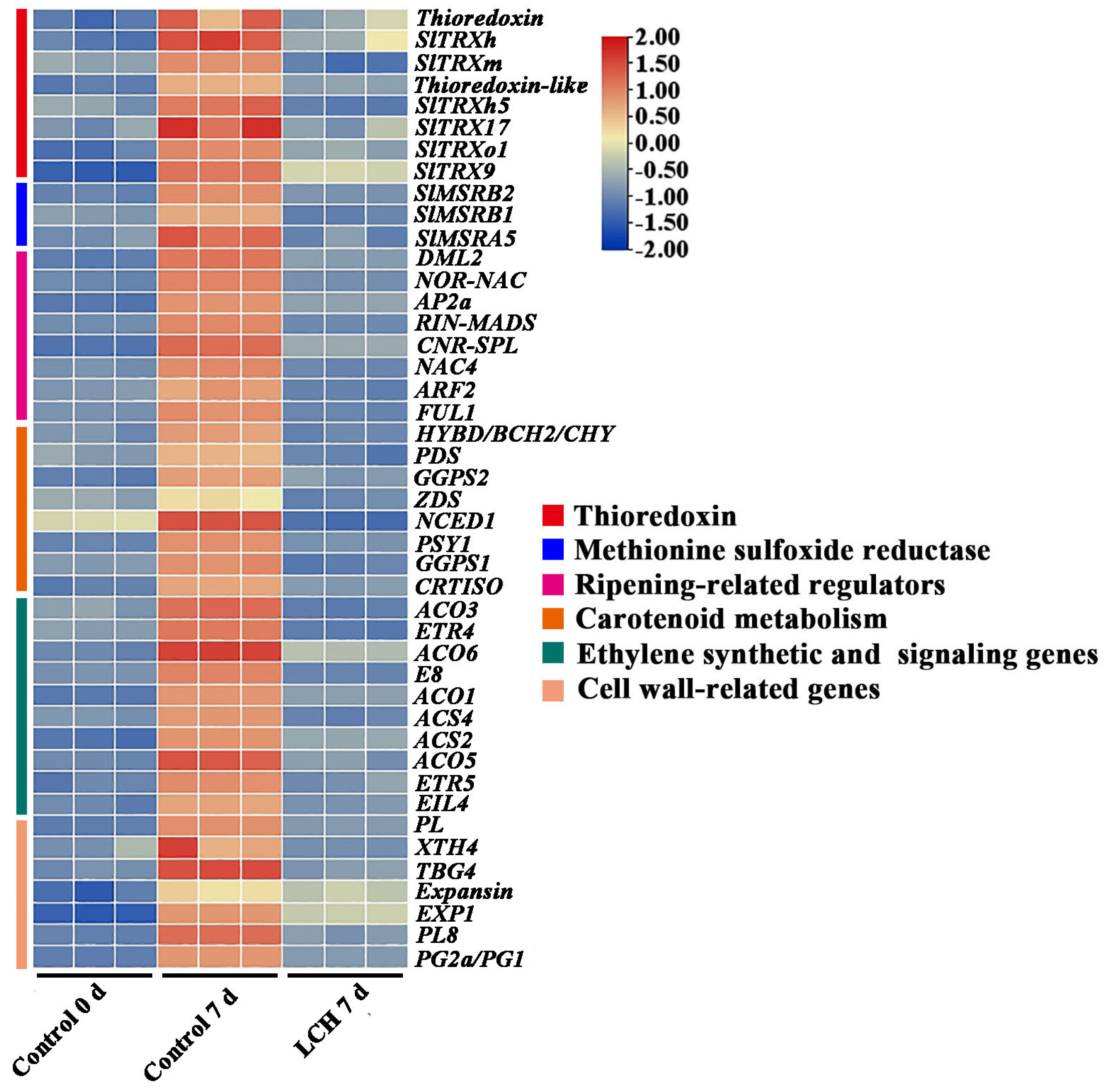
3.3. Transcriptome Profiling of Tomato Fruit in Response to LCH
4. Discussion
4.1. LCH Treatment Delays the Ripening of Harvested Tomato Fruit
4.2. LCH Treatment Influences Redox Balance of Harvested Tomato Fruit
4.3. LCH Treatment Suppresses the Expression of Ripening-Related Genes in Harvested Tomato Fruit
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giovannoni, J.; Cuong, N.; Ampofo, B.; Zhong, S.; Fei, Z. The epigenome and transcriptional dynamics of fruit ripening. Annu. Rev. Plant Biol. 2017, 68, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Qin, G.; Li, B. Reactive oxygen species involved in regulating fruit senescence and fungal pathogenicity. Plant Mol. Biol. 2013, 82, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhong, S.; Grierson, D. Recent advances in ethylene research. J. Exp. Bot. 2009, 60, 3311–3336. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; How-Kit, A.; Stammitti, L.; Teyssier, E.; Rolin, D.; Mortain-Bertrand, A.; Halle, S.; Liu, M.; Kong, J.; Wu, C.; et al. A DEMETER-like DNA demethylase governs tomato fruit ripening. Proc. Natl. Acad. Sci. USA 2015, 112, 10804–10809. [Google Scholar] [CrossRef]
- Lang, Z.; Wang, Y.; Tang, K.; Tang, D.; Datsenka, T.; Cheng, J.; Zhang, Y.; Handa, A.K.; Zhu, J.-K. Critical roles of DNA demethylation in the activation of ripening-induced genes and inhibition of ripening-repressed genes in tomato fruit. Proc. Natl. Acad. Sci. USA 2017, 114, E4511–E4519. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, G.; Liu, X.; Ding, X.; Zhang, D.; Wang, X.; Zhou, Y.; Yan, H.; Li, T.; Wu, K.; et al. Histone demethylase SlJMJ6 promotes fruit ripening by removing H3K27 methylation of ripening-related genes in tomato. New Phytol. 2020, 227, 1138–1156. [Google Scholar] [CrossRef]
- Ding, X.; Liu, X.; Jiang, G.; Li, Z.; Song, Y.; Zhang, D.; Jiang, Y.; Duan, X. SlJMJ7 orchestrates tomato fruit ripening via crosstalk between H3K4me3 and DML2-mediated DNA demethylation. New Phytol. 2022, 233, 1202–1219. [Google Scholar] [CrossRef]
- Ugarte, N.; Petropoulos, I.; Friguet, B. Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxid. Redox. Signal. 2010, 13, 539–549. [Google Scholar] [CrossRef]
- Grimaud, R.; Ezraty, B.; Mitchell, J.K.; Lafitte, D.; Briand, C.; Derrick, P.J.; Barras, F. Repair of oxidized proteins—Identification of a new methionine sulfoxide reductase. J. Biol. Chem. 2001, 276, 48915–48920. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, D.; Zhao, L.; Liu, Y.; Wang, C.; Farid, M.S.; Gu, Y.; Li, J.; Li, T.; Sun, Y.; et al. L-cysteine treatment delayed the quality deterioration of fresh-cut button mushrooms by regulating oxygen metabolism, inhibiting water loss, and stimulating endogenous H2S production. J. Agric. Food Chem. 2022, 71, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, H.; Fan, Z.; Wang, H.; Lin, M.; Chen, Y.; Hung, Y.-C.; Lin, Y. Inhibitory effect of propyl gallate on pulp breakdown of longan fruit and its relationship with ROS metabolism. Postharvest Biol. Tec. 2020, 168, 111272. [Google Scholar] [CrossRef]
- Su, Z.; Hu, M.; Gao, Z.; Li, M.; Yun, Z.; Pan, Y.; Zhang, Z.; Jiang, Y. Apple polyphenols delay senescence and maintain edible quality in litchi fruit during storage. Postharvest Biol. Technol. 2019, 157, 110976. [Google Scholar] [CrossRef]
- Dai, H.; Li, B.; Song, Z.; Tian, J.; Wei, B.; Lang, X.; Zhou, Q. Anthocyanin treatment delays the senescence of blueberry fruit by regulating abscisic acid and anthocyanin synthesis processes. Food Qual. Saf. 2024, 8, fyad053. [Google Scholar] [CrossRef]
- He, M.; Wu, Y.; Hong, M.; Yun, Z.; Li, T.; Jiang, Y. α-Lipoic acid treatment alleviates postharvest pericarp browning of litchi fruit by regulating antioxidant ability and energy metabolism. Postharvest Biol. Technol. 2021, 180, 111629. [Google Scholar] [CrossRef]
- Pace, B.; Capotorto, I.; Ventura, M.; Cefola, M. Evaluation of L-cysteine as anti-browning agent in fresh-cut lettuce processing. J. Food Process. Pres. 2015, 39, 985–993. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Dusemund, B.; Durjava, M.K.; Kouba, M.; Lopez-Alonso, M.; Puente, S.L.; Marcon, F.; et al. Safety and efficacy of l-cysteine monohydrochloride monohydrate produced by fermentation using Escherichia coli KCCM 80109 and Escherichia coli KCCM 80197 for all animal species. EFSA J. 2020, 18, e06101. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Dusemund, B.; Kouba, M.; Durjava, M.K.; Lopez-Alonso, M.; Puente, S.L.; Marcon, F.; et al. Safety and efficacy of L-cysteine hydrochloride monohydrate produced by fermentation using Escherichia coli KCCM 80180 and Escherichia coli KCCM 80181 as a flavouring additive for all animal species. EFSA J. 2020, 18, e06003. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, X.; Zheng, W.; Li, F.; Xiao, S.; Duan, X. BTH treatment delays the senescence of postharvest pitaya fruit in relation to enhancing antioxidant system and phenylpropanoid pathway. Foods 2021, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Xiao, L.; Yan, H.; Zhang, D.; Wu, F.; Liu, X.; Su, X.; Dong, X.; Wang, J.; Duan, X.; et al. Redox regulation of methionine in calmodulin affects the activity levels of senescence-related transcription factors in litchi. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Satekge, T.K.; Magwaza, L.S. Postharvest application of 1-methylcyclopropene (1-MCP) on climacteric fruits: Factors affecting efficacy. Int. J. Fruit Sci. 2022, 22, 595–607. [Google Scholar] [CrossRef]
- Wu, F.; Jiang, G.; Yan, H.; Xiao, L.; Liang, H.; Zhang, D.; Jiang, Y.; Duan, X. Redox regulation of glutathione peroxidase by thioredoxin in longan fruit in relation to senescence and quality deterioration. Food Chem. 2021, 345, 128664. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Jiang, G.; Wu, F.; Li, Z.; Xiao, L.; Jiang, Y.; Duan, X. Sulfoxidation regulation of transcription factor NAC42 influences its functions in relation to stress-induced fruit ripening in banana. J. Exp. Bot. 2021, 72, 682–699. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, M.; Wang, H.; Zhou, C.; Yuan, J.; Li, X.; Pan, Y. Isothermal storage delays the senescence of post-harvest apple fruit through the regulation of antioxidant activity and energy metabolism. Foods 2023, 12, 1765. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Rabiei, V.; Kakavand, F.; Zaare-Nahandi, F.; Razavi, F.; Aghdam, M.S. Nitric oxide and γ-aminobutyric acid treatments delay senescence of cornelian cherry fruits during postharvest cold storage by enhancing antioxidant system activity. Sci. Hortic. 2019, 243, 268–273. [Google Scholar] [CrossRef]
- Nie, Z.; Huang, Q.; Chen, C.; Wan, C.; Chen, J. Chitosan coating alleviates postharvest juice sac granulation by mitigating ROS accumulation in harvested pummelo (Citrus grandis L. Osbeck) during room temperature storage. Postharvest Biol. Technol. 2020, 169, 111309. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, J.; Wang, X.; Yu, M.; Ma, R.; Yu, Z. Synergy of nitric oxide and 1-methylcyclopropene treatment in prolong ripening and senescence of peach fruit. Foods 2021, 10, 2956. [Google Scholar] [CrossRef]
- Fan, L.; Shi, J.; Zuo, J.; Gao, L.; Lv, J.; Wang, Q. Methyl jasmonate delays postharvest ripening and senescence in the non-climacteric eggplant (Solanum melongena L.) fruit. Postharvest Biol. Technol. 2016, 120, 76–83. [Google Scholar] [CrossRef]
- Alexander, L.; Grierson, D. Ethylene biosynthesis and action in tomato: A model for climacteric fruit ripening. J. Exp. Bot. 2002, 53, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-E. Histone deacetylase gene SlHDT1 regulates tomato fruit ripening by affecting carotenoid accumulation and ethylene biosynthesis. Plant Sci. 2022, 318, 111235. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-E.; Hu, Z.; Li, F.; Zhang, L.; Yu, X.; Tang, B.; Chen, G. Silencing of histone deacetylase SlHDT3 delays fruit ripening and suppresses carotenoid accumulation in tomato. Plant Sci. 2017, 265, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Z.; Yao, Q.; Guo, X.; Nguyen, V.; Li, F.; Chen, G. A tomato MADS-box protein, SlCMB1, regulates ethylene biosynthesis and carotenoid accumulation during fruit ripening. Sci. Rep. 2018, 8, 3413. [Google Scholar] [CrossRef]
- Tao, X.; Wu, Q.; Li, J.; Wang, D.; Nassarawa, S.S.; Ying, T. Ethylene biosynthesis and signal transduction are enhanced during accelerated ripening of postharvest tomato treated with exogenous methyl jasmonate. Sci. Hortic. 2021, 281, 109965. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, Y.; Liu, C.; Chen, L.; Ma, J.; Sheng, J.; Shen, L. Inhibition of nitric oxide synthesis delayed mature-green tomato fruits ripening induced by inhibition of ethylene. Sci. Hortic. 2016, 211, 95–101. [Google Scholar] [CrossRef]
- Fujisawa, M.; Nakano, T.; Shima, Y.; Ito, Y. A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell 2013, 25, 371–386. [Google Scholar] [CrossRef]
- Barry, C.S.; Giovannoni, J.J. Ethylene and fruit ripening. J. Plant Growth Regul. 2007, 26, 143–159. [Google Scholar] [CrossRef]
- Jiang, G.; Li, Z.; Ding, X.; Zhou, Y.; Lai, H.; Jiang, Y.; Duan, X. WUSCHEL-related homeobox transcription factor SlWOX13 regulates tomato fruit ripening. Plant Physiol. 2023, kiad623. [Google Scholar] [CrossRef]
- Fu, M.; Li, F.; Zhou, S.; Guo, P.; Chen, Y.; Xie, Q.; Chen, G.; Hu, Z. Trihelix transcription factor SlGT31 regulates fruit ripening mediated by ethylene in tomato. J. Exp. Bot. 2023, 74, 5709–5721. [Google Scholar] [CrossRef]
- Wang, W.; Wang, P.; Li, X.; Wang, Y.; Tian, S.; Qin, G. The transcription factor SlHY5 regulates the ripening of tomato fruit at both the transcriptional and translational levels. Hortic. Res. 2021, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, X.; Liu, H.; Zhang, M.; Liao, W. DNA methylation in tomato fruit ripening. Physiol. Plantarum 2022, 174, e13627. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shao, Z.; Zhang, M.; Wang, Q. Regulation of carotenoid metabolism in tomato. Mol. Plant 2015, 8, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena—An overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Eda, M.; Matsumoto, T.; Ishimaru, M.; Tada, T. Structural and functional analysis of tomato-galactosidase4: Insight into the substrate specificity of the fruit softening-related enzyme. Plant J. 2016, 86, 300–307. [Google Scholar] [CrossRef]
- Uluisik, S.; Chapman, N.H.; Smith, R.; Poole, M.; Adams, G.; Gillis, R.B.; Besong, T.M.D.; Sheldon, J.; Stiegelmeyer, S.; Perez, L.; et al. Genetic improvement of tomato by targeted control of fruit softening. Nat. Biotechnol. 2016, 34, 950–952. [Google Scholar] [CrossRef]
- Su, G.; Lin, Y.; Wang, C.; Lu, J.; Liu, Z.; He, Z.; Shu, X.; Chen, W.; Wu, R.; Li, B.; et al. Expansin SlExp1 and endoglucanase SlCel2 synergistically promote fruit softening and cell wall disassembly in tomato. Plant Cell 2023, 36, 709–726. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Liang, H.; Peng, J.; Ding, S.; Duan, X.; Shan, Y. Application of L-Cysteine Hydrochloride Delays the Ripening of Harvested Tomato Fruit. Foods 2024, 13, 841. https://doi.org/10.3390/foods13060841
Song Y, Liang H, Peng J, Ding S, Duan X, Shan Y. Application of L-Cysteine Hydrochloride Delays the Ripening of Harvested Tomato Fruit. Foods. 2024; 13(6):841. https://doi.org/10.3390/foods13060841
Chicago/Turabian StyleSong, Yunbo, Hanzhi Liang, Jiechun Peng, Shenghua Ding, Xuewu Duan, and Yang Shan. 2024. "Application of L-Cysteine Hydrochloride Delays the Ripening of Harvested Tomato Fruit" Foods 13, no. 6: 841. https://doi.org/10.3390/foods13060841
APA StyleSong, Y., Liang, H., Peng, J., Ding, S., Duan, X., & Shan, Y. (2024). Application of L-Cysteine Hydrochloride Delays the Ripening of Harvested Tomato Fruit. Foods, 13(6), 841. https://doi.org/10.3390/foods13060841





