Grain Chalkiness Is Decreased by Balancing the Synthesis of Protein and Starch in Hybrid Indica Rice Grains under Nitrogen Fertilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Plant Material
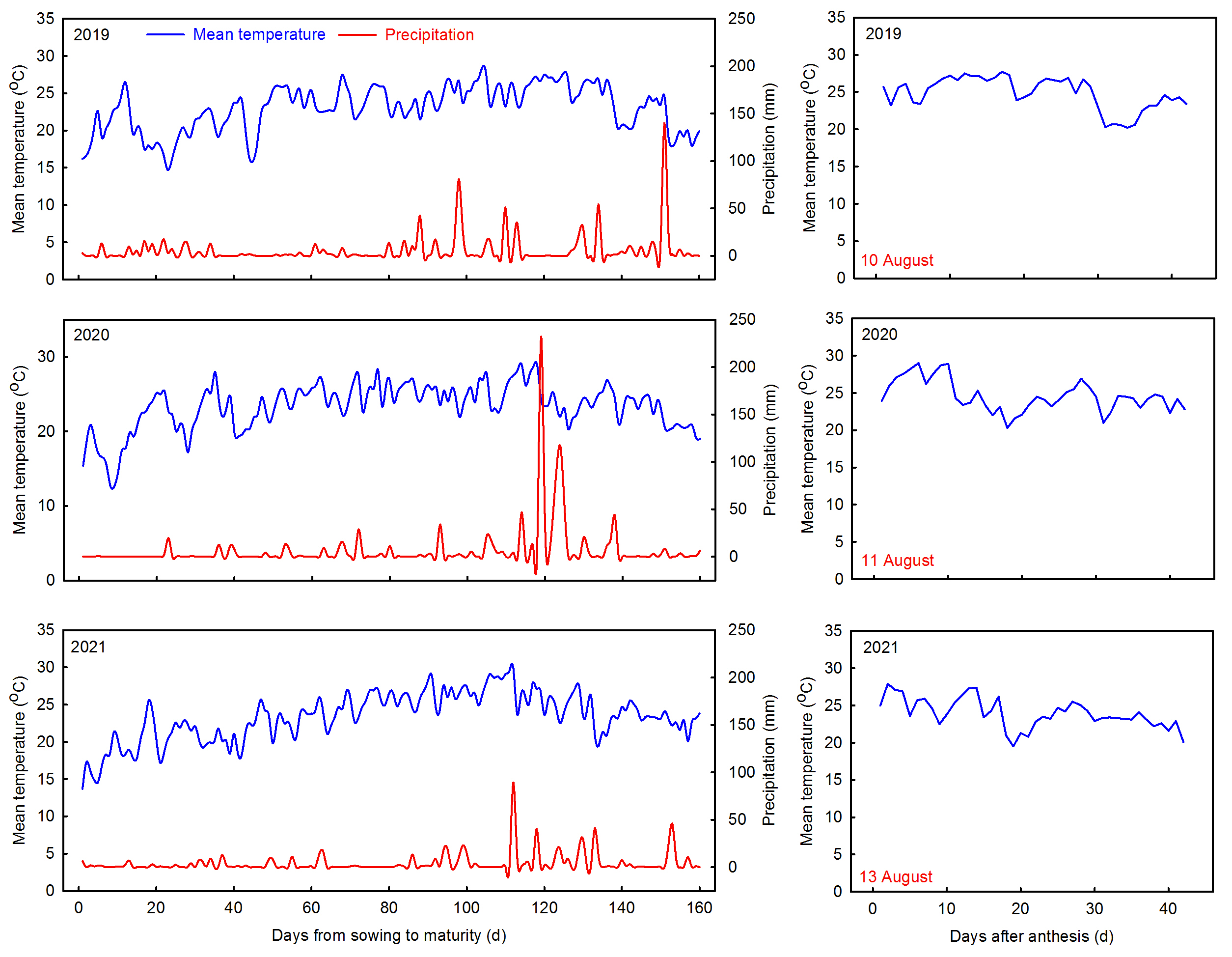
2.2. Experimental Design and Field Management
2.3. Experimental Conditions and Procedures
2.3.1. Sample Preparation
2.3.2. Dynamic Analysis of Key Enzyme Activities in C Metabolism
2.3.3. Dynamic Analysis of Key Enzyme Activities in N Metabolism
2.3.4. Dynamic Analysis of Endogenous Hormones
2.3.5. Dynamic Analysis of Assimilates
2.3.6. Determination of Grain Chalkiness
2.3.7. Determination of Protein Fractions and Amino Acid Composition
2.3.8. Statistical Analysis
3. Results
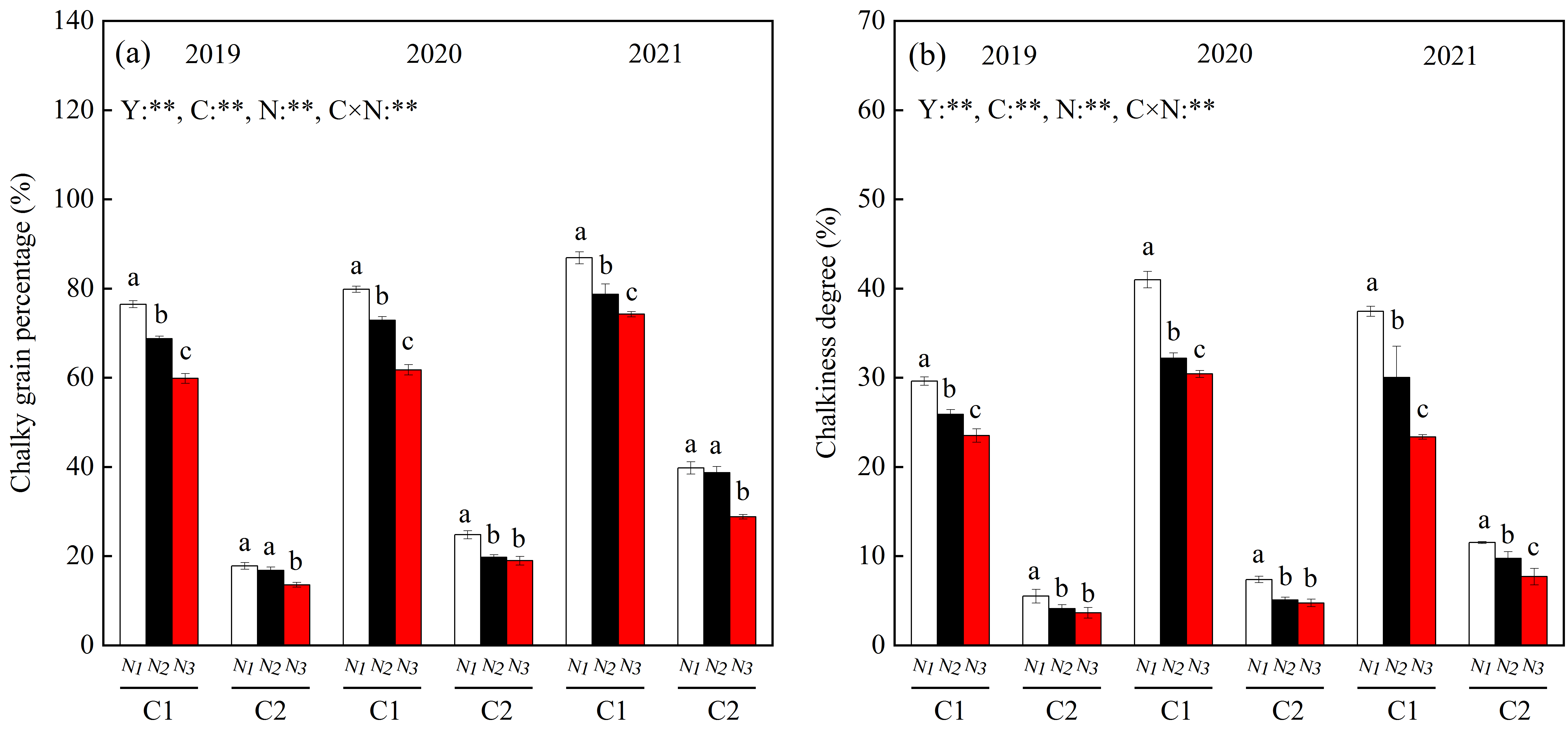
3.1. Rice Appearance Quality
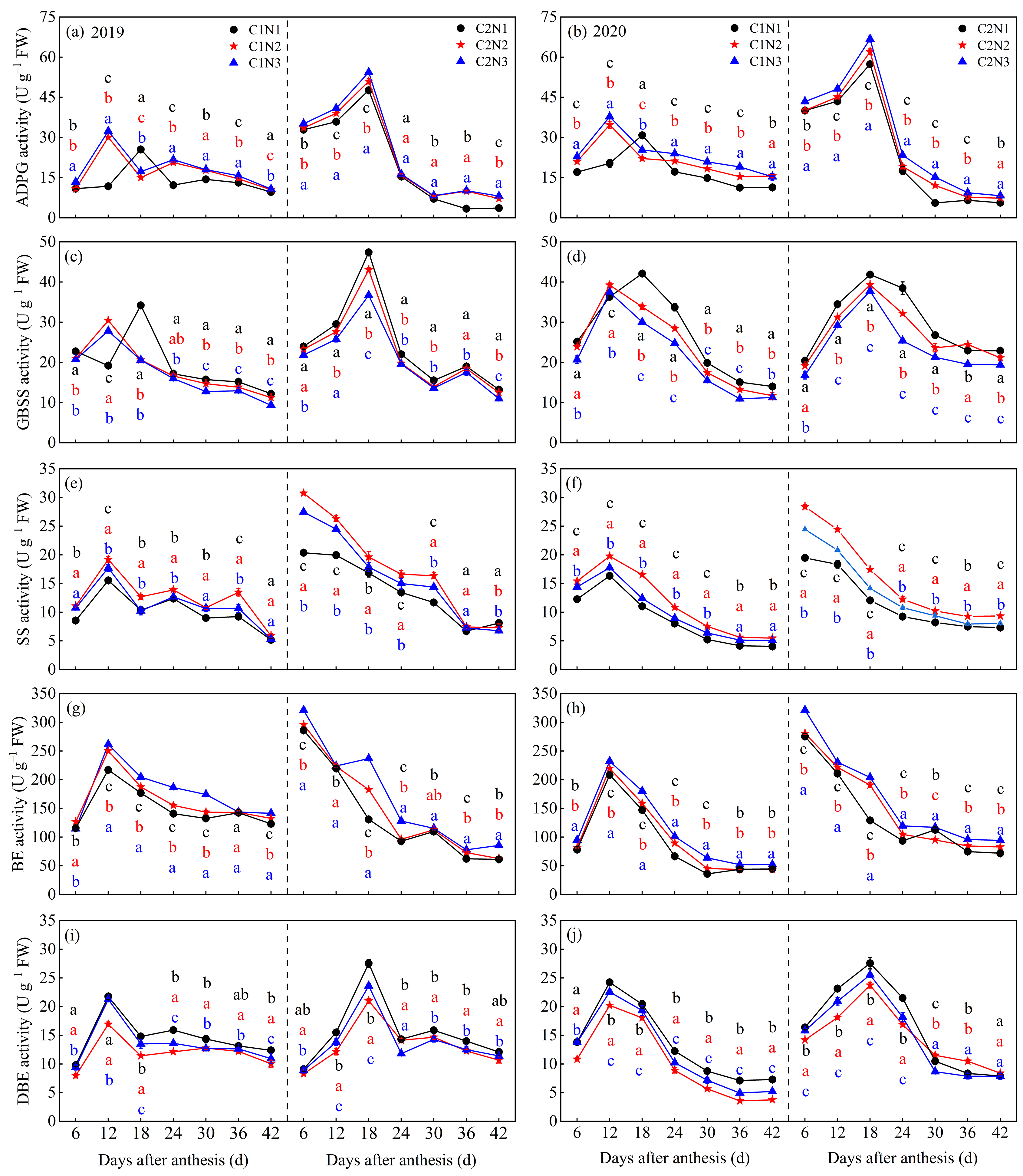
3.2. Dynamic Changes in the Activity of Key Enzymes Involved in Grains C Metabolism
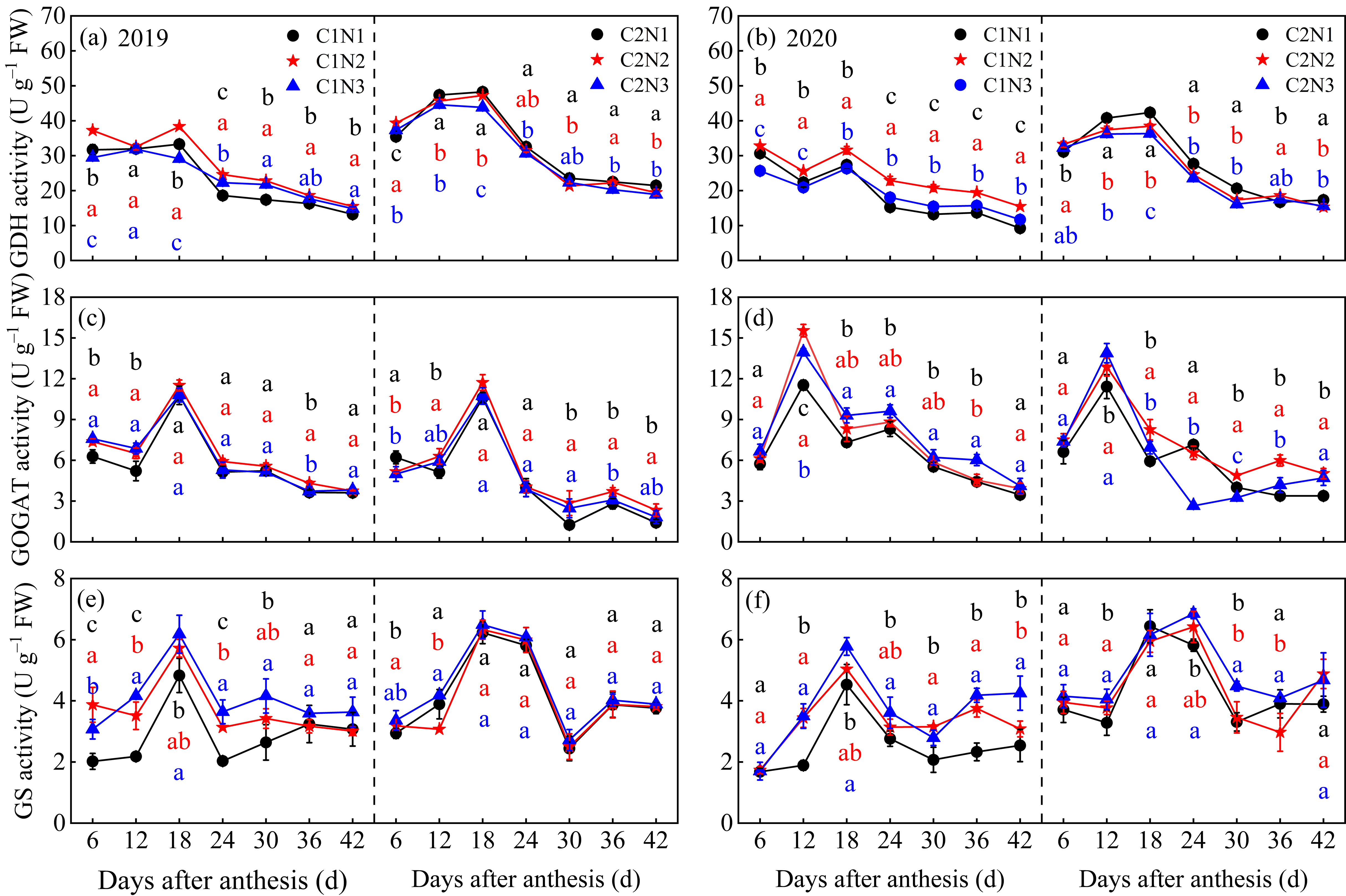
3.3. Dynamic Changes in the Activity of Key Enzymes Involved in Grains N Metabolism
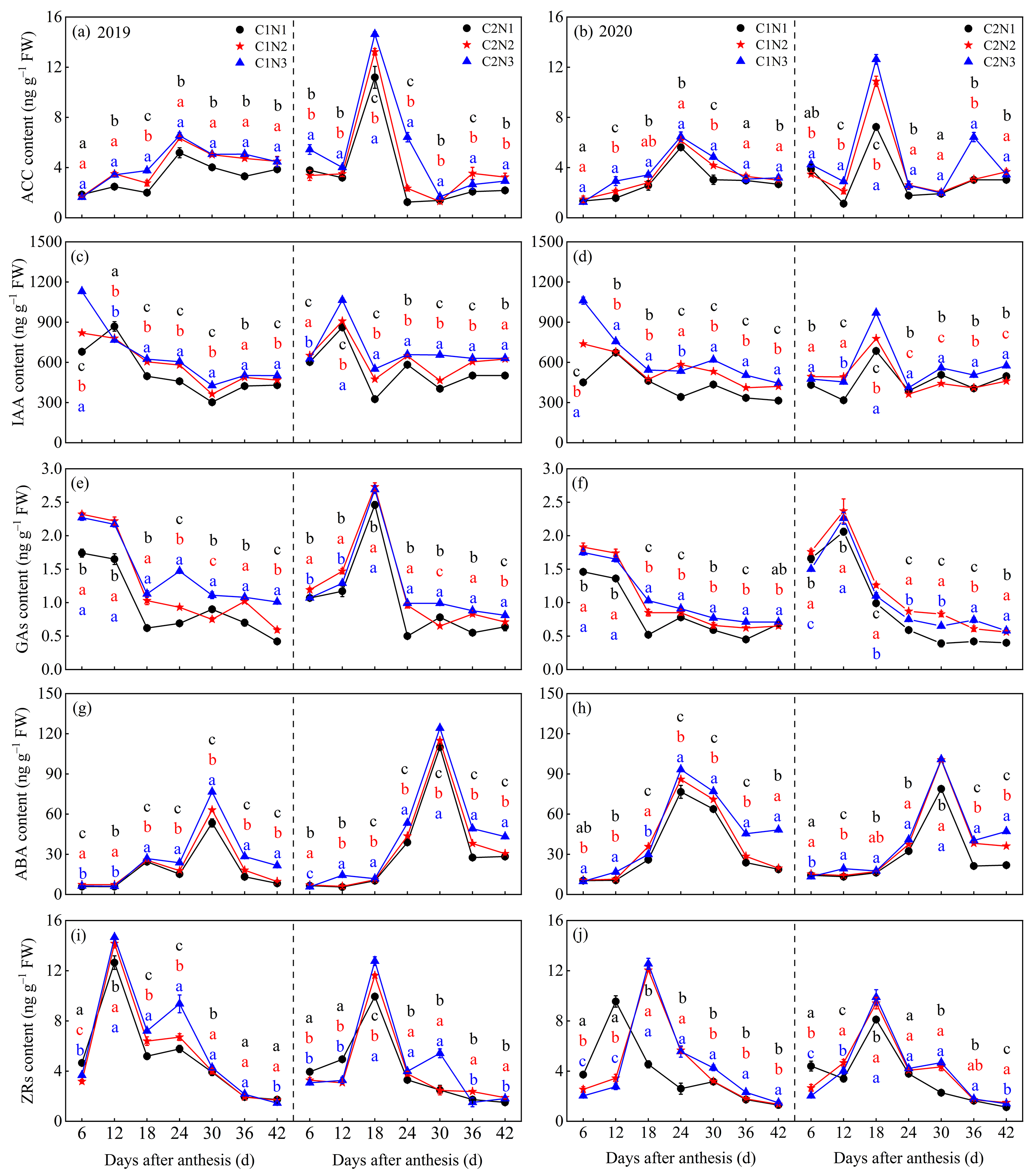
3.4. Dynamic Changes in Endogenous Hormones in Grains
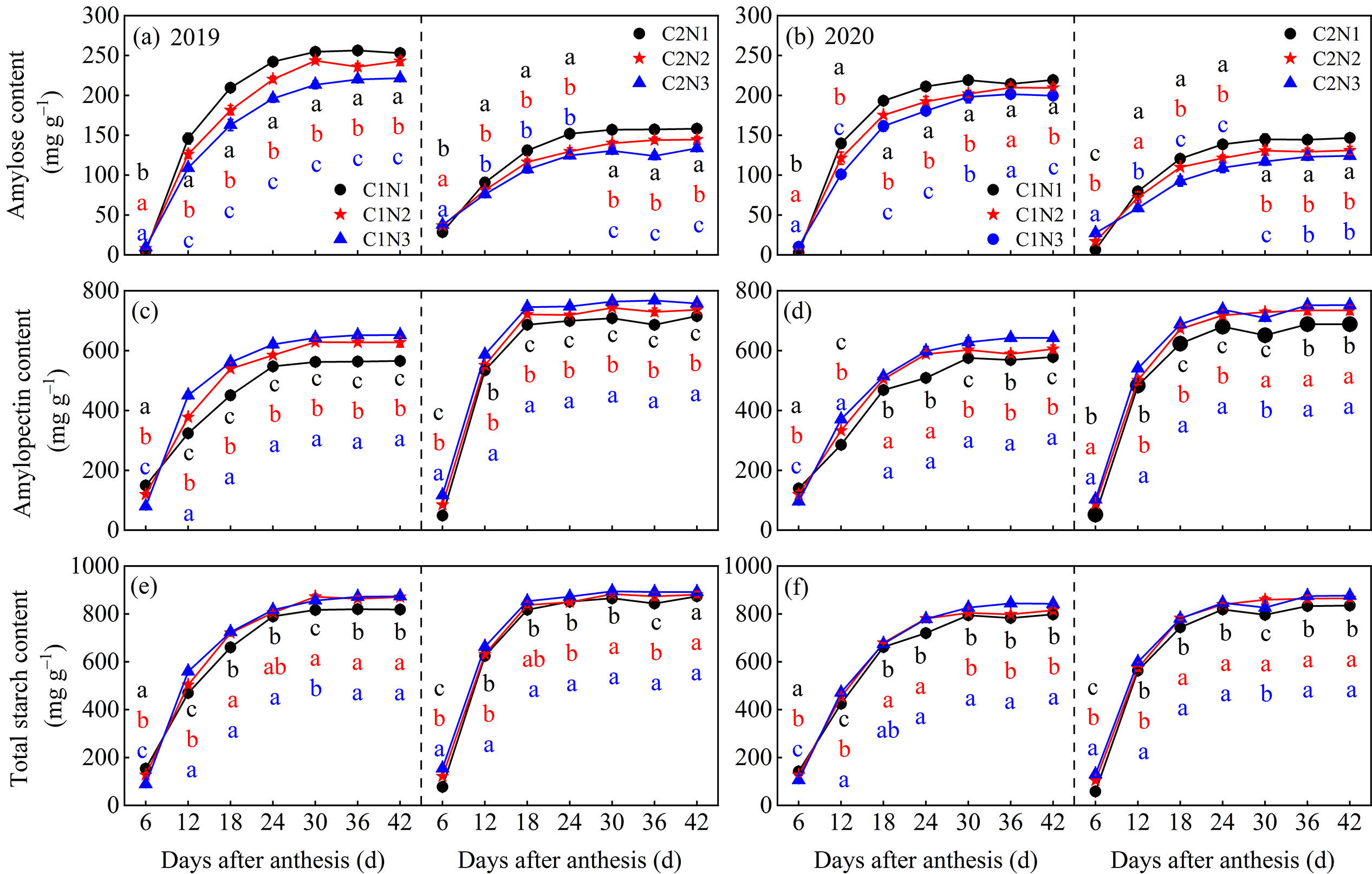
3.5. Dynamic Changes in Starch and Its Composition in Grains
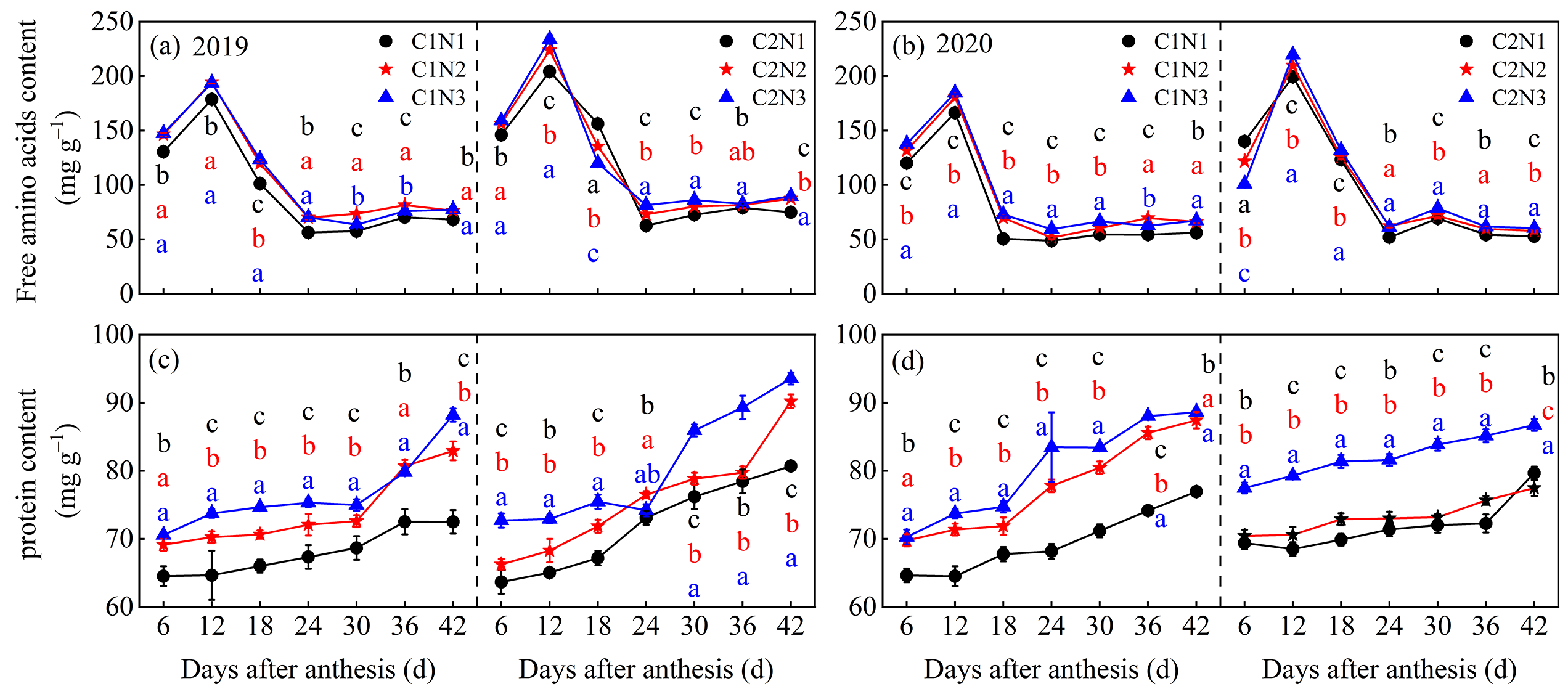
3.6. Dynamic Changes in Free Amino Acids and Total Protein in Grains
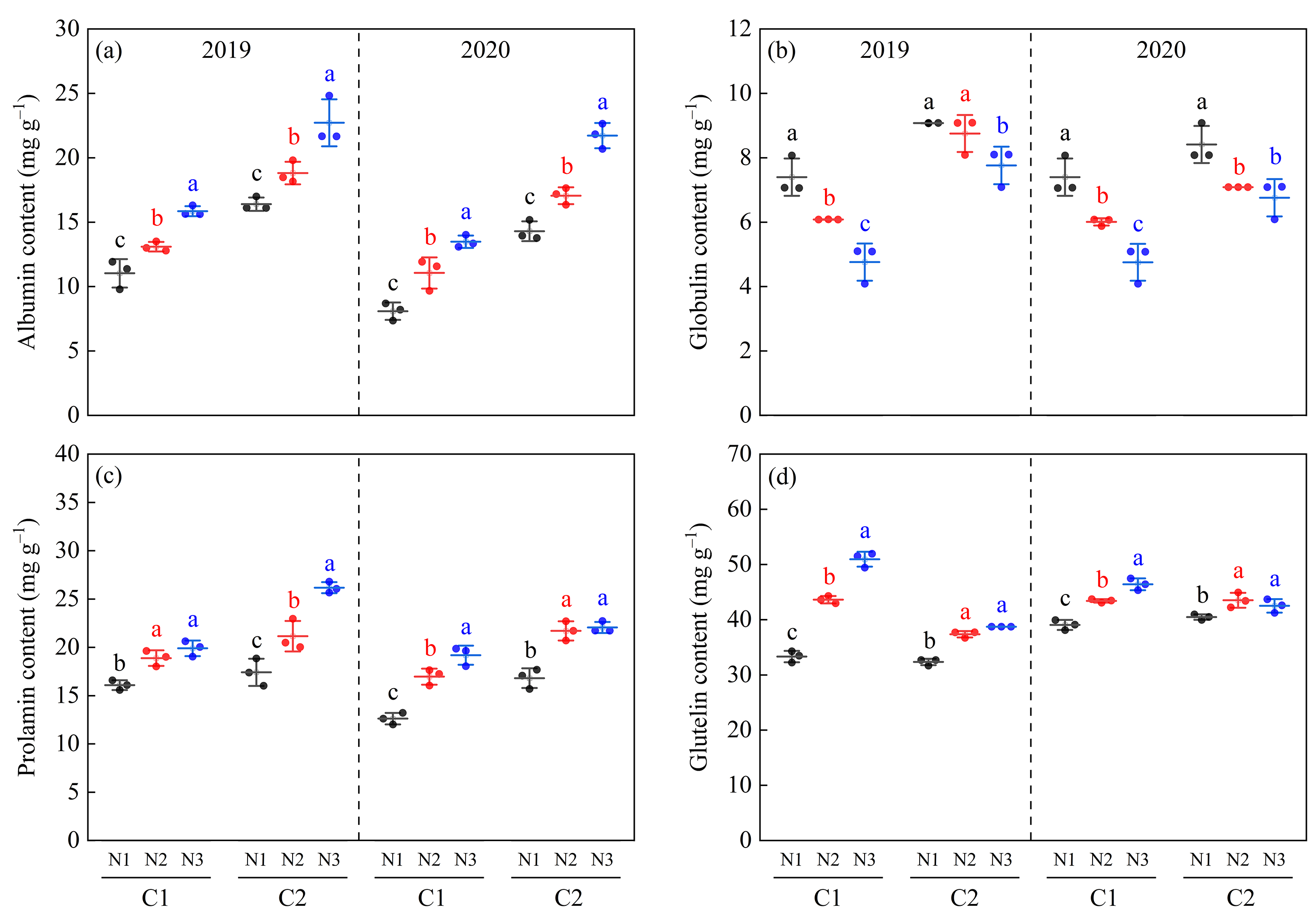
3.7. Rice Protein Fractions
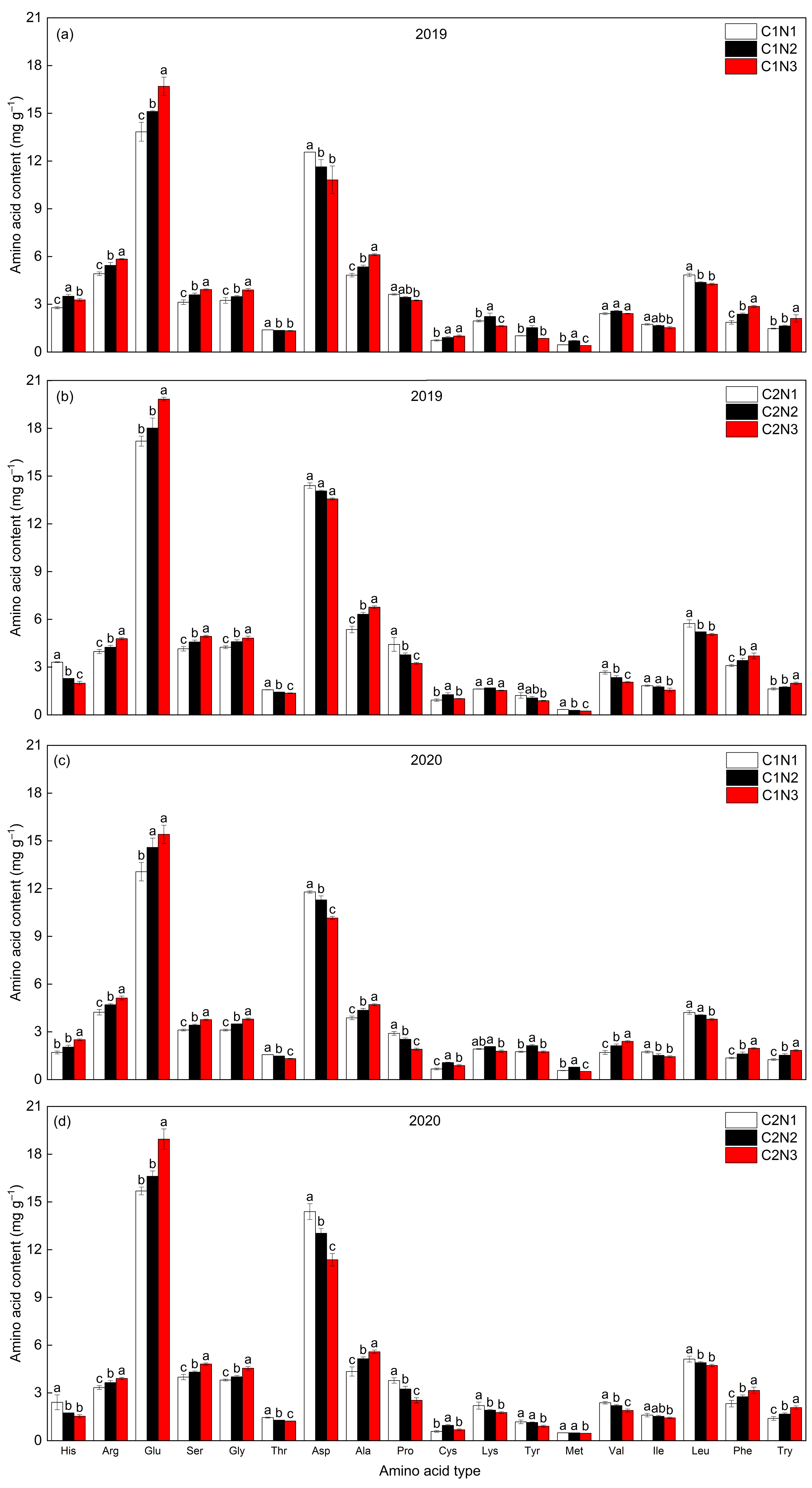
3.8. Rice Amino Acid Composition
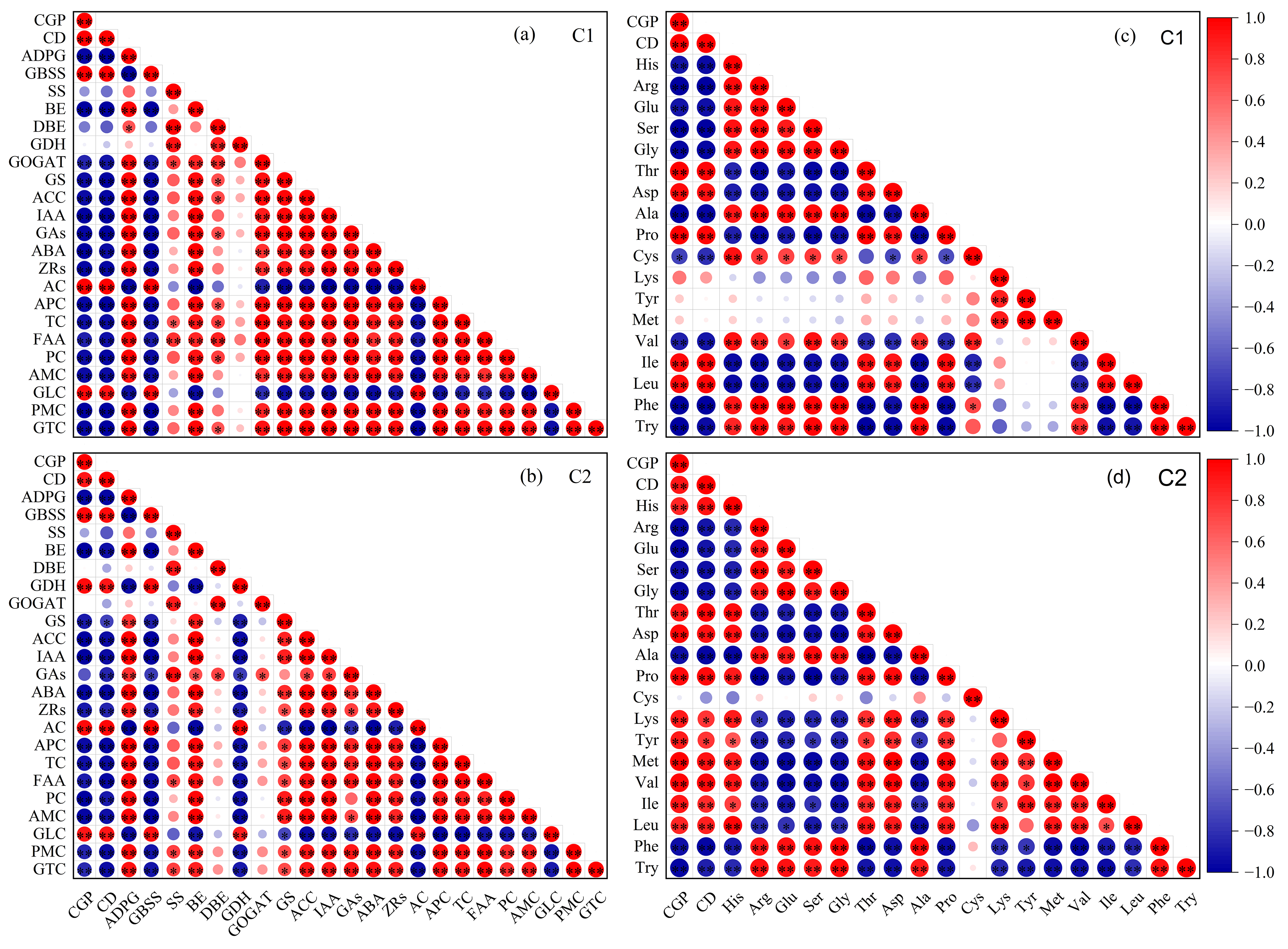
3.9. Relationship between Rice Chalkiness and Key Enzyme Activities of Grain C and N Metabolism, Endogenous Hormones, and Amino Acid Composition
4. Discussion
4.1. Responses of Rice Chalkiness to N Fertilization
4.2. Relationship between Rice Chalkiness Formation and Grain C and N Metabolism
4.3. Relationship between Rice Chalkiness Formation and Secondary Metabolites of Proteins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, F.; Yang, F.; Li, Q.; Zeng, Y.; Li, B.; Zhong, X.; Lu, H.; Wang, L.; Chen, H.; Chen, Y.; et al. Differences in starch structural and physicochemical properties and texture characteristics of cooked rice between the main crop and ratoon rice. Food Hydrocoll. 2021, 116, 106643. [Google Scholar] [CrossRef]
- Huang, M.; Cao, J.; Zhang, R.; Chen, J.; Cao, F.; Liu, L.; Huang, S.; Zhang, M. Delayed sowing does not improve palatability-related traits in high-quality rice. Food Chem. Adv. 2022, 1, 100096. [Google Scholar] [CrossRef]
- Chen, Z.; Li, P.; Xiao, J.; Jiang, Y.; Cai, M.; Wang, J.; Li, C.; Zhan, M.; Cao, C. Dry cultivation with ratoon system impacts rice quality using rice flour physicochemical traits, fatty and amino acids contents. Food Res. Int. 2021, 150, 110764. [Google Scholar] [CrossRef]
- Syahariza, Z.A.; Sar, S.; Hasjim, J.; Tizzotti, M.J.; Gilbert, R.G. The importance of amylose and amylopectin fine structures for starch digestibility in cooked rice grains. Food Chem. 2013, 136, 742–749. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, H.; Guo, B.; Xu, K.; Dai, Q.; Wei, C.; Zhou, G.; Huo, Z. Effects of nitrogen level on structure and physicochemical properties of rice starch. Food Hydrocoll. 2017, 63, 525–532. [Google Scholar] [CrossRef]
- Deng, F.; Li, Q.; Cheng, H.; Zeng, Y.; Li, B.; Zhong, X.; Wang, L.; Ren, W. Relationship between chalkiness and the structural and thermal properties of rice starch after shading during grain-filling stage. Carbohydr. Polym. 2021, 252, 117212. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.M.; Zhong, L.J.; Wang, F.; Zhang, G.P. Differences in cooking and eating properties between chalky and translucent parts in rice grains. Food Chem. 2005, 90, 39–46. [Google Scholar] [CrossRef]
- Impa, S.M.; Raju, B.; Hein, N.T.; Sandhu, J.; Prasad, P.V.V.; Walia, H.; Jagadish, S.V.K. High night temperature effects on wheat and rice: Current status and way forward. Plant Cell Environ. 2021, 44, 2049–2065. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Wang, L.; Pu, S.; Mei, X.; Li, S.; Li, Q.; Ren, W. Shading stress increases chalkiness by postponing caryopsis development and disturbing starch characteristics of rice grains. Agric. For. Meteorol. 2018, 263, 49–58. [Google Scholar] [CrossRef]
- Liu, W.; Yin, T.; Zhao, Y.; Wang, X.; Wang, K.; Shen, Y.; Ding, Y.; Tang, S. Effects of high temperature on rice grain development and quality formation based on proteomics comparative analysis under field warming. Front. Plant Sci. 2021, 12, 746180. [Google Scholar] [CrossRef]
- Li, Y.; Fan, C.; Xing, Y.; Yun, P.; Luo, L.; Yan, B.; Peng, B.; Xie, W.; Wang, G.; Li, X.; et al. Chalk 5 encodes a vacuolar H+-translocating pyrophosphatase influencing grain chalkiness in rice. Nat. Genet. 2014, 46, 398. [Google Scholar] [CrossRef]
- Guo, C.; Wuza, R.; Tao, Z.; Yuan, X.; Luo, Y.; Li, F.; Yang, G.; Chen, Z.; Yang, Z.; Sun, Y.; et al. Effects of elevated nitrogen fertilizer on the multi-level structure and thermal properties of rice starch granules and their relationship with chalkiness traits. J. Sci. Food Agric. 2023, 103, 7302–7313. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.J.; Jones, R.L. (Eds.) Biochemistry & molecular biology of plants. Plant Growth Regul. 2001, 35, 105–106. [Google Scholar]
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent advances in carbon and nitrogen metabolism in C3 plants. Int. J. Mol. Sci. 2021, 22, 318. [Google Scholar] [CrossRef]
- Xi, M.; Wu, W.; Xu, Y.; Zhou, Y.; Chen, G.; Ji, Y.; Sun, X. Grain chalkiness traits is affected by the synthesis and dynamic accumulation of the storage protein in rice. J. Sci. Food Agric. 2021, 101, 6125–6133. [Google Scholar] [CrossRef]
- Fei, L.; Yang, S.; Ma, A.; Lunzhu, C.; Wang, M.; Wang, G.; Guo, S. Grain chalkiness is reduced by coordinating the biosynthesis of protein and starch in fragrant rice (Oryza sativa L.) grain under nitrogen fertilization. Field Crops Res. 2023, 302. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Y.; Sun, Y.; Luo, Y.; Guo, C.; Li, B.; Li, F.; Xing, M.; Yang, Z.; Ma, J. Effects of water and nitrogen on grain filling characteristics, canopy microclimate with chalkiness of directly seeded rice. Agriculture 2022, 12, 122. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Dong, S.; Liu, J.; Wang, Y.; Hussain, S.; Wei, H.; Huo, Z.; Xu, K.; Dai, Q. Response of rice yield and grain quality to combined nitrogen application rate and planting density in saline area. Agriculture 2022, 12, 1788. [Google Scholar] [CrossRef]
- Guo, C.; Yuan, X.; Yan, F.; Xiang, K.; Wu, Y.; Zhang, Q.; Wang, Z.; He, L.; Fan, P.; Yang, Z.; et al. Nitrogen application rate affects the accumulation of carbohydrates in functional leaves and grains to improve grain filling and reduce the occurrence of chalkiness. Front. Plant Sci. 2022, 13, 921130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Song, N.; Chen, Q.; Sun, H.; Peng, T.; Huang, S.; Zhao, Q. Response of grain-filling rate and grain quality of mid-season indica rice to nitrogen application. J. Integr. Agric. 2021, 20, 1465–1473. [Google Scholar] [CrossRef]
- Suriyasak, C.; Harano, K.; Tanamachi, K.; Matsuo, K.; Tamada, A.; Iwaya-Inoue, M.; Ishibashi, Y. Reactive oxygen species induced by heat stress during grain filling of rice (Oryza sativa L.) are involved in occurrence of grain chalkiness. J. Plant Physiol. 2017, 216, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, K.; Yin, T.; Zhao, Y.; Liu, W.; Shen, Y.; Ding, Y.; Tang, S. Nitrogen fertilizer regulated grain storage protein synthesis and reduced chalkiness of rice under actual field warming. Front. Plant Sci. 2021, 12, 715436. [Google Scholar] [CrossRef] [PubMed]
- Xi, M.; Zhao, Y.; Lin, Z.; Zhang, X.; Ding, C.; Tang, S.; Liu, Z.; Wang, S.; Ding, Y. Comparison of physicochemical characteristics between white-belly and white-core rice grains. J. Cereal Sci. 2016, 69, 392–397. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, A.; Xie, L.; Ramachandran, S. Improving protein content and quality by over-expressing artificially synthetic fusion proteins with high lysine and threonine constituent in rice plants. Sci. Rep. 2016, 6, 34427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ran, X.; Yin, T.; Guo, H.; Zhang, X.; Shen, Y.; Liu, W.; Ding, Y.; Tang, S. Nitrogen alleviated the deterioration of rice quality by affecting the accumulation of grain storage protein under elevated temperature. J. Plant Growth Regul. 2022, 42, 3388–3404. [Google Scholar] [CrossRef]
- Zhou, L.; Liang, S.; Ponce, K.; Marundon, S.; Ye, G.; Zhao, X. Factors affecting head rice yield and chalkiness in indica rice. Field Crops Res. 2015, 172, 1–10. [Google Scholar] [CrossRef]
- Gu, J.; Chen, J.; Chen, L.; Wang, Z.; Zhang, H.; Yang, J. Grain quality changes and responses to nitrogen fertilizer of japonica rice cultivars released in the Yangtze River Basin from the 1950s to 2000s. Crop J. 2015, 3, 285–297. [Google Scholar] [CrossRef]
- Yang, Z.; Li, N.; Ma, J.; Sun, Y.; Xu, H. High-yielding traits of heavy panicle varieties under triangle planting geometry: A new plant spatial configuration for hybrid rice in China. Field Crops Res. 2014, 168, 135–147. [Google Scholar] [CrossRef]
- Chen, Z.; Li, P.; Du, Y.; Jiang, Y.; Cai, M.; Cao, C. Dry cultivation and cultivar affect starch synthesis and traits to define rice grain quality in various panicle parts. Carbohydr. Polym. 2021, 269, 118336. [Google Scholar] [CrossRef]
- Schaffer, A.; Petreikov, M. Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiol. 1997, 113, 739–746. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yuki, K.; Park, S.; Ohya, T. Carbohydrate metabolism in the developing endosperm of rice grains. Plant Cell Physiol. 1989, 30, 833–839. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, S.; Gao, R.; Li, Y. Comparison of starch synthesis and related enzyme activities in developing grains among different types of maize. J. Plant Physiol. Mol. Biol. 2007, 33, 25–32. [Google Scholar]
- Jia, Y.; Zou, D.; Wang, J.; Liu, H.; Inayat, M.; Sha, H.; Zheng, H.; Sun, J.; Zhao, H. Effect of low water temperature at reproductive stage on yield and glutamate metabolism of rice (Oryza sativa L.) in China. Field Crops Res. 2015, 175, 16–25. [Google Scholar] [CrossRef]
- Balcke, G.; Handrick, V.; Bergau, N.; Fichtner, M.; Henning, A.; Stellmach, H.; Tissier, A.; Hause, B.; Frolov, A. An UPLC-MS/MS method for highly sensitive high-throughput analysis of phytohormones in plant tissues. Plant Methods 2012, 8, 47. [Google Scholar] [CrossRef]
- Glauser, G.; Grund, B.; Gassner, A.; Menin, L.; Henry, H.; Bromirski, M.; Schutz, F.; McMullen, J.; Rochat, B. Validation of the mass-extraction-window for quantitative methods using liquid chromatography high resolution mass spectrometry. Anal. Chem. 2016, 88, 3264–3271. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Lan, Y.; Sui, X.; Wang, J.; Duan, Q.; Wu, C.; Ding, C.; Li, T. Effects of nitrogen application rate on protein components and yield of low-gluten rice. Agriculture 2021, 11, 302. [Google Scholar] [CrossRef]
- Zhang, C. A Guide to Modern Plant Physiology Experiments; Science Press: Beijing, China, 1999; ISBN 7-03-008099-8. [Google Scholar]
- Idowu, O.; Katsube-Tanaka, T.; Shiraiwa, T. Nitrogen fertilizer application does not always improve available carbohydrate per spikelet but decreases chalkiness under high temperature in rice (Oryza sativa L.) grains. Field Crops Res. 2023, 290. [Google Scholar] [CrossRef]
- Chen, C.; Huang, J.L.; Zhu, L.Y.; Shah, F.; Nie, L.X.; Cui, K.H.; Peng, S.B. Varietal difference in the response of rice chalkiness to temperature during ripening phase across different sowing dates. Field Crops Res. 2013, 151, 85–91. [Google Scholar] [CrossRef]
- Wada, H.; Hatakeyama, Y.; Onda, Y.; Nonami, H.; Nakashima, T.; Erra-Balsells, R.; Morita, S.; Hiraoka, K.; Tanaka, F.; Nakano, H. Multiple strategies for heat adaptation to prevent chalkiness in the rice endosperm. J. Exp. Bot. 2019, 70, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Leethanapanich, K.; Mauromoustakos, A.; Wang, Y. Impact of soaking and drying conditions on rice chalkiness as revealed by scanning electron microscopy. Cereal Chem. 2016, 93, 478–481. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Y.; Chen, L.; Yuan, L.; Wang, Z.; Yang, J. Changes in enzyme activities involved in starch synthesis and hormone concentrations in superior and inferior spikelets and their association with grain filling of super rice. Rice Sci. 2013, 20, 120–128. [Google Scholar] [CrossRef]
- Xie, Q.; Xu, J.; Huang, K.; Su, Y.; Tong, J.; Huang, Z.; Huang, C.; Wei, M.; Lin, W.; Xiao, L. Dynamic formation and transcriptional regulation mediated by phytohormones during chalkiness formation in rice. BMC Plant Biol. 2021, 21, 308. [Google Scholar] [CrossRef]
- Champagne, E.; Bett-Garber, K.; Thomson, J.; Fitzgerald, M. Unraveling the impact of nitrogen nutrition on cooked rice flavor and texture. Cereal Chem. 2009, 86, 274–280. [Google Scholar] [CrossRef]
- Wu, M.; Liu, J.; Bai, X.; Chen, W.; Ren, Y.; Liu, J.; Chen, M.; Zhao, H.; Yao, X.; Zhang, J.; et al. Transcription factors NAC20 and NAC26 interact with RPBF to activate albumin accumulations in rice endosperm. Plant Biotechnol. J. 2023, 21, 890–892. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Li, C.; Lin, S.; Yang, F.; Huang, J.; Liu, Y.; Lur, H. Influence of high temperature during grain filling on the accumulation of storage proteins and grain quality in rice (Oryza sativa L.). J. Agric. Food Chem. 2010, 58, 10545–10552. [Google Scholar] [CrossRef] [PubMed]
| Year | pH Value | P (mg kg−1) | K (mg kg−1) | N (mg kg−1) | Organic Matter (g kg−1) | Total N (g kg−1) |
|---|---|---|---|---|---|---|
| 2019 | 6.55 | 28.49 | 86.32 | 101.31 | 21.30 | 1.53 |
| 2020 | 6.76 | 28.15 | 83.33 | 92.75 | 19.76 | 1.32 |
| 2021 | 6.74 | 26.90 | 82.60 | 95.81 | 19.83 | 1.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Zhang, L.; Jiang, P.; Yang, Z.; Chen, Z.; Xu, F.; Guo, X.; Sun, Y.; Ma, J. Grain Chalkiness Is Decreased by Balancing the Synthesis of Protein and Starch in Hybrid Indica Rice Grains under Nitrogen Fertilization. Foods 2024, 13, 855. https://doi.org/10.3390/foods13060855
Guo C, Zhang L, Jiang P, Yang Z, Chen Z, Xu F, Guo X, Sun Y, Ma J. Grain Chalkiness Is Decreased by Balancing the Synthesis of Protein and Starch in Hybrid Indica Rice Grains under Nitrogen Fertilization. Foods. 2024; 13(6):855. https://doi.org/10.3390/foods13060855
Chicago/Turabian StyleGuo, Changchun, Lin Zhang, Peng Jiang, Zhiyuan Yang, Zongkui Chen, Fuxian Xu, Xiaoyi Guo, Yongjian Sun, and Jun Ma. 2024. "Grain Chalkiness Is Decreased by Balancing the Synthesis of Protein and Starch in Hybrid Indica Rice Grains under Nitrogen Fertilization" Foods 13, no. 6: 855. https://doi.org/10.3390/foods13060855






