Preparation, Biological Activities, and Potential Applications of Hen Egg-Derived Peptides: A Review
Abstract
1. Introduction
2. Bioactive Peptides Derived from Hen Egg Proteins
2.1. Peptide Preparation
2.1.1. Enzymatic Hydrolysis
2.1.2. Microbial Fermentation
2.1.3. Chemical Synthesis
2.2. Purification and Identification of Peptides
3. Biological Activities of Hen Egg-Derived Peptides
3.1. Antioxidant Activity
3.2. Enzyme Inhibitors
3.3. Antibacterial Activity
3.4. Other Activities
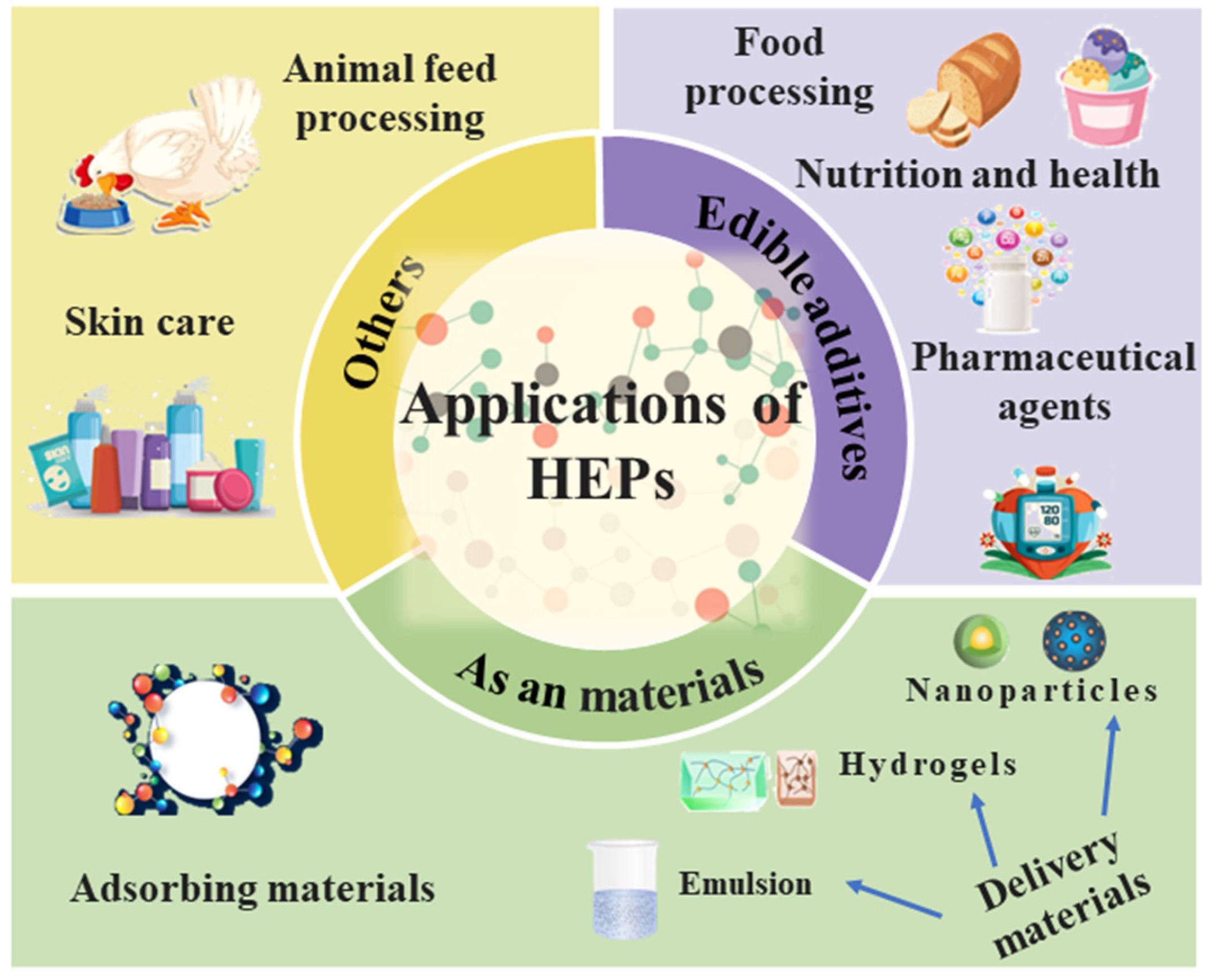
4. Potential Applications of Peptides
4.1. Intervention in Intestinal Health
4.2. Encapsulant Materials for the Delivery of Bioactives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Hen Egg Proteins | HEPs |
| Phosvitin Phosphopeptides | PPPs |
| Egg White Protein | EWP |
| Ovalbumin | OVA |
| Ovotransferrin | OTF |
| Ovomucoid | OM |
| Ovomucin | OV |
| Lysozyme | LM |
| Phosvitin | PV |
| Immunoglobulin Y | IgY |
| Lysozyme Peptides | LMPs |
| Ovotransferrin-derived Peptides | OTFPs |
| Ovalbumin-derived Peptides | OVAPs |
| Pulsed Electric Field | PEF |
| Generally Regarded as Safe | GRAS |
| Molecular Weight Distribution | MWD |
| Ovomucin-derived Peptides | OMPs |
| Egg Yolk Peptides | EYPs |
| EWP Peptide | EWPP |
| Gastrointestinal | GI |
| Eggshell Membrane | ESM |
| Trolox Equivalents | TEs |
| High-temperature and Mild-pressure | HTMP |
| Lactic Acid Bacteria | LAB |
| Synthesis and Solid-Phase Synthesis | SSPS |
| Reverse Phase-High-Performance Liquid Chromatography | RP-HPLC |
| Mass Spectrometry | MS |
| Time Of Flight | TOF |
| Matrix-assisted Laser Deionization Time-of-flight | MALDI-TOF |
| Liquid Chromatography Triple Quadrupole Mass Spectrometry | LC-MS/MS |
| Electrospray Ionization Mass Spectrometry | ESI-MS |
| Triple Quadrupole Mass Spectrometer | TQ-MS |
| Quantitative Structure–Activity Relationship | QSAR |
| Reactive Oxygen Species | ROS |
| Cardiovascular Diseases | CVDs |
| Oxygen Radical Absorbance Capacity-Fluorescein | ORAC-FL |
| Ferric Ion-Reducing Antioxidant Potential | FRAP |
| 2,2’-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid | ABTS |
| 2,2-diphenyl-1-picrylhydrazyl | DPPH |
| Gln-Ser-Leu-Val-Ser-Val-Pro-Gly-Met-Ser | QSLVSVPGMS |
| Angiotensin-I-Converting Enzyme | ACE, EC 3.4.15.1 |
| Dipeptidyl Peptidase IV | DPP-IV, EC 3.4.14.5 |
| Aminopeptidase N | APN, EC 3.4.11.2 |
| Xanthine Oxidase | XO, EC 1.17.3.2,5 |
| Renin–Angiotensin System | RAS |
| Kallikrein–kinin System | KKS |
| Gln-Ile-Gly-Leu-Phe | QIGLF |
| Phe-Glu-Ser-Asn-Thr-Gln-Ala-Thr-Asn-Arg | FESNFNTQATNR |
| Spontaneously Hypertensive Rats | SHRs |
| Nitric Oxide | NO |
| Dipeptidyl-peptidase IV Inhibitor | DPP-IVi |
| Glucagon-like Peptide-1 | GLP-1 |
| Glucose-dependent Insulinotropic Polypeptide | GIP |
| Ala-Asp-Phe | ADF |
| Met-Gly-Arg | MIR |
| Phe-Gly-Arg | FGR |
| Ile-Arg-Asp-Leu-Leu-Glu-Arg | IRDLLER |
| Try-Ala-Glu-Glu-Arg-Tyr-Pro | YAEERYP |
| Ile-Arg-Asn-Val-Leu-Gln-Pro-Ser | IRNVLQPS |
| Arg-Val-Pro-Ser-Leu-Met | RVPSLM |
| Cys-Asn-Arg | CNR |
| Cys-Asp-Arg | CDR |
| Cys-Glu-Phe | GEF |
| Lys-Ser-Trp-Lys-Lys-His-Val-Val-Ser-Gly-Phe-Phe-Leu-Arg | KSWKKHVVSGFFLR |
| Lys-Ser-Trp-Lys-Lys-His-Val-Val-Ser-Gly-Phe-Phe-Leu-Arg-Leu-Trp-Val-His-Lys-Lys | KSWKKHVVSGFFLRLWVHKK |
| Ovomucin Glycopeptide | OGP |
| Asn-Thr-Asp-Gly-Ser-Thr-Asp-Tyr-Gly-Ile-Leu-Gln-Ile-Asn-Ser-Arg | NTDGSTDYGILQINSR |
| Lys-Gly-Gly-Asp-Leu-Gly-Leu-Phe-Glu-Pro-Thr-Leu | KGGDLGLFEPTL |
| Mitogen-activated Protein Kinase | MAPK |
| Janus kinase-signal Transduction and Transcription Activator | JAK-STAT |
| Inducible Nitric Oxide Synthase | iNOS |
| IIe-Arg-Trp | IRW |
| IIe-Gln-Trp | IQW |
| Tumor Necrosis Factor | TNF |
| Vascular Cell Adhesion Molecule-I | VCAM-1 |
| Asp-Gu-Gu-Gu-Asn-Asp-Gln-Val-Lys | DEEENDQVK |
| Thr-ASN-Gly-IIe-Arg | TNGIIR |
| Arg-Val-Pro-Ser-Leu | RVPSL |
| Gln-IIe-Gly-Leu-Phe | QIGLF |
| Leu-Gln-Lys | LQK |
| Leu-Leu | LL |
| Leu-Leu-Phe | LLF |
| Leu-Gln-Lys-Trp | LQKW |
| Angiotensin I | Ang I |
| Angiotensin II | Ang II |
| Angiotensin-Converting Enzyme 2 | ACE2, EC 3.4.17.23 |
| Renin-Angiotensin-Aldosterone System | RAAS |
| Alcalase | EC 3.4.21.62 |
| Thermolysin | EC 3.4.24.27 |
| Neutral proteases | EC 3.4.24.4 |
| Protease N | EC 3.4.24.28 |
| Protease A | EC 3.4.24.39 |
| Trypsin | EC 3.4.21.4 |
| Papain | EC 3.4.22.2 |
| Pepsin | EC 3.4.23.1 |
| α-chymotrypsin | EC 3.4.21.1 |
| Chymotrypsin | 232-671-2 |
| Alkaline phosphatase | E.C.3.1.3.1 |
| Neutrase | 232-752-2 |
| α-glucosidase | EC 3.2.1.1 |
| Tyrosinase | EC 1.14.18.1 |
References
- OECD; Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2023–2032; OECD-FAO Agricultural Outlook; OECD: Paris, France, 2023; ISBN 978-92-64-61933-3. [Google Scholar]
- Lesnierowski, G.; Stangierski, J. What’s New in Chicken Egg Research and Technology for Human Health Promotion?—A Review. Trends Food Sci. Technol. 2018, 71, 46–51. [Google Scholar] [CrossRef]
- Puglisi, M.J.; Fernandez, M.L. The Health Benefits of Egg Protein. Nutrients 2022, 14, 2904. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Diep, T.; Hudson, H.-A.; Hincke, M.T. High Value Applications and Current Commercial Market for Eggshell Membranes and Derived Bioactives. Food Chem. 2022, 382, 132270. [Google Scholar] [CrossRef]
- Sarantidi, E.; Ainatzoglou, A.; Papadimitriou, C.; Stamoula, E.; Maghiorou, K.; Miflidi, A.; Trichopoulou, A.; Mountzouris, K.C.; Anagnostopoulos, A.K. Egg White and Yolk Protein Atlas: New Protein Insights of a Global Landmark Food. Foods 2023, 12, 3470. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Huang, X.; Ma, B.; Chen, Y.; Batool, Z.; Fu, X.; Jin, Y. Modification Methods and Applications of Egg Protein Gel Properties: A Review. Comp Rev Food Sci Food Safe 2022, 21, 2233–2252. [Google Scholar] [CrossRef] [PubMed]
- Marcet, I.; Sáez-Orviz, S.; Rendueles, M.; Díaz, M. Egg Yolk Granules and Phosvitin. Recent Advances in Food Technology and Applications. LWT 2022, 153, 112442. [Google Scholar] [CrossRef]
- Makkar, S.; Liyanage, R.; Kannan, L.; Packialakshmi, B.; Lay, J.O.; Rath, N.C. Chicken Egg Shell Membrane Associated Proteins and Peptides. J. Agric. Food Chem. 2015, 63, 9888–9898. [Google Scholar] [CrossRef] [PubMed]
- Pimchan, T.; Tian, F.; Thumanu, K.; Rodtong, S.; Yongsawatdigul, J. Isolation, Identification, and Mode of Action of Antibacterial Peptides Derived from Egg Yolk Hydrolysate. Poult. Sci. 2023, 102, 102695. [Google Scholar] [CrossRef] [PubMed]
- Benedé, S.; Molina, E. Chicken Egg Proteins and Derived Peptides with Antioxidant Properties. Foods 2020, 9, 735. [Google Scholar] [CrossRef]
- Liu, J.; Jin, Y.; Lin, S.; Jones, G.S.; Chen, F. Purification and Identification of Novel Antioxidant Peptides from Egg White Protein and Their Antioxidant Activities. Food Chem. 2015, 175, 258–266. [Google Scholar] [CrossRef]
- Yeon Cho, H.Y.; Lee, J.-E.; Lee, J.H.; Ahn, D.; Paik, H.-D. The Immune-Enhancing Activity of Egg White Ovalbumin Hydrolysate Prepared with Papain via MAPK Signaling Pathway in RAW 264.7 Macrophages. J. Funct. Foods 2023, 103, 105487. [Google Scholar] [CrossRef]
- Wakabayashi, A.; Kumagai, Y.; Watari, E.; Shimizu, M.; Utsuyama, M.; Hirokawa, K.; Takahashi, H. Importance of Gastrointestinal Ingestion and Macromolecular Antigens in the Vein for Oral Tolerance Induction. Immunology 2006, 119, 167–177. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, L.; Shen, Q.; Wang, R.; Guo, Y.; Zhang, C.; Richel, A. Comparative Analysis of the Bioactive Compounds in Chicken Cartilage: Protective Effects of Chondroitin Sulfate and Type II Collagen Peptides Against Osteoarthritis Involve Gut Microbiota. Front. Nutr. 2022, 9, 843360. [Google Scholar] [CrossRef]
- Bouglé, D.; Bouhallab, S. Dietary Bioactive Peptides: Human Studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 335–343. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food Proteins from Animals and Plants: Differences in the Nutritional and Functional Properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Matsuoka, R.; Takahashi, Y.; Kimura, M.; Masuda, Y.; Kunou, M. Heating Has No Effect on the Net Protein Utilisation from Egg Whites in Rats. ScientificWorldJournal 2017, 2017, 6817196. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Rimón, M.; López-Expósito, I.; López-Fandiño, R.; Miguel, M. Egg White Hydrolysates with in vitro Biological Multiactivities to Control Complications Associated with the Metabolic Syndrome. Eur. Food Res. Technol. 2016, 242, 61–69. [Google Scholar] [CrossRef]
- Greengard, O.; Sentenac, A.; Mendelsohn, N. Phosvitin, the Iron Carrier of Egg Yolk. Biochim. Biophys. Acta 1964, 90, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Mine, Y. Phosvitin Phosphopeptides Increase Iron Uptake in a Caco-2 Cell Monolayer Model. Int. J. Food Sci. Technol. 2006, 41, 455–458. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Jo, C.; Suh, J.W.; Ahn, D.U. Enzymatic Hydrolysis of Ovomucin and the Functional and Structural Characteristics of Peptides in the Hydrolysates. Food Chem. 2016, 192, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.R.; Thomas, U.; Pellegrini, A. A Helix-Loop-Helix Peptide at the Upper Lip of the Active Site Cleft of Lysozyme Confers Potent Antimicrobial Activity with Membrane Permeabilization Action. J. Biol. Chem. 2001, 276, 43767–43774. [Google Scholar] [CrossRef]
- Zheng, J.; Bu, T.; Liu, L.; He, G.; Li, S.; Wu, J. Naturally Occurring Low Molecular Peptides Identified in Egg White Show Antioxidant Activity. Food Res. Int. 2020, 138, 109766. [Google Scholar] [CrossRef]
- Chen, L.; Kang, J.; Sukigara, S. Preparation and Characterization of Polyurethane/Soluble Eggshell Membrane Nanofibers. Biomed. Mater. Eng. 2014, 24, 1979–1989. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, Y.; Ma, M.; Cai, Z.; Li, T. Chemiluminescence Evaluation of Antioxidant Activity and Prevention of DNA Damage Effect of Peptides Isolated from Soluble Eggshell Membrane Protein Hydrolysate. J. Agric. Food Chem. 2010, 58, 12137–12142. [Google Scholar] [CrossRef]
- Lunge, S.; Thakre, D.; Kamble, S.; Labhsetwar, N.; Rayalu, S. Alumina Supported Carbon Composite Material with Exceptionally High Defluoridation Property from Eggshell Waste. J. Hazard. Mater. 2012, 237, 161–169. [Google Scholar] [CrossRef]
- Ballester, A.M.; Esquinas, N.C. Method for Separating the Internal Membrane from the Egg’s Husk. E.S. Patent 2327087B2, March 2010. Available online: https://patents.google.com/patent/ES2327087B2/en (accessed on 29 January 2024).
- Razi, S.M.; Fahim, H.; Amirabadi, S.; Rashidinejad, A. An Overview of the Functional Properties of Egg White Proteins and Their Application in the Food Industry. Food Hydrocoll. 2023, 135, 108183. [Google Scholar] [CrossRef]
- Chang, C.; Lahti, T.; Tanaka, T.; Nickerson, M.T. Egg Proteins: Fractionation, Bioactive Peptides and Allergenicity. J. Sci. Food Agric. 2018, 98, 5547–5558. [Google Scholar] [CrossRef]
- Amro, W.A.; Al-Qaisi, W.; Al-Razem, F. Production and Purification of IgY Antibodies from Chicken Egg Yolk. J. Genet. Eng. Biotechnol. 2018, 16, 99–103. [Google Scholar] [CrossRef]
- Zambrowicz, A.; Zabłocka, A.; Bobak, Ł.; Macała, J.; Janusz, M.; Polanowski, A.; Trziszka, T. A Simple and Rapid Method of Isolation of Active Polypeptide Complex, Yolkin, from Chicken Egg Yolk. Food Chem. 2017, 230, 705–711. [Google Scholar] [CrossRef]
- Si, K.; Gong, T.; Ding, S.; Liu, H.; Shi, S.; Tu, J.; Zhu, L.; Song, L.; Song, L.; Zhang, X. Binding Mechanism and Bioavailability of a Novel Phosvitin Phosphopeptide (Glu-Asp-Asp-pSer-pSer) Calcium Complex. Food Chem. 2023, 404, 134567. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Fish Protein Hydrolysates: Production, Biochemical, and Functional Properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, N.; Zhu, Z.; Wang, Y.; Wang, J.; Xue, C. Changes of Antioxidative Activities and Peptidomic Patterns of Auxenochlorella Pyrenoidosa Protein Hydrolysates: Effects of Enzymatic Hydrolysis and Decoloration Processes. LWT 2021, 152, 112306. [Google Scholar] [CrossRef]
- Lu, Z.; Sun, N.; Dong, L.; Gao, Y.; Lin, S. Production of Bioactive Peptides from Sea Cucumber and Its Potential Health Benefits: A Comprehensive Review. Food Chem. 2022, 70, 7607–7625. [Google Scholar] [CrossRef]
- Ahmed, T.; Sun, X.; Udenigwe, C.C. Role of Structural Properties of Bioactive Peptides in Their Stability during Simulated Gastrointestinal Digestion: A Systematic Review. Trends Food Sci. Technol. 2022, 120, 265–273. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, T.; Jiang, H.; Zhang, M.; Gong, P.; Liu, J.; Liu, X. Effect of pH Treatment on Egg White Protein Digestion and the Peptidomics of Their in vitro Digests. Food Res. Int. 2023, 173, 113327. [Google Scholar] [CrossRef]
- Palika, R.; Mashurabad, P.C.; Nair, M.K.; Reddy, G.B.; Pullakhandam, R. Characterization of Iron-Binding Phosphopeptide Released by Gastrointestinal Digestion of Egg White. Food Res. Int. 2015, 67, 308–314. [Google Scholar] [CrossRef]
- Young, D.; Fan, M.Z.; Mine, Y. Egg Yolk Peptides Up-Regulate Glutathione Synthesis and Antioxidant Enzyme Activities in a Porcine Model of Intestinal Oxidative Stress. J. Agric. Food Chem. 2010, 58, 7624–7633. [Google Scholar] [CrossRef]
- Memarpoor-Yazdi, M.; Asoodeh, A.; Chamani, J. A Novel Antioxidant and Antimicrobial Peptide from Hen Egg White Lysozyme Hydrolysates. J. Funct. Foods 2012, 4, 278–286. [Google Scholar] [CrossRef]
- Nagamalli, H.; Sitaraman, M.; Kandalai, K.K.; Mudhole, G.R. Chicken Egg Shell as a Potential Substrate for Production of Alkaline Protease by Bacillus Altitudinis GVC11 and Its Applications. 3 Biotech 2017, 7, 185. [Google Scholar] [CrossRef]
- Cheng, M.; Takenaka, S.; Aoki, S.; Murakami, S.; Aoki, K. Purification and Characterization of an Eggshell Membrane Decomposing Protease from Pseudomonas Aeruginosa Strain ME-4. J. Biosci. Bioeng. 2009, 107, 373–378. [Google Scholar] [CrossRef]
- Shi, Y.; Kovacs-Nolan, J.; Jiang, B.; Tsao, R.; Mine, Y. Antioxidant Activity of Enzymatic Hydrolysates from Eggshell Membrane Proteins and Its Protective Capacity in Human Intestinal Epithelial Caco-2 Cells. J. Funct. Foods 2014, 10, 35–45. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Jo, C.; Nam, K.C.; Ahn, D.U. Enzymatic Hydrolysis of Ovalbumin and the Functional Properties of the Hydrolysates. Poult. Sci. 2014, 93, 2678–2686. [Google Scholar] [CrossRef]
- Zheng, Z. Antihypertensive Small Molecular Peptide, and Preparation Method and Application Thereof. China National Intellectual Property Administration. Wuhan, C.N. Patent 201910988102.1, October 2019. [Google Scholar]
- Xu, C.; Chen, X. High-Activity Egg White Antioxidative Peptide Preparing Process. Anhui, C.N. Patent 201610248535.X, April 2016. [Google Scholar]
- Huang, Z. Egg Polypeptide Preparation Method and Egg Polypeptide Beverage. Nanning, C.N. Patent 202010387653.5, September 2020. [Google Scholar]
- Lien, Y.-C.; Lai, S.-J.; Lin, C.-Y.; Wong, K.-P.; Chang, M.S.; Wu, S.-H. High-Efficiency Decomposition of Eggshell Membrane by a Keratinase from Meiothermus Taiwanensis. Sci. Rep. 2022, 12, 14684. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, M.; Li, S.; Ahn, D.U.; Chen, N.; Huang, X. Advances in Preparation and Bioactivity of Phosvitin Phosphopeptides. J. Future Foods 2022, 2, 213–222. [Google Scholar] [CrossRef]
- Goulas, A.; Triplett, E.L.; Taborsky, G. Oligophosphopeptides of Varied Structural Complexity Derived from the Egg Phosphoprotein, Phosvitin. J. Protein Chem. 1996, 15, 1–9. [Google Scholar] [CrossRef]
- Ulug, S.K.; Jahandideh, F.; Wu, J. Novel Technologies for the Production of Bioactive Peptides. Trends Food Sci. Technol. 2021, 108, 27–39. [Google Scholar] [CrossRef]
- Jiang, B.; Mine, Y. Preparation of Novel Functional Oligophosphopeptides from Hen Egg Yolk Phosvitin. J. Agric. Food Chem. 2000, 48, 990–994. [Google Scholar] [CrossRef]
- Samaraweera, H.; Moon, S.H.; Lee, E.; Yang, S.H.; Suh, J.-W.; Ahn, D.U. Chemical Hydrolysis of Phosvitin and the Functional Properties of the Hydrolysates. Int. J. Eng. Res. Technol. 2013, 2, 3373–3386. [Google Scholar] [CrossRef]
- Volk, S.P.; Ahn, D.U.; Zeece, M.; Jung, S. Effects of High-Pressure Processing and Enzymatic Dephosphorylation on Phosvitin Properties. J. Sci. Food Agric. 2012, 92, 3095–3098. [Google Scholar] [CrossRef]
- Huang, X.; Moon, S.H.; Lee, J.; Paik, H.; Lee, E.J.; Min, B.; Ahn, D.U. Effective Preparation Method of Phosphopeptides from Phosvitin and the Analysis of Peptide Profiles Using Tandem Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 14086–14101. [Google Scholar] [CrossRef]
- Samaraweera, H.; Moon, S.H.; Lee, E.J.; Grant, J.; Fouks, J.; Choi, I.; Suh, J.W.; Ahn, D.U. Characterisation of Phosvitin Phosphopeptides Using MALDI-TOF Mass Spectrometry. Food Chem. 2014, 165, 98–103. [Google Scholar] [CrossRef]
- Jain, S.; Anal, A.K. Optimization of Extraction of Functional Protein Hydrolysates from Chicken Egg Shell Membrane (ESM) by Ultrasonic Assisted Extraction (UAE) and Enzymatic Hydrolysis. LWT Food Sci. Technol. 2016, 69, 295–302. [Google Scholar] [CrossRef]
- Lei, B.; Majumder, K.; Shen, S.; Wu, J. Effect of Sonication on Thermolysin Hydrolysis of Ovotransferrin. Food Chem. 2011, 124, 808–815. [Google Scholar] [CrossRef]
- Shen, S.; Chahal, B.; Majumder, K.; You, S.-J.; Wu, J. Identification of Novel Antioxidative Peptides Derived from a Thermolytic Hydrolysate of Ovotransferrin by LC-MS/MS. J. Agric. Food Chem. 2010, 58, 7664–7672. [Google Scholar] [CrossRef]
- Li, Y.; Ding, J.; Dong, L.; Sun, N.; Lin, S. Mechanism of Targeted Regulation of Ovalbumin Epitopes by Pulsed Electric Field-Assisted Alcalase Treatment. J. Agric. Food Chem. 2023, 71, 10417–10426. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Oey, I.; Bremer, P.; Silcock, P.; Carne, A. Proteolytic Pattern, Protein Breakdown and Peptide Production of Ovomucin-Depleted Egg White Processed with Heat or Pulsed Electric Fields at Different pH. Food Res. Int. 2018, 108, 465–474. [Google Scholar] [CrossRef]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of Bioactive Peptides by Lactobacillus Species: From Gene to Application. Front. Microbiol. 2018, 9, 2354. [Google Scholar] [CrossRef]
- Nahariah, N.; Hikmah, H.; Yuliati, F. Antioxidant Activity and Chemical Characteristics in Egg Albumen Fermented by Lactid Acid Bacteria. J. Indones. Trop. Anim. Agric. 2020, 45, 214–221. [Google Scholar] [CrossRef]
- Tian, L.; Xiong, D.; Jia, J.; Liu, X.; Zhang, Y.; Duan, X. Mechanism Study on Enhanced Emulsifying Properties of Phosvitin and Calcium-Binding Capacity of Its Phosphopeptides by Lactic Acid Bacteria Fermentation. LWT 2022, 155, 113002. [Google Scholar] [CrossRef]
- Jain, S.; Anal, A.K. Production and Characterization of Functional Properties of Protein Hydrolysates from Egg Shell Membranes by Lactic Acid Bacteria Fermentation. J. Food Sci. Technol. 2017, 54, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Mäde, V.; Els-Heindl, S.; Beck-Sickinger, A.G. Automated Solid-Phase Peptide Synthesis to Obtain Therapeutic Peptides. Beilstein J. Org. Chem. 2014, 10, 1197–1212. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, Q.; Li, M.; Liu, H.; Wang, Q.; Wu, Y.; Niu, L.; Liu, Z. Isolation of a Novel Calcium-Binding Peptide from Phosvitin Hydrolysates and the Study of Its Calcium Chelation Mechanism. Food Res. Int. 2021, 141, 110169. [Google Scholar] [CrossRef]
- Sun, N.; Jin, Z.; Li, D.; Yin, H.; Lin, S. An Exploration of the Calcium-Binding Mode of Egg White Peptide, Asp-His-Thr-Lys-Glu, and in vitro Calcium Absorption Studies of Peptide–Calcium Complex. J. Agric. Food Chem. 2017, 65, 9782–9789. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, S.; Yan, X.; Zhang, M.; Jiang, Y.; Zhou, Y. Egg White-Derived Peptide KPHAEVVLR Promotes Wound Healing in Rat Palatal Mucosa via PI3K/AKT/mTOR Pathway. Peptides 2023, 168, 171074. [Google Scholar] [CrossRef]
- Feng, X.; Liu, C.; Guo, J.; Bi, C.; Cheng, B.; Li, Z.; Shan, A.; Li, Z. Expression and Purification of an Antimicrobial Peptide, Bovine Lactoferricin Derivative LfcinB-W10 in Escherichia Coli. Curr. Microbiol. 2010, 60, 179–184. [Google Scholar] [CrossRef]
- Cui, L.; Huang, H.; Zhang, H.; Wang, X.; Qin, X.; Tu, T.; Zhang, J.; Su, X.; Yu, H.; Bai, Y.; et al. Recombinant Expression of Hen Egg White Lysozyme with the Assistance of Xylanase Fusion Partner in Pichia Pastoris. Bioengineered 2022, 13, 13860–13871. [Google Scholar] [CrossRef]
- Santana, A.; Melo, A.; Tavares, T.; Ferreira, I.M.P.L.V.O. Biological Activities of Peptide Concentrates Obtained from Hydrolysed Eggshell Membrane Byproduct by Optimisation with Response Surface Methodology. Food Funct. 2016, 7, 4597–4604. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Hano, S.; Cheng, M.; Yoshida, K.; Aoki, K. Organic Solvent-Tolerant Elastase Efficiently Hydrolyzes Insoluble, Cross-Linked, Protein Fiber of Eggshell Membranes. Biotechnol. Lett. 2012, 34, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Nimalaratne, C.; Bandara, N.; Wu, J. Purification and Characterization of Antioxidant Peptides from Enzymatically Hydrolyzed Chicken Egg White. Food Chem. 2015, 188, 467–472. [Google Scholar] [CrossRef]
- Liu, L.; Hao, W.; Dai, X.; Zhu, Y.; Chen, K.; Yang, X. Enzymolysis Kinetics and Structural-Functional Properties of High-Intensity Ultrasound-Assisted Alkali Pretreatment Ovalbumin. Int. J. Food Prop. 2020, 23, 80–94. [Google Scholar] [CrossRef]
- Moon, S.H.; Lee, J.H.; Kim, J.H.; Paik, H.-D.; Ahn, D.U. In Vitro Cytotoxic and ACE-Inhibitory Activities of Promod 278P Hydrolysate of Ovotransferrin from Chicken Egg White. Poult. Sci. 2017, 96, 1982–1987. [Google Scholar] [CrossRef]
- Lee, J.H.; Moon, S.H.; Kim, H.S.; Park, E.; Ahn, D.U.; Paik, H.-D. Antioxidant and Anticancer Effects of Functional Peptides from Ovotransferrin Hydrolysates. J. Sci. Food Agric. 2017, 97, 4857–4864. [Google Scholar] [CrossRef]
- Cho, H.Y.; Lee, J.-E.; Lee, J.H.; Ahn, D.U.; Kim, K.-T.; Paik, H.-D. Anti-Biofilm Effect of Egg White Ovotransferrin and Its Hydrolysates against Listeria Monocytogenes. LWT 2022, 165, 113759. [Google Scholar] [CrossRef]
- Rathnapala, E.C.N.; Ahn, D.U.; Abeyrathne, E.D.N.S. Enzymatic Hydrolysis of Ovotransferrin and the Functional Properties of Its Hydrolysates. Food Sci. Anim. Resour. 2021, 41, 608–622. [Google Scholar] [CrossRef]
- You, S.-J.; Udenigwe, C.C.; Aluko, R.E.; Wu, J. Multifunctional Peptides from Egg White Lysozyme. Food Res. Int. 2010, 43, 848–855. [Google Scholar] [CrossRef]
- Zhao, M.; Ahn, D.U.; Li, S.; Liu, W.; Yi, S.; Huang, X. Effects of Phosvitin Phosphopeptide-Ca Complex Prepared by Efficient Enzymatic Hydrolysis on Calcium Absorption and Bone Deposition of Mice. Food Sci. Human. Wellness 2022, 11, 1631–1640. [Google Scholar] [CrossRef]
- Lee, J.-E.; An, B.J.; Jo, C.; Min, B.; Paik, H.-D.; Ahn, D.U. The Elastase and Melanogenesis Inhibitory and Anti-Inflammatory Activities of Phosvitin Phosphopeptides Produced Using High-Temperature and Mild-Pressure (HTMP) Pretreatment and Enzyme Hydrolysis Combinations. Poult. Sci. 2023, 102, 102680. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yin, Y.; Zhao, W.; Yu, Y.; Liu, B.; Liu, J.; Chen, F. Novel Peptides Derived from Egg White Protein Inhibiting Alpha-Glucosidase. Food Chem. 2011, 129, 1376–1382. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, W.; Liu, J.; Lu, J.; Chen, F. QIGLF, a Novel Angiotensin I-Converting Enzyme-Inhibitory Peptide from Egg White Protein. J. Sci. Food Agric. 2011, 91, 921–926. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, D.; Yu, Z.; Ding, L.; Liu, J. Novel Membrane Peptidase Inhibitory Peptides with Activity against Angiotensin Converting Enzyme and Dipeptidyl Peptidase IV Identified from Hen Eggs. J. Funct. Foods 2020, 64, 103649. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Z.; Zhao, W.; Lin, S.; Wang, E.; Zhang, Y.; Hao, H.; Wang, Z.; Chen, F. Isolation and Identification of Angiotensin-Converting Enzyme Inhibitory Peptides from Egg White Protein Hydrolysates. Food Chem. 2010, 122, 1159–1163. [Google Scholar] [CrossRef]
- Yu, Z.; Cao, Y.; Kan, R.; Ji, H.; Zhao, W.; Wu, S.; Liu, J.; Shiuan, D. Identification of Egg Protein-Derived Peptides as Xanthine Oxidase Inhibitors: Virtual Hydrolysis, Molecular Docking, and in Vitro Activity Evaluation. Food Sci. Human. Wellness 2022, 11, 1591–1597. [Google Scholar] [CrossRef]
- Sun, S.; Niu, H.; Yang, T.; Lin, Q.; Luo, F.; Ma, M. Antioxidant and Anti-Fatigue Activities of Egg White Peptides Prepared by Pepsin Digestion. J. Sci. Food Agric. 2014, 94, 3195–3200. [Google Scholar] [CrossRef] [PubMed]
- Jahandideh, F.; Liu, P.; Wu, J. Purification and Identification of Adipogenic-Differentiating Peptides from Egg White Hydrolysate. Food Chem. 2018, 259, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Eckert, E.; Zambrowicz, A.; Bobak, Ł.; Zabłocka, A.; Chrzanowska, J.; Trziszka, T. Production and Identification of Biologically Active Peptides Derived from By-Product of Hen Egg-Yolk Phospholipid Extraction. Int. J. Pept. Res. Ther. 2019, 25, 669–680. [Google Scholar] [CrossRef]
- Smith, J.B. Peptide Sequencing by Edman Degradation. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001; ISBN 978-0-470-01590-2. [Google Scholar]
- Ketha, H.; Kaur, S.; Grebe, S.K.; Singh, R.J. Clinical Applications of LC-MS Sex Steroid Assays: Evolution of Methodologies in the 21st Century. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 217. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.N.; French, D.; Jannetto, P.J.; Rappold, B.A.; Clarke, W.A. Liquid Chromatography–Tandem Mass Spectrometry for Clinical Diagnostics. Nat. Rev. Methods Primers 2022, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.C.A.; Zhou, Y.; Yao, Z.-P. Algorithms for De-Novo Sequencing of Peptides by Tandem Mass Spectrometry: A Review. Anal. Chim. Acta 2023, 1268, 341330. [Google Scholar] [CrossRef] [PubMed]
- Zambrowicz, A.; Pokora, M.; Setner, B.; Dąbrowska, A.; Szołtysik, M.; Babij, K.; Szewczuk, Z.; Trziszka, T.; Lubec, G.; Chrzanowska, J. Multifunctional Peptides Derived from an Egg Yolk Protein Hydrolysate: Isolation and Characterization. Amino Acids 2015, 47, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, Q.; Offengenden, M.; Wu, J. Preparation and Characterization of Phosphopeptides from Egg Yolk Phosvitin. J. Funct. Foods 2015, 18, 190–197. [Google Scholar] [CrossRef]
- Tu, M.; Cheng, S.; Lu, W.; Du, M. Advancement and Prospects of Bioinformatics Analysis for Studying Bioactive Peptides from Food-Derived Protein: Sequence, Structure, and Functions. TrAC Trends Anal. Chem. 2018, 105, 7–17. [Google Scholar] [CrossRef]
- Du, Z.; Comer, J.; Li, Y. Bioinformatics Approaches to Discovering Food-Derived Bioactive Peptides: Reviews and Perspectives. TrAC Trends Anal. Chem. 2023, 162, 117051. [Google Scholar] [CrossRef]
- Marcet, I.; Delgado, J.; Díaz, N.; Rendueles, M.; Díaz, M. Peptides Recovery from Egg Yolk Lipovitellins by Ultrafiltration and Their in Silico Bioactivity Analysis. Food Chem. 2022, 379, 132145. [Google Scholar] [CrossRef] [PubMed]
- Mohd Salim, M.A.S.; Gan, C.-Y. Dual-Function Peptides Derived from Egg White Ovalbumin: Bioinformatics Identification with Validation Using in Vitro Assay. J. Funct. Foods 2020, 64, 103618. [Google Scholar] [CrossRef]
- Majumder, K.; Wu, J. A New Approach for Identification of Novel Antihypertensive Peptides from Egg Proteins by QSAR and Bioinformatics. Food Res. Int. 2010, 43, 1371–1378. [Google Scholar] [CrossRef]
- Wu, J.; Xia, S.; Kalionis, B.; Wan, W.; Sun, T. The Role of Oxidative Stress and Inflammation in Cardiovascular Aging. BioMed Res. Int. 2014, 2014, e615312. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, K.; Jia, X.; Fu, C.; Yu, H.; Wang, Y. Antioxidant Peptides, the Guardian of Life from Oxidative Stress. Med. Res. Rev. 2024, 44, 275–364. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rodrigues, C.F.; Sharopov, F.; Docea, A.O.; Can Karaca, A.; Sharifi-Rad, M.; Kahveci Karıncaoglu, D.; Gülseren, G.; Şenol, E.; Demircan, E.; et al. Diet, Lifestyle and Cardiovascular Diseases: Linking Pathophysiology to Cardioprotective Effects of Natural Bioactive Compounds. Int. J. Environ. Res. Public. Health 2020, 17, 2326. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Majumder, K.; Wu, J. Oxygen Radical Absorbance Capacity of Peptides from Egg White Protein Ovotransferrin and Their Interaction with Phytochemicals. Food Chem. 2010, 123, 635–641. [Google Scholar] [CrossRef]
- Carrillo, W.; Gómez-Ruiz, J.A.; Miralles, B.; Ramos, M.; Barrio, D.; Recio, I. Identification of Antioxidant Peptides of Hen Egg-White Lysozyme and Evaluation of Inhibition of Lipid Peroxidation and Cytotoxicity in the Zebrafish Model. Eur. Food Res. Technol. 2016, 242, 1777–1785. [Google Scholar] [CrossRef]
- Zambrowicz, A.; Eckert, E.; Pokora, M.; Bobak, Ł.; Dąbrowska, A.; Szołtysik, M.; Trziszka, T.; Chrzanowska, J. Antioxidant and Antidiabetic Activities of Peptides Isolated from a Hydrolysate of an Egg-Yolk Protein by-Product Prepared with a Proteinase from Asian Pumpkin (Cucurbita Ficifolia). RSC Adv. 2015, 5, 10460–10467. [Google Scholar] [CrossRef]
- Dávalos, A.; Miguel, M.; Bartolomé, B.; López-Fandiño, R. Antioxidant Activity of Peptides Derived from Egg White Proteins by Enzymatic Hydrolysis. J. Food Prot. 2004, 67, 1939–1944. [Google Scholar] [CrossRef]
- Yi, J.; Zhao, J.; Wu, J. Egg Ovotransferrin Derived IRW Exerts Protective Effect against H2O2-Induced Oxidative Stress in Caco-2 Cells. J. Funct. Foods 2017, 39, 160–167. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.-J.; Zhao, M.-Y.; Xu, W. Influence of Degree of Hydrolysis on Functional Properties, Antioxidant and ACE Inhibitory Activities of Egg White Protein Hydrolysate. Food Sci Biotechnol 2012, 21, 27–34. [Google Scholar] [CrossRef]
- Yousr, M.; Howell, N. Antioxidant and ACE Inhibitory Bioactive Peptides Purified from Egg Yolk Proteins. Int. J. Mol. Sci. 2015, 16, 29161–29178. [Google Scholar] [CrossRef] [PubMed]
- Young, D.; Nau, F.; Pasco, M.; Mine, Y. Identification of Hen Egg Yolk-Derived Phosvitin Phosphopeptides and Their Effects on Gene Expression Profiling against Oxidative Stress-Induced Caco-2 Cells. J. Agric. Food Chem. 2011, 59, 9207–9218. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Kovacs-Nolan, J.; Jiang, B.; Tsao, R.; Mine, Y. Peptides Derived from Eggshell Membrane Improve Antioxidant Enzyme Activity and Glutathione Synthesis against Oxidative Damage in Caco-2 Cells. J. Funct. Foods 2014, 11, 571–580. [Google Scholar] [CrossRef]
- Zhu, L.; Xiong, H.; Huang, X.; Guyonnet, V.; Ma, M.; Chen, X.; Zheng, Y.; Wang, L.; Hu, G. Identification and Molecular Mechanisms of Novel Antioxidant Peptides from Two Sources of Eggshell Membrane Hydrolysates Showing Cytoprotection against Oxidative Stress: A Combined in Silico and in Vitro Study. Food Res. Int. 2022, 157, 111266. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.-Q.; Ju, T.; Sun, J.; Su, Y.-J.; Xu, R.-R.; Yang, Y.-J. Purification and Characterization of Angiotensin I-Converting Enzyme Inhibitory Peptides from Enzymatic Hydrolysate of Hen Egg White Lysozyme. Food Res. Int. 2012, 46, 127–134. [Google Scholar] [CrossRef]
- Wang, L.; Ding, L.; Du, Z.; Yu, Z.; Liu, J. Hydrolysis and Transport of Egg White-Derived Peptides in Caco-2 Cell Monolayers and Everted Rat Sacs. J. Agric. Food Chem. 2019, 67, 4839–4848. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, D.; Yu, Z.; Ding, L.; Liu, J. Aminopeptidase N Inhibitory Peptides Derived from Hen Eggs: Virtual Screening, Inhibitory Activity, and Action Mechanisms. Food Biosci. 2020, 37, 100703. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.; Liu, C.; Liu, B.; Yu, Y.; Zhang, T. Bifunctional Peptides with Antioxidant and Angiotensin-Converting Enzyme Inhibitory Activity in Vitro from Egg White Hydrolysates. J. Food Biochem. 2020, 44, e13347. [Google Scholar] [CrossRef] [PubMed]
- Abdelhedi, O.; Nasri, M. Basic and Recent Advances in Marine Antihypertensive Peptides: Production, Structure-Activity Relationship and Bioavailability. Trends Food Sci. Technol. 2019, 88, 543–557. [Google Scholar] [CrossRef]
- Miralles, B.; Amigo, L.; Recio, I. Critical Review and Perspectives on Food-Derived Antihypertensive Peptides. J. Agric. Food Chem. 2018, 66, 9384–9390. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Sasaki, R.; Yoshikawa, M. Potentiation of the Antihypertensive Activity of Orally Administered Ovokinin, a Vasorelaxing Peptide Derived from Ovalbumin, by Emulsification in Egg Phosphatidylcholine. Biosci. Biotechnol. Biochem. 1995, 59, 2344–2345. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure−Activity Relationship Study of Di- and Tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Guo, H.; Shiuan, D.; Xia, C.; Zhao, W.; Ding, L.; Zheng, F.; Liu, J. Interaction Mechanism of Egg White- Derived ACE Inhibitory Peptide TNGIIR with ACE and Its Effect on the Expression of ACE and AT1 Receptor. Food Sci. Human. Wellness 2020, 9, 52–57. [Google Scholar] [CrossRef]
- Abreu, E.D.L.; Rodrigues Moro, C.; Hassan Husein Kanaan, S.; de Paula, R.B.; Herrera, C.T.; Costa, P.H.D.; Peçanha, F.M.; Vassallo, D.V.; Rossoni, L.V.; Miguel-Castro, M.; et al. ROS Suppression by Egg White Hydrolysate in DOCA-Salt Rats-An Alternative Tool against Vascular Dysfunction in Severe Hypertension. Antioxidants 2022, 11, 1713. [Google Scholar] [CrossRef]
- Thoma, R.; Löffler, B.; Stihle, M.; Huber, W.; Ruf, A.; Hennig, M. Structural Basis of Proline-Specific Exopeptidase Activity as Observed in Human Dipeptidyl Peptidase-IV. Structure 2003, 11, 947–959. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Enhancing Bioactive Peptide Release and Identification Using Targeted Enzymatic Hydrolysis of Milk Proteins. Anal. Bioanal. Chem. 2018, 410, 3407–3423. [Google Scholar] [CrossRef]
- Ross, S.A.; Gulve, E.A.; Wang, M. Chemistry and Biochemistry of Type 2 Diabetes. Chem. Rev. 2004, 104, 1255–1282. [Google Scholar] [CrossRef]
- Yap, P.-G.; Gan, C.-Y. Chicken Egg White-Advancing from Food to Skin Health Therapy: Optimization of Hydrolysis Condition and Identification of Tyrosinase Inhibitor Peptides. Foods 2020, 9, 1312. [Google Scholar] [CrossRef]
- López-García, G.; Dublan-García, O.; Arizmendi-Cotero, D.; Gómez Oliván, L.M. Antioxidant and Antimicrobial Peptides Derived from Food Proteins. Molecules 2022, 27, 1343. [Google Scholar] [CrossRef]
- Mine, Y.; Ma, F.; Lauriau, S. Antimicrobial Peptides Released by Enzymatic Hydrolysis of Hen Egg White Lysozyme. J. Agric. Food Chem. 2004, 52, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Lin, Y.; Mohammadi, T.N.; Masuda, Y.; Honjoh, K.-I.; Miyamoto, T. Characterization of Novel Antimicrobial Peptides Designed on the Basis of Amino Acid Sequence of Peptides from Egg White Hydrolysate. Int. J. Food Microbiol. 2022, 378, 109802. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Hattori, M.; Hara-Kudo, Y.; Okubo, T.; Yamamoto, S.; Takita, T.; Sugita-Konishi, Y. Glycopeptide Derived from Hen Egg Ovomucin Has the Ability To Bind Enterohemorrhagic Escherichia Coli O157:H7. J. Agric. Food Chem. 2004, 52, 5740–5746. [Google Scholar] [CrossRef] [PubMed]
- Rathnapala, E.C.N.; Ahn, D.U.; Abeyrathne, S. Functional Properties of Ovotransferrin from Chicken Egg White and Its Derived Peptides: A Review. Food Sci. Biotechnol. 2021, 30, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Guo, Y.; Fu, X.; Jin, Y. Identification and Antimicrobial Mechanisms of a Novel Peptide Derived from Egg White Ovotransferrin Hydrolysates. LWT 2020, 131, 109720. [Google Scholar] [CrossRef]
- Choi, I.; Jung, C.; Seog, H.; Choi, H. Purification of Phosvitin from Egg Yolk and Determination of Its Physiochemical Properties. Food Sci. Biotechnol. 2004, 13, 434–437. [Google Scholar]
- Christophi, G.P.; Rong, R.; Holtzapple, P.G.; Massa, P.T.; Landas, S.K. Immune Markers and Differential Signaling Networks in Ulcerative Colitis and Crohn’s Disease. Inflamm. Bowel Dis. 2012, 18, 2342–2356. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, J.H.; Moon, S.H.; Ahn, D.U.; Paik, H.-D. Ovalbumin Hydrolysates Inhibit Nitric Oxide Production in LPS-Induced RAW 264.7 Macrophages. Food Sci. Anim. Resour. 2020, 40, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chakrabarti, S.; Fang, J.; Yin, Y.; Wu, J. Low-Molecular-Weight Fractions of Alcalase Hydrolyzed Egg Ovomucin Extract Exert Anti-Inflammatory Activity in Human Dermal Fibroblasts through the Inhibition of Tumor Necrosis Factor-Mediated Nuclear Factor κB Pathway. Nutr. Res. 2016, 36, 648–657. [Google Scholar] [CrossRef]
- Majumder, K.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Structure and Activity Study of Egg Protein Ovotransferrin Derived Peptides (IRW and IQW) on Endothelial Inflammatory Response and Oxidative Stress. J. Agric. Food Chem. 2013, 61, 2120–2129. [Google Scholar] [CrossRef]
- Meram, C.; Wu, J. Anti-Inflammatory Effects of Egg Yolk Livetins (α, β, and γ-Livetin) Fraction and Its Enzymatic Hydrolysates in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Food Res. Int. 2017, 100, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yang, C.; Yin, Y.; Liu, J.; Mine, Y. Phosphopeptides (PPPs) from Hen Egg Yolk Phosvitin Exert Anti-Inflammatory Activity via Modulation of Cytokine Expression. J. Funct. Foods 2012, 4, 718–726. [Google Scholar] [CrossRef]
- Lee, J.-E.; Lee, J.H.; Min, B.; Kim, K.-T.; Ahn, D.U.; Paik, H.-D. Immunostimulatory Effect of Egg Yolk Phosvitin Phosphopeptides Produced by High-Temperature and Mild-Pressure Pretreatment and Enzyme Combinations in RAW 264.7 Cells via TLR2/MAPK Signaling Pathway. J. Funct. Foods 2022, 98, 105264. [Google Scholar] [CrossRef]
- Rzhepakovsky, I.; Anusha Siddiqui, S.; Avanesyan, S.; Benlidayi, M.; Dhingra, K.; Dolgalev, A.; Enukashvily, N.; Fritsch, T.; Heinz, V.; Kochergin, S.; et al. Anti-Arthritic Effect of Chicken Embryo Tissue Hydrolyzate against Adjuvant Arthritis in Rats (X-Ray Microtomographic and Histopathological Analysis). Food Sci. Nutr. 2021, 9, 5648–5669. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Tu, Y.; Du, H.; Zhou, Y.; Zhu, G. Immunomodulatory Effect of Protease Hydrolysates from Ovotransferrin. Food Funct. 2017, 8, 1452–1459. [Google Scholar] [CrossRef]
- Kazana, W.; Jakubczyk, D.; Pacyga-Prus, K.; Leszczyńska, K.; Górska, S.; Siednienko, J.; Macała, J.; Piechowiak, G.; Zabłocka, A. A Novel Mechanism of Macrophage Activation by the Natural Yolkin Polypeptide Complex from Egg Yolk. Int. J. Mol. Sci. 2022, 23, 3125. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.-E.; Paik, H.-D. Immunomodulatory Activity of Egg Yolk Protein Hydrolysates Prepared by Novel Two-Step Hydrolysis: A Study of Mechanism and Stability after in Vitro Digestion Model. Poult. Sci. 2022, 101, 101802. [Google Scholar] [CrossRef]
- Polanowski, A.; Zabłocka, A.; Sosnowska, A.; Janusz, M.; Trziszka, T. Immunomodulatory Activity Accompanying Chicken Egg Yolk Immunoglobulin Y. Poult. Sci. 2012, 91, 3091–3096. [Google Scholar] [CrossRef] [PubMed]
- Polanowski, A.; Sosnowska, A.; Zabłocka, A.; Janusz, M.; Trziszka, T. Immunologically Active Peptides That Accompany Hen Egg Yolk Immunoglobulin Y: Separation and Identification. Biol. Chem. 2013, 394, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.J.; Kim, J.Y.; Kim, K.H.; Lee, H.J. Anticancer Activity of Peptide Fractions from Egg White Hydrolysate against Mouse Lymphoma Cells. Food Sci. Biotechnol. 2003, 12, 224–227. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Kelimu, A.; Korma, S.A.; Cacciotti, I.; Xiang, H.; Cui, C. Novel Iron-Chelating Peptide from Egg Yolk: Preparation, Characterization, and Iron Transportation. Food Chem. X 2023, 18, 100692. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, S.; Ahn, D.U.; Huang, X. Phosvitin Phosphopeptides Produced by Pressurized Hea-Trypsin Hydrolysis Promote the Differentiation and Mineralization of MC3T3-E1 Cells via the OPG/RANKL Signaling Pathways. Poult. Sci. 2020, 100, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Liang, Y.; Bai, J.; Wu, W.; Lin, Q.; Wu, J. Spent Hen-Derived ACE Inhibitory Peptide IWHHT Shows Antioxidative and Anti-Inflammatory Activities in Endothelial Cells. J. Funct. Foods 2019, 53, 85–92. [Google Scholar] [CrossRef]
- Jahandideh, F.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Antioxidant Peptides Identified from Ovotransferrin by the ORAC Method Did Not Show Anti-Inflammatory and Antioxidant Activities in Endothelial Cells. J. Agric. Food Chem. 2016, 64, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Y.; Yao, Y.; Xu, M.; Du, H.; Zhang, M.; Tu, Y. Anti-Inflammatory Activity of Di-Peptides Derived from Ovotransferrin by Simulated Peptide-Cut in TNF-α-Induced Caco-2 Cells. J. Funct. Foods 2017, 37, 424–432. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, F.; Su, Y.; Chang, C.; Li, J.; Luping, G.; Yang, Y. Immunomodulatory Effects of Egg White Peptides on Immunosuppressed Mice and Sequence Identification of Immunomodulatory Peptides. Food Biosci. 2022, 49, 101873. [Google Scholar] [CrossRef]
- López-Martínez, M.I.; Moreno-Fernández, S.; Miguel, M. Development of Functional Ice Cream with Egg White Hydrolysates. Int. J. Gastron. Food Sci. 2021, 25, 100334. [Google Scholar] [CrossRef]
- Huang, P.-H.; Hazeena, S.H.; Qiu, Y.-T.; Ciou, J.-Y.; Hsieh, C.-W.; Shih, M.-K.; Chen, M.-H.; Hou, C.-Y. Application of Egg White Hydrolysate (EWH) to Improve Frothing Functionality of Pasteurized Liquid Egg in Large Quantity Production. Heliyon 2023, 9, e12697. [Google Scholar] [CrossRef]
- Kalman, D.S.; Hewlings, S. The Effect of Oral Hydrolyzed Eggshell Membrane on the Appearance of Hair, Skin, and Nails in Healthy Middle-Aged Adults: A Randomized Double-Blind Placebo-Controlled Clinical Trial. J. Cosmet. Dermatol. 2020, 19, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.; Kalman, D.; Schneider, L.V. A Randomized, Double-Blind, Placebo-Controlled, Prospective Clinical Trial Evaluating Water-Soluble Chicken Eggshell Membrane for Improvement in Joint Health in Adults with Knee Osteoarthritis. J. Med. Food 2019, 22, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, M.; Arakawa, T.; Tokunaga, Y.; Sugimoto, Y.; Ishibashi, M. Insoluble Expression of Highly Soluble Halophilic Metal Binding Protein for Metal Ion Biosorption: Application of Aggregation-Prone Peptide from Hen Egg White Lysozyme. Protein Expr. Purif. 2019, 156, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Miner-Williams, W.M.; Stevens, B.R.; Moughan, P.J. Are Intact Peptides Absorbed from the Healthy Gut in the Adult Human? Nutr. Res. Rev. 2014, 27, 308–329. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, L.; Yu, Z.; Zhang, T.; Liu, J. Digestion and Absorption of an Egg White ACE-Inhibitory Peptide in Human Intestinal Caco-2 Cell Monolayers. Int. J. Food Sci. Nutr. 2016, 67, 111–116. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Miguel, M.; Amigo, L.; Aleixandre, M.A.; Recio, I. Effect of Simulated Gastrointestinal Digestion on the Antihypertensive Properties of Synthetic Beta-Lactoglobulin Peptide Sequences. J. Dairy. Res. 2007, 74, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Khueychai, S.; Jangpromma, N.; Choowongkomon, K.; Joompang, A.; Daduang, S.; Vesaratchavest, M.; Payoungkiattikun, W.; Tachibana, S.; Klaynongsruang, S. A Novel ACE Inhibitory Peptide Derived from Alkaline Hydrolysis of Ostrich (Struthio Camelus) Egg White Ovalbumin. Process Biochem. 2018, 73, 235–245. [Google Scholar] [CrossRef]
- Miguel, M.; Aleixandre, M.A.; Ramos, M.; López-Fandiño, R. Effect of Simulated Gastrointestinal Digestion on the Antihypertensive Properties of ACE-Inhibitory Peptides Derived from Ovalbumin. J. Agric. Food Chem. 2006, 54, 726–731. [Google Scholar] [CrossRef]
- Fisher, N.D.L.; Meagher, E.A. Renin Inhibitors. J. Clin. Hypertens. 2011, 13, 662–666. [Google Scholar] [CrossRef]
- Moguel-Concha, D.D.R.; Borges-Martínez, J.E.; Cid-Gallegos, M.S.; Juárez-Chairez, M.F.; Gómez-Gómez, A.L.; Téllez-Medina, D.I.; Jiménez-Martínez, C. Antioxidant and Renin Inhibitory Activities of Peptides from Food Proteins on Hypertension: A Review. Plant Foods Hum. Nutr. 2023, 78, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yin, Y.; Zhao, W.; Chen, F.; Liu, J. Antihypertensive Effect of Angiotensin-Converting Enzyme Inhibitory Peptide RVPSL on Spontaneously Hypertensive Rats by Regulating Gene Expression of the Renin-Angiotensin System. J. Agric. Food Chem. 2014, 62, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Lezutekong, J.N.; Chen, X.; Oudit, G.Y. Recombinant Human ACE2 and the Angiotensin 1-7 Axis as Potential New Therapies for Heart Failure. Can. J. Cardiol. 2017, 33, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A Novel Angiotensin-Converting Enzyme–Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin 1-9. Circ. Res. 2000, 87, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Bhullar, K.S.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Egg White-Derived Tripeptide IRW (Ile-Arg-Trp) Is an Activator of Angiotensin Converting Enzyme 2. J. Agric. Food Chem. 2018, 66, 11330–11336. [Google Scholar] [CrossRef]
- Cencic, A.; Chingwaru, W. The Role of Functional Foods, Nutraceuticals, and Food Supplements in Intestinal Health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of Functional Food Components on Gut Health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Saibandith, B.; Yupanqui, C.T.; Wichienchot, S. Human Colonic Microbiota Modulation and Branched Chain Fatty Acids Production Affected by Soy Protein Hydrolysate. Int. J. Food Sci. Technol. 2019, 54, 141–148. [Google Scholar] [CrossRef]
- Malinowski, J.; Klempt, M.; Clawin-Rädecker, I.; Lorenzen, P.C.; Meisel, H. Identification of a NFκB Inhibitory Peptide from Tryptic β-Casein Hydrolysate. Food Chem. 2014, 165, 129–133. [Google Scholar] [CrossRef]
- Sun, X.; Gänzle, M.; Field, C.J.; Wu, J. Effect of Proteolysis on the Sialic Acid Content and Bifidogenic Activity of Ovomucin Hydrolysates. Food Chem. 2016, 212, 78–86. [Google Scholar] [CrossRef]
- Lyu, S.; Pan, F.; Ge, H.; Yang, Q.; Duan, X.; Feng, M.; Liu, X.; Zhang, T.; Liu, J. Fermented Egg-Milk Beverage Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice through the Modulation of Intestinal Flora and Short-Chain Fatty Acids. Food Funct. 2022, 13, 702–715. [Google Scholar] [CrossRef]
- Requena, T.; Miguel, M.; Garcés-Rimón, M.; Martínez-Cuesta, M.C.; López-Fandiño, R.; Peláez, C. Pepsin Egg White Hydrolysate Modulates Gut Microbiota in Zucker Obese Rats. Food Funct. 2017, 8, 437–443. [Google Scholar] [CrossRef]
- Raymond, D.M.; Nilsson, B.L. Multicomponent Peptide Assemblies. Chem. Soc. Rev. 2018, 47, 3659–3720. [Google Scholar] [CrossRef]
- Delfi, M.; Sartorius, R.; Ashrafizadeh, M.; Sharifi, E.; Zhang, Y.; De Berardinis, P.; Zarrabi, A.; Varma, R.S.; Tay, F.R.; Smith, B.R.; et al. Self-Assembled Peptide and Protein Nanostructures for Anti-Cancer Therapy: Targeted Delivery, Stimuli-Responsive Devices and Immunotherapy. Nano Today 2021, 38, 101119. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Rostamabadi, H.; Samborska, K.; Mirarab, S.; Rashidinejhad, A.; Jafari, S.M. Protein-Polysaccharide Interactions for the Fabrication of Bioactive-Loaded Nanocarriers: Chemical Conjugates and Physical Complexes. Pharmacol. Res. 2022, 178, 106164. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhang, H.; Shi, X.; Li, S.; Yang, M.; Zhang, T.; Xiao, H.; Du, Z. A Comprehensive Review of Self-Assembled Food Protein-Derived Multicomponent Peptides: From Forming Mechanism and Structural Diversity to Applications. J. Agric. Food Chem. 2023, 71, 11304–11319. [Google Scholar] [CrossRef]
- Liu, W.-J.; Li, X.-L.; Xu, B.-C.; Zhang, B. Self-Assembled Micellar Nanoparticles by Enzymatic Hydrolysis of High-Density Lipoprotein for the Formation and Stability of High Internal Phase Emulsions. J. Agric. Food Chem. 2021, 69, 11015–11025. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Ma, S.; Yang, M.; Zhang, H.; Zhang, T.; Yu, Y.; Du, Z. Co-Assembly of Egg White-Derived Peptides and Protein-Polysaccharide Complexes for Curcumin Encapsulation: The Enhancement of Stability, Redispersibility, and Bioactivity. Food Chem. 2022, 394, 133496. [Google Scholar] [CrossRef]
- Du, Z.; Li, Q.; Li, J.; Su, E.; Liu, X.; Wan, Z.; Yang, X. Self-Assembled Egg Yolk Peptide Micellar Nanoparticles as a Versatile Emulsifier for Food-Grade Oil-in-Water Pickering Nanoemulsions. J. Agric. Food Chem. 2019, 67, 11728–11740. [Google Scholar] [CrossRef]
- Xu, M.; Du, Z.; Liang, H.; Yang, Y.; Li, Q.; Wan, Z.; Yang, X. Adsorption and Foaming Properties of Edible Egg Yolk Peptide Nanoparticles: Effect of Particle Aggregation. Curr. Res. Food Sci. 2021, 4, 270–278. [Google Scholar] [CrossRef]
- Yang, M.; Liu, J.; Li, Y.; Yang, Q.; Liu, C.; Liu, X.; Zhang, B.; Zhang, H.; Zhang, T.; Du, Z. Co-Encapsulation of Egg-White-Derived Peptides (EWDP) and Curcumin within the Polysaccharide-Based Amphiphilic Nanoparticles for Promising Oral Bioavailability Enhancement: Role of EWDP. J. Agric. Food Chem. 2022, 70, 5126–5136. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, S.; Guo, W.; Feng, Y.; Guo, T.; Wu, H. Formation of Calcium Phosphate Nanoparticles Mediated by Animal Protein Hydrolysates Enhances Calcium Absorption by Murine Small Intestine Ex Vivo. Food Funct. 2019, 10, 6666–6674. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, K.; Cui, P.; Bao, Z.; Wang, T.; Lin, S.; Sun, N. Egg-White-Derived Antioxidant Peptide as an Efficient Nanocarrier for Zinc Delivery through the Gastrointestinal System. J. Agric. Food Chem. 2020, 68, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lin, S.; Ge, R.; Xiong, C.; Xu, L.; Zhao, M.; Fan, J. Buckwheat Self-Assembling Peptide-Based Hydrogel: Preparation, Characteristics and Forming Mechanism. Food Hydrocoll. 2022, 125, 107378. [Google Scholar] [CrossRef]
| Methods | Protein | Preparation | Evaluation Indicator | Results | References |
|---|---|---|---|---|---|
| Enzymatic hydrolysis | Eggshell membrane protein | Alcalase: E/S 1% w/w at pH 10, 55 °C for 4 h. Proteases S: E/S 2% w/w, pH 10 at 55 °C for 12 h. Alcalase + Protease S | Total nitrogen recovery DPPH radical scavenging activity Hydroxyl radical scavenging activity | 65.60 ± 0.43% 42.74% 25.5% | [43] |
| Alcalase from Bacillus licheniformis: E/S (v/v) 2.2%, pH 7.6, 55 °C, 6 h. Viscozyme L: E/S (v/v) 1.90, pH 4.6, 50 °C, 6.61 h. Protease from Bacillus amyloliquefaciens: E/S (v/v) 5.3%, pH 6.6 h, 50 °C, 2.90. | ACE inhibitory activity | IC50 (μg/mL: 43.0 ± 8.5 63.0 ± 4.2 43.0 ± 8.5 | [72] | ||
| Recombinant LasB_ME4 (Pseudomonasaeruginosa) E/S 3, pH 6.5, 50 °C for 24 h, with shaking at 140 rpm. | Soluble peptides and proteins (mg/mL) | 0.502 ± 0.016 | [73] | ||
| Egg white protein | Papain: pH 6.0, 50 °C, 5 h. | DPPH scavenging activity The recovery of the 3 h papain hydrolysate | 73.14% 50.62% | [11] | |
| Protease P: E/S (w/w) 25:1, pH 7.5, 45 °C, 3 h. | DH ORAC (μmol TE/mg) ABTS (μmol TE/mg) | 93.3% 1.28 ± 0.06 1.61 ± 0.00 | [74] | ||
| Ultrasound-assisted alkali treatment: pH 8.5, 55 °C, 20 min. Alcalase: E/S 5% (w/v), pH 8.5, 55 °C, 5 h. | Foaming ability Foam stability Oil absorption capacity Water absorption capacity | Compared to unpretreated enzymolysis, these properties increased by 88.5%, 228.7%, 102.6%, and 67.4%, respectively | [75] | ||
| Ovotransferrin | Promod 278P: E/S (w/w) 1:50, 45 °C, 3 h. | ACE-inhibitory activity Cytotoxic activities | 76.82 ± 1.28% IC50 (mg/mL) MCF-7 cells: 10.05 ± 1.55 HeLa cells: 3.45 ± 0.94 HepG2 cells: 4.43 ± 1.87 HT-29 cells: 4.92 ± 0.63 oVo cells: 10.43 ± 3.91 | [76] | |
| Sonication pretreatment (60 Hz for 30 s) Thermolysin: 5% (w/v), pH 8, 60 °C, 3 h. Thermolysin with sonication | ORAC (μmol/mg) | 1.95 ± 0.02 | [59] | ||
| Promod 278P + Thermolysin: E/S 1:50 (w/w), 6 h. | Cytotoxic activity | IC50 (mg/mL) AGS, LoVo, HT-29, and HeLa: 0.79, 0.78, 0.92, and 0.78, respectively | [77] | ||
| Papain: E/S (w/w) 5%, pH 7.0, 50 °C for 6 h. | Biofilm eradication activities | Compared to the control, the treatment of OTFP (500 μg/mL) reduced the biofilm formation rates by 13.76%, 19.18%, and 39.80% and inhibited the metabolic activities by 27.84%, 57.85%, and 65.71% in L. monocytogenes ATCC 15313, H7962, and NADC Scott A, respectively | [78] | ||
| Elastase: E/S 1% (w/v), pH 7.8, 25 °C, 24 h. α-chymotrypsin: E/S 1% (w/v), pH 6.5, 37 °C, 3 h. | Fe3+-chelating activities | 1.06 ± 0.88% 1.25 ± 0.24% | [79] | ||
| Lysozyme | Alcalase: E/S (w/w) 0.5%, pH 7.0, 37 °C, 6 h. | Oxygen radical absorbance capacity-fluorescein (ORAC-FL) assay DPPH radical scavenging activity | IC50 0.463 μmol TE/moL 1.698 μmol TE/mg | [80] | |
| Trypsin + Papain E/S (w/w) 1:20, pH 7.5, 37 °C, 2 h. | DPPH radical scavenging activity Radical scavenging assay (μmol (TE)/mg) | Trypsin + Papain 64.2 ± 2.95% 2.82 ±0.14 μmol TE/mg | [40] | ||
| Phosvitin | Enzymatic treatments: Phosphatase: pH 4.8, 37 °C for 15 h. Pancreatin: pH 7.5. Pepsin A: pH 2.3. | The degree of dephosphorylation Angiotensin-converting enzyme (ACE)-inhibitory antioxidant activity | 63% Dephosphorylated phosvitin: increased by 52% Protease-treated phosvitin: increased by 39% | [54] | |
| Alkaline treatment: 0.1 mol/L NaOH, at 37 °C for 4 h. Trypsin: E/S (w/w) 1:50, pH 8.0, 37 °C, 24 h. | Amino acid peptides Phosphate retention of phosvitin Phosphopeptides | 10~20 35% | [52] | ||
| Trypsin: pH 8.0, 40 °C, 6 h. Multifect 14 L: pH 8.0, 70 °C, 6 h. Trypsin + Multifect 14 L: The substrate/enzyme ratio was 50:1. | DH Number of peptides | 26.01 ± 1.23% 164 | [55] | ||
| High-temperature and mild pressure (HTMP): 121 °C at 0.1 MPa, 30 min. Trypsin: pH 8.0, 37 °C. Thermolysin: pH 8.0, 68 °C. Trypsin + thermolysin: The same enzyme: E/S (1:50, m/m) and incubation time (8 h). | DH The calcium-binding rate of phosvitin Phosphopeptides | 45.55% 43.01% | [81] | ||
| HTMP: 121 °C and 1.5 atm for 60 min. Trypsin: pH 8.0, 37 °C. Trypsin—sterilization hydrolysis: The same enzyme: E/S (1:50, m/m) and incubation time (6 h)- | Effects of phosvitin phosphopeptides on the melanin synthesis in α-MSH-stimulated-B16F10 cells Effects of phosvitin phosphopeptides on the elastase activity Effects of phosvitin phosphopeptides on the NO production in LPS-stimulated RAW 264.7 cells | HTMP-T-S at the concentration of 3 mg/mL suppressed the melanin content by 38.58% HTMP-T-S at a concentration of 50 mg/mL inhibited the elastase activities by 70.67% HTMP-T-S at the concentration of 5 mg/mL reduced NO production by 76.69% | [82] | ||
| Microbial fermentation | Eggshell membrane protein | Lactobacillus plantarum: E/S (W/V) 5%, 30 °C at 120 rpm initial pH 8.0, 30 °C, 30 h. | DH Foaming capacity Emulsification activity | 25.1% 36.7% 94.6 m2/g | [65] |
| Egg white protein | Lactobacillus sp E/S (v/v) 1:1, 37 °C. Addition of different-level milk powder 2% and fermentation times, 24 h. | Antioxidant activity | 3.92 ± 0.56% | [63] | |
| Phosvitin | Streptococcus thermophiles to Lactobacillus bulgaricus was 1:1. E/S (w/w) 1%, pH 6.7, 42 °C, 6 and 9 h. | Surface hydrophobicity Emulsifying properties The consumption of NaOH | Fermentation time: 6 h 2.04 60.49 min Fermentation time: 9 h 0.90 mL | [64] | |
| Chemical synthesis | Egg white protein | AAPPTEC 396 Automated Peptide Synthesizer. The 9-Fluorenylmethoxycarbonyloxy (Fmoc)-protected amino acid synthesis method peptide (RVPSLM). | α-glucosidase inhibitory activity | IC50 23.07 μmol/L | [83] |
| Fmoc-protected amino acid synthesis method. AAPPTEC: Apex 396 automated peptide synthesizer. Peptide QIGLF. | ACE-inhibitory activity | IC50 75 µmol/L | [84] | ||
| Fmoc solid-phase peptide conditions. AAPPTEC: Apex 396 peptide synthesizer. Peptide MIR. | Dipeptidyl peptidase IV-inhibitory activity | IC50 24.97 ± 0.80 mmol/L | [85] | ||
| Ovotransferrin | Solid.phase peptide procedure. FMOC-protected amino acids synthesis method. Peptide RVPSL (328–333). | ACE-inhibitory activity | IC50 20 μmol/L | [86] | |
| Ovalbumin | AAPPTEC: Apex 396 peptide synthesizer. Peptide EEK. | XO-inhibitory activity | IC50 141 μmol/L | [87] | |
| Lysozyme | AEERYP | ORAC (μmolTE/mg) | IC50 4.35 ± 0.09 | [74] | |
| Phosvitin | Solid-phase peptide synthesis method. Peptide DEEENDQVK. | Calcium-binding capacity | 151.1 mg/g | [67] |
| Protein | Peptide Sequence | Test Method | Experimental Results | References |
|---|---|---|---|---|
| Ovalbumin | AEERYP DEDTQAMP | Oxygen radical absorbance capacity | 4.35 ± 0.09 μmolTE/μmol 3.47 ± 0.12 μmolTE/μmol | [74] |
| YAEERYPIL SALAM | The mouse macrophage cell line RAW 264.7: radical-scavenging activity | 3.81 µmol Trolox/mg protein 2.66 µmol Trolox/mg protein | [18] | |
| FFGFN MPDAHL | DPPH radical scavenging activity | IC50 80 mmol/L 60 mmol/L | [11] | |
| Ovotransferrin | WNIP GWNI | Oxygen radical absorbance capacity | 15.47 ± 0.68 μmolTE/μml 13.90 ± 1.05 μmolTE/μmol | [59] |
| IRW | Oxygen radical absorbance capacity | 6.63 ± 0.37 μmol TE/μmol | [105] | |
| Lysozyme | WRNRCKGTD AWIRGCRL WIRGCRL VAWRNRCKGTD | Zebrafish larvae: oxygen radical absorbance capacity-fluorescein assay | IC50 3123 µmol TE/µmol 2743 µmol TE/µmol 2393 µmol TE/µmol 1970 µmol TE/µmol | [106] |
| Apovitellenin-1 | YINQMPQKSRE | DPPH radical scavenging activity Ferric-reducing antioxidant power | 2.3 µmol Troloxeq/mg protein 37.4 µg Fe2+/mg | [95] |
| Egg yolk protein | LAPSLPGKPKPD | Free radical scavenging activity Ferric-reducing antioxidant power | 6.03 µmol Troloxeq/mg protein 296.07 μg Fe2+/mg protein | [107] |
| Enzyme-Inhibitory Activity | Protein | Peptide Sequence | Test Method | Experimental Results | References | |
|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (ACE)-inhibitory activity | Myosin Myosin Ovalbumin | ADF MIR FGR | ACE-inhibitory activity assay | IC50 27.75 ± 0.90 mmol/L 24.97 ± 0.80 mmol/L 66.98 ± 1.40 μmol/L | [85] | |
| Ovotransferrin | RVPSL | ACE activity assay | IC50 20 μmol/L | [86] | ||
| Lysozyme | MKR RGY VAY | Stability of the synthetic peptide-inhibiting ACE | IC50 25.7 ± 0.2 μmol/L 61.9 ± 0.1 μmol/L 2.86 ± 0.08 μmol/L | [115] | ||
| Dipeptidyl peptidase IV (DPP-IV)-inhibitory activity | Myosin Myosin Ovalbumin Lysozyme | MIR ADF CDR FGR | DPP-IV-inhibitory assay | IC 50 4.86 mmol/L 16.83 mmol/L 24.49 mmol/L 46.22 mmol/L | [85] | |
| Egg white protein | IRDLLER YAEERYP IRNVLQPS | DPP-IV-inhibitory activity assay | 186.23 ±15.25μmol/L 340.62 ± 4.73 μmol/L 598.28 ± 15.12 μmol/L | [116] | ||
| α-glucosidase-inhibitory activity | Ovotransferrin | RVPSLM TPSPR | α-glucosidase-inhibitory activity | IC 50 23.07 μmol/L 40.02 μmol/L | [83] | |
| Aminopeptidase N (APN)-inhibitory activity | Ovotransferrin Ovomucoid Avidin | CDR CNR GEF | APN-inhibitory assay | IC 50 8.94 mmol/L 6.42 mmol/L 61.7 mmol/L | [117] | |
| Xanthine oxidase (XO)-inhibitory activity | Ovalbumin | EEK EEAK WPPKN ADIYTE | XO-inhibitory activity assay | IC50 0.4 mg/mL 0.58 mg/mL 17.75 mg/mL 19.01 mg/mL | [87] | |
| Tyrosinase-inhibitory activity | Monophenolase inhibitory activity | Ovalbumin Ovalbumin Ovalbumin-related protein Y | ADHPF AFKDEDTKAMPF ILELPFASGDLLML | The p-values of mushroom tyrosinase-peptide-binding interactions A smaller p-value signifies a higher potential of the peptide binding to the enzyme | 0.002658 0.02053 0.03464 | [82] |
| Diphenolase activity | Ovotransferrin Ovomucin Ovomucin | SDFHLFGPPGK FDGRSR FNCSSAGPGAIGSEC | 0.009412 0.01312 0.01614 | |||
| Protein | Peptide Sequence | Test Method | Experimental Results (IC50) | References |
|---|---|---|---|---|
| Ovotransferrin | AGLAPYKLKPIA | Escherichia coli ATCC 25922 (Gram-negative bacteria) Staphylococcus aureus ATCC 25923 (Gram-positive bacteria) Minimal inhibitory concentration of peptide (MIC) values | 32 μg/mL | [134] |
| Lysozyme | IVSDGDMNAW HGLDNYA | Staphylococcus aureus 23–394 (Gram-positive bacteria), Escherichia coli K-12 (Gram-negative bacteria) (MIC) values | 400 μg/mL | [130] |
| NTDGSTDYGILQINSR | Escherichia coli Leuconostoc mesenteroides bacteria (MIC) values | 355.64 ± 2.2 μg/mL 442.25 ± 2.8 μg/mL | [40] | |
| Gallus gallus apolipoprotein B | KGGDLGLFEPTL | Staphylococcus aureus ATCC29213 (MIC) values | 2 mmol/L | [9] |
| Other Activities | Protein | Peptide Sequence | Test Method | Experimental Results | References |
|---|---|---|---|---|---|
| Anti-inflammatory activity | Ovotransferrin | IWHHT | The expression of vascular cell adhesion molecule-1 (VCAM-1) | IC50 μmol/L | [152] |
| IRW IQW | Expression of adhesion molecules (ICAM-1 and VCAM-1) | Pretreatment with IRW (50 μmol/L) significantly inhibited the TNF-induced increased expression of both ICAM and VCAM-1 Pretreatment with IQW (50 μmol/L) significantly blocked the TNF-induced increased expression of ICAM-1 | [138] | ||
| GW | Inflammatory molecule expression (ICAM-1 and VCAM-1) | Reduction in TNF-α-induced VCAM-1 expression (64.3 ± 20.6%) | [153] | ||
| Ovotransferrin | CR FL HC LL MK | Determination of IL-8 | Reduction in IL-8 | [154] | |
| Immunomodulatory activity | Egg white protein | Egg white protein peptides (200 Da–500 Da) | Male BALB/c mice: The number of white blood cells in the peripheral blood Levels of serum cytokines | Supplementation with 750 mg/kg/d egg white protein peptides: Increase in IL-2, IL-6, TNF-α, and the number of white blood cells | [155] |
| Anti-cancer activity | Ovotransferrin | Ovotransferrin peptides prepared with promod 278P + thermolysin combination | MTT assay AGS LoVo HT-29 HeLa | IC50 (mg/mL) 0.79 0.78 0.92 0.78 | [154] |
| Chelated minerals | Egg white protein | DHTKE | Calcium-binding capacity Binding sites of calcium on the DHTKE peptide | DHTKE: peptide = 1:1 The calcium-binding site corresponded to the carboxyl oxygen, amino nitrogen, and imidazole nitrogen atoms | [68] |
| DKLPGFGDS(PO4)IEAQ | 59Fe uptake in Caco-2 cells Ferritin expression in Caco-2 cells | Iron: synthetic peptide = 1:1, 80 ± 6.9% Increase in ferritin synthesis | [38] | ||
| Phosvitin | DEEENDQVK | Calcium-binding capacity | 151.1 mg Ca/g peptide | [67] | |
| EDDpSpS | Calcium-binding capacity | 468 ± 2.80 mg Ca/g peptide | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Chen, Y.; Liu, H.; Zhang, X. Preparation, Biological Activities, and Potential Applications of Hen Egg-Derived Peptides: A Review. Foods 2024, 13, 885. https://doi.org/10.3390/foods13060885
Song L, Chen Y, Liu H, Zhang X. Preparation, Biological Activities, and Potential Applications of Hen Egg-Derived Peptides: A Review. Foods. 2024; 13(6):885. https://doi.org/10.3390/foods13060885
Chicago/Turabian StyleSong, Li, Yi Chen, Huiping Liu, and Xiaowei Zhang. 2024. "Preparation, Biological Activities, and Potential Applications of Hen Egg-Derived Peptides: A Review" Foods 13, no. 6: 885. https://doi.org/10.3390/foods13060885
APA StyleSong, L., Chen, Y., Liu, H., & Zhang, X. (2024). Preparation, Biological Activities, and Potential Applications of Hen Egg-Derived Peptides: A Review. Foods, 13(6), 885. https://doi.org/10.3390/foods13060885







