Degradation Mechanism of Aflatoxin M1 by Recombinant Catalase from Bacillus pumilusE-1-1-1: Food Applications in Milk and Beer
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, and Chemicals
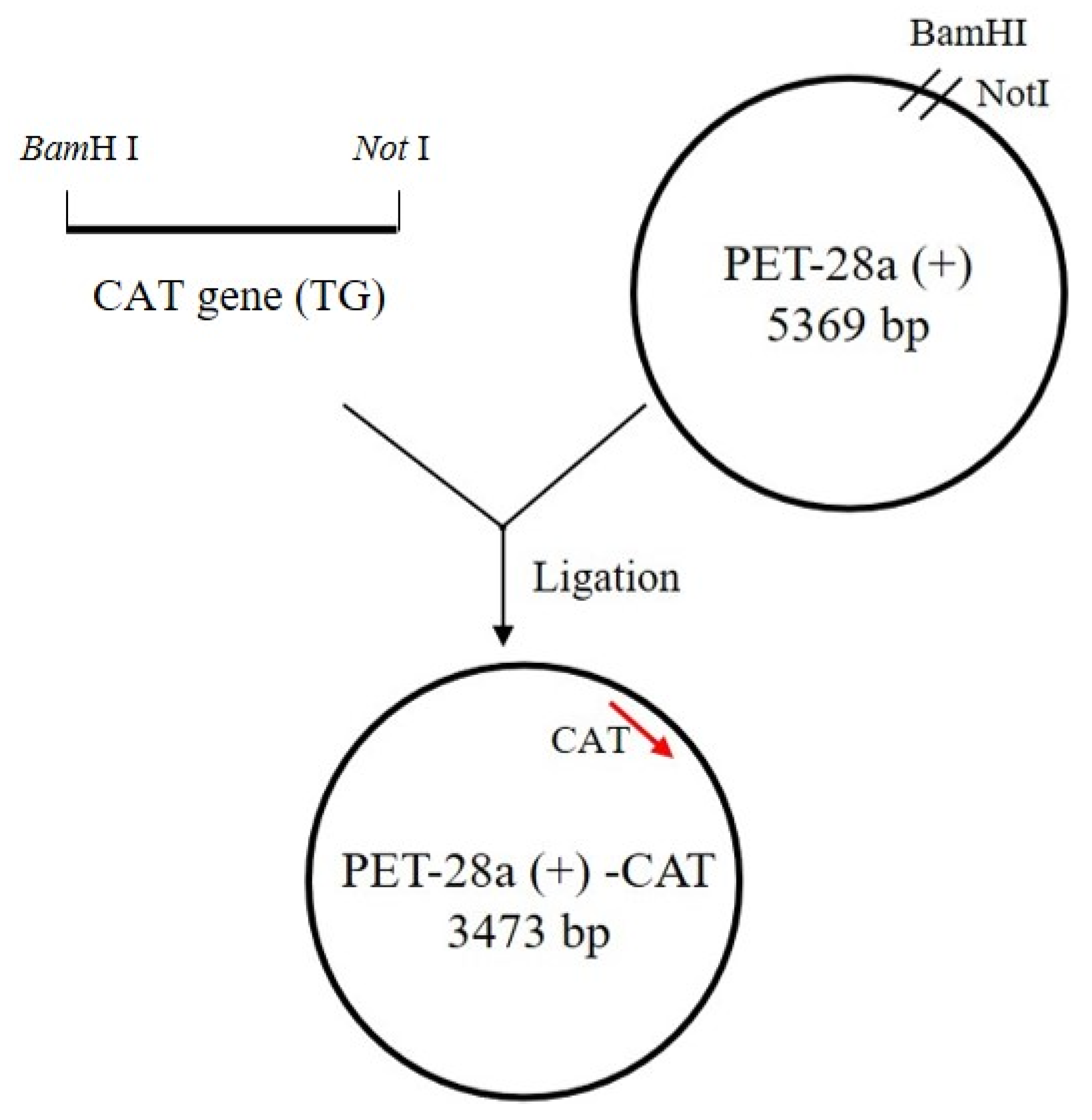
2.2. PCR Amplification of the CAT Gene and Cloning into an Expression Vector
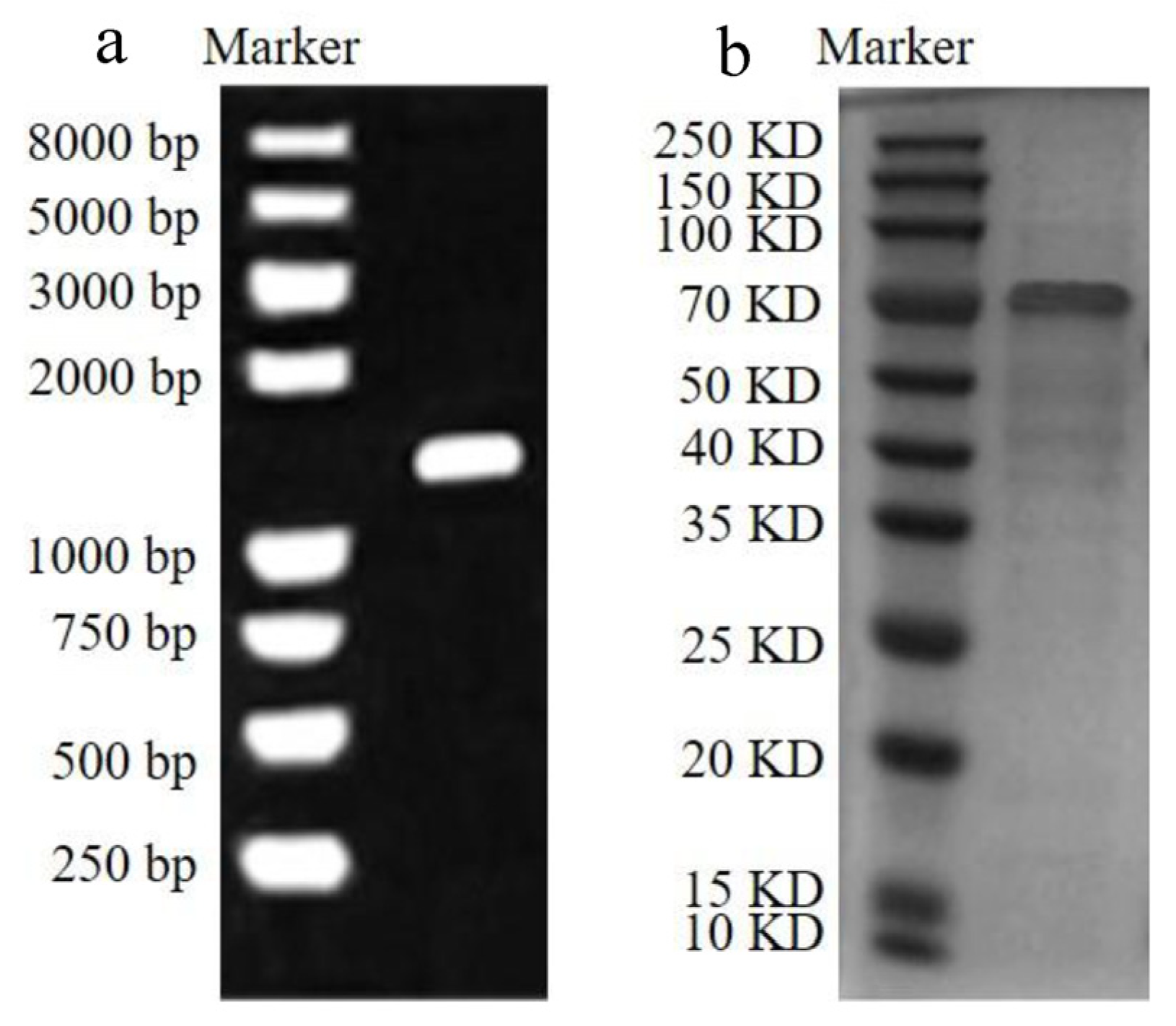
2.3. Quantitative Analysis of rCAT
2.4. Measurement of AFM1 Concentrations by HPLC
2.5. AFM1 Degradation by rCAT
2.6. Degradation of AFM1 Times
2.7. Measurement of Kinetic Parameters
2.8. Liquid Chromatography–Mass Spectrometry (LC–MS) Analysis for the Identification of Degraded Products
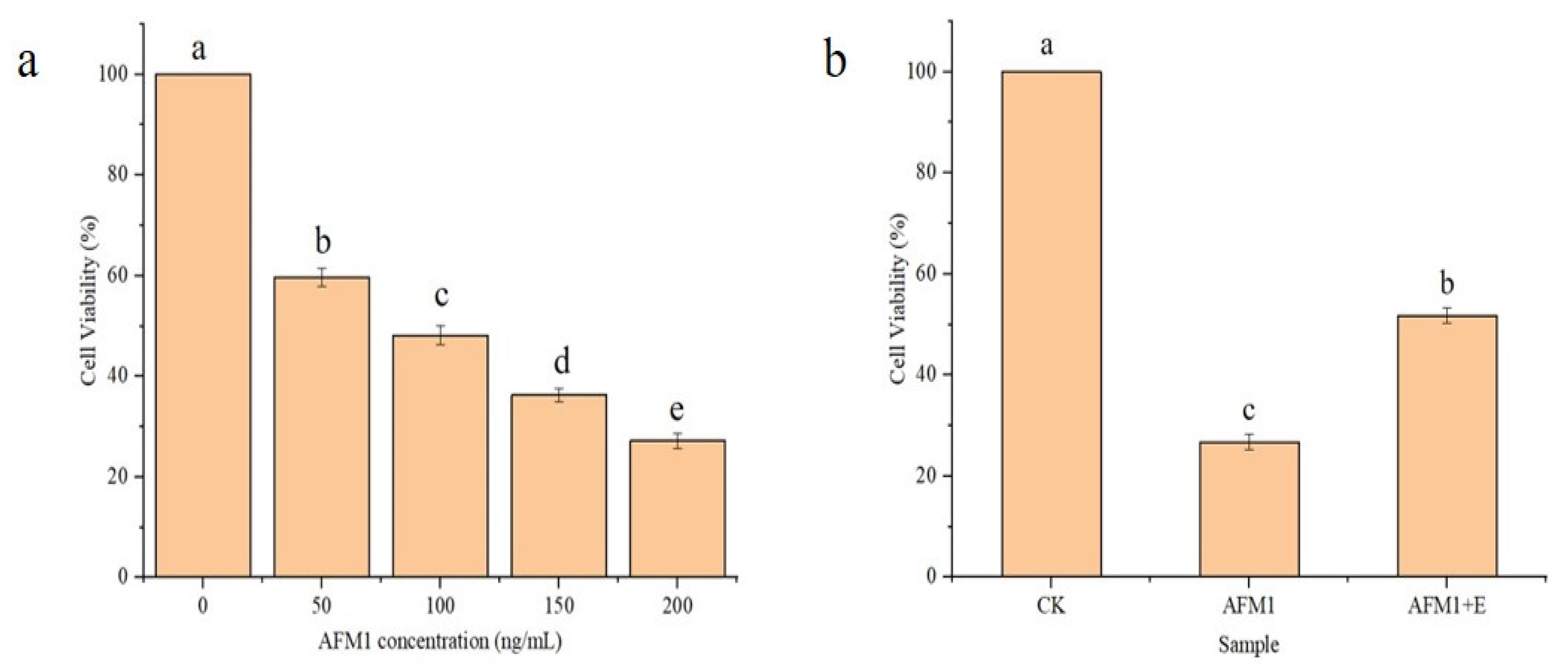
2.9. Cell Viability Assay
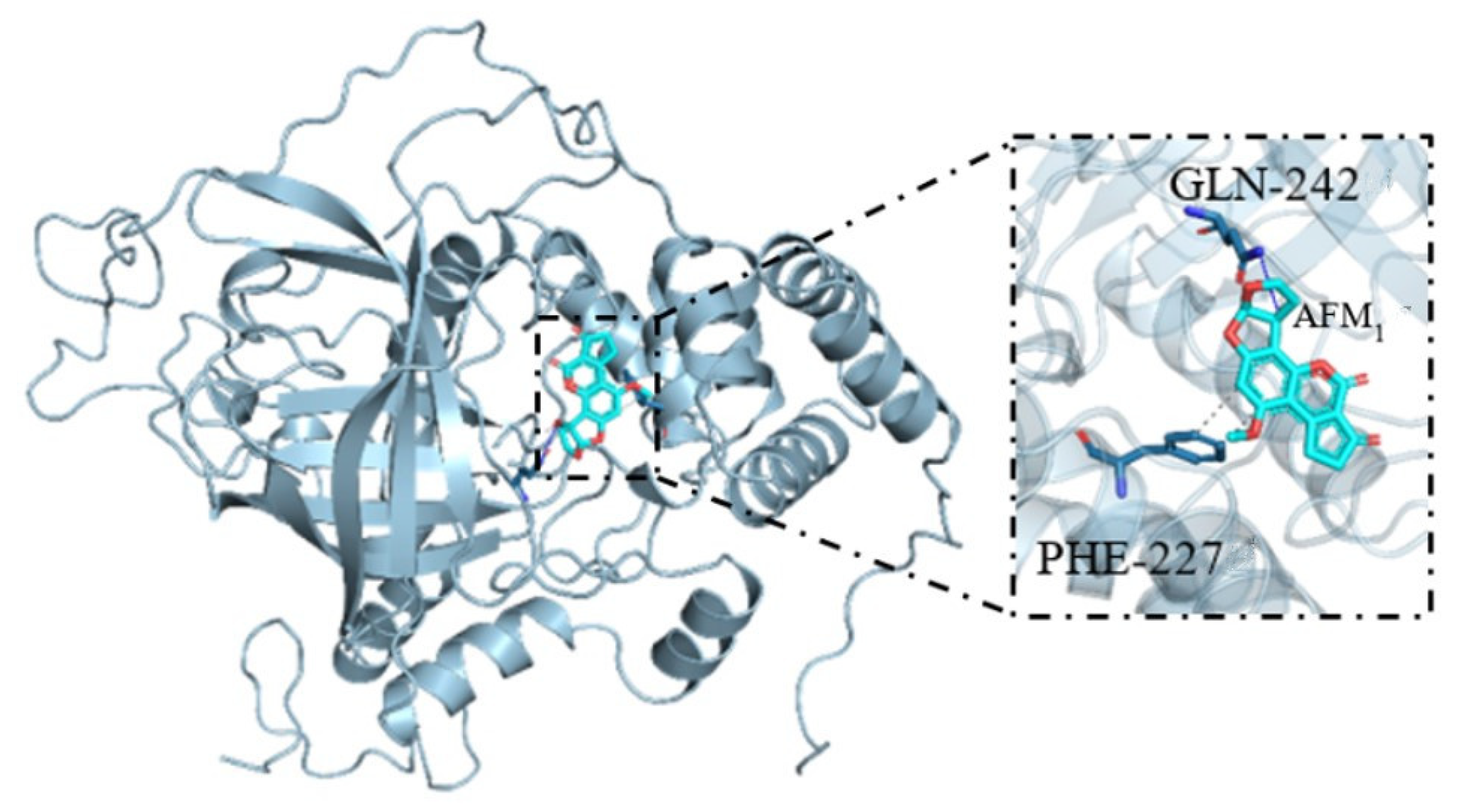
2.10. Homology Modeling and Molecular Docking
2.11. AFM1 Degradation in Beer and Milk
2.12. Statistical Analysis
3. Results and Discussion
3.1. Purified Proteins, Concentrations, and Enzyme Activity of CAT
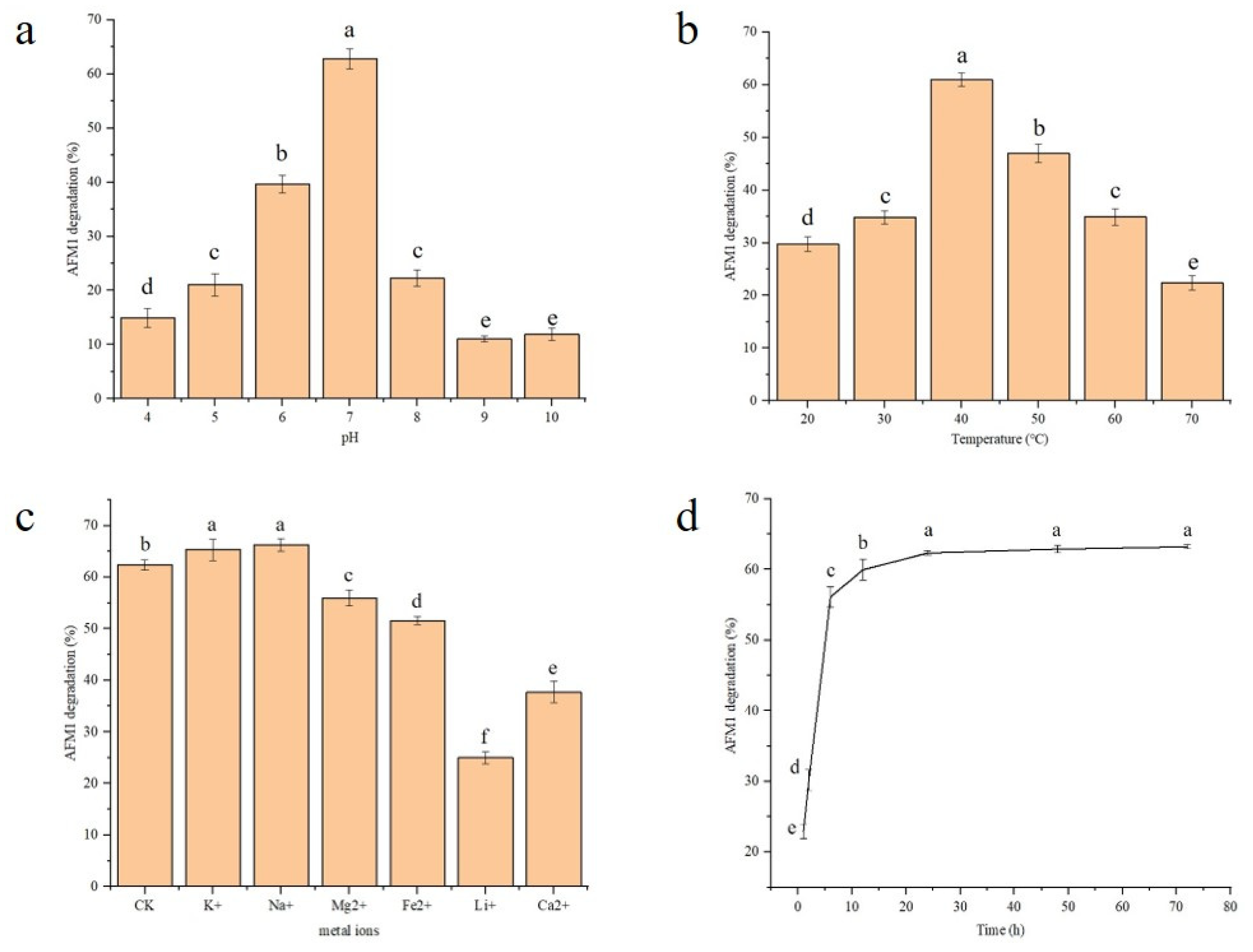
3.2. Characterization of AFM1 Degradation by rCAT in Pattern Solution
3.2.1. pH
3.2.2. Temperature
3.2.3. The Effects of Metal Ion Addition
3.3. Degradation Times of AFM1 by rCAT
3.4. Determination of Kinetic Parameters
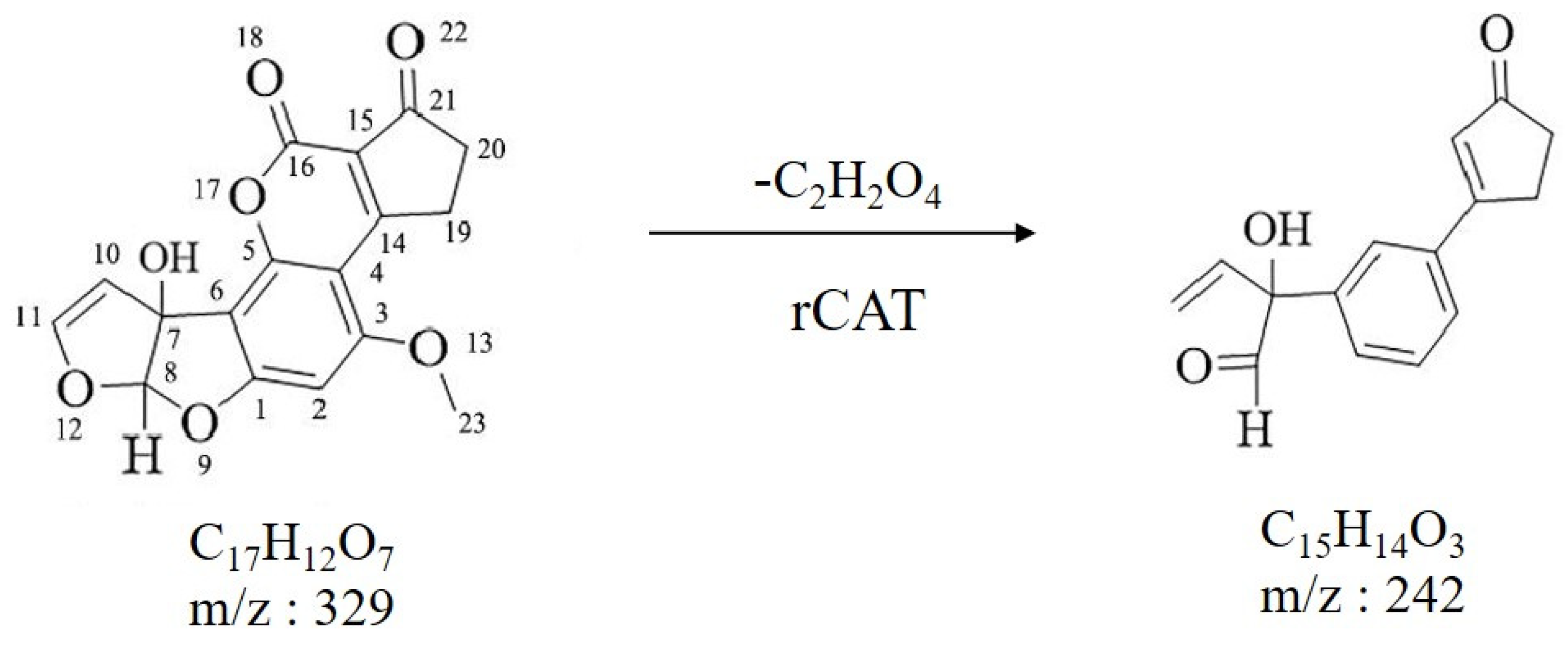
3.5. Identification of Degraded Products by LC–MS
3.6. Toxicity Analysis of CAT-Mediated AFM1 Oxidation Products
3.7. Molecular Docking and Binding Model of AFM1 and CAT
3.8. Degradation of AFM1 in Milk and Beer
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| rCAT | Recombinant catalase |
| E. coli | Escherichia coli |
| Km | Kinetic Momentum |
| Vmax | Virtual Machine Maximum value |
| Hep-G2 | Human hepatocarcinoma cells |
References
- Cao, H.; Liu, D.L.; Mo, X.M. A fungal enzyme with the ability of aflatoxin B1 conversion: Purification and ESI-MS/MS identification. Microbiol. Res. 2011, 166, 475–483. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Iordache, F.; Stanca, L. Comprehensive overview and critical perspective on the analytical techniques applied to aflatoxin determination—A review paper. Microchem. J. 2023, 191, 108770. [Google Scholar] [CrossRef]
- Giacometti, F.; Tomasello, F.; Savini, F. Impact of the implementation of tailored management strategies to reduce the occurrence of aflatoxin M1 in milk-supply chain in Italy. Food Control. 2023, 149, 109720. [Google Scholar] [CrossRef]
- Zhou, W.; Wieczorek, M.N.; Pawliszyn, J. High throughput and automated solid-phase microextraction and determination by liquid chromatography-mass spectrometry for the analysis of mycotoxins in beer. Food Chem. 2023, 426, 136557. [Google Scholar] [CrossRef] [PubMed]
- Hasninia, D.; Salimi, G.; Bahrami, G.; Sharafi, K.; Omer, A.K.; Rezaie, M.; Kiani, A. Human health risk assessment of aflatoxin M1 in raw and pasteurized milk from the Kermanshah province, Iran. J. Food Compos. Anal. 2022, 110, 104568. [Google Scholar] [CrossRef]
- Martínez, M.; Magnoli, A.P.; Pereyra, M.; Cavaglieri, L.J.T. Probiotic bacteria and yeasts adsorb aflatoxin M1 in milk and degrade it to less toxic AFM1-metabolites. Toxicon 2019, 172, 1–7. [Google Scholar] [CrossRef]
- Jiang, M.M.; Ji, J.M.; Zhang, Y.X. Removal of aflatoxins in peanut oils by activated carbon functionalized with sodium dodecyl sulfonate. Food Control 2023, 153, 109935. [Google Scholar] [CrossRef]
- Torshizi MA, K.; Sedaghat, A. A consortium of detoxifying bacteria mitigates the aflatoxin B1 toxicosis on performance, health, and blood constituents of laying hens. Poult. Sci. 2023, 102, 102601. [Google Scholar] [CrossRef]
- Guo, Y.P.; Zhao, L.H.; Ma, Q.G. Novel strategies for degradation of aflatoxins in food and feed: A review. Food Res. Int. 2021, 140, 109878. [Google Scholar] [CrossRef]
- Sheen, A.; Agarwal, Y.; Cheah, K.M. Tumor-localized catalases can fail to alter tumor growth and transcriptional profiles in subcutaneous syngeneic mouse tumor models. Redox Biol. 2023, 64, 102766. [Google Scholar] [CrossRef]
- Qi, L.G.; Ma, Y.L.; Cai, R. Degradation of aflatoxins in apple juice by pulsed light and the analysis of their degradation products. Food Control. 2023, 148, 109648. [Google Scholar] [CrossRef]
- Li, W.; Li, W.J.; Zhang, C. Study on the mechanism of aflatoxin B1 degradation by Tetragenococcus halophilus. LWT-Food Sci. Technol. 2023, 180, 114662. [Google Scholar] [CrossRef]
- Jiang, M.T.; Liu, Y.X.; Xue, H.J. Expression and biochemical characterization of a Bacillus subtilis catalase in Pichia pastoris X-33. Protein Expr. Purif. 2023, 208–209, 106277. [Google Scholar] [CrossRef]
- Taheur, F.B.; Mansour, C.; Jeddou, K.B. Aflatoxin B1 degradation by microorganisms isolated from Kombucha culture. Toxicon 2020, 179, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, A.; Kumar, V.; Ramalingam, S. Zingiber officinale mediated aflatoxins cleanup in aqueous medium: Kinetics, toxicity and cytoprotective effect. J. Clean. Prod. 2022, 381, 135155. [Google Scholar] [CrossRef]
- Shi, X.L.; Shi, Z.H.; Feng, M.Q. High expression of recombinant human catalase and its immunomodulatory effects on H1N1 influenza virus infection. Process Biochem. 2013, 48, 588–592. [Google Scholar] [CrossRef]
- Nagy, J.M.; Cass, A.E.; Brown, K.A. Purification and Characterization of Recombinant Catalase-Peroxidase, Which Confers Isoniazid Sensitivity in Mycobacterium tuberculosis. J. Biol. Chem. 1997, 272, 31265–31271. [Google Scholar] [CrossRef]
- Shaeer, A.; Aslam, M.; Aziz, F. Looking into a highly thermostable and efficient recombinant manganese-catalase from Geobacillus thermopakistaniensis. J. Biosci. Bioeng. 2022, 133, 34642121. [Google Scholar] [CrossRef]
- Shaeer, A.; Rashid, M.A.N. Structural and functional analyses of a novel manganese-catalase from Bacillus subtilis R5. Int. J. Biol. Macromoclecules 2021, 180, 222–233. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Chen, M.R.; Chen, Y. Characterization and Exploration of Recombinant Wheat Catalase for Improvement of Wheat-Flour-Processing Quality. J. Agric. Food Chem. 2019, 67, 2660–2669. [Google Scholar] [CrossRef]
- Yang, H.Y.; Zhang, X.; Ma, Z. Expression, purification and characterization of catalase from Corynebacterium glutamicum. Sheng Wu Gong Cheng Xue Bao 2020, 36, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Philibert, T.; Rao, Z.M.; Yang, T.W. Heterologous expression and characterization of a new heme-catalase in Bacillus subtilis 168. J. Ind. Microbiol. Biotechnol. 2016, 43, 27016935. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.L.; Hou, Y.; Li, S.H. Purification, cloning, expression, and biochemical characterization of a monofunctional catalase, KatP, from Pigmentiphaga sp. DL-8. Protein Expr. Purif. 2015, 108, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.O.; Sibaja KV, M.; Nogueira, W.V.; Feltrin AC, P.; Pinheiro DF, A.; Cerqueira, M.B.R.; Badiale Furlong, E.; Garda-Buffon, J. Peroxidase as a simultaneous degradation agent of ochratoxin A and zearalenone applied to model solution and beer. Food Res. Int. 2020, 131, 109039. [Google Scholar] [CrossRef]
- Nguyen, T.; Palmer, J.; Loo, T. Investigation of UV light treatment (254 nm) on the reduction of aflatoxin M1 in skim milk and degradation products after treatment. Food Chem. 2022, 390, 133165. [Google Scholar] [CrossRef]
- Wong, J.J.; Hsieh, D.P. Mutagenicity of aflatoxins related to their metabolism and carcinogenic potential. Proc. Natl. Acad. Sci. USA 1976, 73, 2241–2244. [Google Scholar] [CrossRef]
- Nikmaram, N.; Brückner, L.; Cramer, B. Degradation products of aflatoxin M1 (AFM1) formed by high voltage atmospheric cold plasma (HVACP) treatment. Toxicon 2023, 230, 107160. [Google Scholar] [CrossRef]
- Shen, B.; Wei, K.; Ding, Y.H.; Zhang, J.S. Molecular cloning, mRNA expression and functional characterization of a catalase from Chinese black sleeper (Bostrychus sinensis). Fish Shellfish. Immunol. 2020, 103, 310–320. [Google Scholar] [CrossRef]
| Name | Sequence | Length |
|---|---|---|
| Forward | CGGGATCCATGAAAGAAGATCAACACCCTAAG | 32 |
| Reverse | TTGCGGCCGCTTAATAAGGATCTGATGGTGTG | 32 |
| Name | Degradation |
|---|---|
| beer | 31.3% |
| milk | 47.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhao, F.; Wang, X.; Peng, K.; Kang, C.; Sang, Y. Degradation Mechanism of Aflatoxin M1 by Recombinant Catalase from Bacillus pumilusE-1-1-1: Food Applications in Milk and Beer. Foods 2024, 13, 888. https://doi.org/10.3390/foods13060888
Liu X, Zhao F, Wang X, Peng K, Kang C, Sang Y. Degradation Mechanism of Aflatoxin M1 by Recombinant Catalase from Bacillus pumilusE-1-1-1: Food Applications in Milk and Beer. Foods. 2024; 13(6):888. https://doi.org/10.3390/foods13060888
Chicago/Turabian StyleLiu, Xiaoyu, Fangkun Zhao, Xianghong Wang, Kaige Peng, Chunyu Kang, and Yaxin Sang. 2024. "Degradation Mechanism of Aflatoxin M1 by Recombinant Catalase from Bacillus pumilusE-1-1-1: Food Applications in Milk and Beer" Foods 13, no. 6: 888. https://doi.org/10.3390/foods13060888
APA StyleLiu, X., Zhao, F., Wang, X., Peng, K., Kang, C., & Sang, Y. (2024). Degradation Mechanism of Aflatoxin M1 by Recombinant Catalase from Bacillus pumilusE-1-1-1: Food Applications in Milk and Beer. Foods, 13(6), 888. https://doi.org/10.3390/foods13060888





