Mechanical Damage Caused by Compression and Its Effects on Storage Quality of Mandarin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Pretreatment
2.2. Damage Sample Preparation
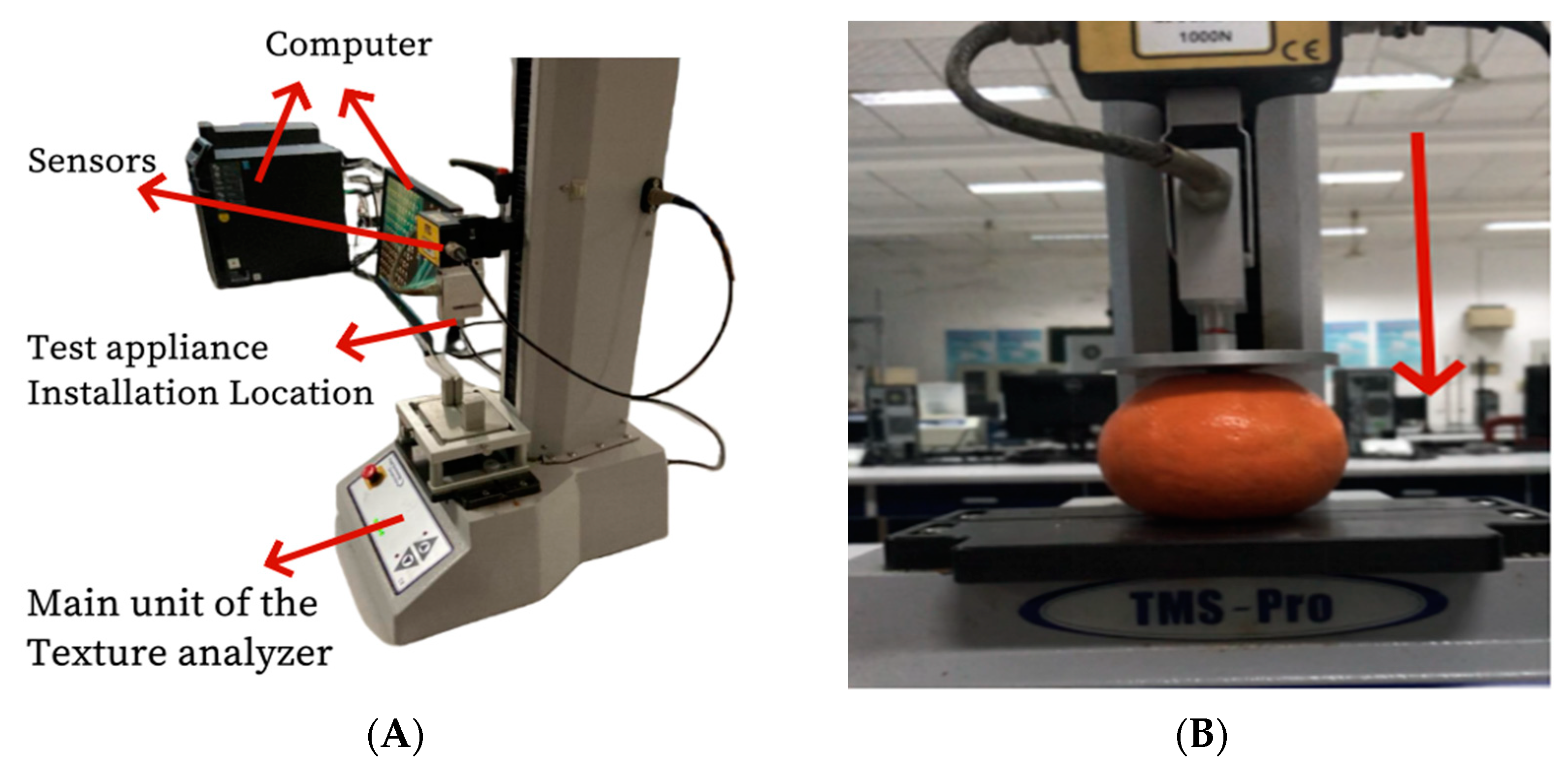
2.3. Image Acquisition of Mandarin Internal Structure
2.4. Microscopic Image Acquisition of Exocarp
2.5. Electron Microscope Image Acquisition of Exocarp
2.6. Storage Test
2.7. The Establishment Method of Prediction Model
2.8. Statistical Methods
3. Results and Discussion
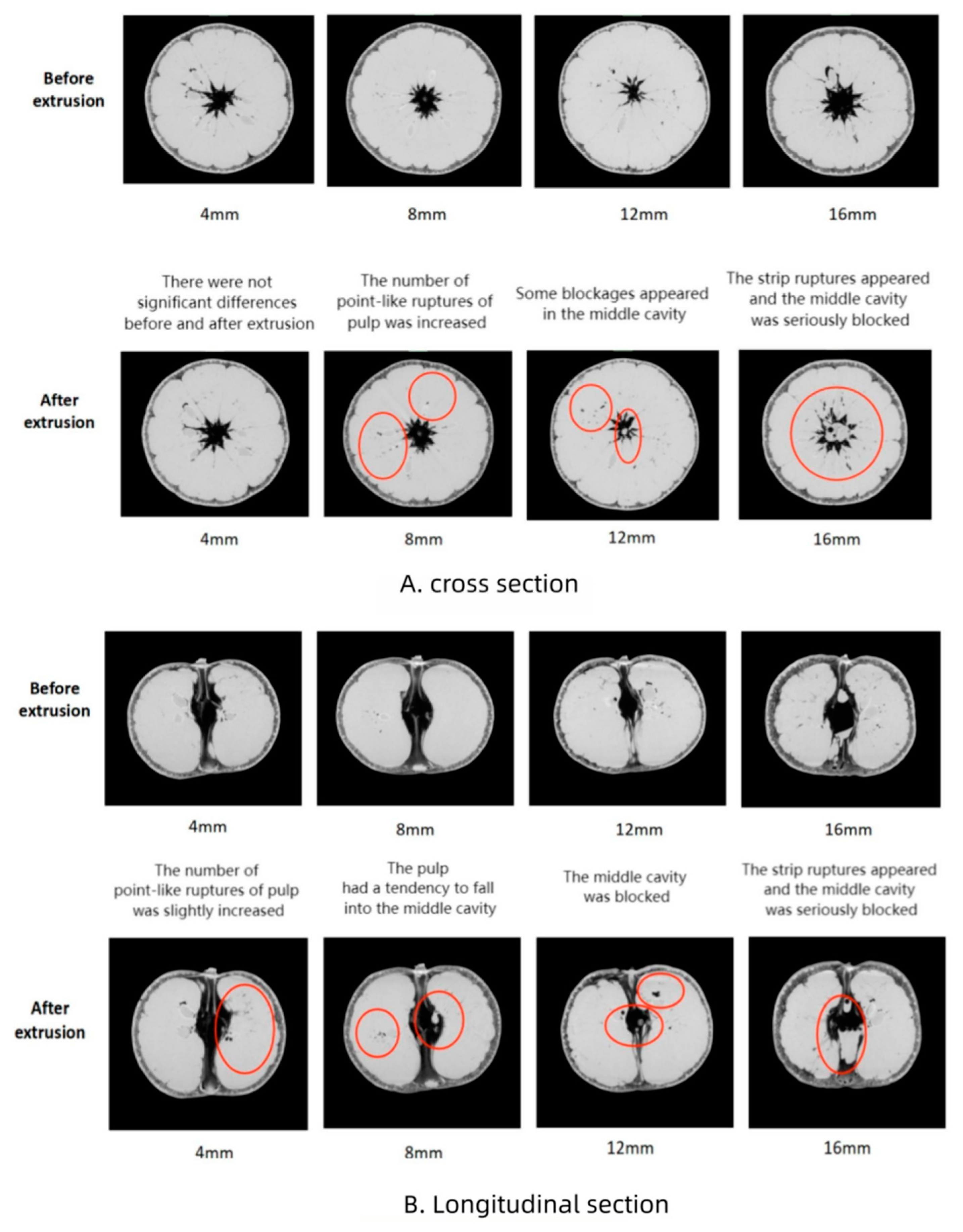
3.1. Image Features of Mandarin Internal Structure
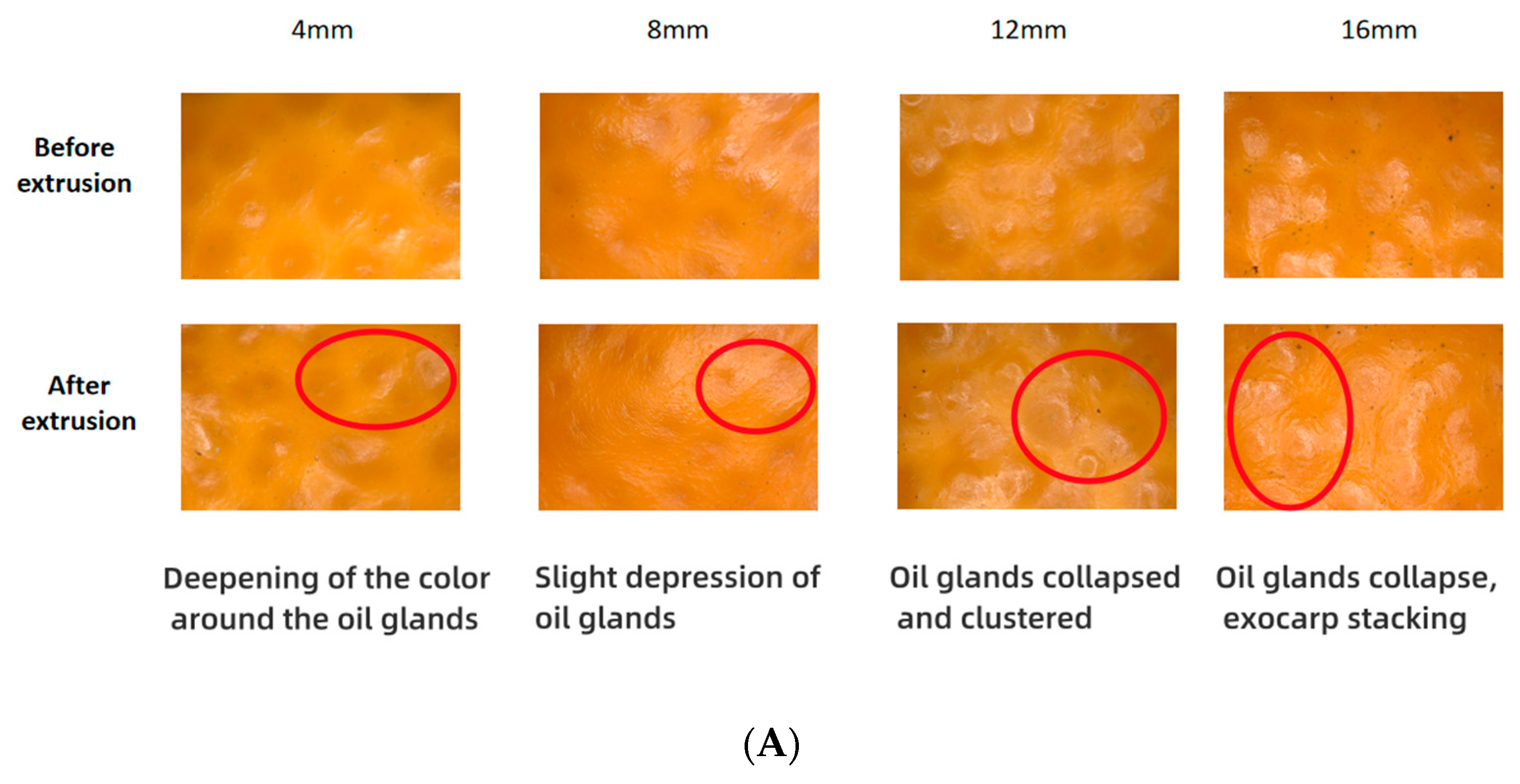
3.2. Magnified Image Characterization of Mandarin Exocarp
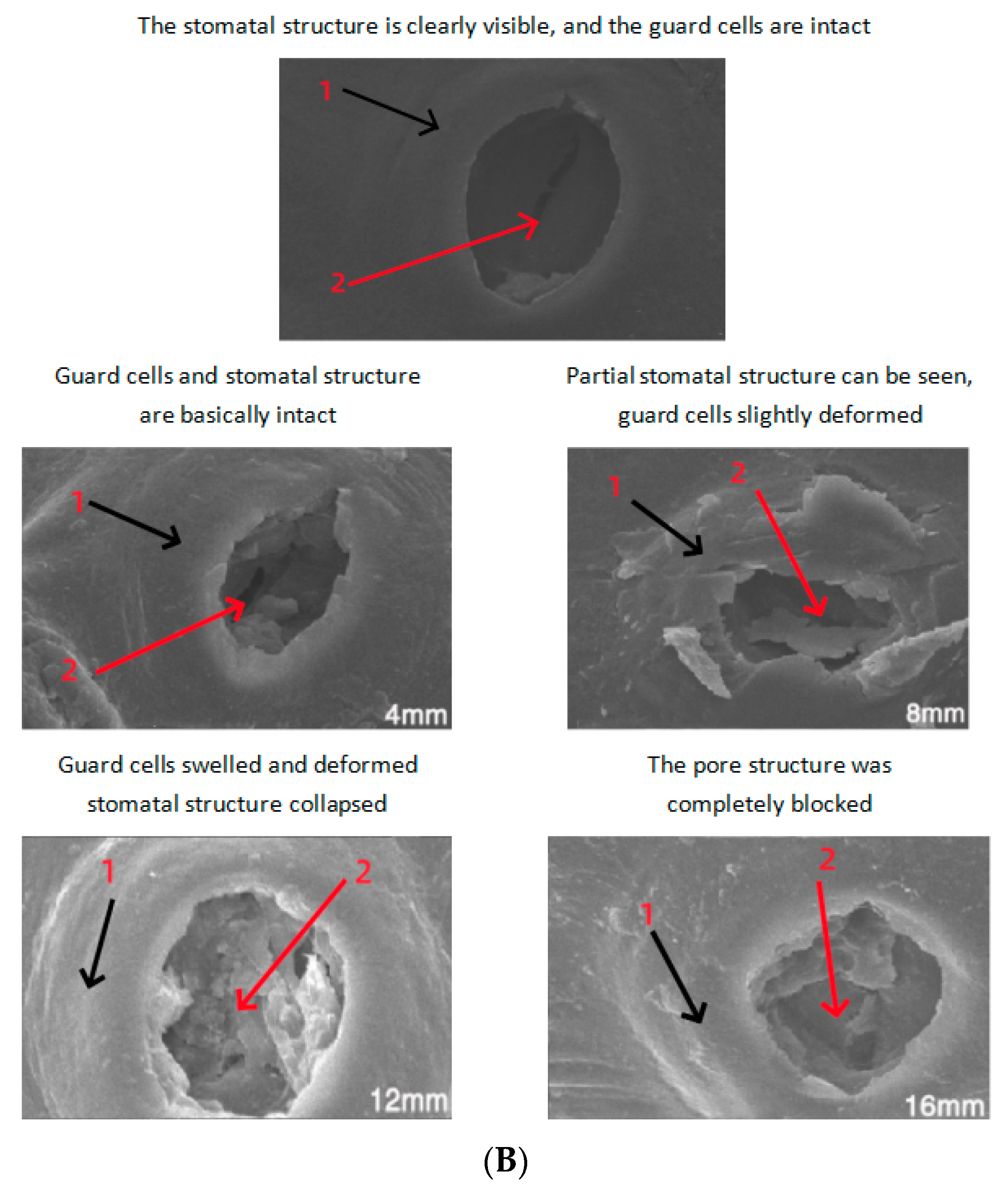
3.3. Electron Microscope Image Characteristics of Exocarp
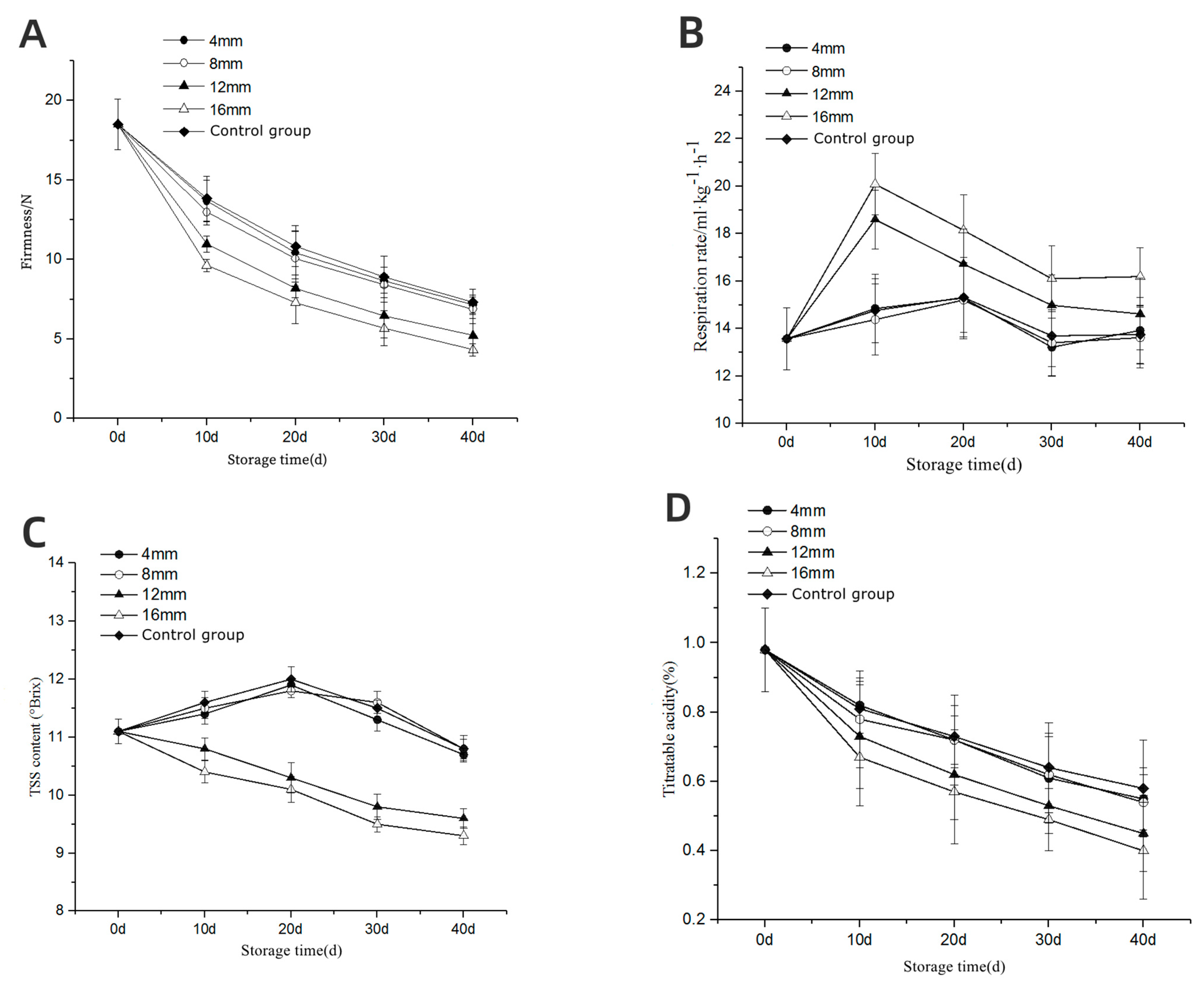
3.4. Quality of Storage—Firmness
3.5. Respiration Rate
3.6. Total Soluble Solids (TSS)
3.7. Titratable Acid (TA) Content
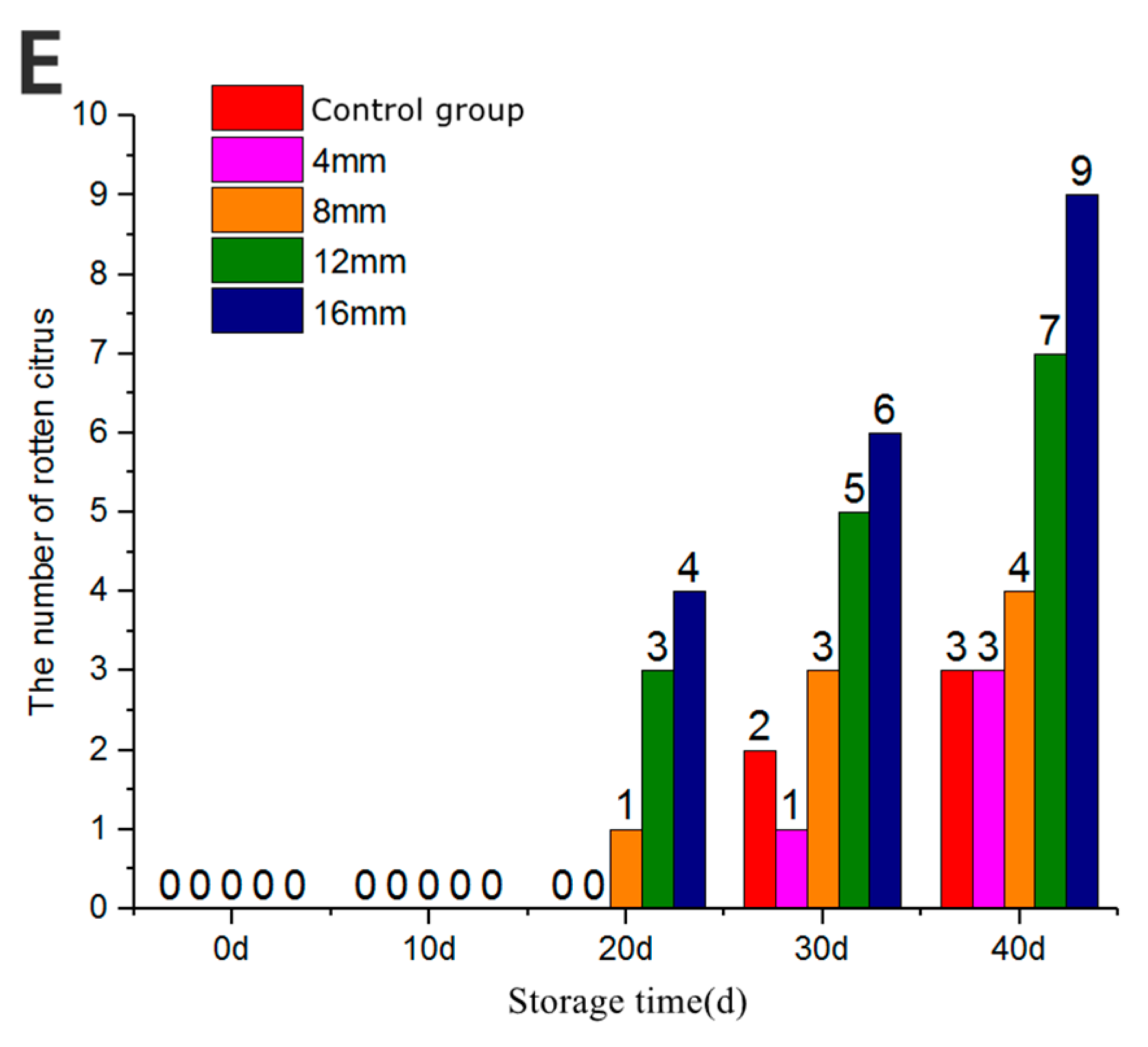
3.8. The Number of Decay
3.9. Prediction Model of Mandarin Decay Rate Based on Image Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, C.X.; Wang, P.X.; Jia, M.; Gui, Y.; Ma, Y.Q. Evaluation of juice-making quality of three main Chinese Mandarin (Citrus reticulate) varieties. Food Ferment. Ind. 2019, 45, 173–178. [Google Scholar]
- Bao, Y.; Yang, C.; Zhao, Y.; Liu, X.; Guo, Y. Collision damage assessment of mechanically harvested blueberry fruits based on collision deformation energy. J. Agric. Eng. 2017, 33, 283–292. [Google Scholar]
- Berk, Z. Citrus Fruit Processing; Academic Press: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Opara, U.L.; Pathare, P.B. Bruise damage measurement and analysis of fresh horticultural produce—A review. Postharvest Biol. Technol. 2014, 91, 9–24. [Google Scholar] [CrossRef]
- Lu, L. Special Preface to Fruits and Vegetables Preservation Technology. Packag. Eng. 2017, 38, 10. [Google Scholar]
- Liu, L.; Chen, H.; Li, L.; Peng, S.; Liu, Y.X. Study on mechanical properties of Citrus reticulata blanco during storage based on mechanical damage. Hubei Agric. Sci. 2019, 58, 124–127. [Google Scholar]
- Lu, S.; Luo, Q.; Lai, S.; Xia, Q. Compression characteristics and damage mechanism of rose orange. Food Mach. 2024, 40, 128–134. [Google Scholar]
- Razavi, M.S.; Asghari, A.; Azadbakh, M.; Shamsabadi, H.-A. Analyzing the pear bruised volume after static loading by Magnetic Resonance Imaging (MRI). Sci. Hortic. 2018, 229, 33–39. [Google Scholar] [CrossRef]
- Komarnicki, P.; Stopa, R.; Szyjewicz, D.; Kuta, Ł.; Klimza, T. Influence of Contact Surface Type on the Mechanical Damages of Apples Under Impact Loads. Food Bioprocess Technol. 2017, 10, 1479–1494. [Google Scholar] [CrossRef]
- Pòlitecnic de Vàlencia, S/N C D V. Citrus Fruit Damage Susceptibility according to Variety, Citrus Type, Season and Date of Harvest. 25 February 2024. [Google Scholar]
- Siregar, T.H.; Ahmad, U.; Sutrisno; Maddu, A. Mechanical Damage Detection of Indonesia Local Citrus Based on Fluorescence Imaging. IOP Conf. Ser. Earth Environ. Sci. 2018, 147, 012006. [Google Scholar] [CrossRef]
- Santos, M.Z.D.; Neitzke, D.F.; Favarão, S.C.M. Injúrias mecânicas na qualidade pós-colheita de laranja ‘Rosa’ armazenada sob condição ambiente. Rev. Campo Digit. 2014, 9. Available online: https://api.semanticscholar.org/CorpusID:123842129 (accessed on 29 January 2024).
- Miranda, M.; Spricigo, P.C.; Ferreira, M.D. Mechanical damage during harvest and loading affect orange postharvest quality. Eng. Agric. 2015, 35, 154–162. [Google Scholar] [CrossRef]
- Shao, X.; Chen, H.; Pan, H.; Ritenour, M.A.; Hu, C.; Xu, Q.; Bao, X. Effect of steam blanching on peelability and quality of Citrus reticulata Blanco. J. Food Sci. Technol. 2021, 58, 3790–3797. [Google Scholar] [CrossRef]
- Firouzjaei, R.A.; Minaei, S.; Beheshti, B. Sweet lemon mechanical damage detection using image processing technique and UV radiation. Food Meas. 2018, 12, 1513–1518. [Google Scholar] [CrossRef]
- Wen, Z.; Cao, L. Damage Pattern Recognition of Citrus reticulate Blanco Based on Multi-fractal Analysis of Image Hue. Nongye Jixie Xuebao/Trans. Chin. Soc. Agric. Mach. 2014, 45, 262–267. [Google Scholar] [CrossRef]
- Doosti-Irani, O.; Golzarian, M.R.; Aghkhani, M.H.; Sadrnia, H.; Doosti-Irani, M. Development of multiple regression model to estimate the apple’s bruise depth using thermal maps. Postharvest Biol. Technol. 2016, 116, 75–79. [Google Scholar] [CrossRef]
- Shao, X. Research on the Mechanism of Compression Damage and Quality Deterioration of Mandarin. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar] [CrossRef]
- Mazidi, H.K.M.; Sadrnia, H. Evaluation of orange mechanical damage during packaging by study of changes in firmness. Int. Food Res. 2016, 23, 899–903. [Google Scholar]
- Li, T. 1-Methylcyclopropene extends the shelf-life of ‘Shatangju’ mandarin (Citrus reticulate Blanco) fruit with attached leaves. Postharvest Biol. Technol. 2012, 67, 92–95. [Google Scholar] [CrossRef]
- Horwitz, W. Acidity titratable of fruit products (AOAC Official Method 942.15). In Official Methods of Analysis of AOAC International, 17th ed.; Horwitz, W., Ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Song, S.; Gao, T. Research on image segmentation algorithm based on threshold. In Proceedings of the 2021 13th International Conference on Measuring Technology and Mechatronics Automation (ICMTMA), Beihai, China, 16–17 January 2021; pp. 306–308. [Google Scholar]
- Li, Z.; Thomas, C. Quantitative evaluation of mechanical damage to fresh fruits. Trends Food Sci. Technol. 2014, 35, 138–150. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, J.; Jiang, Z.; Li, J.; Zou, X.; Wang, J. Finite element prediction and experimental verification for damage on citrus fruit from robot clamping. J. South China Agric. Univ. 2016, 37, 98–102. [Google Scholar]
- Gao, S.; Kang, H.; An, X.; Cheng, Y.; Chen, H.; Chen, Y.; Li, S. Non-destructive Storage Time Prediction of Newhall Navel Oranges Based on the Characteristics of Rind Oil Glands. Front. Plant Sci. 2022, 13, 811630. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.L.; Cui, W.J.; Yao, S.X.; Zeng, K.F. Effect of Mechanical Damage on Organizational Structure of Postharvest Navel Orange Rind. Food Sci. 2019, 40, 118–126. [Google Scholar]
- Knight, T.G.; Andreas, K.; Margaret, S. The Relationship Between Oil Gland and Fruit Development in Washington Navel Orange (Citrus sinensis L. Osbeck). Ann. Bot. 2001, 88, 1039–1047. [Google Scholar] [CrossRef]
- Maia, V.M.; Salomão, L.C.C.; Siqueira, D.L.; Aspiazú, I.; Maia, L.C.B. Physical and metabolic changes induced by mechanical damage in ‘dwarf-prata’ banana fruits kept under cold storage. Aust. J. Crop Sci. 2014, 8, 1029–1037. [Google Scholar]
- Pei, J.; Li, Y.; Cheng, C.; Li, Z. Effects of different calcium agents on fruit firmness and related cell wall metabolites in ‘Hanfu’ apple. J. Fruit Sci. 2018, 35, 1059–1066. [Google Scholar]
- Aguilar-Velazquez, B.; Rosas-Quijano, R.; Vazquez-Ovando, A.; Salvador-Figueroa, M.; Adriano-Anaya, L.; Galvez-Lopeza, D. CpXTH2 and CpXTH5 genes are expressed during fruit development and ripening in Carica papaya L. Fruits 2021, 76, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sila, D.N.; Duvetter, T.; De Baerdemaeker, J.; Hendrickx, M. Effect of mechanical impact-bruising on polygalacturonase and pectinmethylesterase activity and pectic cell wall components in tomato fruit. Postharvest Biol. Technol. 2008, 47, 98–106. [Google Scholar]
- Sediqi, A.G.; Kramchote, S.; Itamura, H.; Esumi, T. Physiological changes in sweet cherry fruit in response to physical damage. Acta Hortic. 2019, 1235, 495–502. [Google Scholar] [CrossRef]
- Mattiuz, B.-H.; Durigan, J.F.; Rossi Junior, O.D. Processamento mínimo em goiabas ‘Paluma’ e ‘Pedro Sato’: 2. Avaliação química, sensorial e microbiológica. Food Sci. Technol. 2003, 23, 409–413. [Google Scholar] [CrossRef]
- Singh, K.K.; Reddy, B.S. Post-harvest physico-mechanical properties of orange peel and fruit. J. Food Eng. 2006, 73, 112–120. [Google Scholar] [CrossRef]
- Sanches, J.; Durigan, J.F.; Durigan, M.F.B. Aplicação de danos mecânicos em abacates e seus efeitos na qualidade dos frutos. Eng. Agrícola 2008, 28, 164–175. [Google Scholar] [CrossRef]
- Burdon, J.; Lallu, N.; Francis, K. The susceptibility of kiwifruit to low temperature breakdown is associated with pre-harvest temperatures and at-harvest soluble solids content. Postharvest Biol. Technol. 2007, 43, 283–290. [Google Scholar] [CrossRef]
- Rapisarda, P.; Bianco, M.L.; Pannuzzo, P.; Timpanaro, N. Effect of cold storage on vitamin c, phenolics and antioxidant activity of five orange genotypes [Citrus sinensis (L.) Osbeck]. Postharvest Biol. Technol. 2008, 49, 348–354. [Google Scholar] [CrossRef]
- Montero, C.R.S.; Schwarz, L.L.; Santos, L.C.d.; Andreazz, C.S.; Kechinski, C.P.; Bender, R.J. Postharvest mechanical damage affects fruit quality of ‘Montenegrina’ and ‘Rainha’ tangerines. Pesqui. Agropecuária Bras. 2009, 44, 1636–1640. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Huang, W. Detection of early bruises on peaches (Amygdalus persica L.) using hyperspectral imaging coupled with improved watershed segmentation algorithm. Postharvest Biol. Technol. 2018, 135, 104–113. [Google Scholar] [CrossRef]
- Chen, G.; Lin, X.; Xu, Y.; Yu, Y.; Wu, J.; Wen, J.; Xiao, G. The damage characteristics of mechanical compression on grapefruit sandbags and the increase of bitter substances. Mod. Food Sci. Technol. 2020, 36, 274–284. [Google Scholar]

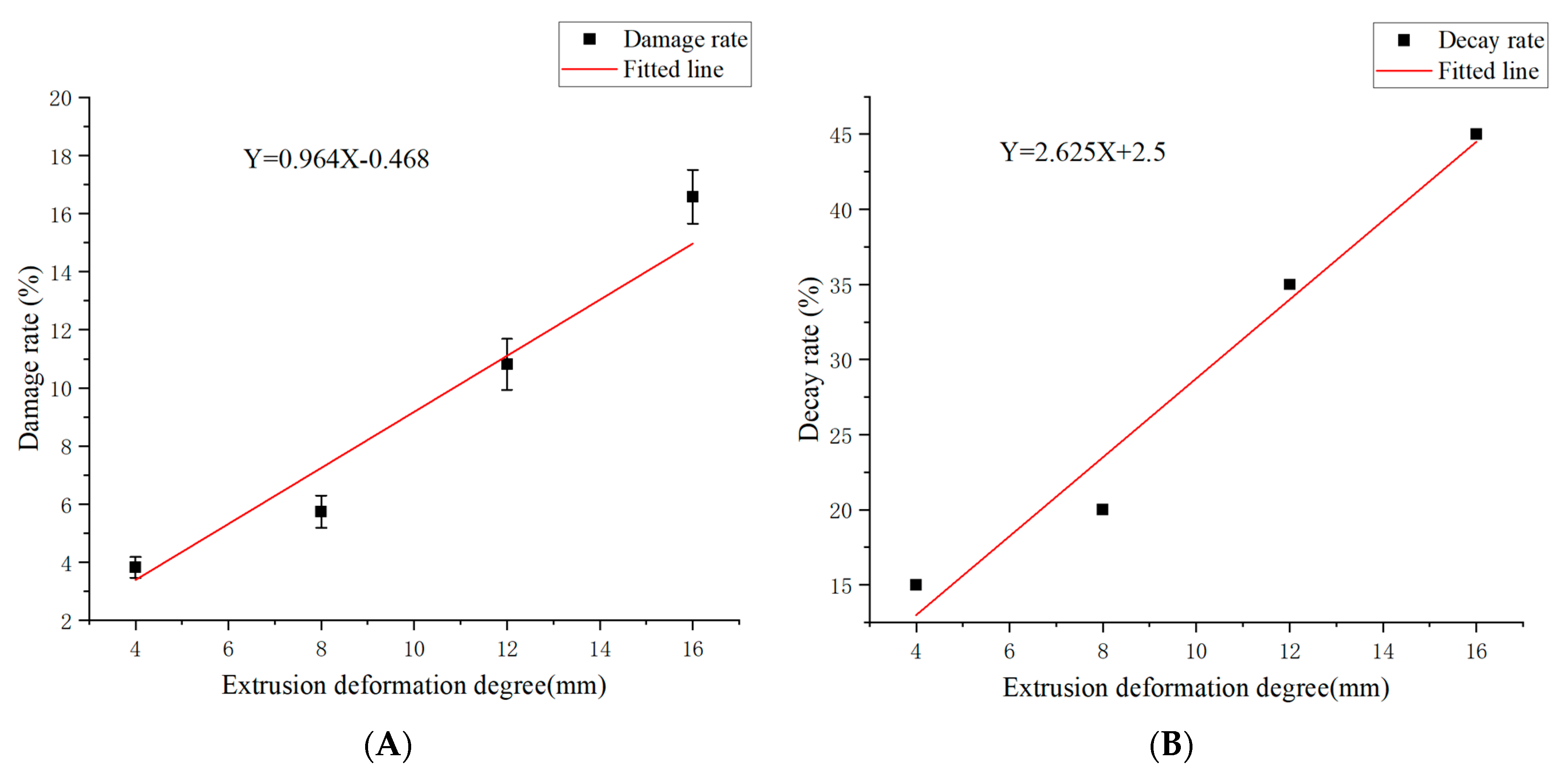
| Compression Deformation Degree (mm) | Damage Rate (%) | Decay Rate (%) |
|---|---|---|
| 4 | 3.82 ± 0.35 a | 15 |
| 8 | 5.74 ± 0.46 b | 20 |
| 12 | 10.81 ± 0.89 c | 35 |
| 16 | 16.58 ± 1.07 d | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, H.; Chen, H.; Li, X. Mechanical Damage Caused by Compression and Its Effects on Storage Quality of Mandarin. Foods 2024, 13, 892. https://doi.org/10.3390/foods13060892
Tian H, Chen H, Li X. Mechanical Damage Caused by Compression and Its Effects on Storage Quality of Mandarin. Foods. 2024; 13(6):892. https://doi.org/10.3390/foods13060892
Chicago/Turabian StyleTian, Haoyu, Hong Chen, and Xiaoxian Li. 2024. "Mechanical Damage Caused by Compression and Its Effects on Storage Quality of Mandarin" Foods 13, no. 6: 892. https://doi.org/10.3390/foods13060892





