Cryoprotective Effects and Quality Maintenance of Antifreeze Proteins and Peptides on Aquatic Products: A Review
Abstract
1. Introduction
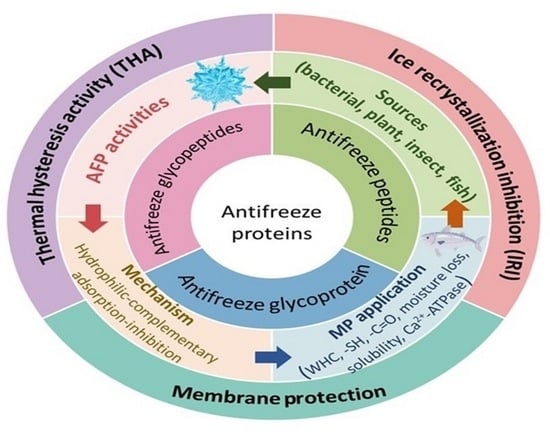
2. Origin of Antifreeze Proteins and Antifreeze Peptides
2.1. Antifreeze Proteins (AFPs)
2.2. Antifreeze Peptides (AFPTs)
3. Model Action of AFPs and AFPTs
3.1. Thermal Hysteresis Activity (THA)
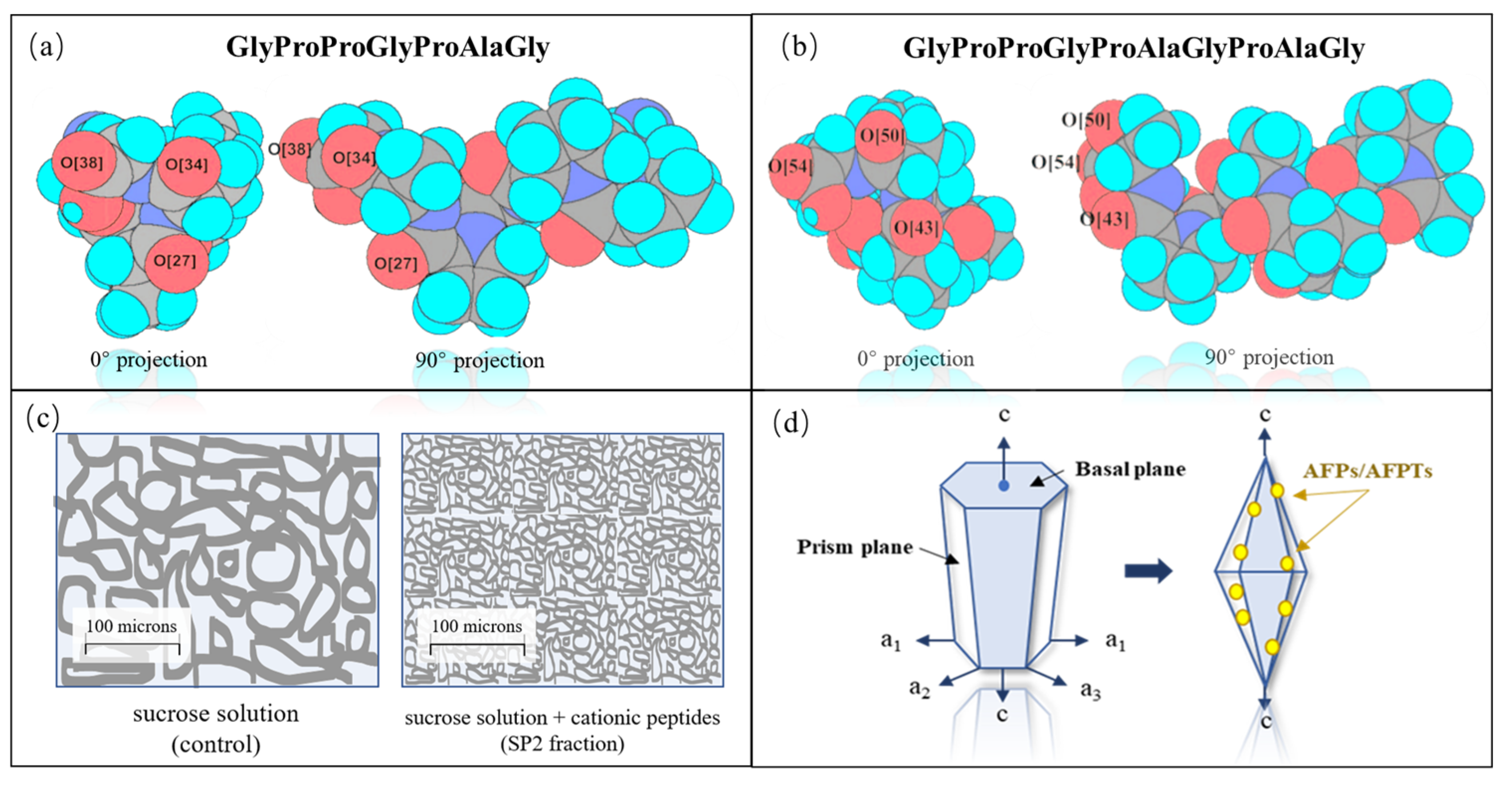
3.2. Inhibition of Ice Recrystallization
3.3. Membrane Protection
3.4. “Hydrophilic-Complementary” Model
3.5. “Adsorption-Inhibition” Theory
4. Freezing Protection of Aquatic Products by AFPs and AFPTs
4.1. Origin Muscle and Fillets
4.2. Mince and Surimi Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, F.J.; Jiang, W.T.; Chen, X.; Wu, J.H.; Huang, J.L.; Cai, X.X.; Wang, S.Y. Investigation on the quality regulating mechanism of antifreeze peptides on frozen surimi: From macro to micro. Food Res. Int. 2023, 163, 112299. [Google Scholar] [CrossRef]
- Huang, X.; Sun, L.; Liu, L.; Wang, G.; Luo, P.; Tang, D.; Huang, Q. Study on the mechanism of mulberry polyphenols inhibiting oxidation of beef myofibrillar protein. Food Chem. 2022, 372, 131241. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhang, L.T.; Gao, S.; Bao, Y.L.; Tan, Y.Q.; Luo, Y.K.; Li, X.M.; Hong, H. Effect of protein oxidation in meat and exudates on the water holding capacity in bighead carp (Hypophthalmichthys nobilis) subjected to frozen storage. Food Chem. 2022, 370, 131079. [Google Scholar] [CrossRef]
- Jiang, Q.Q.; Huang, S.Y.; Du, Y.F.; Xiao, J.B.; Wang, M.F.; Wang, X.C.; Shi, W.Z.; Zhao, Y.L. Quality improvement of tilapia fillets by light salting during repeated freezing-thawing: Contribution of structural rearrangement and molecular interactions. Food Chem. 2023, 406, 135097. [Google Scholar] [CrossRef]
- Zhang, G.P.; Zhu, C.Y.; Walayat, N.; Tang, W.; Tu, Y.G.; Ding, Y.T.; Liu, J.H. Effect of cryoprotectants on physicochemical and structural changes in repeated freeze-thawed egg white protein. Food Biosci. 2023, 55, 102913. [Google Scholar] [CrossRef]
- Du, L.H.; Betti, M. Identification and evaluation of cryoprotective peptides from chicken collagen: Ice-growth inhibition activity compared to that of type I antifreeze proteins in sucrose model systems. J. Agric. Food Chem. 2016, 64, 5232–5240. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Yang, W.G.; Wei, H.M.; Deng, S.G.; Yu, X.X.; Huang, T. The mechanisms and applications of cryoprotectants in aquatic products: An overview. Food Chem. 2023, 408, 135202. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; He, S.F.; Sun, Y.Y.; Pan, D.D.; Zhou, C.Y.; He, J. Effectiveness of l-arginine/l-lysine in retarding deterioration of structural and gelling properties of duck meat myofibrillar protein during freeze-thaw cycles. Food Biosci. 2023, 51, 102302. [Google Scholar] [CrossRef]
- Xiang, H.; Yang, X.H.; Ke, L.; Hu, Y. The properties, biotechnologies, and applications of antifreeze proteins. Int. J. Biol. Macromol. 2020, 153, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, A.; Kaari, M.; Venugopal, G.; Manikkam, R.; Joseph, J.; Bhaskar, P.V. Anti freeze proteins (Afp): Properties, sources and applications-A review. Int. J. Biol. Macromol. 2021, 189, 292–305. [Google Scholar] [CrossRef]
- Tirado-Kulieva, V.A.; Miranda-Zamora, W.R.; Hernández-Martínez, E.; Pantoja-Tirado, L.R.; Bazán-Tantaleán, D.L.; Camacho-Orbegoso, E.W. Effect of antifreeze proteins on the freeze-thaw cycle of foods: Fundamentals, mechanisms of action, current challenges and recommendations for future work. Heliyon 2022, 8, e10973. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Nakamura, N.; Omiya, K.; Nishikawa, J.; Kawahara, H.; Obata, H. Identification of an antifreeze lipoprotein from Moraxella sp. of antarctic origin (microbiology & fermentation technology). Biosci. Biotech. Bioch. 2002, 66, 239–247. [Google Scholar] [CrossRef][Green Version]
- Middleton, A.J.; Marshall, C.B.; Faucher, F.; Maya, B.-D.; Braslavsky, I.; Campbell, R.L.; Walker, V.K.; Davies, P.L. Antifreeze protein from freeze-tolerant grass has a beta-roll fold with an irregularly structured ice-binding site. J. Mol. Biol. 2012, 416, 713–724. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhao, J.; Chen, L.; Zhou, Y.F.; Wu, J.H. Preparation, isolation and hypothermia protection activity of antifreeze peptides from shark skin collagen. LWT-Food Sci. Technol. 2014, 55, 210–217. [Google Scholar] [CrossRef]
- Cao, H.; Zhao, Y.L.; Zhu, Y.B.; Xu, F.; Yu, J.S.; Yuan, M. Antifreeze and cryoprotective activities of ice-binding collagen peptides from pig skin. Food Chem. 2016, 194, 1245–1253. [Google Scholar] [CrossRef]
- Cao, L.; Majura, J.J.; Liu, L.; Cao, W.H.; Chen, Z.Q.; Zhu, G.P.; Gao, J.L.; Zheng, H.N.; Lin, H.S. The cryoprotective activity of tilapia skin collagen hydrolysate and the structure elucidation of its antifreeze peptide. LWT-Food Sci. Technol. 2023, 179, 114670. [Google Scholar] [CrossRef]
- Cao, S.Q.; Cai, J.X.; Wang, X.Z.; Zhou, K.N.; Liu, L.; He, L.Y.; Qi, X.Y.; Yang, H. Cryoprotective effect of collagen hydrolysates from squid skin on frozen shrimp and characterizations of its antifreeze peptides. LWT-Food Sci. Technol. 2023, 174, 114443. [Google Scholar] [CrossRef]
- Damodaran, S.; Wang, S.Y. Ice crystal growth inhibition by peptides from fish gelatin hydrolysate. Food Hydrocolloid. 2017, 70, 46–56. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Gavlighi, H.A.; Xu, X.M.; Regenstein, J.M. Hydrolysates from rainbow trout (Oncorhynchus mykiss) processing by-products: Properties when added to fish mince with different freeze-thaw cycles. Food Biosci. 2019, 30, 100418. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S. Potential application of seafood-derived peptides as bifunctional ingredients, antioxidant–cryoprotectant: A review. J. Funct. Foods. 2015, 19, 753–764. [Google Scholar] [CrossRef]
- Wang, S.Y.; Damodaran, S. Ice-structuring peptides derived from bovine collagen. J. Agric. Food Chem. 2009, 57, 5501–5509. [Google Scholar] [CrossRef]
- Damodaran, S. Inhibition of ice crystal growth in ice cream mix by gelatin hydrolysate. J. Agric. Food Chem. 2007, 55, 10918–10923. [Google Scholar] [CrossRef]
- Nikoo, M.; Regenstein, J.M.; Ghomi, M.R.; Benjakul, S.; Yang, N.; Xu, X.M. Study of the combined effects of a gelatin-derived cryoprotective peptide and a non-peptide antioxidant in a fish mince model system. LWT-Food Sci. Technol. 2015, 60, 358–364. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Ehsani, A.; Li, J.; Wu, F.F.; Yang, N.; Xu, B.C.; Jin, Z.Y.; Xu, X.M. Antioxidant and cryoprotective effects of a tetrapeptide isolated from Amur sturgeon skin gelatin. J. Funct. Foods. 2014, 7, 609–620. [Google Scholar] [CrossRef]
- Cao, W.Q.; Liu, M.Q.; Kong, S.Y.; Wu, M.X.; Zhang, Y.; Yang, P.Y. Recent advances in software tools for more generic and precise intact glycopeptide analysis. Mol. Cell. Proteom. 2021, 20, 100060. [Google Scholar] [CrossRef] [PubMed]
- Ochi, R. Antifreeze glycopeptide-functionalized amphiphilic molecules showing self-assembly and ice recrystallization inhibition activity. Trends. Glycosci. Glyc. 2019, 31, E137–E138. [Google Scholar] [CrossRef]
- Adam, M.K.; Jarrett-Wilkins, C.; Beards, M.; Staykov, E.; Macfarlane, L.R.; Bell, T.D.M.; Matthews, J.M.; Manners, I.; Faul, C.F.J.; Moens, P.D.J. 1D self-assembly and ice recrystallization inhibition activity of antifreeze glycopeptide-functionalized perylene bisimides. Chem.-Eur. J. 2018, 24, 7834–7839. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.Y. Cryoprotective effect of antifreeze glycopeptide analogues obtained by nonenzymatic glycation on Streptococcus thermophilus and its possible action mechanism. Food Chem. 2019, 288, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Abed-Elmdoust, A.; Uysal, O.; Rahimi, R.; Farahmand, Y. Antifreeze proteins are robust cryoprotectants for sperm cryopreservation in fishes: A systematic review and meta-analysis. Aquaculture 2021, 534, 736250. [Google Scholar] [CrossRef]
- Li, L.F.; Liang, X.X. The influence of adsorption orientation on the statistical mechanics model of type I antifreeze protein: The coverage rate. Phys. A 2015, 421, 355–359. [Google Scholar] [CrossRef]
- Jia, Z.C.; Davies, P.L. Antifreeze proteins: An unusual receptor-ligand interaction. Trends Biochem. Sci. 2002, 27, 101–106. [Google Scholar] [CrossRef]
- Nicodemus, J.; O’Tousa, J.E.; Duman, J.G. Expression of a beetle, Dendroides canadensis, antifreeze protein in Drosophila melanogaster. J. Insect. Physiol. 2006, 52, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Takamichi, M.; Nishimiya, Y.; Miura, A.; Tsuda, S. Effect of annealing time of an ice crystal on the activity of type III antifreeze protein. Febs J. 2007, 274, 6469–6476. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Fomich, M.; Dia, V.P.; Wang, T. Succinylation of zein and gelatin hydrolysates improved their ice recrystallization inhibition activity. Food Chem. 2023, 424, 136431. [Google Scholar] [CrossRef] [PubMed]
- Monalisa, K.; Shibata, M.; Hagiwara, T. Ice recrystallization inhibition behavior by wheat flour and its synergy effect with antifreeze protein III. Food Hydrocolloid. 2023, 143, 108882. [Google Scholar] [CrossRef]
- Leiter, A.; Rau, S.; Winger, S.; Muhle-Goll, C.; Luy, B.; Gaukel, V. Influence of heating temperature, pressure and pH on recrystallization inhibition activity of antifreeze protein type III. J. Food Eng. 2016, 187, 53–61. [Google Scholar] [CrossRef]
- Gaukel, V.; Leiter, A.; Spieß, W.E.L. Synergism of different fish antifreeze proteins and hydrocolloids on recrystallization inhibition of ice in sucrose solutions. J. Food Eng. 2014, 141, 44–50. [Google Scholar] [CrossRef]
- Tatsuro, K.; Mami, S.; Ai, M.; Yoshiyuki, N.; Sakae, T.; Matsuo, U. Antifreeze Protein Prolongs the Life-Time of Insulinoma Cells during Hypothermic Preservation. PLoS ONE 2013, 8, e73643. [Google Scholar] [CrossRef]
- Tomczak, M.M.; Hincha, D.K.; Estrada, S.D.; Wolkers, W.F.; Crowe, J.H. A mechanism for stabilization of membranes at low temperatures by an antifreeze protein. Biophys. J. 2002, 82, 874–881. [Google Scholar] [CrossRef]
- Raymond, J.A.; Devries, A.L. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc. Natl. Acad. Sci. USA 1977, 74, 2589–2593. [Google Scholar] [CrossRef]
- Kong, C.H.Z.; Hamid, N.; Liu, T.T.; Sarojini, V. Effect of antifreeze peptide pretreatment on ice crystal size, drip loss, texture, and volatile compounds of frozen carrots. J. Agric. Food Chem. 2016, 64, 4327–4335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.T.; Shan, Y.k.; Hong, H.; Luo, Y.K.; Hong, X.H.; Ye, W.J. Prevention of protein and lipid oxidation in freeze-thawed bighead carp (Hypophthalmichthys nobilis) fillets using silver carp (Hypophthalmichthys molitrix) fin hydrolysates. LWT-Food Sci. Technol. 2020, 123, 109050. [Google Scholar] [CrossRef]
- Luo, W.; Yuan, C.Z.; Wu, J.H.; Liu, Y.L.; Wang, F.X.; Li, X.H.; Wang, S.Y. Inhibition mechanism of membrane-separated silver carp hydrolysates on ice crystal growth obtained through experiments and molecular dynamics simulation. Food Chem. 2023, 414, 135695. [Google Scholar] [CrossRef]
- Nian, L.Y.; Cao, A.L.; Cai, L.Y. Investigation of the antifreeze mechanism and effect on quality characteristics of largemouth bass (Micropterus salmoides) during F-T cycles by hAFP. Food Chem. 2020, 325, 126918. [Google Scholar] [CrossRef]
- Jenkelunas, P.J.; Li-Chan, E.C.Y. Production and assessment of Pacific hake (Merluccius productus) hydrolysates as cryoprotectants for frozen fish mince. Food Chem. 2018, 239, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Cao, S.A.; Zhu, Z.X.; Hu, B.; Chen, H.; Tu, M.L.; Tan, Z.J.; Du, M.; Li, T.T. Characterization and the mechanism underlying the cryoprotective activity of a peptide from large yellow croaker (Pseudosciaena crocea). Food Chem. 2024, 435, 137512. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.J.; Liu, Y.L.; Yu, J.; Wang, F.X.; Li, X.H. Identification and characterization of cryoprotective peptides extracted from silver carp (Hypophthalmichthys molitrix) hydrolysates. Int. J. Food Prop. 2019, 22, 1011–1023. [Google Scholar] [CrossRef]
- Cai, L.Y.; Nian, L.Y.; Zhao, G.H.; Zhang, Y.H.; Sha, L.; Li, J.R. Effect of herring antifreeze protein combined with chitosan magnetic nanoparticles on quality attributes in red sea bream (Pagrosomus major). Food Bioprocess. Tech. 2019, 12, 409–421. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, Z.X.; Tu, M.L.; Chang, J.L.; Han, S.Y.; Han, L.Y.; Chen, H.; Tan, Z.J.; Du, M.; Li, T.T. Characterizations and the mechanism underlying cryoprotective activity of peptides from enzymatic hydrolysates of Pseudosciaena crocea. Foods. 2023, 12, 875. [Google Scholar] [CrossRef]
- Nian, L.Y.; Cao, A.L.; Cai, L.Y.; Ji, H.W.; Liu, S.C. Effect of vacuum impregnation of red sea bream (Pagrosomus major) with herring AFP combined with CS@Fe3O4 nanoparticles during freeze-thaw cycles. Food Chem. 2019, 291, 139–148. [Google Scholar] [CrossRef]
- Yasemi, M. Prevention of denaturation of freshwater crayfish muscle subjected to different freeze-thaw cycles by gelatin hydrolysate. Food Chem. 2017, 234, 199–204. [Google Scholar] [CrossRef]
- Tian, H.; Yang, F.J.; Chen, X.; Guo, L.; Wu, X.P.; Wu, J.H.; Huang, J.L.; Wang, S.Y. Investigation and effect on 3D printing quality of surimi ink during freeze-thaw cycles by antifreeze peptides. J. Food Eng. 2023, 337, 111234. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Cryoprotective effect of gelatin hydrolysate from blacktip shark skin on surimi subjected to different freeze-thaw cycles. LWT-Food Sci. Technol. 2012, 47, 437–442. [Google Scholar] [CrossRef]
- Tian, H.; Chen, X.; Chen, C.R.; Wu, J.H.; Huang, J.L.; Zhao, L.; Wang, S.Y. Analysis of the shape retention ability of antifreeze peptide-based surimi 3D structures: Potential in freezing and thawing cycles. Food Chem. 2023, 405, 134780. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.Z.; Yang, F.J.; Wu, J.H.; Huang, D.; Huang, J.L.; Wang, S.Y. Effects and mechanism of antifreeze peptides from silver carp scales on the freeze-thaw stability of frozen surimi. Food Chem. 2022, 396, 133717. [Google Scholar] [CrossRef]
- Zhou, W.J.; Wang, F.X.; Yu, J.; Li, X.H.; Liu, Y.L. Cryoprotective effects of protein hydrolysates prepared from by-products of silver carp (hypophthalmichthys molitrix) on freeze-thawed surimi. Appl. Sci. 2019, 9, 563. [Google Scholar] [CrossRef]
- Shen, X.L.; Li, T.; Li, X.H.; Wang, F.X.; Liu, Y.L.; Wu, J.H. Dual cryoprotective and antioxidant effects of silver carp (Hypophthalmichthys molitrix) protein hydrolysates on unwashed surimi stored at conventional and ultra-low frozen temperatures. LWT-Food Sci. Technol. 2022, 153, 112563. [Google Scholar] [CrossRef]
- Lin, J.; Hong, H.; Zhang, L.T.; Zhang, C.; Luo, Y.K. Antioxidant and cryoprotective effects of hydrolysate from gill protein of bighead carp (Hypophthalmichthys nobilis) in preventing denaturation of frozen surimi. Food Chem. 2019, 298, 124868. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.J.; Jiang, W.T.; Chen, X.; Chen, X.; Wu, J.H.; Huang, J.L.; Cai, X.X.; Wang, S.Y. Identification of novel antifreeze peptides from Takifugu obscurus skin and molecular mechanism in inhibiting ice crystal growth. J. Agric. Food Chem. 2020, 70, 14148–14156. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, M.; Benjakul, S.; Xu, X.M. Antioxidant and cryoprotective effects of Amur sturgeon skin gelatin hydrolysate in unwashed fish mince. Food Chem. 2015, 181, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.T.; Li, Q.; Hong, H.; Luo, Y.K. Prevention of protein oxidation and enhancement of gel properties of silver carp (Hypophthalmichthys molitrix) surimi by addition of protein hydrolysates derived from surimi processing by-products. Food Chem. 2020, 316, 126343. [Google Scholar] [CrossRef] [PubMed]

| AFPs/AFPTs Resource | Application | Treatment | Concentration | Function | Mechanism | References |
|---|---|---|---|---|---|---|
| Large yellow croaker (Pseudosciaena crocea) meat hydrolysates | Fresh turbot (Scophthalmus maximus) cubes | The F-T cycles (stored at −20 °C for 1 day and thawed at 4 °C for 12 h and ended when the core temperature reached 0–4 °C) three times | 0.5, 1.0, 2.0 mg/mL | Inhibited ice crystal growth and muscle fiber damage and decreased sulfhydryl content and Ca2+-ATPase activity | Reduced MP oxidation caused by ice crystals | [46,49] |
| Squid skin (Dosidicus gigas) collagen hydrolysates | Shrimp (Penaeus penicillatus) muscle | The F-T cycles (frozen at −25 °C for 24 h and thawed at 4 °C) 2, 6, 10 and 14 times | 1% (w/w) | Inhibited effects on the denaturation and structural changes of myofibrillar protein; partially retained the ability to bind water; and offered protection for mechanical injury caused by ice crystals | Retarded protein oxidation | [17] |
| Tilapia (Oreochromis mossambicus) skin collagen hydrolysate | Scallop (Chlamys farreri) adductor | Snap frozen at −75 °C for 2 h and stored at −18 °C for 8 weeks | 0.5, 1, 2, 3 g/100 g | Increased thermal hysteresis activity, exhibited a higher salt soluble protein concentration, total sulfhydryl content, Ca2+-ATPase activity, and water-holding capacity | Reduced MP denaturation | [16] |
| Herring (Clupea harengus) skin type I AFP | Red sea bream meat pieces (Pagrosomus major) | The F-T cycles (frozen at −20 °C for 24 h and thawed at 4 °C for 12 h) once, thrice, and five times | 0.1% (w/v) | Improved thermal stability and viscoelasticity and restricted water mobility and distribution | Inhibited MP oxidation and aggregation | [50] |
| Largemouth bass (Micropterus salmoides) fillets | Soaked at 4 °C for 12 h, frozen at −20 °C for 24 h, and thawed at 4 °C overnight | 0.05, 0.08, 0.1, 0.5% (w/v) | Inhibited ice crystal growth, modified ice crystal sharpness, increased thermal stability, and improved the spatial network structure of protein | Prevented MP oxidation and aggregation | [48] | |
| The F-T cycles (frozen at −20 °C for 24 h and thawed at 4 °C for 12 h) 3 times | 0.1% (w/v) | Reduced the freezing point and generated thermal hysteresis | Delayed MP degradation | [44] | ||
| Silver carp (Hypophthalmichthys molitrix) fin hydrolysates | Bighead carp (Hypophthalmichthys nobilis) fillets | The F-T cycles (stored at −18 °C for 1 week and thawed at 4 °C until the core temperature to 0 °C) 0, 2, 4, and 6 times | 2% (w/v) | Exhibited in vitro scavenging activity (ABTS radicals) and chelating activity to ferrous ions and inhibited the formation of carbonyls and disulfide bonds and the loss of Ca2+-ATPase activity | Reduced protein/lipid oxidation and degradation | [42] |
| Beluga sturgeon (Huso huso) skin gelatin hydrolysates | Freshwater crayfish (Astacus leptodactylus) muscle | The F-T cycles (stored at −18 °C) 6 times | 8% (w/v) | Reduced secondary lipid oxidation and the loss of in sulfhydryl groups and Ca2+-ATPase activity; scavenged free radicals and chelated ferrous ions | Inhibited myosin heavy chain denaturation and impeded lipid oxidation | [51] |
| AFPs/AFPTs Resource | Application | Treatment | Concentration | Function | Mechanism | References |
|---|---|---|---|---|---|---|
| Silver carp (Hypophthalmichthys molitrix) scale hydrolysates | Grass carp (Ctenopharyngodon idella) surimi | The F-T cycles (stored at −20 °C for 72 h and thawed at 25 °C for 1 h) 5 times | 2, 4, 6, 8% | Delayed sulfhydryl oxidation, the carbonylation of amino acids, and the exposure of hydrophobic amino acids by inhibiting ice crystal growth | Delayed MP degradation | [55] |
| Grass/Sliver carp surimi ink for 3D printing | The F-T cycles (stored at −20 °C for 5 days and thawed at 25 °C for 4 h) 4 times | 1, 2, 4, 6, 8% (w/w) | Protected the rheological properties of surimi ink (viscosity, τ0, shear-thinning characteristics, viscosity recovery, temperature recovery, and modulus) | Provided more interaction points for protein | [52,54] | |
| Sliver carp meat hydrolysates | Sliver carp surimi | The F-T cycles (stored at −25 ± 1 °C for 24 h and thawed at 4 ± 1 °C for 12 h) 6 times | 2 g/100 g | Displayed lower salt-soluble protein extractability loss, less actomyosin Ca2+-ATPase activity decrease, and unfrozen water content decrease | Protected against MP denaturation; absorbed the ice surface; and inhibited ice crystallization | [47] |
| Sliver carp by-product (fish meat leftovers on bones and heads) hydrolysates | 2, 4, 6 g/100 g | Displayed higher actomyosin extractability, Ca2+-ATPase activity, and, correspondingly, lower surface hydrophobicity of actomyosin; presented comparable textures for gels | Decreased protein denaturation/aggregation and improved gel-forming capacity | [56] | ||
| Sliver carp protein hydrolysates | Unwashed sliver carp surimi | The F-T cycles (stored at −18/−60 °C for 12 h and thawed at 4 °C for 12 h) 3 and 6 times | 0.4, 0.8 g/100 mL MP solution | Alleviated carbonyl content for MP, decreased free sulfhydryl content, and maintained the protein bands’ stability; slowed down lipid peroxide malondialdehyde formation and flavor compound change rates in unwashed surimi; and interacted with the proteins to alleviate the water loss and structural collapse of the gel | Inhibited MP oxidation, aggregation and denaturation, and lipid oxidation | [57] |
| Bighead carp (Hypophthalmichthys nobilis) gill protein hydrolysates | Silver carp surimi | Frozen at −18 °C for 4 months | 1, 2% (w/w) | Improved the texture, and the properties reduced the decrease in salt-soluble protein content and Ca2+-ATPase activity | Scavenged free radicals and chelate metal ions | [58] |
| Takifugu obscurus skin | Grass carp surimi | The F-T cycles (frozen at −25 °C for 3 days and thawed at 25 °C water bath) 5 times | 2, 4, 6, 8% (w/w) | Inhibited the growth and recrystallization of ice crystals, prevented protein denaturation, and maintained the spatial structure and water retention ability of proteins | Inhibited protein freezing-induced oxidation | [1,59] |
| Pacific hake (Merluccius productus) protein hydrolysates | Pacific cod (Gadus macrocephalus) mince and fish ball | The F-T cycles (stored at −25 °C for 18 h and thawed at 4 °C for 6 h) 6 times | 2, 4, 6, 8% (w/w) | Improved water-holding capacity and thermal stability, retarded salt extractable protein degeneration, and enhanced flavor and texture | Indicated the protein stabilization and cryoprotective effect | [45] |
| Amur sturgeon (Acipenser schrenckii) skin gelatin hydrolysate | Japanese sea bass (Lateolabrax japonicas) unwashed mince | The F-T cycles (frozen at −18 °C for 20 h and thawed at 4 °C for 4 h) 3 and 6 times | 8% (w/w) | Stabilized the water associated with myofibrils, retarded protein carbonyl formation, and caused lower loss of sulfhydryl content | Protected against protein and lipid oxidation | [60] |
| Amur sturgeon skin gelatin tetrapeptide (Pro-Ala-Gly-Tyr) | Japanese sea bass mince | The F-T cycles (frozen at −14 °C for 18 h and thawed at 4 °C for 6 h) 6 times | 25, 50, 100, 200 ppm | Scavenged activity against DPPH, ABTS, and hydroxyl radicals, prevented lipid oxidation, influenced water distribution, and decreased myosin and actin denaturation | Showed antioxidative and cryoprotective effects | [24] |
| Rainbow trout (Oncorhynchus mykiss) processing by-product hydrolysates | Asian seabass (Lates calcarifer) mince | Pre-frozen at −18 °C for 24 h and underwent F-T cycles (frozen at −18 °C for 18 h and thawed at 4 °C for 6 h) 6 times | 4, 8% | Decreased the loss of total sulfhydryl groups and protein solubility and protein carbonyl formation | Maintained the thermal properties and water bonding ability of the MP | [19] |
| Surimi processing by-products (fish head, skin, scale, bone, dark muscle, etc.) hydrolysates | Silver carp surimi | Surimi paste frozen at −18 °C 3 months | 2% | Retarded sulfhydryl oxidation, carbonylation, myosin denaturation, and the exposure of hydrophobic amino acids and improved the gelation properties and water-holding capacity of gels | Chemical antioxidants and cryoprotectants | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Geng, W.; Li, M.; Wu, Z.; Ma, Y.; Li, Z.; Benjakul, S.; Zhao, Q. Cryoprotective Effects and Quality Maintenance of Antifreeze Proteins and Peptides on Aquatic Products: A Review. Foods 2024, 13, 917. https://doi.org/10.3390/foods13060917
Fan X, Geng W, Li M, Wu Z, Ma Y, Li Z, Benjakul S, Zhao Q. Cryoprotective Effects and Quality Maintenance of Antifreeze Proteins and Peptides on Aquatic Products: A Review. Foods. 2024; 13(6):917. https://doi.org/10.3390/foods13060917
Chicago/Turabian StyleFan, Xinru, Wenhao Geng, Meng Li, Zixuan Wu, Yongsheng Ma, Zhibo Li, Soottawat Benjakul, and Qiancheng Zhao. 2024. "Cryoprotective Effects and Quality Maintenance of Antifreeze Proteins and Peptides on Aquatic Products: A Review" Foods 13, no. 6: 917. https://doi.org/10.3390/foods13060917
APA StyleFan, X., Geng, W., Li, M., Wu, Z., Ma, Y., Li, Z., Benjakul, S., & Zhao, Q. (2024). Cryoprotective Effects and Quality Maintenance of Antifreeze Proteins and Peptides on Aquatic Products: A Review. Foods, 13(6), 917. https://doi.org/10.3390/foods13060917