Resistant Starch from Purple Sweet Potatoes Alleviates Dextran Sulfate Sodium-Induced Colitis through Modulating the Homeostasis of the Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Starch Isolation
2.3. Preparation of Resistant Starch
2.4. Chemical Composition
2.5. Physicochemical Properties
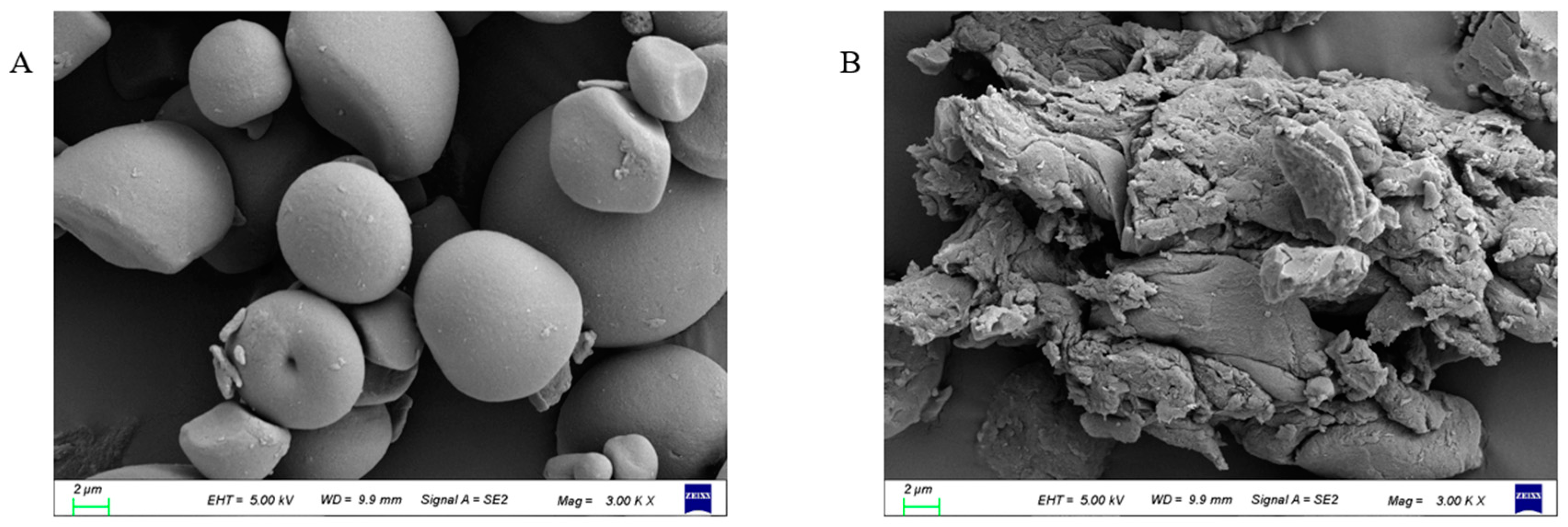
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Color of Starch
2.5.3. Water-Binding Capacity (WBC)
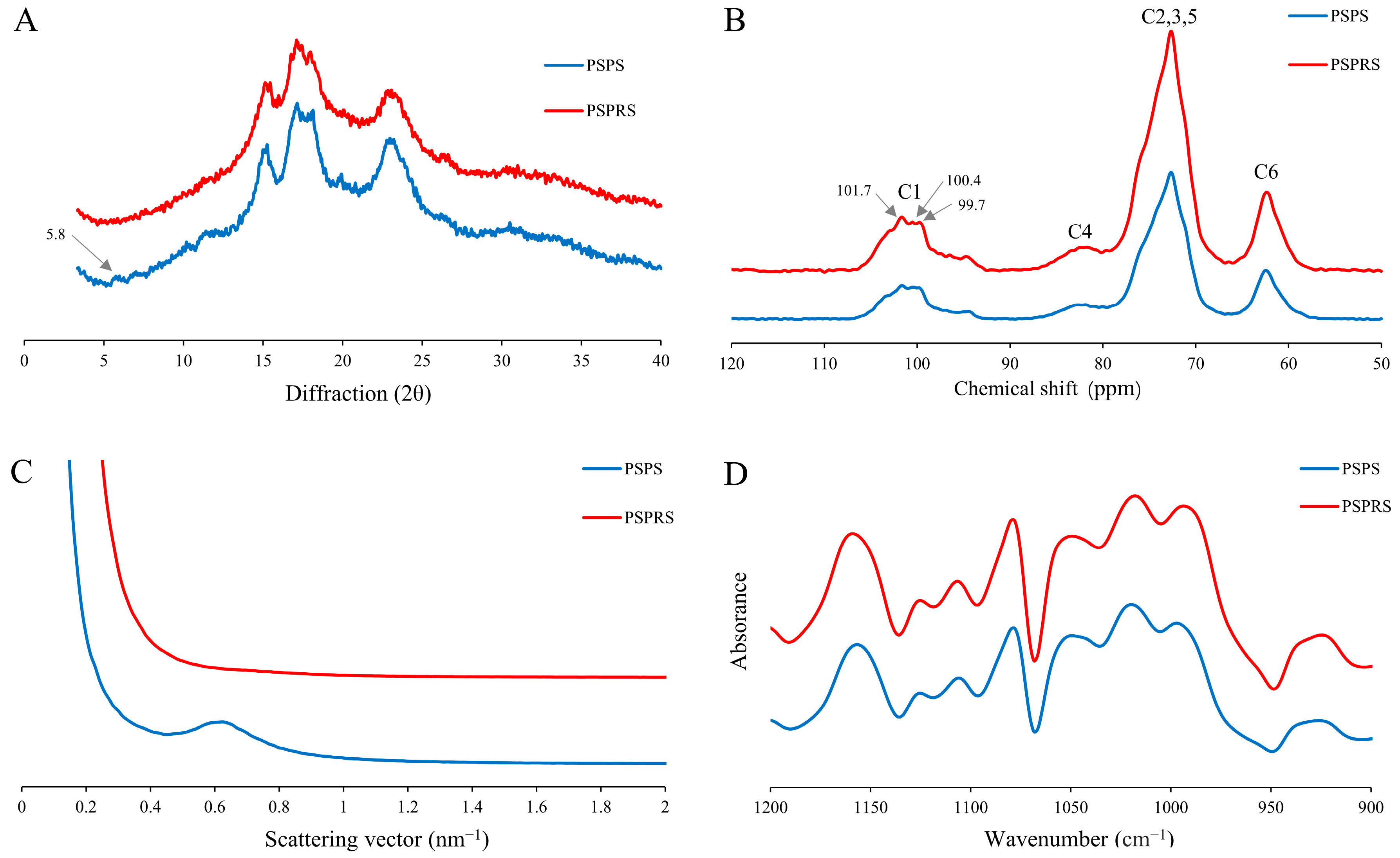
2.5.4. X-ray Powder Diffractometry (XRD)
2.5.5. Solid-State Cross-Polarization Magic Angle Spinning Nuclear Magnetic Resonance (13C CP/MAS NMR)
2.5.6. Small-Angle X-ray Scattering (SAXS)
2.5.7. Fourier-Transform Infrared Spectroscopy (FTIR)
2.6. Animals and Treatments
2.6.1. Animal Experimental Design
2.6.2. Immune Organ Index Analysis
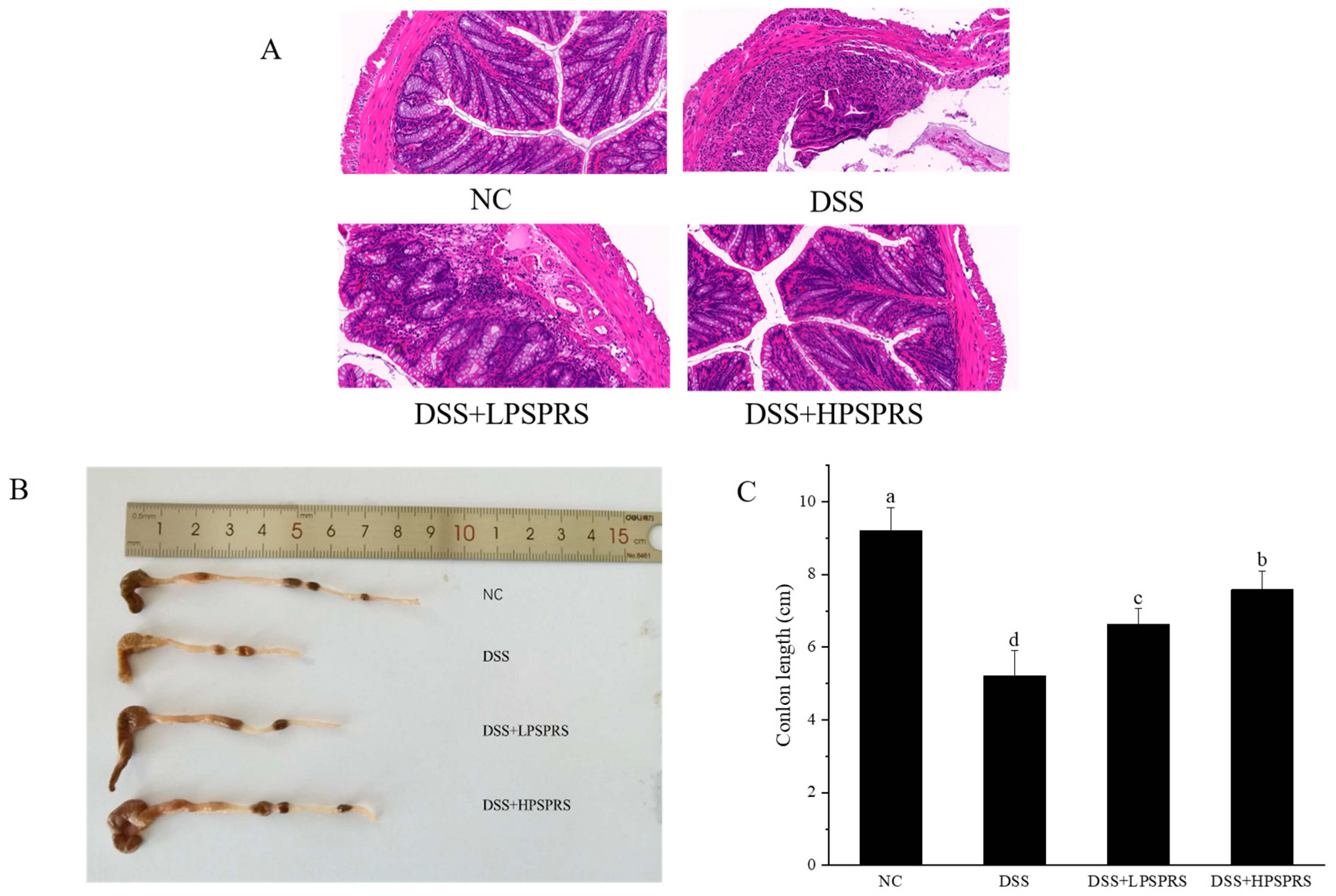
2.6.3. Histological Analysis
2.6.4. Measurement of Cytokines and IgA in the Colon
2.7. Short-Chain Fatty Acid Analysis
2.8. High-Throughput 16S rRNA Sequencing
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Color Parameters
3.3. Morphological Characteristics
3.4. Structural Characterization
3.5. Effects of PSPRS on Immune Organ Indices
3.6. Histological Characterization
3.7. Effects of PSPRS on Inflammatory Cytokines in DSS-Induced Colitis Mice
3.8. Effects of PSPRS on the Production of SCFAs in DSS-Induced Colitis Mice
3.9. Effect of PSPRS on the Gut Microbiota in DSS-Induced Colitis Mice
3.9.1. Operational Taxonomic Unit (OTU) Analysis
3.9.2. PLS-DA Analysis
3.9.3. Alpha Diversity Analysis
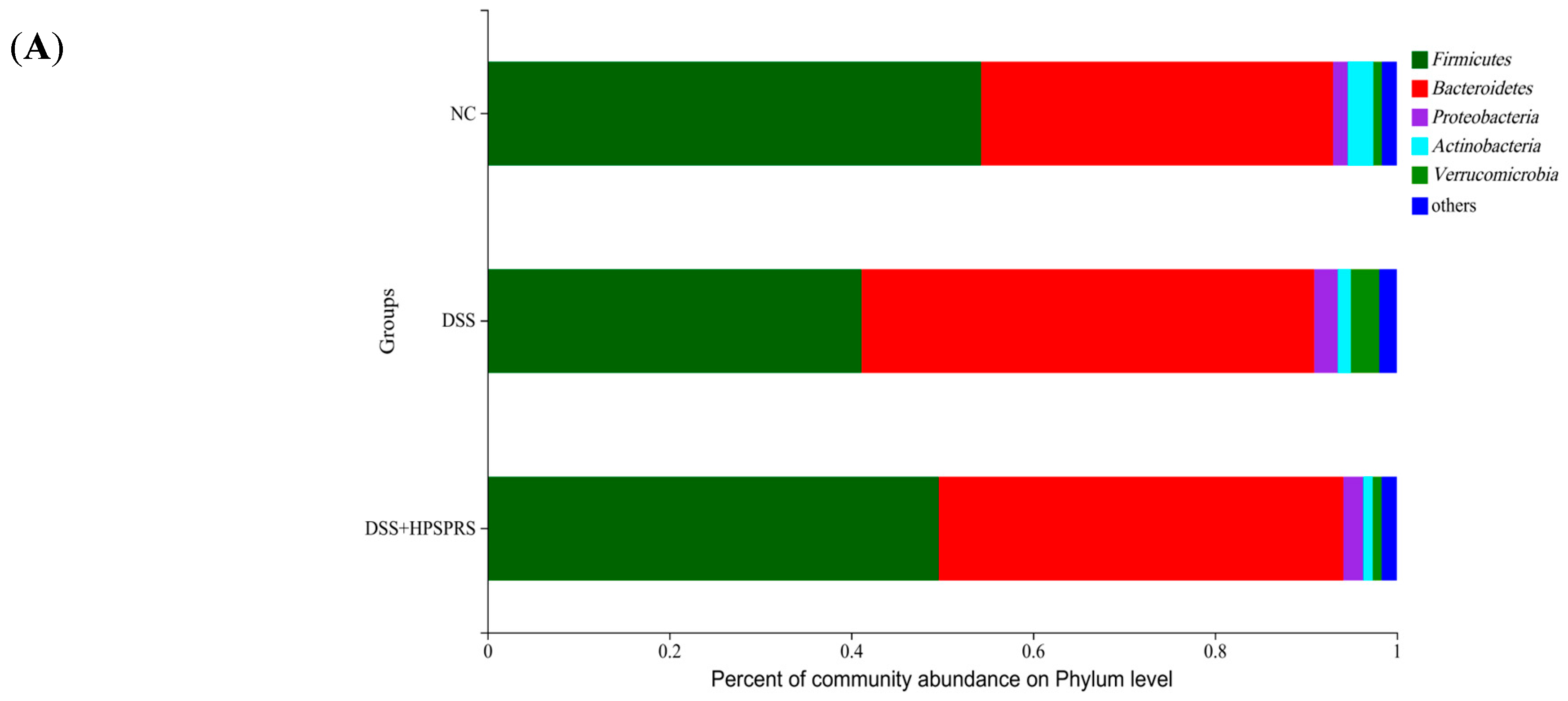
3.9.4. Composition and Abundance of Gut Microflora at the Phylum Level
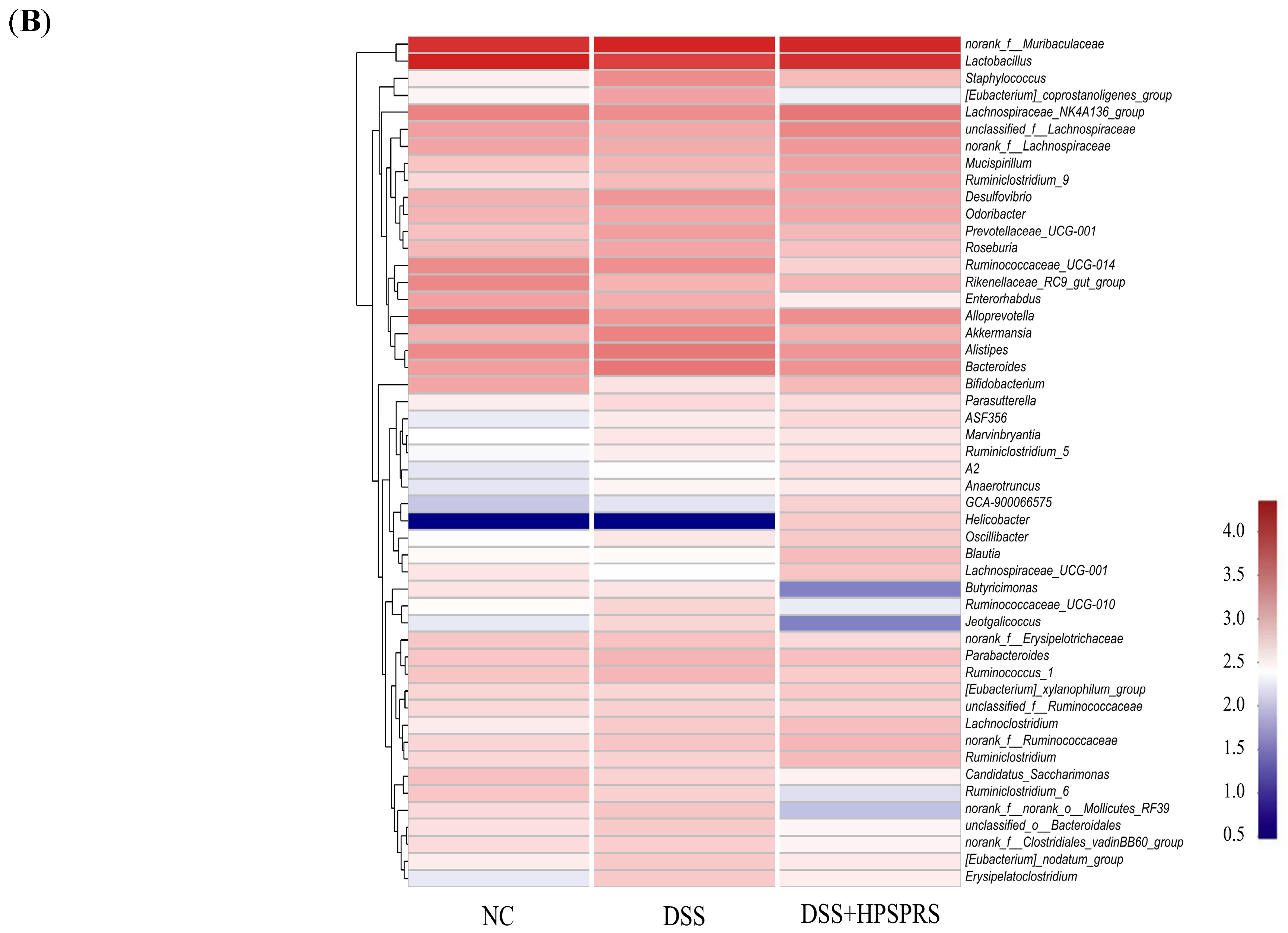
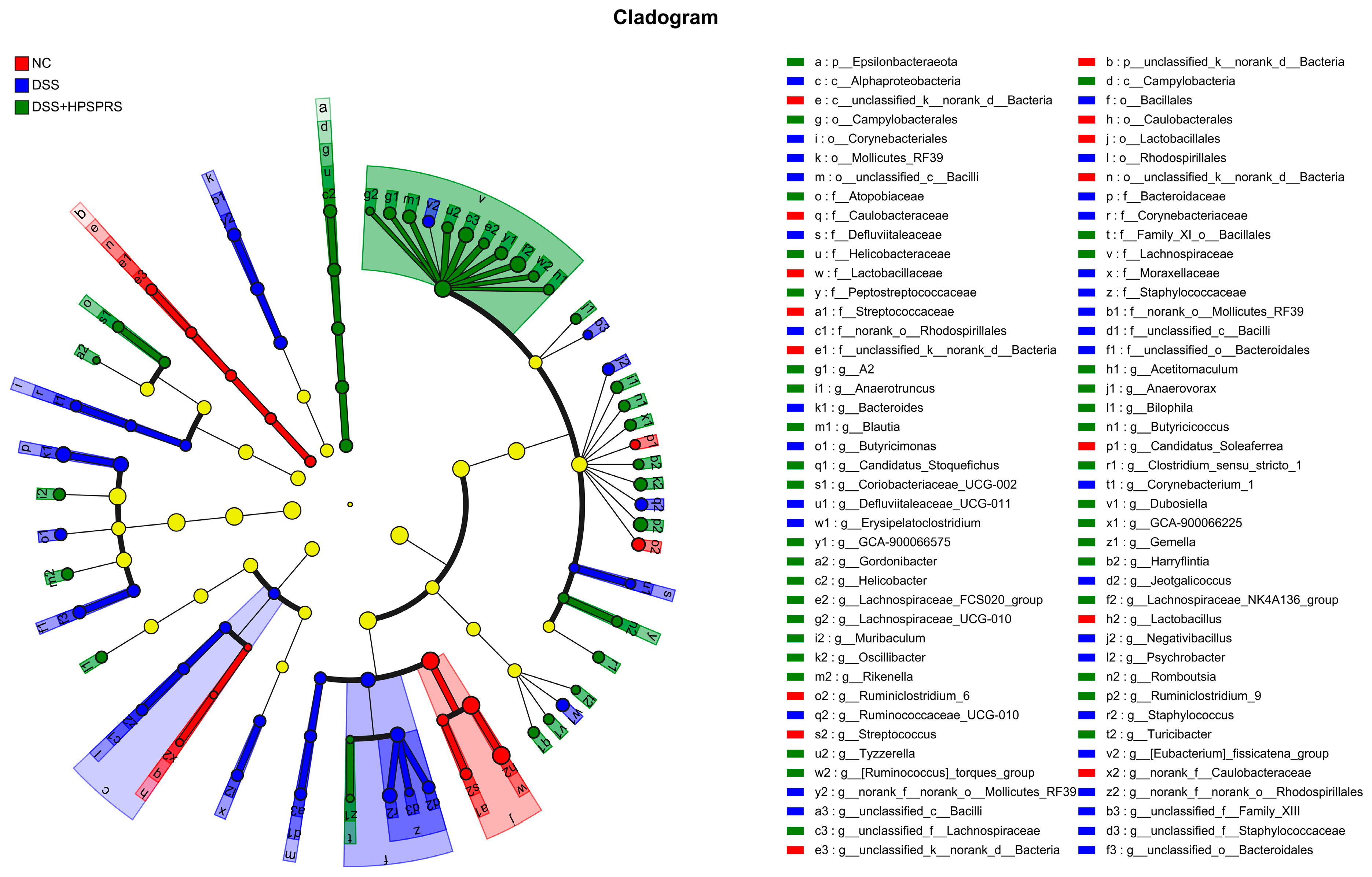
3.9.5. Analysis of Gut Microflora at the Genus Level
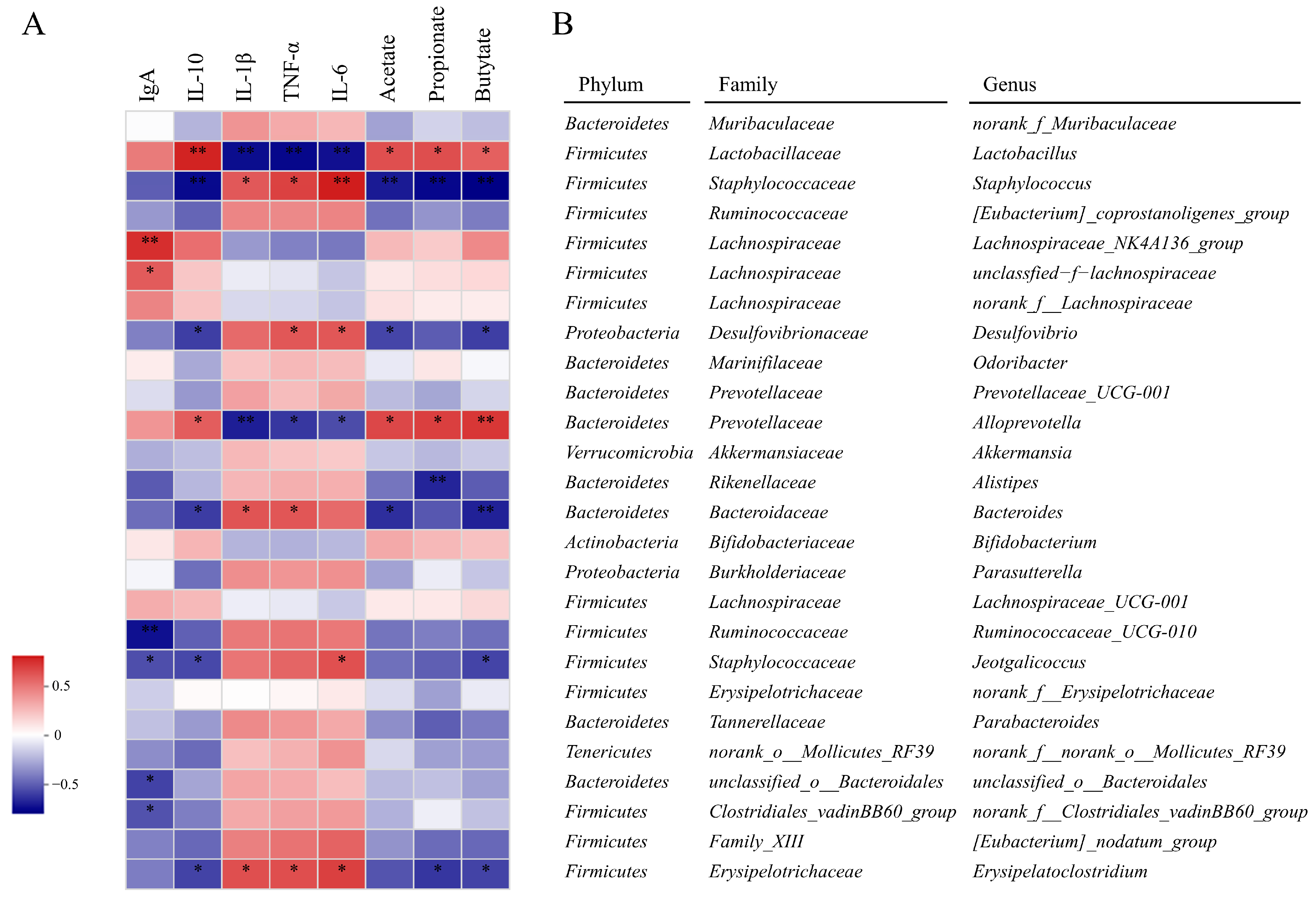
3.9.6. Correlations between the Gut Microbiota and UC Parameters
3.10. Analysis of 16S Function Prediction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, Z.; Xie, Z.; Chen, G.; Sun, Y.; Zeng, X.; Liu, Z. Anti-inflammatory and gut microbiota modulatory effects of polysaccharides from Fuzhuan brick tea on colitis in mice induced by dextran sulfate sodium. Food Funct. 2022, 13, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Ng, S.C. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017, 152, 313–321. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, Z.; He, Y.; Gu, C.; Zhu, K.; Li, S.; Li, Y.; Lu, X.; Liu, J.; Zhang, N.; et al. Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage. Life Sci. 2018, 196, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Liu, C.; Jiang, S. Cross Talk between gut microbiota and intestinal mucosal immunity in the development of ulcerative colitis. Infect. Immun. 2021, 89, e00014-21. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cheng, Z.; Sun, X.; Si, X.; Gong, E.; Wang, Y.; Tian, J.; Shu, C.; Ma, F.; Meng, X. Lonicera caerulea L. polyphenols alleviate oxidative stress-induced intestinal environment imbalance and lipopolysaccharide-induced liver injury in HFD-fed rats by regulating the Nrf2/HO-1/NQO1 and MAPK pathways. Mol. Nutr. Food Res. 2020, 64, 1901315. [Google Scholar] [CrossRef]
- Montroy, J.; Berjawi, R.; Lalu, M.M.; Podolsky, E.; Peixoto, C.; Sahin, L.; Stintzi, A.; Mack, D.; Fergusson, D.A. The effects of resistant starches on inflammatory bowel disease in preclinical and clinical settings: A systematic review and meta-analysis. BMC Gastroenterol. 2020, 20, 372. [Google Scholar] [CrossRef]
- Niu, W.; Chen, X.; Xu, R.; Dong, H.; Yang, F.; Wang, Y.; Zhang, Z.; Ju, J. Polysaccharides from natural resources exhibit great potential in the treatment of ulcerative colitis: A review. Carbohydr. Polym. 2021, 254, 117189. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Nishida, A.; Nishino, K.; Sakai, K.; Owaki, Y.; Noda, Y.; Imaeda, H. Can control of gut microbiota be a future therapeutic option for inflammatory bowel disease? World J. Gastroenterol. 2021, 27, 3317–3326. [Google Scholar] [CrossRef]
- Barberio, B.; Facchin, S.; Patuzzi, I.; Ford, A.C.; Massimi, D.; Valle, G.; Sattin, E.; Simionati, B.; Bertazzo, E.; Zingone, F.; et al. A specific microbiota signature is associated to various degrees of ulcerative colitis as assessed by a machine learning approach. Gut Microbes 2022, 14, 2028366. [Google Scholar] [CrossRef]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Q.; Ferdinand, U.; Gong, X.; Qu, Y.; Gao, W.; Ivanistau, A.; Feng, B.; Liu, M. Isolation and characterization of starch from light yellow, orange, and purple sweet potatoes. Int. J. Biol. Macromol. 2020, 160, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, T.; Wu, B.; Fu, W.; Xu, B.; Pamuru, R.R.; Kennett, M.; Vanamala, J.K.P.; Reddivari, L. Anthocyanin-containing purple potatoes ameliorate DSS-induced colitis in mice. J. Nutr. Biochem. 2021, 93, 108616. [Google Scholar] [CrossRef] [PubMed]
- Bie, N.; Duan, S.; Meng, M.; Guo, M.; Wang, C. Regulatory effect of non-starch polysaccharides from purple sweet potato on intestinal microbiota of mice with antibiotic-associated diarrhea. Food Funct. 2021, 12, 5563–5575. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Deng, L.; Chen, J.; Zhou, S.; Liu, S.; Fu, Y.; Yang, C.; Liao, Z.; Chen, M. An analytical pipeline to compare and characterise the anthocyanin antioxidant activities of purple sweet potato cultivars. Food Chem. 2016, 194, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, B.; Tang, C.; Gou, Y.; Chen, H.; Wang, Y.; Jin, C.; Liu, J.; Niu, F.; Kan, J.; et al. Characterization, antioxidant activity and hepatoprotective effect of purple sweetpotato polysaccharides. Int. J. Biol. Macromol. 2018, 115, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Fan, J.; Tian, Z.; Ma, L.; Meng, Y.; Yang, Z.; Zeng, X.; Liu, X.; Kang, L.; Nan, X. Effects of treatment methods on the formation of resistant starch in purple sweet potato. Food Chem. 2022, 367, 130580. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Chen, T.; Green, S.J.; Mutlu, E.; Martin, B.R.; Rumpagaporn, P.; Patterson, J.A.; Keshavarzian, A.; Hamaker, B.R. Physical inaccessibility of a resistant starch shifts mouse gut microbiota to butyrogenic Firmicutes. Mol. Nutr. Food. Res. 2019, 63, 1801012. [Google Scholar] [CrossRef]
- Jacobasch, G.; Schmiedl, D.; Kruschewski, M.; Schmehl, K. Dietary resistant starch and chronic inflammatory bowel diseases. Int. J. Biol. Colorectal Dis. 1999, 14, 201–211. [Google Scholar] [CrossRef]
- Guo, K.; Liu, T.; Xu, A.; Zhang, L.; Bian, X.; Wei, C. Structural and functional properties of starches from root tubers of white, yellow, and purple sweet potatoes. Food Hydrocoll. 2019, 89, 829–836. [Google Scholar] [CrossRef]
- Gani, A.; Ashwar, B.A.; Akhter, G.; Gani, A.; Shah, A.; Masoodi, F.A.; Wani, I.A. Resistant starch from five Himalayan rice cultivars and Horse chestnut: Extraction method optimization and characterization. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.P.; Carpiné, D.; da Silva Bambirra Alves, F.E.; Barbi, R.C.T.; de Melo, A.M.; Ikeda, M.; Ribani, R.H. Thermal, structural, morphological and bioactive characterization of acid and neutral modified loquat (Eriobotrya japonica lindl.) seed starch and its by-products. J. Therm. Anal. Calorim. 2022, 147, 6721–6737. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists International, 18th ed.; AOAC: Rockville, MD, USA, 2005. [Google Scholar]
- McCleary, B.V.; Monaghan, D.A. Measurement of resistant starch. J. AOAC. Int. 2002, 85, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, M.; Chen, H.; Zhang, B. Effects of the combination of repeated heat-moisture treatment and compound enzymes hydrolysis on the structural and physicochemical properties of porous wheat starch. Food Chem. 2019, 274, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.; Liu, J.; Yong, H.; Liu, Y.; Qin, Y.; Liu, J. Development of active packaging based on chitosan-gelatin blend films functionalized with Chinese hawthorn (Crataegus pinnatifida) fruit extract. Int. J. Biol. Macromol. 2019, 140, 384–392. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, L.; Liu, W.; Liu, Q.; Wang, F.; Zhang, H.; Hu, H.; Blecker, C. Physicochemical and structural characterization of potato starch with different degrees of gelatinization. Foods 2021, 10, 1104. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, N.; Kan, J.; Sun, R.; Tang, S.; Jin, C. Anti-inflammatory activity of alkali-soluble polysaccharides from arctium lappa l. and its effect on gut microbiota of mice with inflammation. Int. J. Biol. Macromol. 2020, 154, 773–787. [Google Scholar] [CrossRef]
- Feng, L.; Lu, C.; Yang, Y.; Lu, Y.; Li, Q.; Huang, L.; Fan, X.; Liu, Q.; Zhang, C. The physicochemical properties of starch are affected by wxlv in indica rice. Foods 2021, 10, 3089. [Google Scholar] [CrossRef]
- Kan, J.; Chen, C.; Huo, T.; Xie, W.; Hui, Y.; Liu, J.; Jin, C. Polyphenolic-enriched peach peels extract regulates lipid metabolism and improves the gut microbiota composition in high fat diet-fed mice. J. Funct Foods 2020, 72, 104082. [Google Scholar] [CrossRef]
- Liu, M.Y.; Yun, S.J.; Cao, J.L.; Cheng, F.; Chang, M.C.; Meng, J.L.; Liu, J.; Cheng, Y.; Xu, L.; Xue-ran Geng, X.; et al. The fermentation characteristics of Sparassis crispa polysaccharides and their effects on the intestinal microbes in mice. Chem. Biol. Technol. Agric. 2021, 8, 27. [Google Scholar] [CrossRef]
- Demirkesen-Bicak, H.; Tacer-Caba, Z.; Nilufer-Erdil, D. Pullulanase treatments to increase resistant starch content of black chickpea (Cicer arietinum L.) starch and the effects on starch properties. Int. J. Biol. Macromol. 2018, 111, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Chen, J.; Jin, X.; Chen, J.; Ding, Y.; Shi, M.; Guo, X.; Yan, Y. Effect of inulin on thermal properties, pasting, rheology, and in vitro digestion of potato starch. Starch Stärke 2023, 75, 220021. [Google Scholar] [CrossRef]
- Sang, Y.; Bean, S.; Seib, P.A.; Pedersen, J.; Shi, Y.C. Structure and functional properties of sorghum starches differing in amylose content. J. Agric. Food Chem. 2008, 56, 6680–6685. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Gani, A.; Jhan, F.; Jenno, J.L.H.; Dar, M.A. Resistant starch type 2 from lotus stem: Ultrasonic effect on physical and nutraceutical properties. Ultrason. Sonochem. 2021, 76, 105655. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Wang, X.; Sun, J.; Fang, Y.; Liu, J.; Jin, C. Comparison of the structural characterization and physicochemical properties of starches from seven purple sweet potato varieties cultivated in China. Int. J. Biol. Macromol. 2018, 120, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, F.; Du, C.; Li, M.; Leng, Z.; Yu, X.; Du, S.-K. Optimization of corn resistant starch preparation by dual enzymatic modification using response surface methodology and its physicochemical characterization. Foods 2022, 11, 2223. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhan, J.; Lu, H.; Chang, R.; Tian, Y. Interactions between recrystallized rice starch and flavor molecules. Food Hydrocoll. 2022, 124, 107271. [Google Scholar] [CrossRef]
- Paixão e Silva, G.D.L.; Bento, J.A.C.; Ribeiro, G.O.; Lião, L.M.; Soares Júnior, M.S.; Caliari, M. Application potential and technological properties of colored sweet potato starches. Starch-Stärke 2021, 73, 2000100. [Google Scholar] [CrossRef]
- Xu, J.; Ma, Z.; Li, X.; Liu, L.; Hu, X. A more pronounced effect of type III resistant starch vs. type II resistant starch on ameliorating hyperlipidemia in high fat diet-fed mice is associated with its supramolecular structural characteristics. Food Funct. 2020, 11, 1982–1995. [Google Scholar] [CrossRef]
- Jiang, F.; Du, C.; Guo, Y.; Fu, J.; Jiang, W.; Du, S.K. Physicochemical and structural properties of starches isolated from quinoa varieties. Food Hydrocolloid 2020, 101, 105515. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Hasei, S.; Yamamotoya, T.; Honda, H.; Kushiyama, A.; Sakoda, H.; Fujishiro, M.; Ono, H.; Ito, H.; Okabe, T.; et al. Pathological role of Pin1 in the development of DSS-induced colitis. Cells 2021, 10, 1230. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Xu, J.; Adhikari, B.; Lv, W. Microwave-assisted enzymatic extraction of flavonoids from Armeniaca mume Sieb. Blossom and their immunomodulating effect in mice with DSS-induced colitis. Molecules 2021, 26, 855. [Google Scholar] [CrossRef] [PubMed]
- El-naseery, N.I.; Mousa, H.S.E.; Noreldin, A.E.; El-Far, A.H.; Elewa, Y.H.A. Aging-associated immunosenescence via alterations in splenic immune cell populations in rat. Life Sci. 2020, 241, 117168. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.R.; Park, H.J.; Seo, B.I.; Roh, S.S. New approach of medicinal herbs and sulfasalazine mixture on ulcerative colitis induced by dextran sodium sulfate. World J. Gastroenterol. 2020, 26, 5272. [Google Scholar] [CrossRef]
- Sun, J.; Chen, H.; Kan, J.; Gou, Y.; Liu, J.; Zhang, X.; Wu, X.; Tang, S.; Sun, R.; Qian, C. Nianfeng zhang, fuxiang niu, changhai jin, anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in dss-induced colitis mice. Int. J. Biol. Macromol. 2020, 153, 708–722. [Google Scholar] [CrossRef]
- Palmano, K.P.; MacGibbon, A.K.H.; Gunn, C.A.; Schollum, L.M. In vitro and in vivo anti-inflammatory activity of bovine milkfat globule (mfgm)-derived complex lipid fractions. Nutrients 2020, 12, 2089. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Gao, Y.; Xue, Y.; Yan, Y.; Guo, X. Metagenomic analysis of gut microbiota modulatory effects of jujube (Ziziphus jujuba mill.) polysaccharides in a colorectal cancer mouse model. Food Funct. 2020, 11, 163–173. [Google Scholar] [CrossRef]
- Uchiyama, T.; Itaya-Hironaka, A.; Yamauchi, A.; Makino, M.; Sakuramoto-Tsuchida, S.; Shobatake, R.; Ota, H.; Takeda, M.; Ohbayashi, C.; Takasawa, S. Intermittent hypoxia up-regulates CCL2, RETN, and TNFα mRNAs in adipocytes via down-regulation of miR-452. Int. J. Mol. Sci. 2019, 20, 1960. [Google Scholar] [CrossRef]
- Mitani, T.; Yoshioka, Y.; Furuyashiki, T.; Yamashita, Y.; Shirai, Y.; Ashida, H. Enzymatically synthesized glycogen inhibits colitis through decreasing oxidative stress. Free Radical Biol. Med. 2017, 106, 355–367. [Google Scholar] [CrossRef]
- Perriot, S.; Mathias, A.; Perriard, G.; Canales, M.; Jonkmans, N.; Merienne, N.; Meunier, C.; Kassar, L.E.; Anselme, L.; Perrier, A.L.; et al. Human induced pluripotent stem cell-derived astrocytes are differentially activated by multiple sclerosis-associated cytokines. Stem. Cell Rep. 2018, 11, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, J.Y.; Xiong, F.; Wu, B.H.; Luo, M.H.; Yu, Z.C.; Liu, T.T.; Li, D.F.; Tang, Q.; Li, Y.X.; et al. Curcumin ameliorates DSS-induced colitis in mice by regulating the Treg/Th17 signaling pathway. Mol. Med. Rep. 2021, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Luck, H.; Khan, S.; Kim, J.H.; Copeland, J.K.; Revelo, X.S.; Tsai, S.; Chakraborty, M.; Cheng, K.; Chan, Y.T.; Mark, K.; et al. Gut-associated IgA+ immune cells regulate obesity-related insulin resistance. Nat. Commun. 2019, 10, 3650. [Google Scholar] [CrossRef] [PubMed]
- Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Beale, D.J.; Karpe, A.V.; Shastri, S.; Basheer, W.; Southam, R.; Eri, R.; et al. Synbiotic supplementation with prebiotic green banana resistant starch and probiotic Bacillus coagulans spores ameliorates gut inflammation in mouse model of inflammatory bowel diseases. Eur. J. Nutr. 2020, 59, 3669–3689. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Han, D.H.; Jang, Y.J.; Park, S.; Jang, S.J.; Lee, G.; Han, H.S.; Ko, G. Alleviation of DSS-induced colitis via Lactobacillus acidophilus treatment in mice. Food Funct. 2021, 12, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Li, G.; Zhu, K.; Sun, P.; Feng, X.; Zhao, X. Effect of resistant starch on HCl/ethanol-induced gastric injury in rats. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 613–619. [Google Scholar] [CrossRef]
- Lkhagva, E.; Chung, H.J.; Hong, J.; Tang, W.H.W.; Lee, S.I.; Hong, S.T.; Lee, S. The regional diversity of gut microbiome along the GI tract of male C57BL/6 mice. BMC Microbiol. 2021, 21, 44. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Liang, H.; Ye, L.; Lan, L.; Lu, F.; Wang, Q.; Lei, T.; Yang, X.; Cui, P.; et al. Differences in alpha diversity of gut microbiota in neurological diseases. Front. Neurosci. 2022, 16, 879318. [Google Scholar] [CrossRef]
- Yang, F.; Yang, D.; Liu, S.; Xu, S.; Wang, F.; Chen, H.; Liu, Y. Use of high-throughput sequencing to identify fungal communities on the surface of citri reticulatae pericarpium during the 3-year aging process. Curr. Microbiol. 2021, 78, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhang, X.; Hao, H.; Liu, Q.; Zhou, Z.; Liang, X.; Liu, T.; Gong, P.; Zhang, L.; Zhai, Z.; et al. Lactobacillus rhamnosus gg derived extracellular vesicles modulate gut microbiota and attenuate inflammatory in dss-induced colitis mice. Nutrients 2021, 13, 3319. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Sun, J.; Liu, J.; Chen, H.; Kan, J.; Qian, C.; Zhang, N.; Jin, C. Structural characterization of a water-soluble purple sweet potato polysaccharide and its effect on intestinal inflammation in mice. J. Funct. Foods 2019, 61, 103502. [Google Scholar] [CrossRef]
- Peng, J.; Li, X.; Zheng, L.; Duan, L.; Gao, Z.; Hu, D.; Li, J.; Li, X.; Shen, X.; Xiao, H. Ban-lan-gen granule alleviates dextran sulfate sodium-induced chronic relapsing colitis in mice via regulating gut microbiota and restoring gut SCFA derived-GLP-1 production. J. Inflamm. Res. 2022, 15, 1457–1470. [Google Scholar] [CrossRef] [PubMed]
- Li, A.L.; Ni, W.W.; Zhang, Q.M.; Li, Y.; Zhang, X.; Wu, H.Y.; Du, P.; Hou, J.C.; Zhang, Y. Effect of cinnamon essential oil on gut microbiota in the mouse model of dextran sodium sulfate-induced colitis. Microbiol. Immunol. 2020, 64, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.L.; Tseng, C.H.; Ho, H.J.; Cheung, C.K.; Lin, J.Y.; Chen, Y.J.; Chen, F.C.; Hsu, Y.C.; Lin, J.T.; El-Omar, E.M.; et al. Fecal microbiota transplantation confers beneficial metabolic effects of diet and exercise on diet-induced obese mice. Sci. Rep. 2018, 8, 15625. [Google Scholar] [CrossRef] [PubMed]
- Whon, N.R.; Shin, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar]
- Sheng, K.; Xu, Y.; Kong, X.; Wang, J.; Zha, X.; Wang, Y. Probiotic Bacillus cereus alleviates dextran sulfate sodium-induced colitis in mice through improvement of the intestinal barrier function, anti-inflammation, and gut microbiota modulation. J. Agric. Food Chem. 2021, 69, 14810–14823. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordevi, D.; Vítězová, M.; Kollár, P. Cross-correlation analysis of the Desulfovibrio growth parameters of intestinal species isolated from people with colitis. Biologia 2018, 73, 1137–1143. [Google Scholar] [CrossRef]
- Chen, B.; Luo, J.; Han, Y.; Du, H.; Liu, J.; He, W.; Zhu, J.; Xiao, J.; Wang, J.; Cao, Y.; et al. Dietary tangeretin alleviated dextran sulfate sodium-induced colitis in mice via inhibiting inflammatory response, restoring intestinal barrier function, and modulating gut microbiota. J. Agric. Food Chem. 2021, 69, 7663–7674. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Liu, J.; Zheng, Z.; Li, Q.; Wang, H.; Chen, Z.; Wang, K. Identification of the core active structure of a Dendrobium officinale polysaccharide and its protective effect against dextran sulfate sodium-induced colitis via alleviating gut microbiota dysbiosis. Food Res. Int. 2020, 137, 109641. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Li, D.; Ma, C.; Feng, Y.; Hu, X.; Chen, F. Barley leaf insoluble dietary fiber alleviated dextran sulfate sodium-induced mice colitis by modulating gut microbiota. Nutrients 2021, 13, 846. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cai, M.; Yang, K.; Sun, P.; Xu, J.; Li, Z.; Tian, B. Phenolics from dendrobium officinale leaf ameliorate dextran sulfate sodium-induced chronic colitis by regulating gut microbiota and intestinal barrier. J. Agric. Food Chem. 2023, 71, 16630–16646. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, W.; Zhang, Y.; Zhang, D.; Qiu, B.; Wang, X.; Liu, J.; Liu, L. Therapeutic and prebiotic effects of five different native starches on dextran sulfate sodium-induced mice model of colonic colitis. Mol. Nutr. Food Res. 2021, 65, 2000922. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, M.; Liu, Q.; Zhao, Q.; Zeng, J.; Wang, Q.; Zhao, Y.; Du, F.; Chen, Y.; Shen, J.; et al. Starch from Pueraria lobata and the amylose fraction alleviates dextran sodium sulfate induced colitis in mice. Carbohydr. Polym. 2023, 302, 120329. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, N.; Kan, J.; Zhang, X.; Wu, X.; Sun, R.; Tang, S.; Liu, J.; Qian, C.; Jin, C. Structural characterization of water-soluble polysaccharide from Arctium lappa and its effects on colitis mice. Carbohydr. Polym. 2019, 213, 89–99. [Google Scholar] [CrossRef]
- Feng, X.; Du, C.; Wang, C. Structural characterization of polysaccharide from yellow sweet potato and ameliorates DSS-induced mice colitis by active GPR41/MEK/ERK 1/2 signaling pathway. Int. J. Biol. Macromol. 2021, 192, 278–288. [Google Scholar] [CrossRef]

| Sample 1 | Moisture (%) | Ash (%) | Protein (%) | Starch (%) | Amylose (%) | Resistant Starch (%) | WBC (%) |
|---|---|---|---|---|---|---|---|
| PSPS | 9.38 ± 0.35 a | 1.07 ± 0.43 a | 0.85 ± 0.08 a | 92.30 ± 0.51 a | 24.42 ± 0.61 b | 28.12 ± 1.25 b | 283.74 ± 4.73 a |
| PSPRS | 7.79 ± 0.13 b | 1.38 ± 0.29 a | 0.06 ± 0.04 b | 89.83 ± 0.58 b | 38.69 ± 0.53 a | 71.64 ± 2.67 a | 222.48 ± 3.27 b |
| Sample | Color Parameters 1 | Relative Crystallinity | SAXS Parameter 2 | IR Ratio of 1047 cm−1/1022 cm−1 | ||||
|---|---|---|---|---|---|---|---|---|
| L | a | b | Smax (nm−1) | d (nm) | Imax | |||
| PSPS | 95.28 ± 0.42 a | −1.24 ± 0.14 a | 3.70 ± 0.57 a | 34.78 | 0.626 | 10.031 | 187.138 | 0.723 |
| PSPRS | 91.78 ± 0.98 b | −0.69 ± 0.05 b | 4.52 ± 0.73 a | 30.51 | -- 3 | -- | -- | 0.689 |
| Sample Groups 1 | Initial Body Weight (g) | Final Body Weight (g) | Thymus Index (mg/g) | Spleen Index (mg/g) |
|---|---|---|---|---|
| NC | 30.07 ± 0.71 a | 36.22 ± 1.44 a | 1.57 ± 0.10 a | 3.08 ± 0.19 c |
| DSS | 30.47 ± 0.62 a | 31.36 ± 1.09 c | 1.10 ± 0.10 c | 3.84 ± 0.16 a |
| DSS+LPSPRS | 30.13 ± 0.70 a | 32.64 ± 0.53 bc | 1.22 ± 0.06 c | 3.55 ± 0.09 b |
| DSS+HPSPRS | 30.58 ± 0.93 a | 34.08 ± 0.78 b | 1.34 ± 0.04 b | 3.25 ± 0.16 c |
| Sample Groups 1 | IL-6 (pg/mL) | TNF-α (pg/mL) | IL-1β (pg/mL) | IL-10 (pg/mL) | IgA (µg/mL) |
|---|---|---|---|---|---|
| NC | 54.17 ± 4.71 d | 501.64 ± 40.77 d | 67.53 ± 7.42 d | 620.93 ± 33.39 a | 3.15 ± 0.18 ab |
| DSS | 97.86 ± 8.03 a | 856.81 ± 50.64 a | 114.13 ± 4.53 a | 256.47 ± 13.85 d | 2.63 ± 0.12 c |
| DSS+LPSPRS | 75.33 ± 4.71 b | 704.88 ± 27.61 b | 101.94 ± 5.78 b | 361.37 ± 28.13 c | 2.85 ± 0.18 bc |
| DSS+ HPSPRS | 64.53 ± 4.90 c | 629.35 ± 35.79 c | 87.95 ± 8.99 c | 540.26 ± 26.82 b | 3.33 ± 0.31 a |
| Sample Groups 1 | Acetate | Propionate | Butyrate |
|---|---|---|---|
| NC | 6.35 ± 0.78 a | 0.86 ± 0.18 a | 1.02 ± 0.36 a |
| DSS | 2.32 ± 0.43 c | 0.27 ± 0.07 c | 0.27 ± 0.12 b |
| DSS+ HPSPRS | 3.96 ± 0.39 b | 0.57 ± 0.11 b | 0.74 ± 0.13 a |
| Sample Groups 1 | Sobs | Shannon | Simpson | ACE | Coverage |
|---|---|---|---|---|---|
| NC | 110.00 ± 3.79 a | 2.62 ± 0.27 a | 0.17 ± 0.038 b | 113.49 ± 5.01 a | 0.9995 ± 0.0002 a |
| DSS | 95.30 ± 3.35 c | 1.93 ± 0.25 b | 0.31 ± 0.061 a | 104.47 ± 4.39 b | 0.9994 ± 0.0005 a |
| DSS+HPSPRS | 104.30 ± 3.59 b | 2.52 ± 0.31 a | 0.19 ± 0.052 b | 109.37 ± 3.67 ab | 0.9995 ± 0.0001 a |
| Sample Groups 1 | Firmicutes (%) | Bacteroidetes (%) | Proteobacteria (%) | Actinobacteria (%) | Verrucomicrobia (%) | The Ratio of Firmicutes to Bacteroidetes |
|---|---|---|---|---|---|---|
| NC | 54.29 ± 5.78 a | 38.69 ± 5.40 b | 1.62 ± 0.39 b | 2.83 ± 1.30 a | 0.92 ± 0.54 b | 1.42 ± 0.19 a |
| DSS | 41.14 ± 4.25 b | 49.78 ± 6.74 a | 2.59 ± 0.32 a | 1.45 ± 0.85 ab | 3.12 ± 1.69 a | 0.85 ± 0.16 c |
| DSS+HPSPRS | 49.63 ± 3.69 a | 44.49 ± 3.06 ab | 2.10 ± 0.37 ab | 1.04 ± 0.54 b | 0.97 ± 0.77 b | 1.12 ± 0.12 b |
| Gut Microbiota 1 | Relative Abundance (%) | Dynamic Change in Relative Abundance 2 | |||
|---|---|---|---|---|---|
| NC | DSS | DSS+HPSPRS | DSS vs. NC | DSS+HPSPRS vs. DSS | |
| norank_f_Muribaculaceae | 25.86 ± 6.26 a | 34.66 ± 9.17 a | 33.67 ± 7.55 a | ↑ | ↓ |
| Lactobacillus | 36.87 ± 6.63 a | 17.64 ± 5.36 c | 26.89 ± 3.42 b | ↓ * | ↑ * |
| Staphylococcus | 0.17 ± 0.10 b | 2.40 ± 0.51 a | 0.69 ± 0.22 b | ↑ * | ↓ * |
| [Eubacterium]_coprostanoligenes_group | 0.14 ± 0.09 ab | 1.39 ± 1.27 a | 0.07 ± 0.04 b | ↑ | ↓ * |
| Lachnospiraceae_NK4A136_group | 3.09 ± 0.26 b | 2.41 ± 0.38 c | 4.62 ± 0.84 a | ↓ * | ↑ * |
| unclassfied_f_lachnospiraceae | 1.45 ± 0.47 b | 1.17 ± 0.56 b | 2.84 ± 0.77 a | ↓ | ↑ * |
| norank_f_Lachnospiraceae | 1.31 ± 0.21 ab | 1.06 ± 0.54 b | 1.83 ± 0.46 a | ↓ | ↑ * |
| Desulfovibrio | 0.93 ± 0.29 b | 1.88 ± 0.51 a | 1.23 ± 0.25 ab | ↑ * | ↓ |
| Odoribacter | 0.84 ± 0.16 a | 1.23 ± 0.59 a | 1.22 ± 0.46 a | ↑ | ↓ |
| Prevotellaceae_UCG-001 | 0.60 ± 0.40 a | 1.52 ± 0.96 a | 0.77 ± 0.45 a | ↑ | ↓ |
| Alloprevotella | 3.90 ± 1.12 a | 1.76 ± 0.67 b | 2.31 ± 0.55 ab | ↓ * | ↑ |
| Akkermansia | 0.92 ± 0.55 b | 3.12 ± 1.69 a | 0.97 ± 0.77 b | ↑ * | ↓ * |
| Alistipes | 2.57 ± 0.97 ab | 4.33 ± 1.73 a | 1.90 ± 0.78 b | ↑ | ↓ * |
| Bacteroides | 1.50 ± 0.71 b | 4.36 ± 1.32 a | 2.10 ± 0.45 b | ↑ * | ↓ * |
| Bifidobacterium | 1.27 ± 0.51 a | 0.24 ± 0.15 b | 0.69 ± 0.34 ab | ↓ * | ↑ |
| Parasutterella | 0.18 ± 0.05 a | 0.31 ± 0.16 a | 0.29 ± 0.09 a | ↑ | ↓ |
| Lachnospiraceae_UCG-001 | 0.22 ± 0.15 ab | 0.10 ± 0.04 b | 0.55 ± 0.34 a | ↓ | ↑ * |
| Ruminococcaceae_UCG-010 | 0.12 ± 0.06 b | 0.36 ± 0.17 a | 0.06 ± 0.02 b | ↑ * | ↓ * |
| Jeotgalicoccus | 0.06 ± 0.05 b | 0.34 ± 0.26 a | 0.01 ± 0.01 b | ↑ * | ↓ * |
| norank_f_Erysipelotrichaceae | 0.50 ± 0.32 a | 0.57 ± 0.21 a | 0.32 ± 0.19 a | ↑ | ↓ |
| Parabacteroides | 0.51 ± 0.25 a | 0.85 ± 0.27 a | 0.62 ± 0.24 a | ↑ | ↓ |
| norank_f_norank_o_Mollicutes_RF39 | 0.31 ± 0.11 b | 0.54 ± 0.10 a | 0.02 ± 0.01 c | ↑ * | ↓ * |
| unclassified_o_Bacteroidales | 0.26 ± 0.11 b | 0.46 ± 0.07 a | 0.14 ± 0.12 b | ↑ * | ↓ * |
| norank_f_Clostridiales_vadinBB60_group | 0.29 ± 0.19 a | 0.42 ± 0.23 a | 0.14 ± 0.07 a | ↑ | ↓ |
| [Eubacterium]_nodatum_group | 0.18 ± 0.13 a | 0.47 ± 0.28 a | 0.20 ± 0.09 a | ↑ | ↓ |
| Erysipelatoclostridium | 0.06 ± 0.04 c | 0.47 ± 0.11 a | 0.18 ± 0.09 b | ↑ * | ↓ * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Gao, M.; Kan, J.; Cheng, Q.; Chen, X.; Tang, C.; Chen, D.; Zong, S.; Jin, C. Resistant Starch from Purple Sweet Potatoes Alleviates Dextran Sulfate Sodium-Induced Colitis through Modulating the Homeostasis of the Gut Microbiota. Foods 2024, 13, 1028. https://doi.org/10.3390/foods13071028
Wang Z, Gao M, Kan J, Cheng Q, Chen X, Tang C, Chen D, Zong S, Jin C. Resistant Starch from Purple Sweet Potatoes Alleviates Dextran Sulfate Sodium-Induced Colitis through Modulating the Homeostasis of the Gut Microbiota. Foods. 2024; 13(7):1028. https://doi.org/10.3390/foods13071028
Chicago/Turabian StyleWang, Zhihao, Min Gao, Juan Kan, Qingyang Cheng, Xiaotong Chen, Chao Tang, Dan Chen, Shuai Zong, and Changhai Jin. 2024. "Resistant Starch from Purple Sweet Potatoes Alleviates Dextran Sulfate Sodium-Induced Colitis through Modulating the Homeostasis of the Gut Microbiota" Foods 13, no. 7: 1028. https://doi.org/10.3390/foods13071028
APA StyleWang, Z., Gao, M., Kan, J., Cheng, Q., Chen, X., Tang, C., Chen, D., Zong, S., & Jin, C. (2024). Resistant Starch from Purple Sweet Potatoes Alleviates Dextran Sulfate Sodium-Induced Colitis through Modulating the Homeostasis of the Gut Microbiota. Foods, 13(7), 1028. https://doi.org/10.3390/foods13071028







