Reformulation of Tunisian Sun-Dried Merguez with Camel Meat: Characterization of Physicochemical and Compositional Changes in Organic Acids, Fatty Acids, Volatile Compounds, and Minerals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Formulation and Manufacturing of Dry Merguez
2.3. Environmental Conditions, Drying Weight Loss, Water Activity, and pH
2.4. Microbiological Analysis
2.5. Chemical Composition
2.5.1. Moisture, Protein, and Fat Determination
2.5.2. Organic Acid Analysis
2.5.3. Fatty Acid Identification
2.5.4. Volatile Compounds
2.5.5. Mineral Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Weight Loss, Water Activity, and pH
3.2. Microbiological Change
3.3. Change in Chemical Composition
3.3.1. Moisture, Protein, and Fat Composition
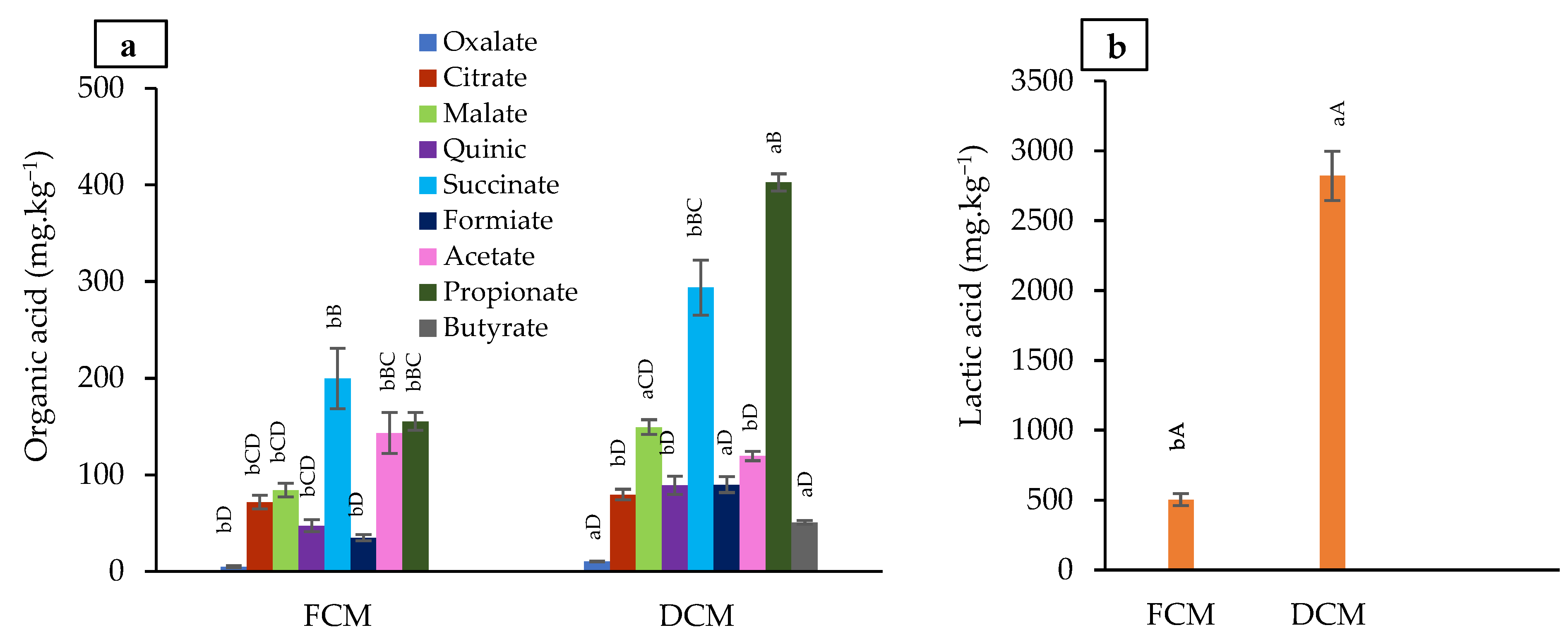
3.3.2. Organic Acid Composition
3.3.3. Fatty Acids
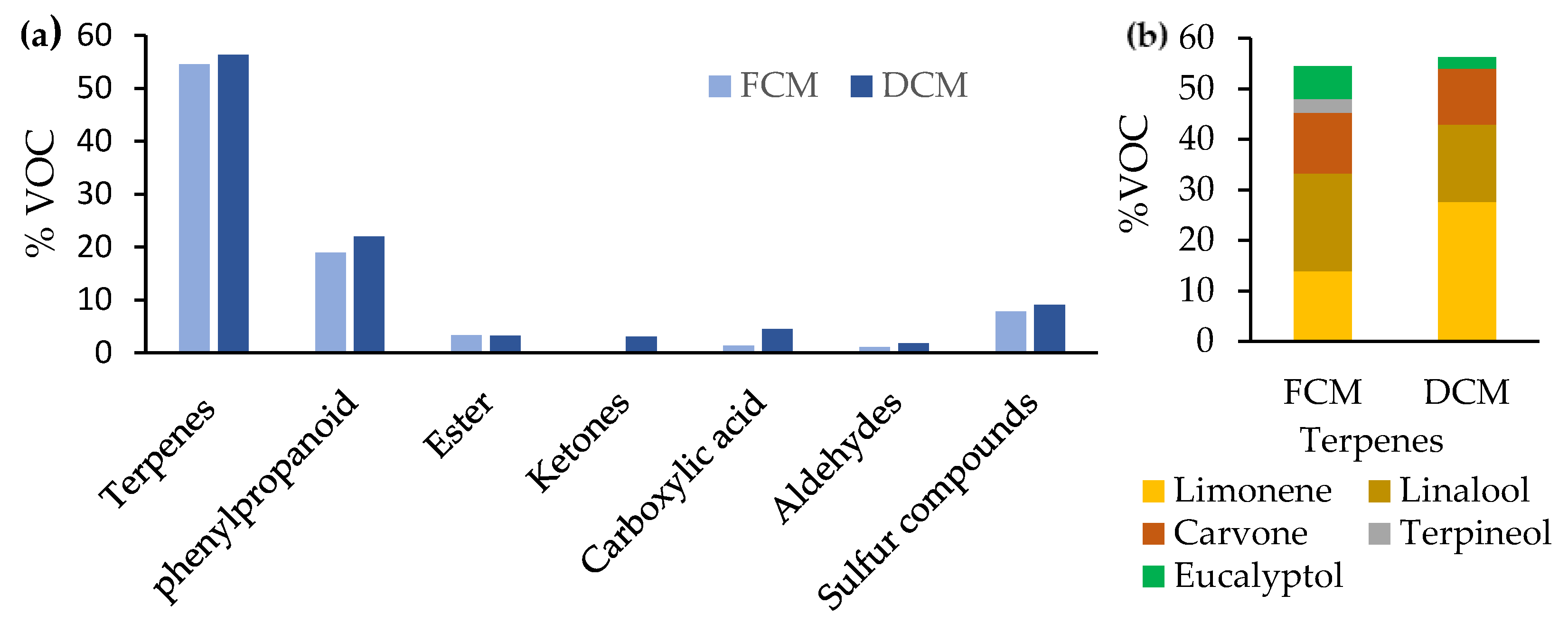
3.3.4. Volatile Compounds
3.3.5. Mineral Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gagaoua, M.; Boudechicha, H.R. Ethnic Meat Products of the North African and Mediterranean Countries: An Overview. J. Ethn. Foods. 2018, 5, 83–98. [Google Scholar] [CrossRef]
- Triki, M.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Effect of Preformed Konjac Gels, with and without Olive Oil, on the Technological Attributes and Storage Stability of Merguez Sausage. Meat Sci. 2013, 93, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Romano Young, K. Tunisia, Food and drink. In World and Its Peoples: Middle East, Western Asia and Northern Africa; Marshall Cavendish: New York, NY, USA, 2007; Volume 9, pp. 1282–1283. [Google Scholar]
- Gobert, E.G. Usages et Rites Alimentaires Des Tunisiens, Leur Aspect Domestique, Physiologique et Social; L’Institute Pasteur: Paris, France, 1940; Tome XXIX; pp. 475–589. [Google Scholar]
- Bader, R.; Becila, S.; Ruiz, P.; Djeghim, F.; Sanah, I.; Boudjellal, A.; Gatellier, P.; Portanguen, S.; Talon, R.; Leroy, S. Physicochemical and Microbiological Characteristics of El-Guedid from Meat of Different Animal Species. Meat Sci. 2021, 171, 108277. [Google Scholar] [CrossRef]
- Chemache, L.; Kehal, F.; Namoune, H.; Chaalal, M.; Gagaoua, M. Couscous: Ethnic Making and Consumption Patterns in the Northeast of Algeria. J. Ethn. Foods 2018, 5, 211–219. [Google Scholar] [CrossRef]
- Flores, M.; Piornos, J.A. Fermented Meat Sausages and the Challenge of Their Plant-Based Alternatives: A Comparative Review on Aroma-Related Aspects. Meat Sci. 2021, 182, 108636. [Google Scholar] [CrossRef] [PubMed]
- Kadim, I.T.; Al-Amri, I.S.; Alkindi, A.Y.; Haq, Q.M.I. Nutritional Values and Health Benefits of Dromedary Camel Meat. Anim. Front. 2022, 12, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Dib, A.L.; Bererhi, E.-H. Recent Advances in Dromedary Camels and Their Products. Animals 2022, 12, 162. [Google Scholar] [CrossRef]
- Kadim, I.T.; Mahgoub, O.; Mbaga, M. Potential of Camel Meat as a Nontraditional High-Quality Source of Protein for Human Consumption. Anim. Front. 2014, 4, 13–17. [Google Scholar] [CrossRef]
- Li, Q.; Yang, L.; Li, R.; Chen, G.; Dong, J.; Wu, L.; Fu, Y.; Yang, J. Lipid Analysis of Meat from Bactrian Camel (Camelus bacterianus), Beef, and Tails of Fat-Tailed Sheep Using UPLC-Q-TOF/MS Based Lipidomics. Front. Nutr. 2023, 10, 1053116. [Google Scholar] [CrossRef]
- El Adab, S.; Ben Wadda, W.; Tekiki, A.; Ben Moussa, O.; Boulares, M.; Sadok, S.; Hassouna, M. Effect of Mixed Starter Cultures on Biogenic Amine Formation during the Ripening of Tunisian Dry Fermented Camel Meat Sausage. Ital. J. Food Sci. 2020, 32, 321–336. [Google Scholar] [CrossRef]
- Mejri, L.; Ziadi, A.; El Adab, S.; Boulares, M.; Essid, I.; Hassouna, M. Effect of Commercial Starter Cultures on Physicochemical, Microbiological and Textural Characteristics of a Traditional Dry Fermented Sausage Reformulated with Camel Meat and Hump Fat. J. Food Meas. Charact. 2017, 11, 758–767. [Google Scholar] [CrossRef]
- Kargozari, M.; Moini, S.; Akhondzadeh Basti, A.; Emam-Djomeh, Z.; Ghasemlou, M.; Revilla Martin, I.; Gandomi, H.; Carbonell-Barrachina, Á.A.; Szumny, A. Development of Turkish Dry-Fermented Sausage (Sucuk) Reformulated with Camel Meat and Hump Fat and Evaluation of Physicochemical, Textural, Fatty Acid and Volatile Compound Profiles during Ripening. LWT Food Sci. Technol. 2014, 59, 849–858. [Google Scholar] [CrossRef]
- Ayyash, M.; Olaimat, A.; Al-Nabulsi, A.; Liu, S.-Q. Bioactive Properties of Novel Probiotic Lactococcus Lactis Fermented Camel Sausages: Cytotoxicity, Angiotensin Converting Enzyme Inhibition, Antioxidant Capacity, and Antidiabetic Activity. Food. Sci. Anim. Resour. 2020, 40, 155–171. [Google Scholar] [CrossRef]
- Kadirvel, G.; Banerjee, B.B.; Meitei, S.; Doley, S.; Sen, A.; Muthukumar, M. Market Potential and Opportunities for Commercialization of Traditional Meat Products in North East Hill Region of India. Vet. World 2018, 11, 118–124. [Google Scholar] [CrossRef] [PubMed]
- ISO 18787; Foodstuffs—Determination of Water Activity. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 2917; Meat and Meat Products—Measurement of PH. International Organization of Standardization: Geneva, Switzerland, 1999.
- ISO 4833-1; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 15214; Microbiology of Food and Animal Feeding Stuffs Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria Colony-Count Technique at 30 Degrees C. International Organization for Standardization: Geneva, Switzerland, 1998.
- NFV 08 059; Microbiology of Food Animal Feeding Stuffs—Enumeration of Yeasts and Moulds by Colony-Count Technique at 25 Degrees C—Routine Method. AFNOR: Paris, France, 2002.
- NFV 08-054; Microbiology of Food and Animal Feeding Stuffs—Enumeration of Presumptive Enterobacteria by Colony Count Technique at 30 °C or 37 °C. AFNOR: Paris, France, 2009.
- ISO 16649-2; Microbiology of Food and Animal Feeding Stuff—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 Degrees C Using 5-Bromo-4-Chloro-3-Indolyl Beta-D-Glucuronide. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 15213; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Sulfite-Reducing Bacteria Growing under Anaerobic Conditions. International Organization for Standardization: Geneva, Switzerland, 2003.
- NFV 08 057-1; Microbiology of Food and Animal Feeding Stuffs—Routine Method for Enumeration of Coagulase Positive Staphylococcus by Colony-Count Technique at 37 °C—Part 1: Technique with Confirmation of the Colonies. AFNOR: Paris, France, 2004.
- ISO 11290-1; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria Spp.—Part 1: Detection Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2002. [Google Scholar]
- ISO 1443; Meat and Meat Products—Determination of Total Fat Content. International Organization for Standardization: Geneva, Switzerland, 1973.
- Dursun, A.; Güler, Z.; Şekerli, Y.E. Characterization of Volatile Compounds and Organic Acids in Ultra-High-Temperature Milk Packaged in Tetra Brik Cartons. Int. J. Food Prop. 2017, 20, 1511–1521. [Google Scholar] [CrossRef]
- Hara, A.; Radin, N.S. Lipid Extraction of Tissues with a Low-Toxicity Solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Jrad, Z.; Oussaief, O.; Zaidi, S.; Khorchani, T.; El-Hatmi, H. Co-Fermentation Process Strongly Affect the Nutritional, Texture, Syneresis, Fatty Acids and Aromatic Compounds of Dromedary UF-Yogurt. J. Food Sci. Technol. 2021, 58, 1727–1739. [Google Scholar] [CrossRef]
- ISO 6869; Animal Feeding Stuffs—Determination of the Contents of Calcium, Copper, Iron, Magnesium, Manganese, Potassium, Sodium and Zinc—Method Using Atomic Absorption Spectrometry. International Organization for Standardization: Geneva, Switzerland, 2000.
- Grau, R.; Andres, A.; Barat, J.M. Principles of Drying. In Handbook of Fermented Meat and Poultry; Toldrá, F., Hui, Y.H., Astiasaran, I., Sebranek, J., Talon, R., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2014; pp. 31–38. [Google Scholar] [CrossRef]
- Cavalheiro, C.P.; Ruiz-Capillas, C.; Herrero, A.M.; Jiménez-Colmenero, F.; Pintado, T.; de Menezes, C.R.; Fries, L.L.M. Effect of Different Strategies of Lactobacillus plantarum Incorporation in Chorizo Sausages. J. Sci. Food Agric. 2019, 99, 6706–6712. [Google Scholar] [CrossRef]
- Guo, F.; Si, R.; He, J.; Yuan, L.; Hai, L.; Ming, L.; Yi, L.; Ji, R. Comprehensive Transcriptome Analysis of Adipose Tissue in the Bactrian Camel Reveals Fore Hump Has More Specific Physiological Functions in Immune and Endocrine Systems. Livest. Sci. 2019, 228, 195–200. [Google Scholar] [CrossRef]
- Suliman, G.M.; Alowaimer, A.N.; Hussein, E.O.S.; Ali, H.S.; Abdelnour, S.A.; El-Hack, M.E.A.; Swelum, A.A. Chemical Composition and Quality Characteristics of Meat in Three One-Humped Camel (Camelus dromedarius) Breeds as Affected by Muscle Type and Post-Mortem Storage Period. Animals 2019, 9, 834. [Google Scholar] [CrossRef]
- Păucean, A.; Kádár, C.B.; Simon, E.; Vodnar, D.C.; Ranga, F.; Rusu, I.E.; Vișan, V.-G.; Socaci, S.-A.; Man, S.; Chiș, M.S.; et al. Freeze-Dried Powder of Fermented Chili Paste—New Approach to Cured Salami Production. Foods 2022, 11, 3716. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs; European Commission: Brussels, Belgium, 2005; pp. 1–26.
- Fédération du Commerce et de la Distribution. Criteres Microbiologiques Applicables a Partir de 2022 aux Activites de Fabrication, Preparation, Decoupe ou Simple Manipulation de Denrees Nues en Rayon “A La Coupe” et en Atelier en Magasin; Fédération du Commerce et de la Distribution: Paris, France, 2022; pp. 1–31. Available online: https://www.fcd.fr/ (accessed on 15 October 2023).
- Sallam, K.I.; Abd-Elrazik, Y.; Raslan, M.T.; Imre, K.; Morar, A.; Herman, V.; Zaher, H.A. Cefotaxime-, Ciprofloxacin-, and Extensively Drug-Resistant Escherichia coli O157:H7 and O55:H7 in Camel Meat. Foods 2023, 12, 1443. [Google Scholar] [CrossRef] [PubMed]
- Fayez, M.; El-Ghareeb, W.R.; Elmoslemany, A.; Alsunaini, S.J.; Alkafafy, M.; Alzahrani, O.M.; Mahmoud, S.F.; Elsohaby, I. Genotyping and Antimicrobial Susceptibility of Clostridium Perfringens and Clostridioides Difficile in Camel Minced Meat. Pathogens 2021, 10, 1640. [Google Scholar] [CrossRef] [PubMed]
- Halagarda, M.; Wójciak, K.M. Health and Safety Aspects of Traditional European Meat Products. A Review. Meat Sci. 2022, 184, 108623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.H.; Xu, X.L.; Liu, Y. Preservation Technologies for Fresh Meat—A Review. Meat Sci. 2010, 86, 119–128. [Google Scholar] [CrossRef]
- Delgado, J.; Rondán, J.J.; Núñez, F.; Rodríguez, A. Influence of an Industrial Dry-Fermented Sausage Processing on Ochratoxin A Production by Penicillium nordicum. Int. J. Food Microbiol. 2021, 339, 109016. [Google Scholar] [CrossRef] [PubMed]
- De Melo Nazareth, T.; Calpe, J.; Luz, C.; Mañes, J.; Meca, G. Manufacture of a Potential Antifungal Ingredient Using Lactic Acid Bacteria from Dry-Cured Sausages. Foods 2023, 12, 1427. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Suri, S.; Trif, M.; Ozogul, F. Organic Acids Production from Lactic Acid Bacteria: A Preservation Approach. Food Biosci. 2022, 46, 101615. [Google Scholar] [CrossRef]
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef]
- Dittoe, D.K.; Ricke, S.C.; Kiess, A.S. Organic Acids and Potential for Modifying the Avian Gastrointestinal Tract and Reducing Pathogens and Disease. Front. Vet. Sci. 2018, 5, 216. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Kim, J.-Y.; Yoo, J.-H.; Lee, S.-Y. Development of a Selective Medium for the Enumeration of Lactic Acid Bacteria and Bifidobacteria in Food Products. Food Sci. Biotechnol. 2023, 32, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Barcenilla, C.; Puente, A.; Cobo-Díaz, J.F.; Alexa, E.-A.; Garcia-Gutierrez, E.; O’Connor, P.M.; Cotter, P.D.; González-Raurich, M.; López, M.; Prieto, M.; et al. Selection of Lactic Acid Bacteria as Biopreservation Agents and Optimization of Their Mode of Application for the Control of Listeria monocytogenes in Ready-to-Eat Cooked Meat Products. Int. J. Food Microbiol. 2023, 403, 110341. [Google Scholar] [CrossRef]
- Döding, A.; Hüfner, M.; Nachtsheim, F.; Iffarth, V.; Bölter, A.; Bastian, A.; Symmank, J.; Andreas, N.; Schädel, P.; Thürmer, M.; et al. Mediterranean Diet Component Oleic Acid Increases Protective Lipid Mediators and Improves Trabecular Bone in a Porphyromonas gingivalis Inoculation Model. J. Clin. Periodontol. 2023, 50, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Hamed Hammad Mohammed, H.; Jin, G.; Ma, M.; Khalifa, I.; Shukat, R.; Elkhedir, A.E.; Zeng, Q.; Noman, A.E. Comparative Characterization of Proximate Nutritional Compositions, Microbial Quality and Safety of Camel Meat in Relation to Mutton, Beef, and Chicken. LWT Food Sci. Technol. 2020, 118, 108714. [Google Scholar] [CrossRef]
- Borrajo, P.; Karwowska, M.; Lorenzo, J.M. The Effect of Salvia hispanica and Nigella sativa Seed on the Volatile Profile and Sensory Parameters Related to Volatile Compounds of Dry Fermented Sausage. Molecules 2022, 27, 652. [Google Scholar] [CrossRef] [PubMed]
- Dippong, T.; Senila, L.; Muresan, L.E. Preparation and Characterization of the Composition of Volatile Compounds, Fatty Acids and Thermal Behavior of Paprika. Foods 2023, 12, 2041. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.C.T.; Dunkel, A.; Frank, O.; McCormack, B.; Dowd, E.; Didzbalis, J.; Dawid, C.; Hofmann, T. A High Throughput Toolbox for Comprehensive Flavor Compound Mapping in Mint. Food Chem. 2021, 365, 130522. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Arraouadi, S.; Noumi, E.; Aouadi, K.; Adnan, M.; Khan, M.A.; Kadri, A.; Snoussi, M. Antimicrobial, Antioxidant, Anti-Acetylcholinesterase, Antidiabetic, and Pharmacokinetic Properties of Carum carvi L. and Coriandrum sativum L. Essential Oils Alone and in Combination. Molecules 2021, 26, 3625. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A Review of Its Botany, Phytochemistry, Pharmacology, Contemporary Application, and Toxicology. Biomed. Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Bin Emran, T.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Sommer, S.; Lang, L.M.; Drummond, L.; Buchhaupt, M.; Fraatz, M.A.; Zorn, H. Odor Characteristics of Novel Non-Canonical Terpenes. Molecules 2022, 27, 3827. [Google Scholar] [CrossRef]
- Abe, K.; Hori, Y.; Myoda, T. Characterization of Key Aroma Compounds in Aged Garlic Extract. Food Chem. 2020, 312, 126081. [Google Scholar] [CrossRef]
- Xia, L.; Qian, M.; Cheng, F.; Wang, Y.; Han, J.; Xu, Y.; Zhang, K.; Tian, J.; Jin, Y. The Effect of Lactic Acid Bacteria on Lipid Metabolism and Flavor of Fermented Sausages. Food Biosci. 2023, 56, 103172. [Google Scholar] [CrossRef]
- Si, R.; Na, Q.; Wu, D.; Wu, X.; Ming, L.; Ji, R. Effects of Age and Muscle Type on the Chemical Composition and Quality Characteristics of Bactrian Camel (Camelus bactrianus) Meat. Foods 2022, 11, 1021. [Google Scholar] [CrossRef]
- Abdelrahman, M.M.; Alhidary, I.A.; Aljumaah, R.S.; Faye, B. Blood Trace Element Status in Camels: A Review. Animals 2022, 12, 2116. [Google Scholar] [CrossRef]
- Sahu, P.K.; Cervera-Mata, A.; Chakradhari, S.; Singh Patel, K.; Towett, E.K.; Quesada-Granados, J.J.; Martín-Ramos, P.; Rufián-Henares, J.A. Seeds as Potential Sources of Phenolic Compounds and Minerals for the Indian Population. Molecules 2022, 27, 3184. [Google Scholar] [CrossRef] [PubMed]


| Ingredients | Composition (%) |
|---|---|
| Camel meat (biceps femoris muscles) | 69 |
| Hump fat | 14 |
| Ground hot red chili pepper | 4.0 |
| Crushed fresh garlic | 3.5 |
| Harissa | 3.5 |
| Tabeul | 1.5 |
| Natural sheep casings (16–18 mm) | 1.5 |
| Salt | 2.0 |
| Powdered fennel seeds | 0.60 |
| Crushed dried mint | 0.40 |
| Samples | FCM (log CFU·g−1) | DCM (log CFU·g−1) |
|---|---|---|
| Mesophilic aerobic bacteria | 7.9 ± 0.1 a | 5.2 ± 0.5 b |
| Lactic acid bacteria | 6.7 ± 0.05 a | 3.7 ± 0.08 b |
| Enterobacteria | 4.8 ± 0.1 a | <2 b |
| Yeast and mold | 4.4 ± 0.2 a | 2.6 ± 0.01 b |
| Escherichia coli β glucuronidase+ | 3.4 ± 0.4 a | <1 b |
| Sulfate-reducing anaerobic bacteria | 2.3 ± 0.06 a | <1 b |
| Positive coagulase staphylococcus | <2 a | <2 a |
| Listeria monocytogenes per 25 g | n.d. | n.d. |
| Components (g/100 g) | FCM | DCM |
|---|---|---|
| Moisture | 60.5 ± 1.0 a | 12.3 ± 0.4 b |
| Protein | 22.6 ± 0.6 b | 29.3 ± 0.9 a |
| Fat | 21.5 ± 0.2 b | 42.5 ± 0.3 a |
| Hump Fat | DCM | |
|---|---|---|
| Saturated Fatty Acids | ||
| Caprilic acid C8:0 | 0.0230 ± 0.0010 | 0.0230 ± 0.0010 |
| Capric acid C10:0 | 0.0740 ± 0.0020 | 0.0630 ± 0.0010 |
| Lauric acid C12:0 | 0.287 ± 0.002 | 0.267 ± 0.013 |
| Tridecanoic acid C13:0 | n.d. | 0.0520 ± 0.0010 |
| Myristic acid C14:0 | 6.22 ± 0.02 | 5.91 ± 0.52 |
| Pentadecanoic acid C15:0 | 0.890 ± 0.030 | 0.960 ± 0.080 |
| Palmitic acid C16:0 | 24.0 ± 0.03 | 25.7 ± 5.1 |
| Heptadecanoic acid C17:0 | 1.31 ± 0.04 | 1.04 ± 0.34 |
| Stearic acid C18:0 | 27.7 ± 0.8 | 24.3± 3.5 |
| Unsaturated Fatty Acids | ||
| Miristoleic acid C14:1 | 0.150 ± 0.090 | 0.125± 0.000 |
| Palmitoleic acid C16:1 | 2.82 ± 0.21 | 3.68 ± 0.01 |
| Heptadecenoic acid C17:1 | 0.600 ± 0.050 | 0.810 ± 0.100 |
| Oleic acid C18:1 (n-9) | 32.6 ± 0.1 | 33.2 ± 0.4 |
| Linoleic acid C18:2 (n-6) | 2.23 ± 0.05 | 3.31 ± 0.20 |
| Eicosenoic acid, C20:1 | 0.190 ± 0.120 | 0.270 ± 0.001 |
| Arachidonic acid C20:4 (n-6) | 0.109 ± 0.001 | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belguith, K.; Jrad, Z.; Oussaief, O.; Debara, M.; Bouhemda, T.; Sebii, H.; Hammadi, M.; El Hatmi, H. Reformulation of Tunisian Sun-Dried Merguez with Camel Meat: Characterization of Physicochemical and Compositional Changes in Organic Acids, Fatty Acids, Volatile Compounds, and Minerals. Foods 2024, 13, 1032. https://doi.org/10.3390/foods13071032
Belguith K, Jrad Z, Oussaief O, Debara M, Bouhemda T, Sebii H, Hammadi M, El Hatmi H. Reformulation of Tunisian Sun-Dried Merguez with Camel Meat: Characterization of Physicochemical and Compositional Changes in Organic Acids, Fatty Acids, Volatile Compounds, and Minerals. Foods. 2024; 13(7):1032. https://doi.org/10.3390/foods13071032
Chicago/Turabian StyleBelguith, Khaoula, Zeineb Jrad, Olfa Oussaief, Mohamed Debara, Talel Bouhemda, Haifa Sebii, Mohamed Hammadi, and Halima El Hatmi. 2024. "Reformulation of Tunisian Sun-Dried Merguez with Camel Meat: Characterization of Physicochemical and Compositional Changes in Organic Acids, Fatty Acids, Volatile Compounds, and Minerals" Foods 13, no. 7: 1032. https://doi.org/10.3390/foods13071032
APA StyleBelguith, K., Jrad, Z., Oussaief, O., Debara, M., Bouhemda, T., Sebii, H., Hammadi, M., & El Hatmi, H. (2024). Reformulation of Tunisian Sun-Dried Merguez with Camel Meat: Characterization of Physicochemical and Compositional Changes in Organic Acids, Fatty Acids, Volatile Compounds, and Minerals. Foods, 13(7), 1032. https://doi.org/10.3390/foods13071032






