Blood-Pressure-Lowering and Endothelium-Dependent Vasorelaxant Effects of Nutgall Tree in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Solution
2.3. Total Polyphenol Content
2.4. Animals and Ethics Statement
2.5. Measurement of the Acute Blood-Pressure-Lowering Effect
2.6. Measurement of the Vasorelaxant Effect in Aortic Rings of SD Rat
2.7. Statistical Analysis
3. Results
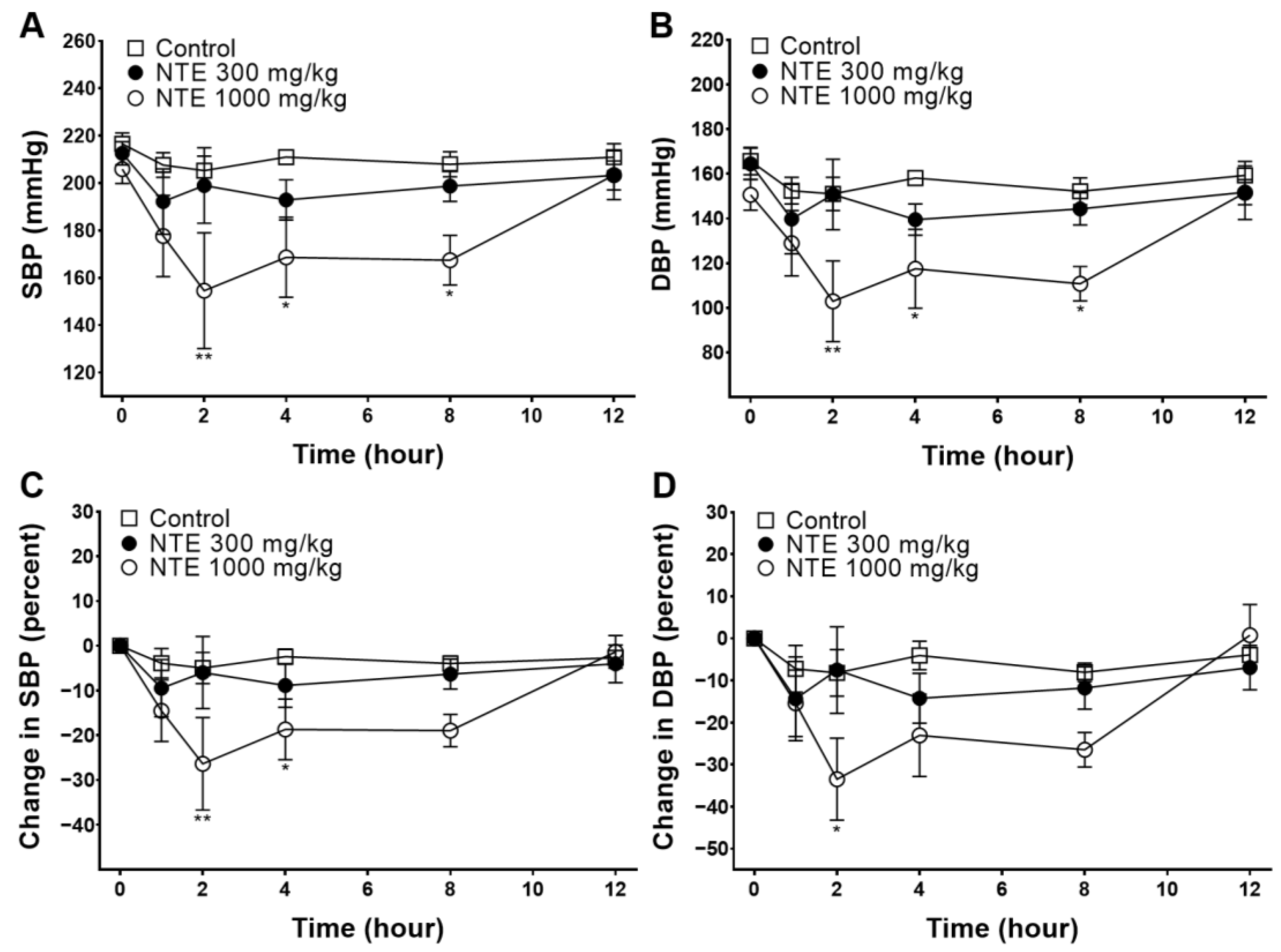
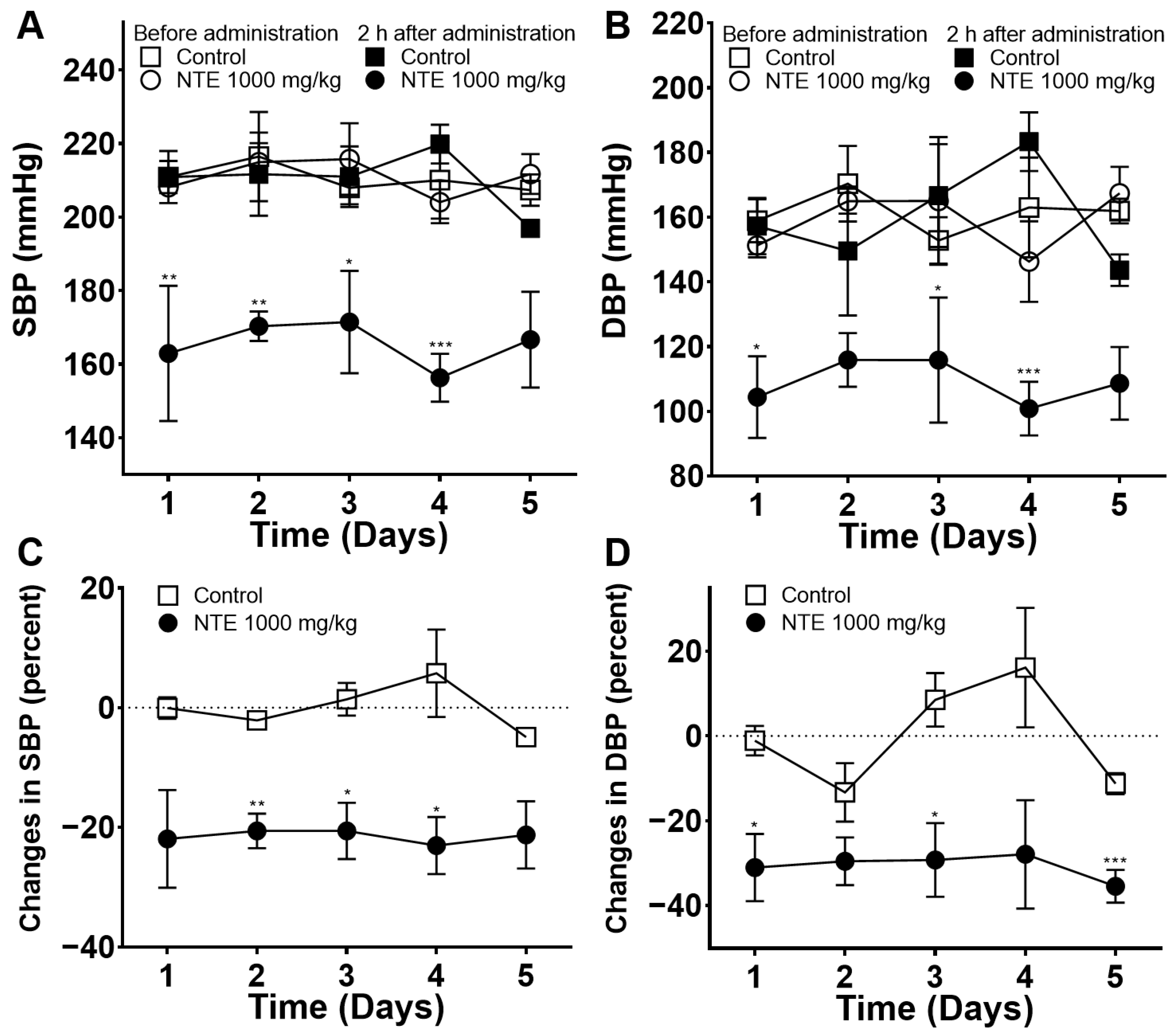
3.1. Acute Blood-Pressure-Lowering Effect of NTE
3.2. Vasorelaxant Effect of NTE
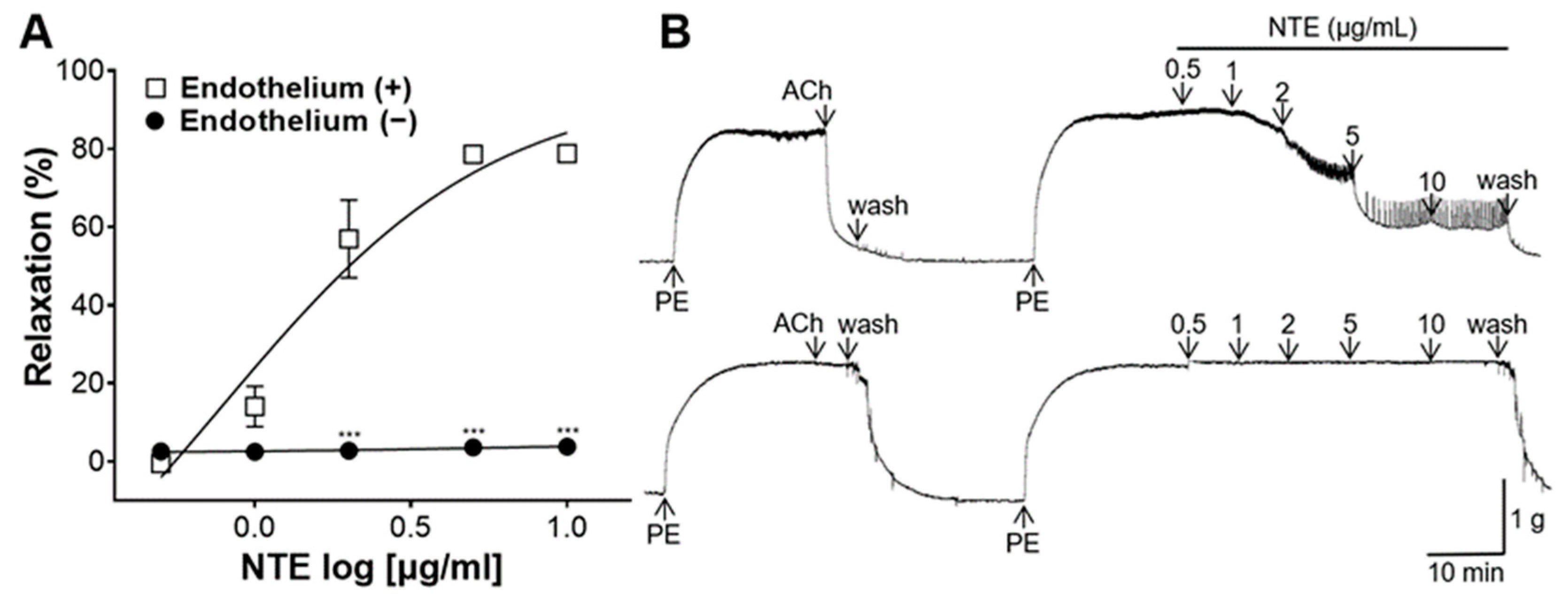
3.3. Vasorelaxant Effect of NTE on Aortic Rings with or without Endothelium
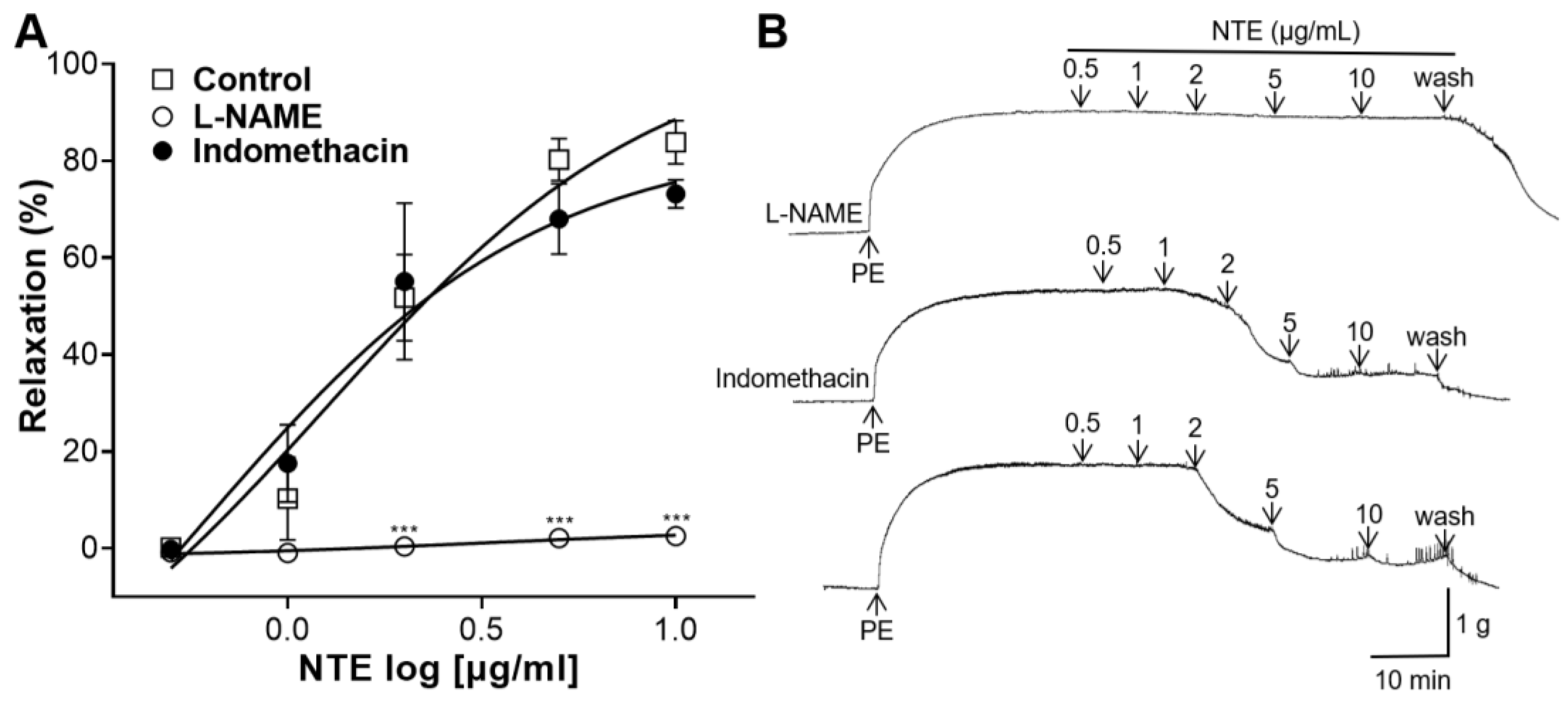
3.4. Effects of L-NAME or Indomethacin Pretreatment on NTE-Induced Vasorelaxation
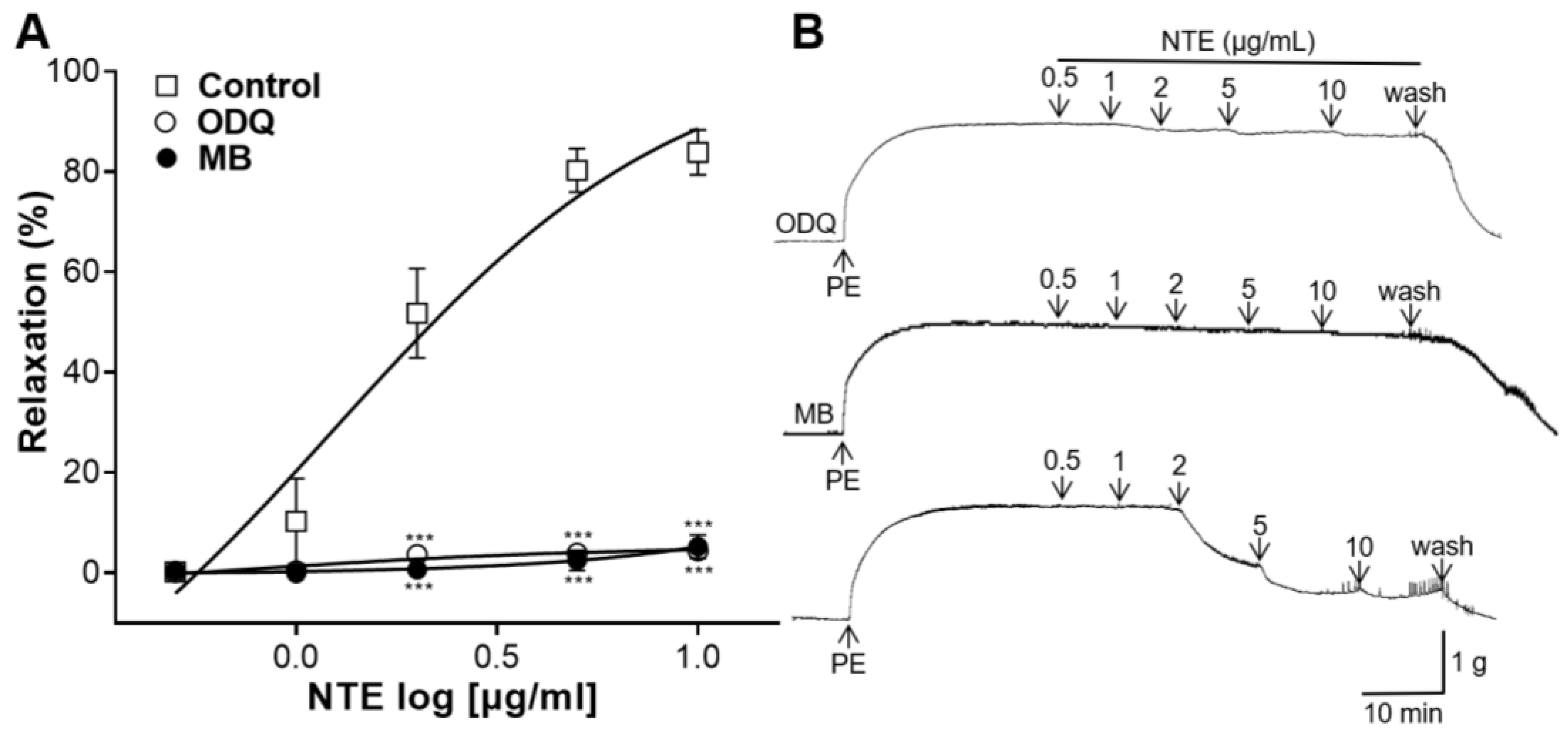
3.5. Effects of ODQ or MB Pretreatment on NTE-Induced Vasorelaxation
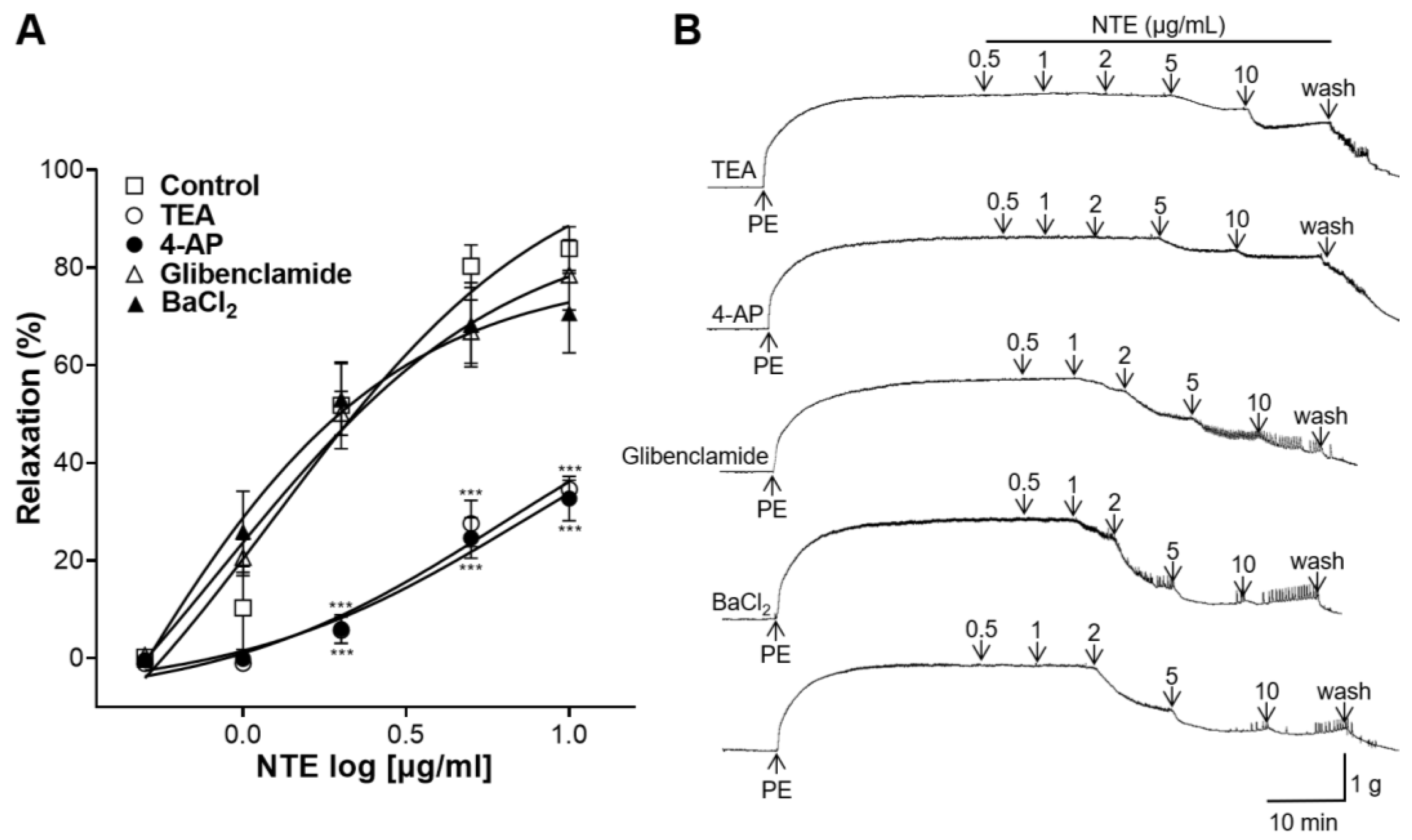
3.6. Effects of K+ Channel Blockers Pretreatment on NTE-Induced Vasorelaxation
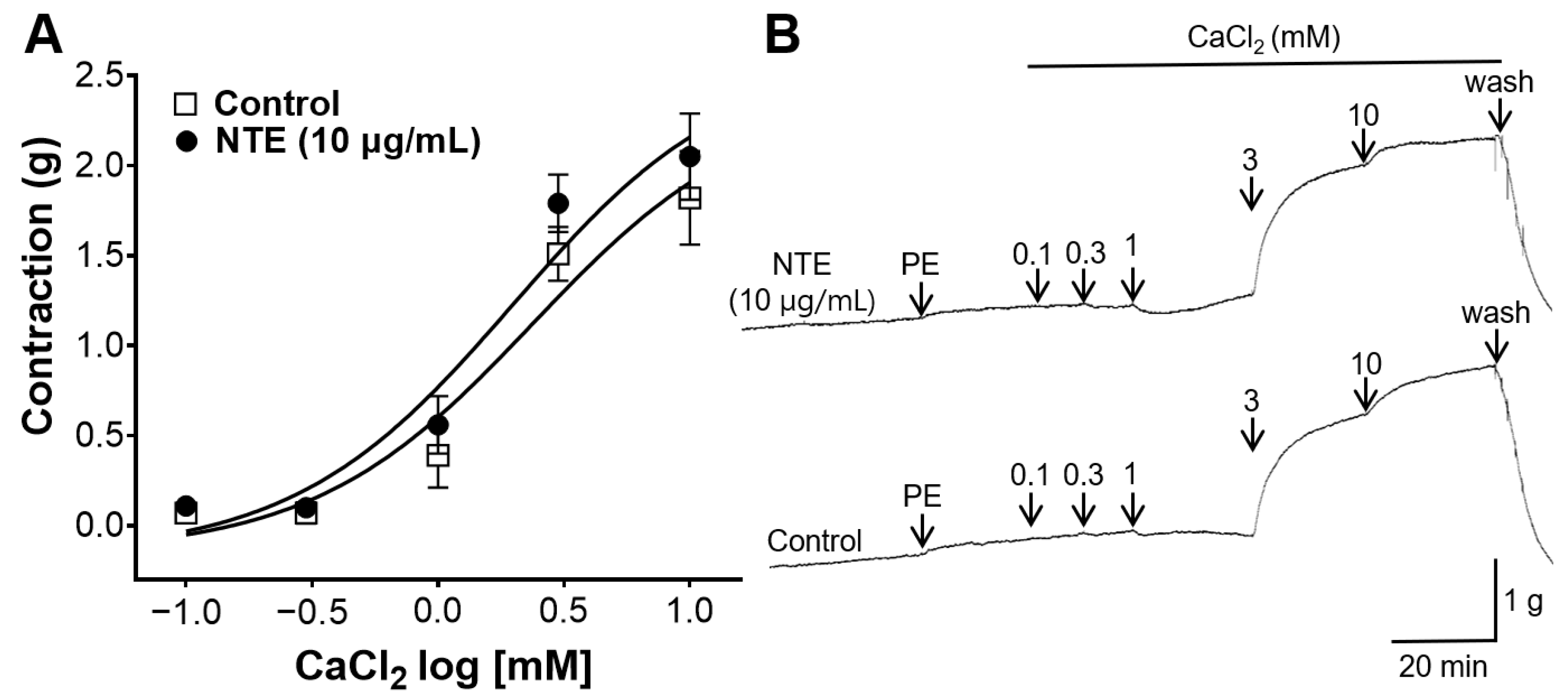
3.7. Inhibitory Effect of NTE on Vasoconstrictive Action of Ca2+
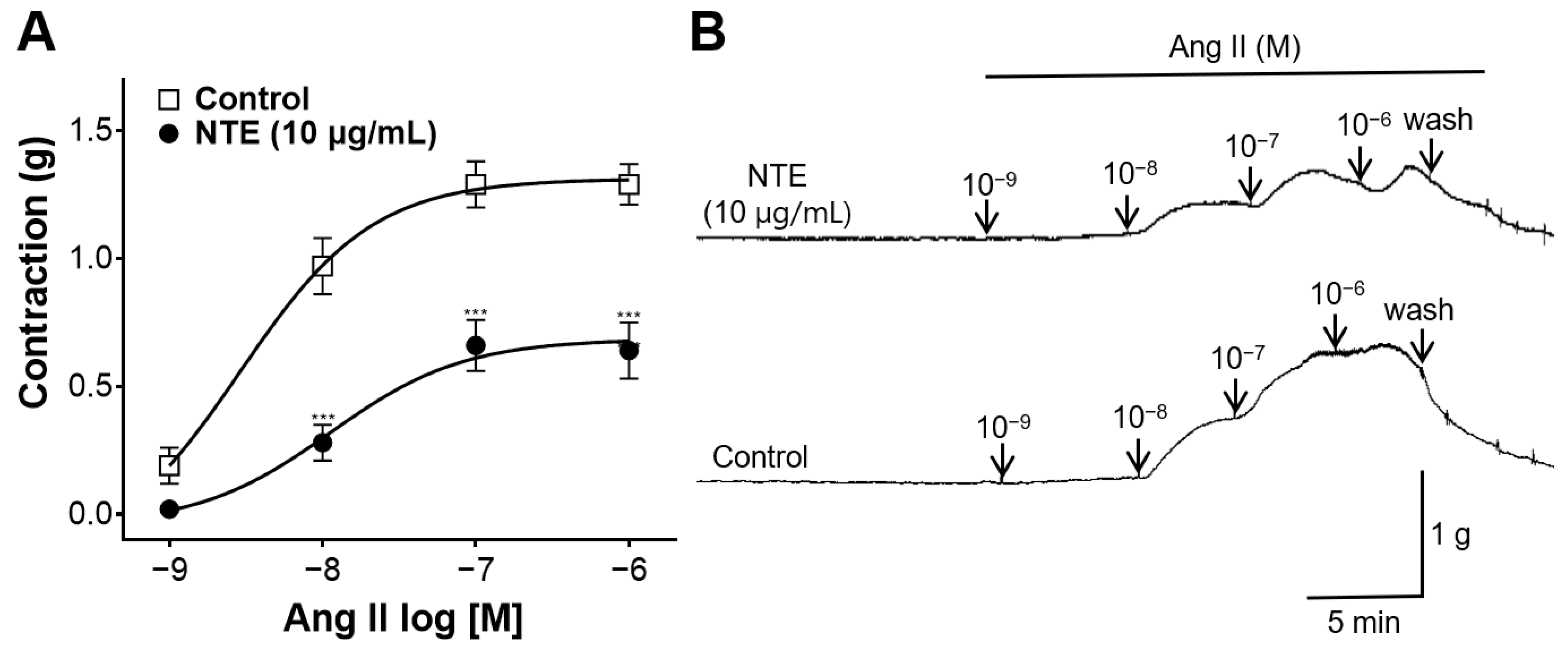
3.8. Inhibitory Effect of NTE on Vasoconstrictive Action of Ang II
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francula-Zaninovic, S.; Nola, I.A. Management of Measurable Variable Cardiovascular Disease’ Risk Factors. Curr. Cardiol. Rev. 2018, 14, 153–163. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, Y.; Zheng, M.; Pan, A.; Wang, M.; Zhao, M.; Li, Y.; Yao, S.; Chen, S.; Wu, S.; et al. Association of Age of Onset of Hypertension With Cardiovascular Diseases and Mortality. J. Am. Coll. Cardiol. 2020, 75, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Blood Pressure Lowering Treatment Trialists’ Collaboration. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: An individual participant-level data meta-analysis. Lancet 2021, 397, 1625–1636. [Google Scholar] [CrossRef]
- Leuci, R.; Brunetti, L.; Poliseno, V.; Laghezza, A.; Loiodice, F.; Tortorella, P.; Piemontese, L. Natural Compounds for the Prevention and Treatment of Cardiovascular and Neurodegenerative Diseases. Foods 2021, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.J. A rational definition for functional foods: A perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef]
- Putnik, P.; Kovačević, D.B. Sustainable Functional Food Processing. Foods 2021, 10, 1438. [Google Scholar] [CrossRef]
- Liao, W.; Sun, G.; Xu, D.; Wang, Y.; Lu, Y.; Sun, J.; Xia, H.; Wang, S. The Blood-Pressure-Lowering Effect of Food-Protein-Derived Peptides: A Meta-Analysis of Recent Clinical Trials. Foods 2021, 10, 2316. [Google Scholar] [CrossRef]
- Festa, J.; Hussain, A.; Al-Hareth, Z.; Singh, H.; Boit, M.D. Anthocyanins and Vascular Health: A Matter of Metabolites. Foods 2023, 12, 1796. [Google Scholar] [CrossRef]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Djakpo, O.; Yao, W. Rhus chinensis and Galla Chinensis—Folklore to modern evidence: Review. Phytother. Res. 2010, 24, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Yi, J.; Cai, S. Phytochemical characteristics and biological activities of Rhus chinensis Mill.: A review. Curr. Opin. Food Sci. 2022, 48, 100925. [Google Scholar] [CrossRef]
- Li, M.; Wang, A.; Zhang, Y.; Han, T.; Guan, L.; Fan, D.; Liu, J.; Xu, Y. A comprehensive review on ethnobotanical, phytochemical and pharmacological aspects of Rhus chinensis Mill. J. Ethnopharmacol. 2022, 293, 115288. [Google Scholar] [CrossRef] [PubMed]
- Devi, H.M.; Singh, N.I. Traditional medicinal uses and pharmacological properties of Rhus chinensis Mill.: A systematic review. Eur. J. Integr. Med. 2018, 21, 43–49. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Park, J.; Choi, H.-Y.; Lee, K. Hypotensive and Endothelium-Dependent Vasorelaxant Effects of Grayblue Spicebush Ethanol Extract in Rats. Foods 2023, 12, 4282. [Google Scholar] [CrossRef]
- Jung, J.; Shin, S.; Park, J.; Lee, K.; Choi, H.-Y. Hypotensive and Vasorelaxant Effects of Sanguisorbae Radix Ethanol Extract in Spontaneously Hypertensive and Sprague Dawley Rats. Nutrients 2023, 15, 4510. [Google Scholar] [CrossRef]
- Doggrell, S.A.; Brown, L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc. Res. 1998, 39, 89–105. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, Y.; Zhao, L.; Cai, S.; Cheng, G. Acute and subchronic toxicities of the ethanol and hot-water extracts from Chinese sumac (Rhus chinensis Mill.) fruits by oral administration in rats. Food Chem. Toxicol. 2018, 119, 14–23. [Google Scholar] [CrossRef]
- Jackson, W.F. Ion channels and vascular tone. Hypertension 2000, 35, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Stankevicius, E.; Kevelaitis, E.; Vainorius, E.; Simonsen, U. Role of nitric oxide and other endothelium-derived factors. Medicina 2003, 39, 333–341. [Google Scholar] [PubMed]
- Sandoo, A.; Zanten, J.J.C.S.V.v.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, T.L.; Pryzwansky, K.B.; Wyatt, T.A.; Lincoln, T.M. Regulation of Sarcoplasmic-Reticulum Protein-Phosphorylation by Localized Cyclic Gmp-Dependent Protein-Kinase in Vascular Smooth-Muscle Cells. Mol. Pharmacol. 1991, 40, 923–931. [Google Scholar] [PubMed]
- Flavahan, N.A. Balancing prostanoid activity in the human vascular system. Trends Pharmacol. Sci. 2007, 28, 106–110. [Google Scholar] [CrossRef]
- Jackson, W.F. Potassium channels and regulation of the microcirculation. Microcirculation 1998, 5, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wen, J.; Wang, N.; Wang, C.; Xu, Q.; Yang, Y. Ion Channels and Vascular Diseases. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e146–e156. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Syed, A.U.; Prada, M.P.; Nystoriak, M.A.; Santana, L.F.; Nieves-Cintrón, M.; Navedo, M.F. Calcium Channels in Vascular Smooth Muscle. Adv. Pharmacol. 2017, 78, 49–87. [Google Scholar]
- Schmieder, R.E.; Hilgers, K.F.; Schlaich, M.P.; Schmidt, B.M.W. Renin-angiotensin system and cardiovascular risk. Lancet 2007, 369, 1208–1219. [Google Scholar] [CrossRef]
- Touyz, R.M. The role of angiotensin II in regulating vascular structural and functional changes in hypertension. Curr. Hypertens. Rep. 2003, 5, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Zhuo, J.; Mendelsohn, F.A.O. Localization and function of angiotensin AT1 receptors. Am. J. Hypertens. 2000, 13, 31s–38s. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Wu, J. Molecular Targets of Antihypertensive Peptides: Understanding the Mechanisms of Action Based on the Pathophysiology of Hypertension. Int. J. Mol. Sci. 2015, 16, 256–283. [Google Scholar] [CrossRef] [PubMed]
- Koumallos, N.; Sigala, E.; Milas, T.; Baikoussis, N.G.; Aragiannis, D.; Sideris, S.; Tsioufis, K. Angiotensin Regulation of Vascular Homeostasis: Exploring the Role of ROS and RAS Blockers. Int. J. Mol. Sci. 2023, 24, 12111. [Google Scholar] [CrossRef]
- Adefegha, S.A. Functional Foods and Nutraceuticals as Dietary Intervention in Chronic Diseases; Novel Perspectives for Health Promotion and Disease Prevention. J. Diet. Suppl. 2018, 15, 977–1009. [Google Scholar] [CrossRef]
- McNeill, J.R.; Jurgens, T.M. A systematic review of mechanisms by which natural products of plant origin evoke vasodilatation. Can. J. Physiol. Pharm. 2006, 84, 803–821. [Google Scholar] [CrossRef]

| Total Phenolic | Total Flavonoid | Total Anthocyanin |
|---|---|---|
| 187.04 ± 2.69 | 90.20 ± 0.59 | 1.30 ± 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Park, J.; Choi, H.-Y.; Bu, Y.; Lee, K. Blood-Pressure-Lowering and Endothelium-Dependent Vasorelaxant Effects of Nutgall Tree in Rats. Foods 2024, 13, 1041. https://doi.org/10.3390/foods13071041
Shin S, Park J, Choi H-Y, Bu Y, Lee K. Blood-Pressure-Lowering and Endothelium-Dependent Vasorelaxant Effects of Nutgall Tree in Rats. Foods. 2024; 13(7):1041. https://doi.org/10.3390/foods13071041
Chicago/Turabian StyleShin, Sujin, Junkyu Park, Ho-Young Choi, Youngmin Bu, and Kyungjin Lee. 2024. "Blood-Pressure-Lowering and Endothelium-Dependent Vasorelaxant Effects of Nutgall Tree in Rats" Foods 13, no. 7: 1041. https://doi.org/10.3390/foods13071041






