Lactic Acid Bacteria of Marine Origin as a Tool for Successful Shellfish Farming and Adaptation to Climate Change Conditions
Abstract
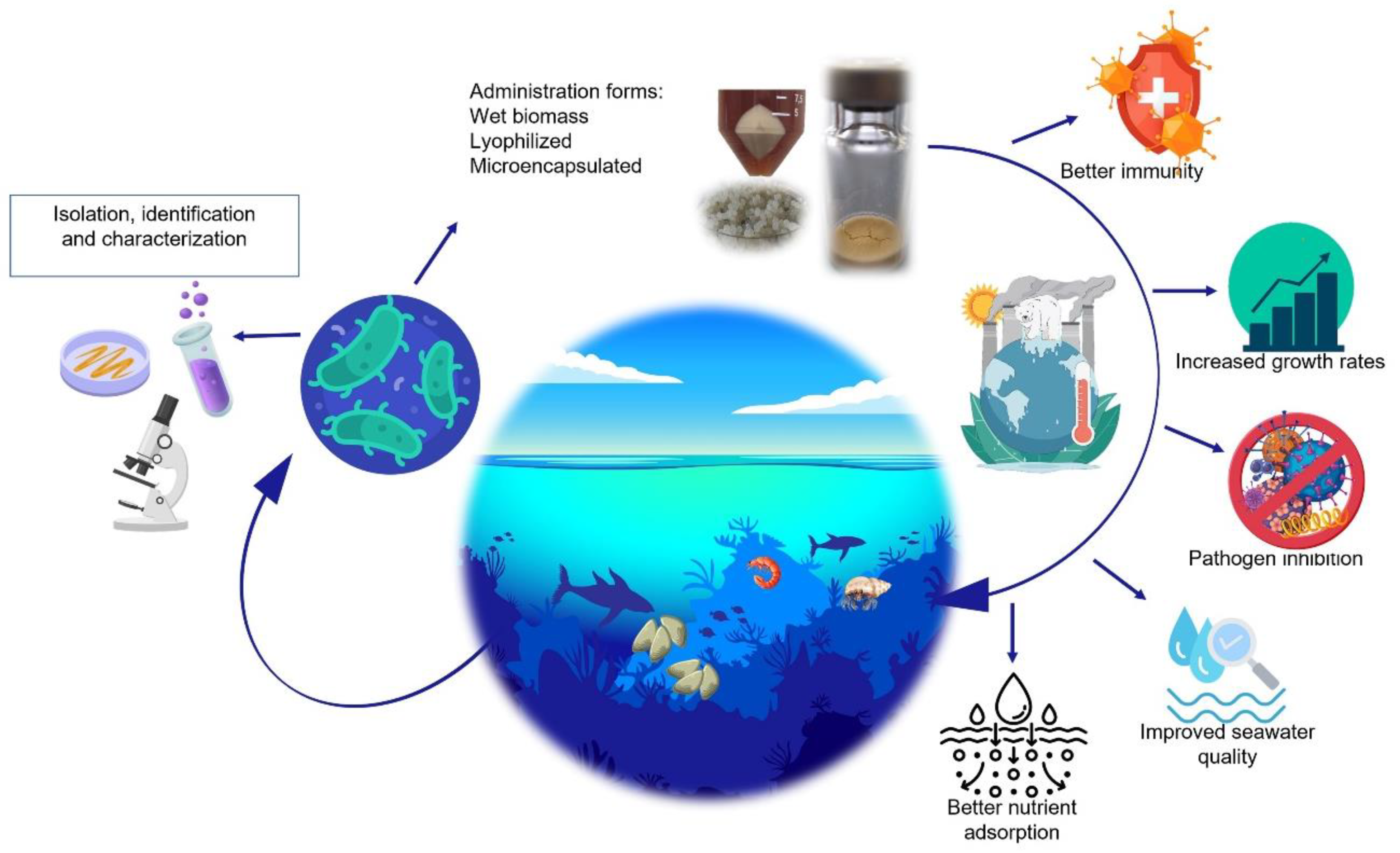
:1. Introduction
2. Lactic Acid Bacteria
Marine Origin Lactic Acid Bacteria
3. Probiotics
4. Application of Probiotics in Sustainable Cultivation of Shellfish
4.1. Growth Promoters
4.2. Digestion of Nutrients
4.3. Seawater Quality
4.4. Stress Tolerance
5. Challenges in Shellfish Cultivation in Climate Change Conditions and Potential Solutions
- -
- Implementing adaptive management strategies: Shellfish farmers can respond to shifting environmental conditions by consistently monitoring water quality, temperature, and pH levels. Using these data, they can adjust their practices such as feed management, stocking densities, and harvest timing to maximize growth and survival rates.
- -
- Adopting sustainable aquaculture practices: Practices like integrated multi-trophic aquaculture systems offer a means to mitigate climate change risks in shellfish farming. These systems involve cultivating various species, like shellfish and seaweed, in tandem. This approach can reduce water nutrient levels, provide supplementary food for shellfish, and potentially counteract ocean acidification through adjacent seaweed photosynthesis.
- -
- Developing resilient shellfish varieties: Research and breeding programs can concentrate on creating shellfish strains that are better equipped to handle changing environmental conditions. These varieties may possess genetic traits or be selectively bred for heightened tolerance to elevated temperatures, lower pH levels, and other climate change-related stressors.
- -
- Implementing conservation and restoration efforts: safeguarding and reviving natural habitats such as seagrass beds, salt marshes, and oyster reefs can serve as natural defenses against climate change impacts on shellfish farming.
- -
- Fostering collaboration between climate change scientists and aquaculture practitioners: by collaborating, these two groups can exchange knowledge and research outcomes and best practices to devise strategies and solutions addressing climate change challenges in shellfish farming.
- -
- Investing in monitoring and research: Sustained research and monitoring endeavors are vital for a deeper understanding of different shellfish species’ vulnerabilities to climate change. This knowledge can guide the development and application of targeted adaptation strategies.
- -
- Implementing policy and regulatory measures: governments and regulatory bodies hold a pivotal role in supporting shellfish farmers through policies fostering sustainable aquaculture practices, incentivizing climate change adaptation, and providing funding for research and infrastructure enhancements [80,81].
- (1)
- Enhanced immune function: Probiotics have the potential to boost the immune system of shellfish, making them more resistant to diseases that may become more prevalent as a result of changing environmental conditions. By fostering a healthy microbial community, probiotics help create a protective barrier against harmful pathogens.
- (2)
- Improved nutrient utilization: Climate change can affect the availability and distribution of nutrients in the water. Probiotics play a role in enhancing the efficiency of nutrient utilization by shellfish, ensuring optimal growth even under suboptimal conditions.
- (3)
- Mitigation of harmful algal blooms: Certain probiotic strains have shown promise in preventing or mitigating harmful algal blooms. By outcompeting harmful algae for nutrients or producing substances that inhibit their growth, probiotics can help maintain a balanced and healthy aquatic environment for shellfish [83].
6. Conclusions and Further Perspective
- -
- Develop formulations of probiotic cultures and models of treatment of fresh shellfish with probiotic cultures, with the aim of ensuring microbiological safety and extended shelf life;
- -
- Develop models of packaging and preservation of shellfish;
- -
- Investigate shellfish treated in this way will be accepted by consumers;
- -
- Conduct scientific research on the positive impact of indigenous probiotic cultures on the microbiological safety and extended durability of shellfish;
- -
- Educate small producers about the advantages and benefits of using bioprotective microbial cultures to improve the health and prolong the shelf life of shellfish;
- -
- Develop ways of feeding certain types of bivalves depending on whether the breeding is carried out in aquariums or cages in the sea;
- -
- Study the interaction of probiotic cultures with other microorganisms in the sea and the possible potential damage to the ecological system and food chain;
- -
- Encourage breeders to add indigenous probiotic cultures, in a form available to the shellfish, during the feeding process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mugwanya, M.; Dawood, M.A.; Kimera, F.; Sewilam, H. Anthropogenic temperature fluctuations and their effect on aquaculture: A comprehensive review. Aquac. Fish. 2022, 7, 223–243. [Google Scholar] [CrossRef]
- Dawood, M.A.; Noreldin, A.E.; Sewilam, H. Long term salinity disrupts the hepatic function, intestinal health, and gills antioxidative status in Nile tilapia stressed with hypoxia. Ecotoxicol. Environ. Saf. 2021, 220, 112412. [Google Scholar] [CrossRef]
- Fazio, F. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 2019, 500, 237–242. [Google Scholar] [CrossRef]
- Ruby, P.; Ahilan, B. An overview of climate change impact in fisheries and aquaculture. Clim. Chang. 2018, 4, 87–94. [Google Scholar]
- Weatherdon, L.V.; Magnan, A.K.; Rogers, A.D.; Sumaila, U.R.; Cheung, W.W. Observed and projected impacts of climate change on marine fisheries, aquaculture, coastal tourism, and human health: An update. Front. Mar. Sci. 2016, 3, 48. [Google Scholar] [CrossRef]
- Maulu, S.; Hasimuna, O.J.; Haambiya, L.H.; Monde, C.; Musuka, C.G.; Makorwa, T.H.; Munganga, B.P.; Phiri, K.J.; Nsekanabo, J.D. Climate change effects on aquaculture production: Sustainability implications, mitigation, and adaptations. Front. Sustain. Food Syst. 2021, 5, 609097. [Google Scholar] [CrossRef]
- Gubbins, M.; Bricknell, I.; Service, M. Impacts of climate change on aquaculture. MCCIP Sci. Rev. 2013, 318–327. [Google Scholar] [CrossRef]
- Frost, M.; Baxter, J.M.; Buckley, P.J.; Cox, M.; Dye, S.R.; Withers Harvey, N. Impacts of climate change on fish, fisheries and aquaculture. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 331–336. [Google Scholar] [CrossRef]
- Collins, C.; Bresnan, E.; Brown, L.; Falconer, L.; Guilder, J.; Jones, L.; Kennerley, A.; Malham, S.; Murray, A.; Stanley, M. Impacts of Climate Change on Aquaculture. (MCCIP Science Review; No. 2020); Marine Climate Change Impacts Partnership: Lowestoft, UK, 2020. [Google Scholar] [CrossRef]
- Lebel, L.; Navy, H.; Jutagate, T.; Akester, M.J.; Sturm, L.; Lebel, P.; Lebel, B. Innovation, practice, and adaptation to climate in the aquaculture sector. Rev. Fish. Sci. Aquac. 2021, 29, 721–738. [Google Scholar] [CrossRef]
- Kovačić, I.; Žunec, A.; Matešković, M.; Burić, P.; Iveša, N.; Štifanić, M.; Frece, J. Commercial quality, biological indices and biochemical composition of queen scallop Aequipecten opercularis in culture. Fishes 2023, 8, 48. [Google Scholar] [CrossRef]
- Čanak, I.; Kovačić, I.; Žunec, A.; Jakopović, Ž.; Kostelac, D.; Markov, K.; Štifanić, M.; Burić, P.; Iveša, N.; Frece, J. Study of the impact of Lactiplantibacillus plantarum I on the health status of queen scallop Aequipecten opercularis. Appl. Sci. 2023, 13, 7723. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Song, Z.; Shan, C.; Zhu, R.; Liu, F. Development of a simple, low-cost and eurytopic medium based on Pleurotus eryngii for lactic acid bacteria. AMB Express 2016, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Šušković, J.; Kos, B.; Beganović, J.; Leboš Pavunc, A.; Habjanič, K.; Matošić, S. Antimicrobial activity–the most important property of probiotic and starter lactic acid bacteria. Food Technol. Biotechnol. 2010, 48, 296–307. [Google Scholar]
- Wu, C.; Huang, J.; Zhou, R. Genomics of lactic acid bacteria: Current status and potential applications. Crit. Rev. Microbiol. 2017, 43, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, K.; Thiruneelakandan, G. Prospects of lactic acid bacteria of marine origin. Indian J. Biotechnol. 2008, 7, 170–177. [Google Scholar]
- Sica, M.G.; Olivera, N.L.; Brugnoni, L.I.; Marucci, P.L.; López-Cazorla, A.C.; Cubitto, M.A. Isolation, identification and antimicrobial activity of lactic acid bacteria from the Bahía Blanca Estuary. Rev. Biol. Mar. Y Oceanogr. 2010, 45, 389–397. [Google Scholar] [CrossRef]
- Muñoz-Atienza, E.; Gómez-Sala, B.; Araújo, C.; Campanero, C.; Del Campo, R.; Hernández, P.E.; Herranz, C.; Cintas, L.M. Antimicrobial activity, antibiotic susceptibility and virulence factors of lactic acid bacteria of aquatic origin intended for use as probiotics in aquaculture. BMC Microbiol. 2013, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Čanak, I.; Markov, K.; Gavrilović, A.; Bosanac, P.; Dujaković, J.; Jakopović, Ž. Mikrobiološki i kemijski parametri ribe i školjkaša. Croat. J. Food Technol. Biotechnol. Nutr. 2018, 13, 44–49. [Google Scholar] [CrossRef]
- Čanak, I.; Markov, K.; Jakopović, Ž.; Kostelac, D.; Čolak, S.; Mejdandžić, D.; Mattea, Ž.; Damir, J.; Frece, J. In vitro characterization of Lactobacillus plantarum O1 isolated from gut of sea bream (Sparus aurata) as potential fish probiotic. In Proceedings of the 4th International Conference of Food and Biosystems Engineering (FaBE 2019), Heraklion, Greece, 30 May–2 June 2019; pp. 170–175. [Google Scholar]
- Merrifield, D.L.; Balcázar, J.L.; Daniels, C.; Zhou, Z.; Carnevali, O.; Sun, Y.Z.; Hoseinifar, S.H.; Ringø, E. Indigenous lactic acid bacteria in fish and crustaceans. Aquac. Nutr. Gut Health Probiotics Prebiotics 2014, 128–168. [Google Scholar] [CrossRef]
- Pinto, A.L.; Fernandes, M.; Pinto, C.; Albano, H.; Castilho, F.; Teixeira, P. Characterization of anti-Listeria bacteriocins isolated from shellfish: Potential antimicrobials to control non-fermented seafood. Int. J. Food Microbiol. 2009, 129, 50–58. [Google Scholar] [CrossRef]
- Pavlova, B.K.; Ibryamova, S.F.; Arhangelova, N.N.; Doichev, D.D.; Ivanov, R.I.; Chipev, N.H.; Natchev, N.D.; Ignatova-Ivanova, T.V. Integrative investigation on the ecology of the black sea mussel Mytilus galloprovincialis Lam. and its habitat. Ecol. Balk. 2020, 3, 10–17. [Google Scholar]
- Lee, H.I.; Hee Kim, M.; Young, K.K.; So, J.S. Screening and selection of stress resistant Lactobacillus spp. isolated from the marine oyster (Crassostrea gigas). Anaerobe 2010, 16, 522–526. [Google Scholar] [CrossRef]
- Fajardo, P.; Atanassova, M.; Garrido-Maestu, A.; Wortner-Smith, T.; Cotterill, J.; Cabado, A.G. Bacteria isolated from shellfish digestive gland with antipathogenic activity as candidates to increase the efficiency of shellfish depuration process. Food Control 2014, 46, 272–281. [Google Scholar] [CrossRef]
- Alonso, S.; Carmen Castro, M.; Berdasco, M.; de la Banda, I.G.; Moreno-Ventas, X.; de Rojas, A.H. Isolation and partial characterization of lactic acid bacteria from the gut microbiota of marine fishes for potential application as probiotics in aquaculture. Probiotics Antimicrob. Proteins 2019, 11, 569–579. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Gaggìa, F.; Mattarelli, P.; Biavati, B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010, 141, S15–S28. [Google Scholar] [CrossRef]
- Ringø, E. Probiotics in shellfish aquaculture. Aquac. Fish. 2020, 5, 1–27. [Google Scholar] [CrossRef]
- Gómez-Sala, B.; Muñoz-Atienza, E.; Sánchez, J.; Basanta, A.; Herranz, C.; Hernández, P.E.; Cintas, L.M. Bacteriocin production by lactic acid bacteria isolated from fish, seafood and fish products. Eur. Food Res. Technol. 2015, 241, 341–356. [Google Scholar] [CrossRef]
- Yang, Q.; Lü, Y.; Zhang, M.; Gong, Y.; Li, Z.; Tran, N.T.; He, Y.; Zhu, C.; Lu, Y.; Zhang, Y.; et al. Lactic acid bacteria, Enterococcus faecalis Y17 and Pediococcus pentosaceus G11, improved growth performance, and immunity of mud crab (Scylla paramamosain). Fish Shellfish. Immunol. 2019, 93, 135–143. [Google Scholar] [CrossRef]
- Labh, S.N.; Shakya, S.R. Application of immunostimulants as an alternative to vaccines for health management in aquaculture. Int. J. Fish. Aquat. Stud. 2014, 2, 153–156. [Google Scholar]
- Elston, R.A.; Ford, S.E. Shellfish diseases and health management. In Shellfish Aquaculture and the Environment; Wiley-Blackwell: Oxford, UK, 2011; pp. 359–394. ISBN 978-0-8138-1413-1. [Google Scholar]
- Carnegie, R.B.; Arzul, I.; Bushek, D. Managing marine mollusc diseases in the context of regional and international comerce: Policy issues and emerging concerns. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150215. [Google Scholar] [CrossRef]
- Aguilar-Macías, O.L.; Ojeda-Ramírez, J.J.; Campa-Córdova, A.I.; Saucedo, P.E. Evaluation of natural and commercial probiotics for improving growth and survival of the pearl oyster, Pinctada mazatlanica, during late hatchery and early field culturing. J. World Aquac. Soc. 2010, 41, 447–454. [Google Scholar] [CrossRef]
- Campa-Córdova, A.I.; González-Ocampo, H.; Luna-González, A.; Mazón-Suástegui, J.M.; Ascencio, F. Growth, survival, and superoxide dismutase activity in juvenile Crassostrea corteziensis (Hertlein, 1951) treated with probiotics. Hidrobiológica 2009, 19, 151–157. [Google Scholar]
- Sohn, S.; Lundgren, K.M.; Tammi, K.; Karim, M.; Smolowitz, R.; Nelson, D.R.; Rowley, D.C.; Gómez-Chiarri, M. Probiotic strains for disease management in hatchery larviculture of the eastern oyster Crassostrea virginica. J. Shellfish. Res. 2016, 35, 307–317. [Google Scholar] [CrossRef]
- Diep, D.B.; Nes, I.F. Ribosomally synthesized antibacterial peptides in Gram positive bacteria. Curr. Drug Targets 2002, 3, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Ringø, E.; Schillinger, U.; Holzapfel, W. Antimicrobial activity of lactic acid bacteria isolated from aquatic animals and the use of lactic acid bacteria in aquaculture. In Biology of Growing Animals; Mosenthin, R., Zentek, J., Żebrowska, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 418–453. ISBN 9780444512321. [Google Scholar]
- Sumon, T.A.; Hussain, M.A.; Sumon, M.A.A.; Jang, W.J.; Abellan, F.G.; Sharifuzzaman, S.M.; Brown, C.L.; Lee, E.-W.; Kim, C.-H.; Hasan, M.T. Functionality and prophylactic role of probiotics in shellfish aquaculture. Aquac. Rep. 2022, 25, 101220. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, M.; Li, X.; Han, Y.; Wu, F.; Liu, Y. The effects of feeding Lactobacillus pentosus on growth, immunity, and disease resistance in Haliotis discus hannai Ino. Fish Shellfish. Immunol. 2018, 78, 42–51. [Google Scholar] [CrossRef]
- Hadi, J.A.; Gutierrez, N.; Alfaro, A.C.; Roberts, R.D. Use of probiotic bacteria to improve growth and survivability of farmed New Zealand abalone (Haliotis iris). N. Z. J. Mar. Freshw. Res. 2014, 48, 405–415. [Google Scholar] [CrossRef]
- Grandiosa, R.; Mérien, F.; Young, T.; Van Nguyen, T.; Gutierrez, N.; Kitundu, E.; Alfaro, A.C. Multi-strain probiotics enhance immune responsiveness and alters metabolic profiles in the New Zealand black-footed abalone (Haliotis iris). Fish Shellfish. Immunol. 2018, 82, 330–338. [Google Scholar] [CrossRef]
- Grandiosa, R.; Young, T.; Van Nguyen, T.; Mérien, F.; Alfaro, A.C. Immune response in probiotic-fed New Zealand black-footed abalone (Haliotis iris) under Vibrio splendidus challenge. Fish Shellfish. Immunol. 2020, 104, 633–639. [Google Scholar] [CrossRef]
- Karim, M.; Zhao, W.; Rowley, D.; Nelson, D.; Gomez-Chiarri, M. Probiotic strains for shellfish aquaculture: Protection of eastern oyster, Crassostrea virginica, larvae and juveniles againsl bacterial challenge. J. Shellfish. Res. 2013, 32, 401–408. [Google Scholar] [CrossRef]
- El-Dakar, A.Y.; Shalaby, S.M.; Saoud, I.P. Assessing the use of a dietary probiotic/prebiotic as an enhancer of spinefoot rabbitfish Siganus rivulatus survival and growth. Aquac. Nutr. 2007, 13, 407–412. [Google Scholar] [CrossRef]
- Muller, J.A.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Manufacture of probiotic bacteria. In Prebiotics and Probiotics Science and Technology; Springer: New York, NY, USA, 2009; pp. 725–759. [Google Scholar]
- Martínez-Cruz, P.; Ibáñez, A.L.; Monroy Hermosillo, O.A.; Ramírez Saad, H.C. Use of probiotics in aquaculture. Int. Sch. Res. Not. 2012, 2012, 916845. [Google Scholar] [CrossRef]
- Lara-Flores, M.; Olvera-Novoa, M.A.; Guzmán-Méndez, B.E.; López-Madrid, W. Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus, and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia (Oreochromis niloticus). Aquaculture 2003, 216, 193–201. [Google Scholar] [CrossRef]
- Ghosh, S.; Sinha, A.; Sahu, C. Dietary probiotic supplementation in growth and health of live-bearing ornamental fishes. Aquac. Nutr. 2008, 14, 289–299. [Google Scholar] [CrossRef]
- Dharmaraj, S.; Dhevendaran, K. Evaluation of Streptomyces as a probiotic feed for the growth of ornamental fish Xiphophorus helleri. Food Technol. Biotechnol. 2010, 48, 497–504. [Google Scholar]
- Macey, B.M.; Coyne, V.E. Improved growth rate and disease resistance in farmed Haliotis midae through probiotic treatment. Aquaculture 2005, 245, 249–261. [Google Scholar] [CrossRef]
- Balcázar, J.L.; Decamp, O.; Vendrell, D.; De Blas, I.; Ruiz-Zarzuela, I. Health and nutritional properties of probiotics in fish and shellfish. Microb. Ecol. Health Dis. 2006, 18, 65–70. [Google Scholar] [CrossRef]
- El-Haroun, E.R.; Goda, A.S.; Kabir Chowdhury, M.A. Effect of dietary probiotic Biogen® supplementation as a growth promoter on growth performance and feed utilization of Nile tilapia Oreochromis niloticus (L.). Aquac. Res. 2006, 37, 1473–1480. [Google Scholar] [CrossRef]
- Lin, H.Z.; Guo, Z.; Yang, Y.; Zheng, W.; Li, Z.J. Effect of dietary probiotics on apparent digestibility coefficients of nutrients of white shrimp Litopenaeus vannamei Boone. Aquac. Res. 2004, 35, 1441–1447. [Google Scholar] [CrossRef]
- Chai, P.C.; Song, X.L.; Chen, G.F.; Xu, H.; Huang, J. Dietary supplementation of probiotic Bacillus PC465 isolated from the gut of Fenneropenaeus chinensis improves the health status and resistance of Litopenaeus vannamei against white spot syndrome virus. Fish Shellfish. Immunol. 2016, 54, 602–611. [Google Scholar] [CrossRef]
- Zhao, J.; Ling, Y.; Zhang, R.; Ke, C.; Hong, G. Effects of dietary supplementation of probiotics on growth, immune responses, and gut microbiome of the abalone Haliotis diversicolor. Aquaculture 2018, 493, 289–295. [Google Scholar] [CrossRef]
- Lalloo, R.; Ramchuran, S.; Ramduth, D.; Görgens, J.; Gardiner, N. Isolation and selection of Bacillus spp. as potential biological agents for enhancement of water quality in culture of ornamental fish. J. Appl. Microbiol. 2007, 103, 1471–1479. [Google Scholar] [CrossRef]
- Wang, Y.B.; Xu, Z.R.; Xia, M.S. The effectiveness of commercial probiotics in northern white shrimp Penaeus vannamei ponds. Fish. Sci. 2005, 71, 1036–1041. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S. Effects of heat killed Lactobacillus plantarum (LP20) supplemental diets on growth performance, stress resistance and immune response of red sea bream, Pagrus major. Aquaculture 2015, 442, 29–36. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S. Effects of partial substitution of fish meal by soybean meal with or without heat-killed Lactobacillus plantarum (LP20) on growth performance, digestibility, and immune response of amberjack, Seriola dumerili Juveniles. BioMed Res. Int. 2015, 2015, 514196. [Google Scholar] [CrossRef]
- Castex, M.; Lemaire, P.; Wabete, N.; Chim, L. Effect of probiotic Pediococcus acidilactici on antioxidant defences and oxidative stress of Litopenaeus stylirostris under Vibrio nigripulchritudo challenge. Fish Shellfish. Immunol. 2010, 28, 622–631. [Google Scholar] [CrossRef]
- Zheng, X.; Duan, Y.; Dong, H.; Zhang, J. Effects of dietary Lactobacillus plantarum in different treatments on growth performance and immune gene expression of white shrimp Litopenaeus vannamei under normal condition and stress of acute low salinity. Fish Shellfish. Immunol. 2017, 62, 195–201. [Google Scholar] [CrossRef]
- Rengpipat, S.; Rukpratanporn, S.; Piyatiratitivorakul, S.; Menasaveta, P. Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture 2000, 191, 271–288. [Google Scholar] [CrossRef]
- Li, H.D.; Tian, X.L.; Dong, S.L. Growth performance, non-specific immunity, intestinal histology and disease resistance of Litopenaeus vannamei fed on a diet supplemented with live cells of Clostridium butyricum. Aquaculture 2019, 498, 470–481. [Google Scholar] [CrossRef]
- Miao, S.; Han, B.; Zhao, C.; Hu, J.; Zhu, J.; Zhang, X.; Sun, L. Effects of dietary Pediococcus acidilactici GY2 single or combined with Saccharomyces cerevisiae or/and β-glucan on the growth, innate immunity response and disease resistance of Macrobrachium rosenbergii. Fish Shellfish. Immunol. 2020, 98, 68–76. [Google Scholar] [CrossRef]
- Amoah, K.; Huang, Q.C.; Dong, X.H.; Tan, B.P.; Zhang, S.; Chi, S.Y.; Yang, Q.-H.; Liu, H.-Y.; Yang, Y.Z. Paenibacillus polymyxa improves the growth, immune and antioxidant activity, intestinal health, and disease resistance in Litopenaeus vannamei challenged with Vibrio parahaemolyticus. Aquaculture 2020, 518, 734563. [Google Scholar] [CrossRef]
- Dash, G.; Raman, R.P.; Prasad, K.P.; Makesh, M.; Pradeep, M.A.; Sen, S. Evaluation of paraprobiotic applicability of Lactobacillus plantarum in improving the immune response and disease protection in giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879). Fish Shellfish. Immunol. 2015, 43, 167–174. [Google Scholar] [CrossRef]
- Kolanchinathan, P.; Kumari, P.R.; Gnanam, T.S.; John, G.; Balasundaram, A. Research article performance evaluation of two probiotic species, on the growth, body composition and immune expression in Penaeus monodon. J. Fish. Aquat. Sci. 2017, 12, 157–167. [Google Scholar] [CrossRef]
- Tepaamorndech, S.; Chantarasakha, K.; Kingcha, Y.; Chaiyapechara, S.; Phromson, M.; Sriariyanun, M.; Kirschke, C.P.; Huang, L.; Visessanguan, W. Effects of Bacillus aryabhattai TBRC8450 on vibriosis resistance and immune enhancement in Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish. Immunol. 2019, 86, 4–13. [Google Scholar] [CrossRef]
- Li, H.; Tian, X.; Zhao, K.; Jiang, W.; Dong, S. Effect of Clostridium butyricum in different forms on growth performance, disease resistance, expression of genes involved in immune responses and mTOR signaling pathway of Litopenaeus vannamai. Fish Shellfish. Immunol. 2019, 87, 13–21. [Google Scholar] [CrossRef]
- Wu, H.J.; Sun, L.B.; Li, C.B.; Li, Z.Z.; Zhang, Z.; Wen, X.B.; Hu, Z.; Zhang, Y.-L.; Li, S.K. Enhancement of the immune response and protection against Vibrio parahaemolyticus by indigenous probiotic Bacillus strains in mud crab (Scylla paramamosain). Fish Shellfish. Immunol. 2014, 41, 156–162. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, A.C.; Angulo, C.; Luna-González, A.; Álvarez-Ruiz, P.; Mazón-Suástegui, J.M.; Campa-Córdova, Á.I. Effect of mixed-Bacillus spp isolated from pustulose ark Anadara tuberculosa on growth, survival, viral prevalence and immune-related gene expression in shrimp Litopenaeus vannamei. Fish Shellfish. Immunol. 2016, 59, 95–102. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Dong, H.; Ding, X.; Liu, Q.; Li, H.; Zhang, J.; Xiong, D. Changes in the intestine microbial, digestive, and immune-related genes of Litopenaeus vannamei in response to dietary probiotic Clostridium butyricum supplementation. Front. Microbiol. 2018, 9, 2191. [Google Scholar] [CrossRef]
- Hao, K.; Liu, J.Y.; Ling, F.; Liu, X.L.; Lu, L.; Xia, L.; Wang, G.X. Effects of dietary administration of Shewanella haliotis D4, Bacillus cereus D7 and Aeromonas bivalvium D15, single or combined, on the growth, innate immunity and disease resistance of shrimp, Litopenaeus vannamei. Aquaculture 2014, 428, 141–149. [Google Scholar] [CrossRef]
- Interaminense, J.A.; Vogeley, J.L.; Gouveia, C.K.; Portela, R.S.; Oliveira, J.P.; Silva, S.M.; Coimbra, M.R.M.; Peixoto, S.M.; Soares, R.B.; Buarque, D.S.; et al. Effects of dietary Bacillus subtilis and Shewanella algae in expression profile of immune-related genes from hemolymph of Litopenaeus vannamei challenged with Vibrio parahaemolyticus. Fish Shellfish. Immunol. 2019, 86, 253–259. [Google Scholar] [CrossRef]
- Thiyagarajan, V.; Ko, G.W.K. Larval growth response of the Portuguese oyster (Crassostrea angulata) to multiple climate change stressors. Aquaculture 2012, 370, 90–95. [Google Scholar] [CrossRef]
- Reid, G.K.; Gurney-Smith, H.J.; Marcogliese, D.J.; Knowler, D.; Benfey, T.; Garber, A.F.; Forster, I.; Chopin, T.; Brewer-Dalton, K.; Moccia, R.; et al. Climate change and aquaculture: Considering biological response and resources. Aquac. Environ. Interact. 2019, 11, 569–602. [Google Scholar] [CrossRef]
- Barton, A.; Waldbusser, G.G.; Feely, R.A.; Weisberg, S.B.; Newton, J.A.; Hales, B.; Cudd, S.; Eudeline, B.; Langdon, C.J.; Jefferds, I.; et al. Impacts of coastal acidification on the Pacific Northwest shellfish industry and adaptation strategies implemented in response. Oceanography 2015, 28, 146–159. [Google Scholar] [CrossRef]
- Buck, B.H.; Nevejan, N.; Wille, M.; Chambers, M.D.; Chopin, T. Offshore and multi-use aquaculture with extractive species: Seaweeds and bivalves. In Aquaculture Perspective of Multi-Use Sites in the Open Ocean: The Untapped Potential for Marine Resources in the Anthropocene; Buck, B.H., Langan, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 23–69. ISBN 978-3-319-51157-3. [Google Scholar]
- Hai, N.V. The use of probiotics in aquaculture. J. Appl. Microbiol. 2015, 119, 917–935. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.; Zhang, D.; Bi, X. Control of harmful blue-green algae in ponds by berberine compound and probiotics. Fish. Sci. 2017, 36, 443–448. [Google Scholar]
- Sun, F.; Wang, Y.; Wang, C.; Zhang, L.; Tu, K.; Zheng, Z. Insights into the intestinal microbiota of several aquatic organisms and association with the surrounding environment. Aquaculture 2019, 507, 196–202. [Google Scholar] [CrossRef]
- Sehnal, L.; Brammer-Robbins, E.; Wormington, A.M.; Blaha, L.; Bisesi, J.; Larkin, I.; Martyniuk, C.J.; Simonin, M.; Adamovsky, O. Microbiome composition and function in aquatic vertebrates: Small organisms making big impacts on aquatic animal health. Front. Microbiol. 2021, 12, 567408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čanak, I.; Kostelac, D.; Jakopović, Ž.; Markov, K.; Frece, J. Lactic Acid Bacteria of Marine Origin as a Tool for Successful Shellfish Farming and Adaptation to Climate Change Conditions. Foods 2024, 13, 1042. https://doi.org/10.3390/foods13071042
Čanak I, Kostelac D, Jakopović Ž, Markov K, Frece J. Lactic Acid Bacteria of Marine Origin as a Tool for Successful Shellfish Farming and Adaptation to Climate Change Conditions. Foods. 2024; 13(7):1042. https://doi.org/10.3390/foods13071042
Chicago/Turabian StyleČanak, Iva, Deni Kostelac, Željko Jakopović, Ksenija Markov, and Jadranka Frece. 2024. "Lactic Acid Bacteria of Marine Origin as a Tool for Successful Shellfish Farming and Adaptation to Climate Change Conditions" Foods 13, no. 7: 1042. https://doi.org/10.3390/foods13071042
APA StyleČanak, I., Kostelac, D., Jakopović, Ž., Markov, K., & Frece, J. (2024). Lactic Acid Bacteria of Marine Origin as a Tool for Successful Shellfish Farming and Adaptation to Climate Change Conditions. Foods, 13(7), 1042. https://doi.org/10.3390/foods13071042






