Structural, Thermal, Pasting and Digestion Properties of Starches from Developing Root Tubers of Sweet Potato
Abstract
:1. Introduction
2. Materials and Methods
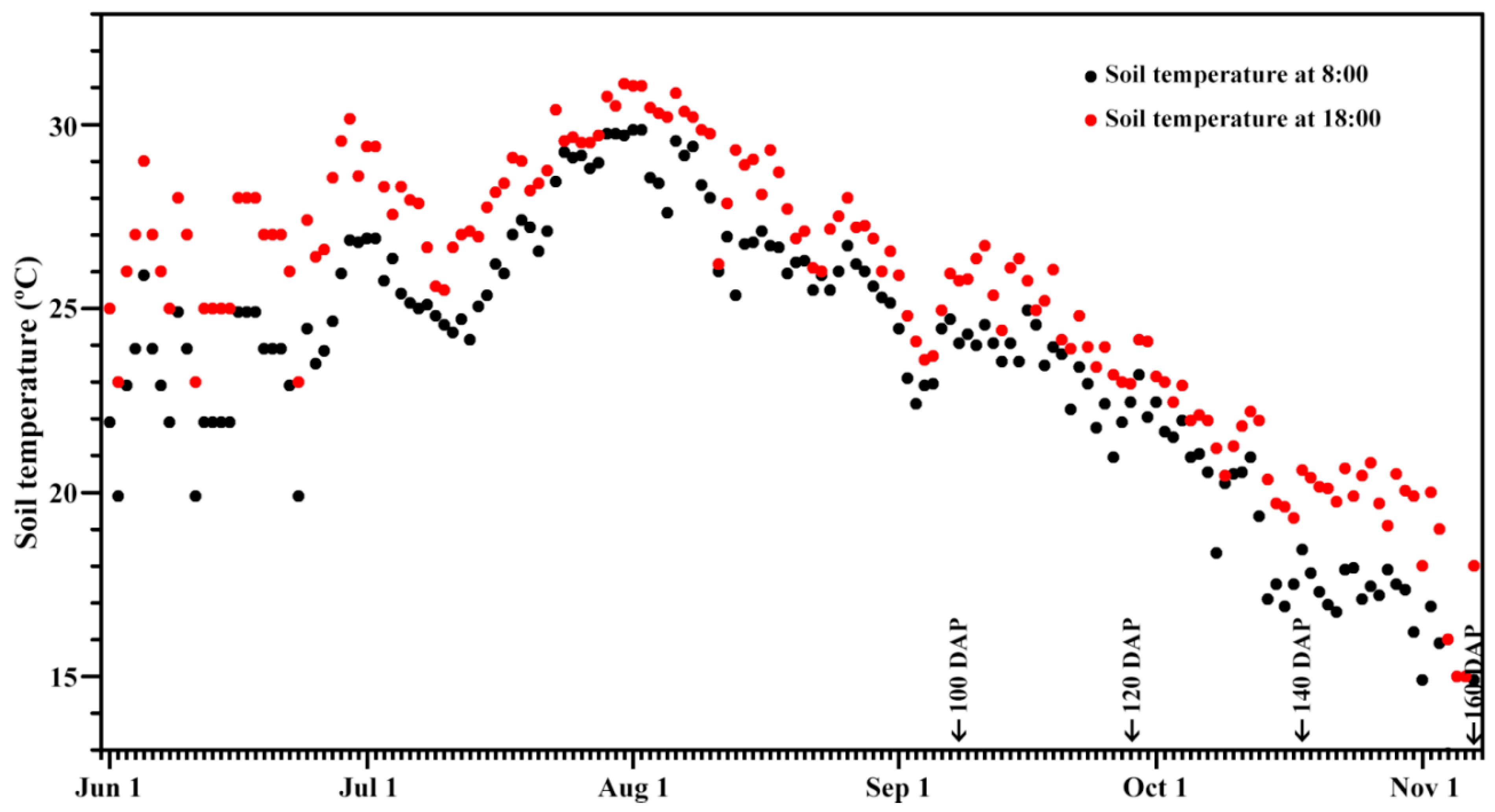
2.1. Plant Materials
2.2. Isolation of Starches from Root Tubers
2.3. Granule Shapes and Sizes of Starch Granules
2.4. Iodine Colorimetry Analysis of Starch
2.5. Crystalline Structure Analysis of Starch
2.6. Short-Ranged Ordered Structure Analysis of Starch
2.7. Lamellar Structure Analysis of Starch
2.8. Thermal Property Analysis of Starch
2.9. Pasting Property Analysis of Starch
2.10. Digestion Analysis of Starch
2.11. Principal Component Analysis
2.12. Statistical Analysis
3. Results and Discussion
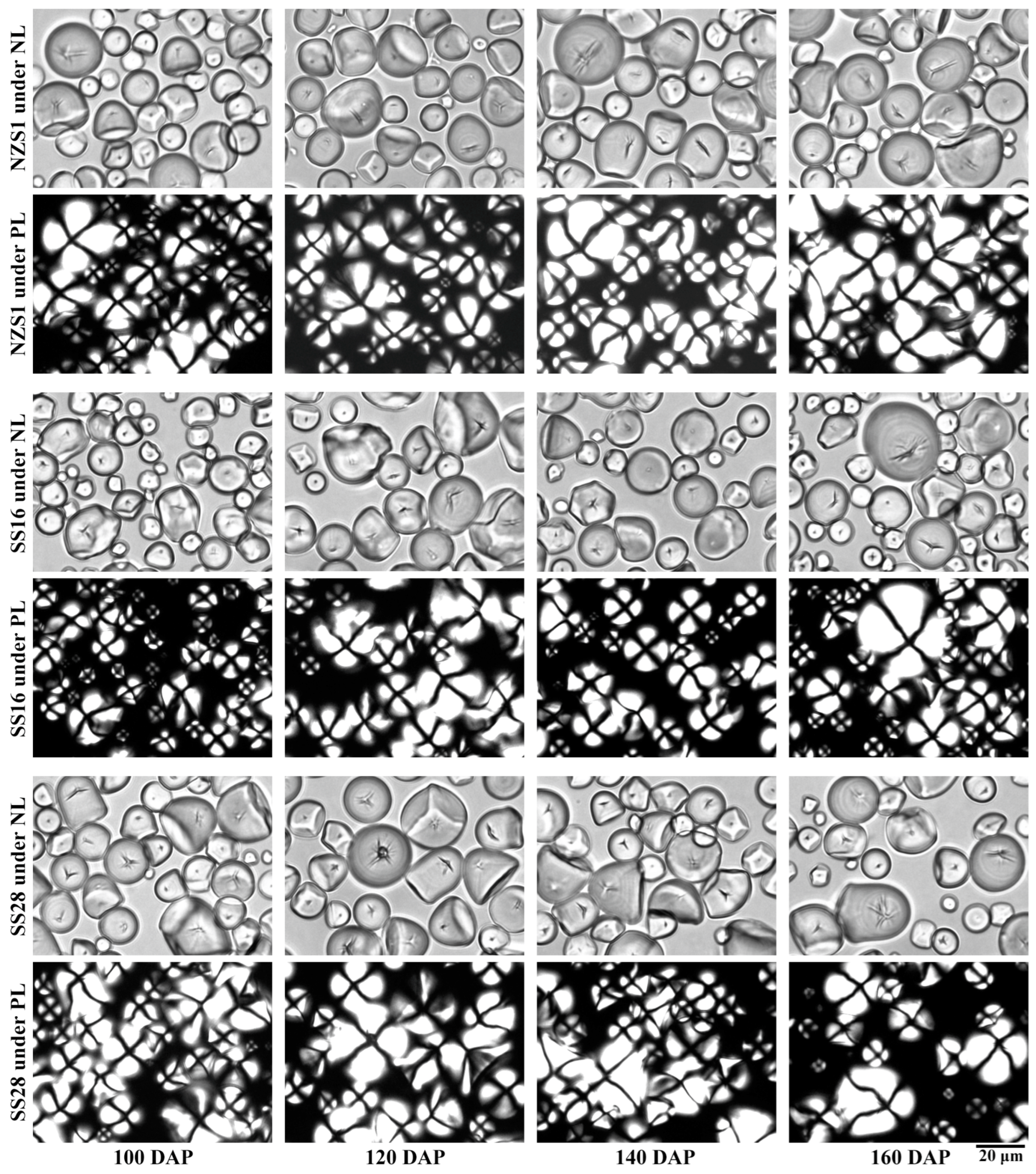
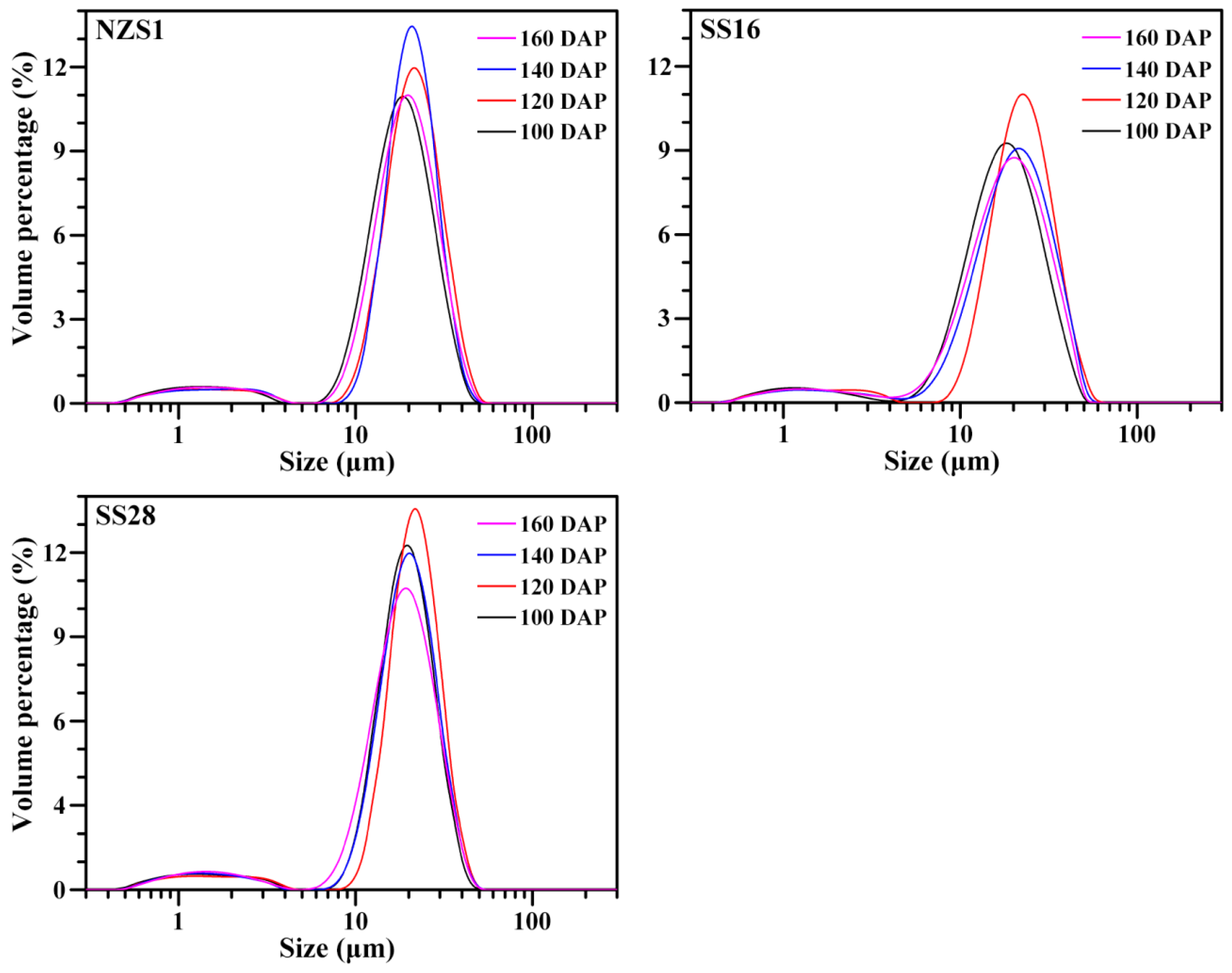
3.1. Granule Shape and Size of Starch
3.2. Iodine Absorption and Amylose Content of Starch
3.3. Crystalline Structure of Starch
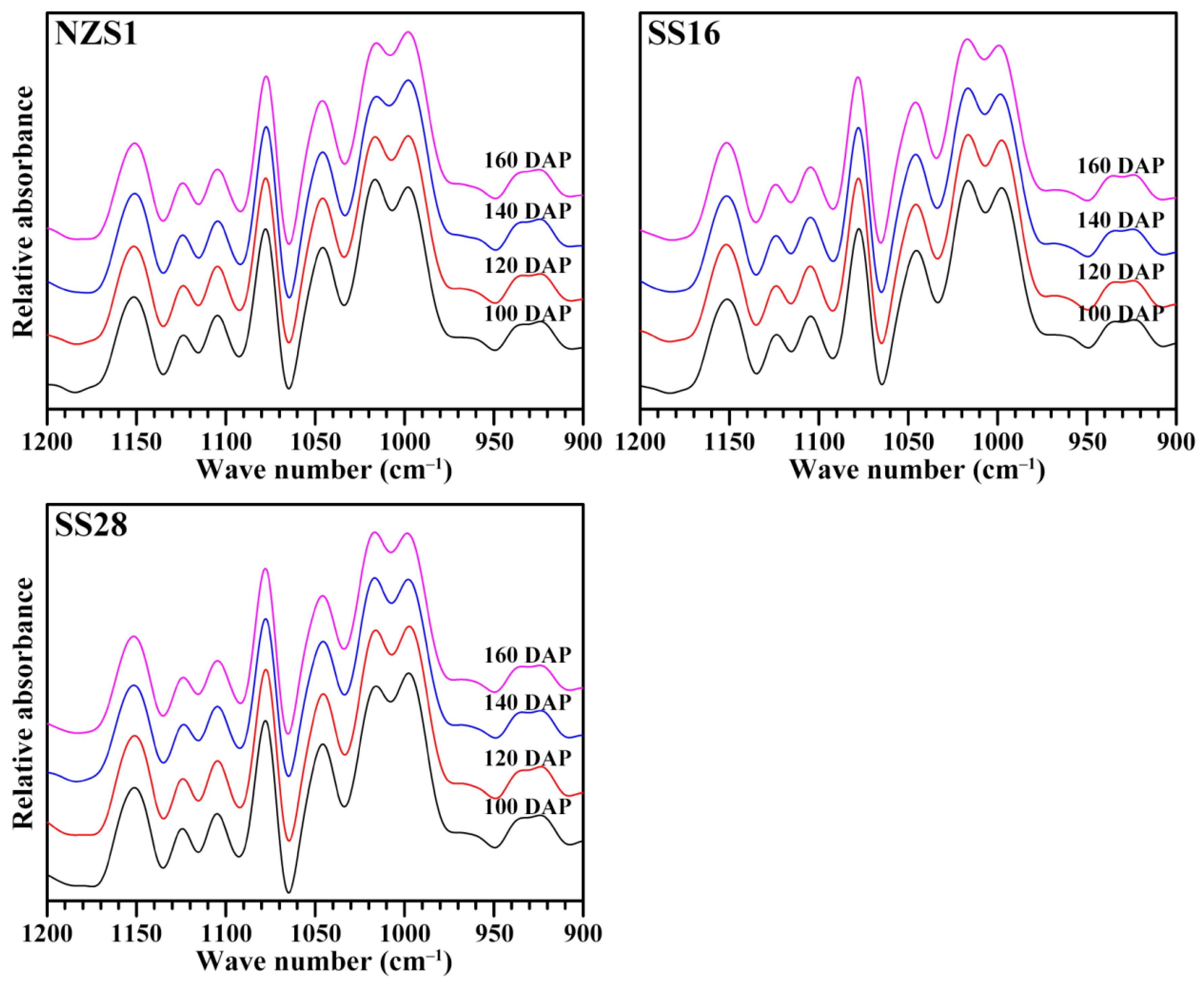
3.4. Short-Ranged Ordered Structure of Starch
3.5. Lamellar Structure of Starch
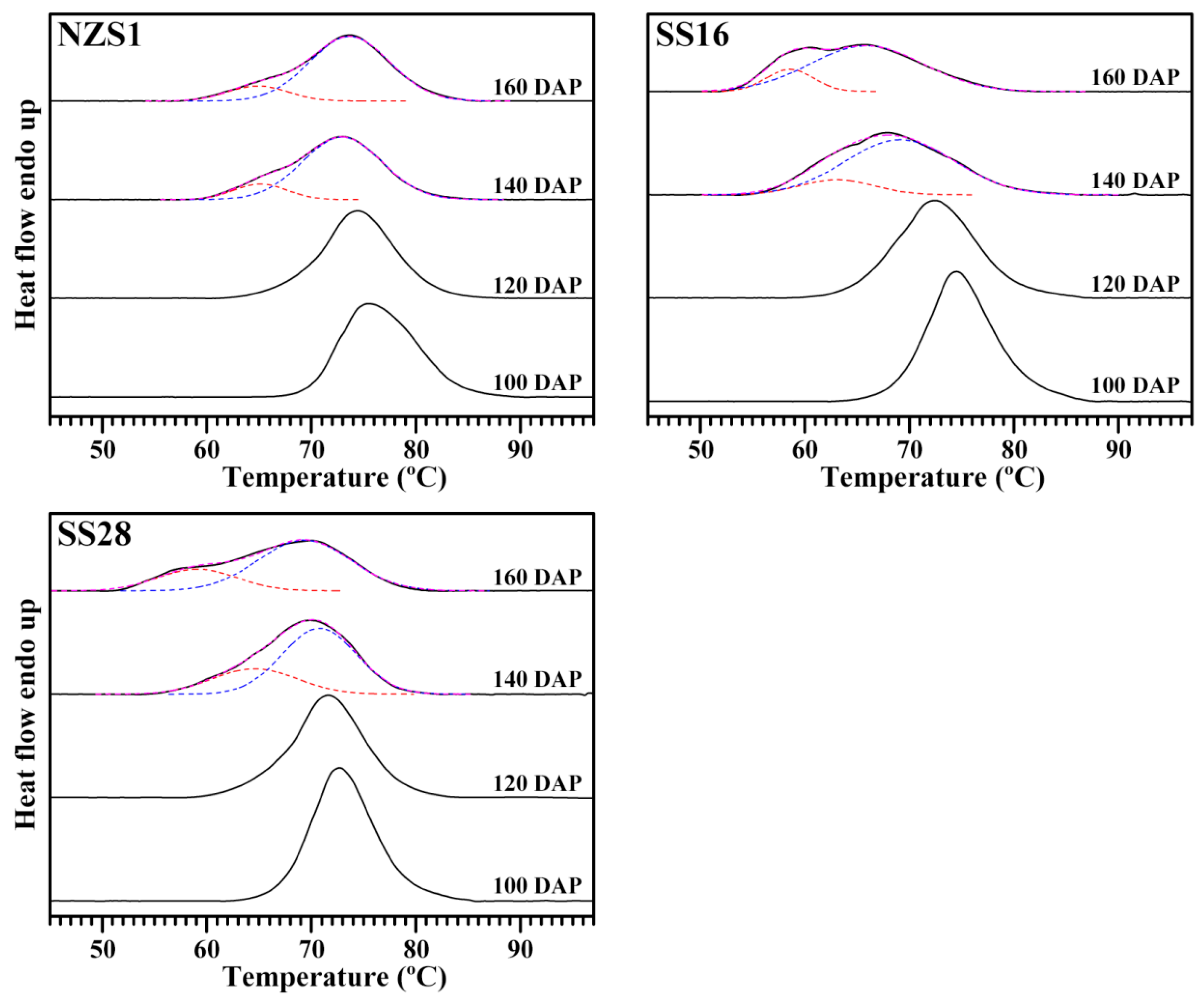
3.6. Thermal Properties of Starch
3.7. Pasting Properties of Starch
3.8. Digestion of Starch
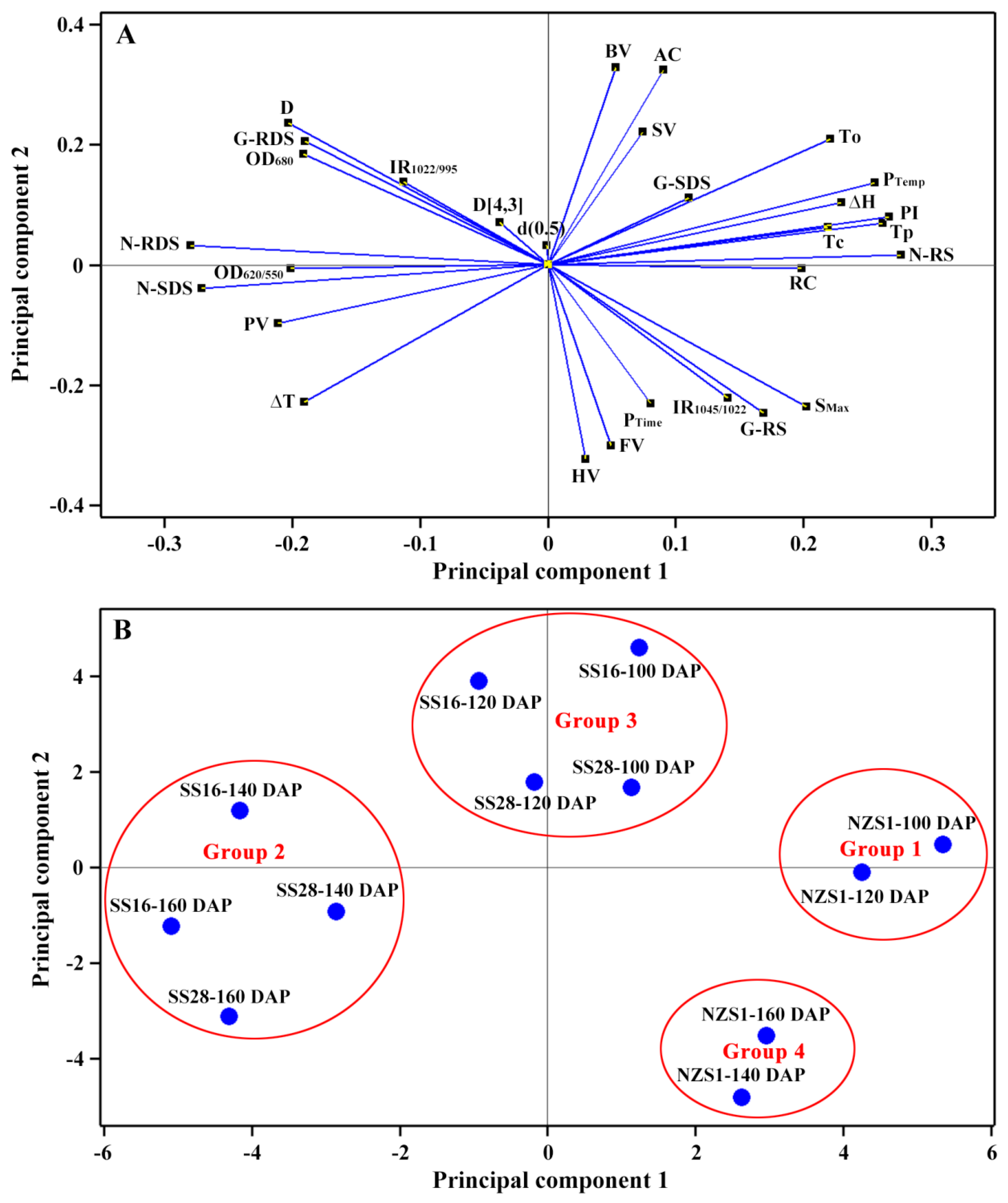
3.9. Principal Component Analysis (PCA) of Starch
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emmambux, M.N.; Taylor, J.R.N. Morphology, physical, chemical, and functional properties of starches from cereals, legumes, and tubers cultivated in Africa: A review. Starch 2013, 65, 715–729. [Google Scholar] [CrossRef]
- Li, M.; Daygon, V.D.; Solah, V.; Dhital, S. Starch granule size: Does it matter? Crit. Rev. Food Sci. Nutr. 2021, 63, 3683–3703. [Google Scholar] [CrossRef] [PubMed]
- Seung, D. Amylose in starch: Towards an understanding of biosynthesis, structure and function. New Phytol. 2020, 228, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kainuma, K. On the cluster structure of amylopectin. Plant Mol. Biol. 2022, 108, 291–306. [Google Scholar] [CrossRef]
- Magallanes-Cruz, P.A.; Duque-Buitrago, L.F.; Martínez-Ruiz, N.D. Native and modified starches from underutilized seeds: Characteristics, functional properties and potential applications. Food Res. Int. 2023, 169, 112875. [Google Scholar] [CrossRef] [PubMed]
- Kringel, D.H.; Halal, S.L.M.E.; Zavareze, E.D.; Dias, A.R.G. Methods for the extraction of roots, tubers, pulses, pseudocereals, and other unconventional starches sources: A review. Starch 2020, 72, 1900234. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Felisberto, M.H.F.; Sanches, E.A.; Campelo, P.H.; Clerici, M.T.P.S. Non-conventional starch sources. Curr. Opin. Food Sci. 2021, 39, 93–102. [Google Scholar] [CrossRef]
- Ren, Y.; Guo, K.; Zhang, B.; Wei, C. Comparison of physicochemical properties of very small granule starches from endosperms of dicotyledon plants. Int. J. Biol. Macromol. 2020, 154, 818–825. [Google Scholar] [CrossRef]
- Ren, Y.; Wei, Q.; Lin, L.; Shi, L.; Cui, Z.; Li, Y.; Huang, C.; Wei, C. Physicochemical properties of a new starch from ramie (Boehmeria nivea) root. Int. J. Biol. Macromol. 2021, 174, 392–401. [Google Scholar] [CrossRef]
- Ren, Y.; Wei, Q.; Chen, H.; Shi, L.; Sheng, W.; Zhang, Z.; Li, Y.; Huang, C.; Wei, C. Characterization of underutilized root starches from eight varieties of ramie (Boehmeria nivea) grown in China. Int. J. Biol. Macromol. 2021, 183, 1475–1485. [Google Scholar] [CrossRef]
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Li, B.; He, H.J.; Mahdi, A.A.; Al-Ansi, W.; Saeed, A. An overview of the isolation, modification, physicochemical properties, and applications of sweet potato starch. Food Bioprocess Technol. 2024, 17, 1–32. [Google Scholar] [CrossRef]
- Guo, K.; Liu, T.; Xu, A.; Zhang, L.; Bian, X.; Wei, C. Structural and functional properties of starches from root tubers of white, yellow, and purple sweet potatoes. Food Hydrocoll. 2019, 89, 829–836. [Google Scholar] [CrossRef]
- Song, H.G.; Choi, I.; Lee, J.S.; Chung, M.N.; Yoon, C.S.; Han, J. Comparative study on physicochemical properties of starch films prepared from five sweet potato (Ipomoea batatas) cultivars. Int. J. Biol. Macromol. 2021, 189, 758–767. [Google Scholar] [CrossRef]
- Truong, V.D.; Avula, R.Y.; Pecota, K.V.; Yencho, G.C. Sweetpotato production, processing, and nutritional quality. In Handbook of Vegetables and Vegetable Processing, 2nd ed.; Siddiq, M., Uebersax, M.A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 811–838. [Google Scholar]
- Noda, T.; Takahata, Y.; Sato, T.; Ikoma, H.; Mochida, H. Combined effects of planting and harvesting dates on starch properties of sweet potato roots. Carbohydr. Polym. 1997, 33, 169–176. [Google Scholar] [CrossRef]
- Noda, T.; Kobayashi, T.; Suda, I. Effect of soil temperature on starch properties of sweet potatoes. Carbohydr. Polym. 2001, 44, 239–246. [Google Scholar] [CrossRef]
- Genkina, N.K.; Noda, T.; Koltisheva, G.I.; Wasserman, L.A.; Tester, R.F.; Yuryev, V.P. Effects of growth temperature on some structural properties of crystalline lamellae in starches extracted from sweet potatoes (Sunnyred and Ayamurasaki). Starch 2003, 55, 350–357. [Google Scholar] [CrossRef]
- Genkina, N.K.; Wasserman, L.A.; Noda, T.; Tester, R.F.; Yuryev, V.P. Effects of annealing on the polymorphic structure of starches from sweet potatoes (Ayamurasaki and Sunnyred cultivars) grown at various soil temperatures. Carbohydr. Res. 2004, 339, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Yang, X.; Cai, Y.Z.; Bertoft, E.; Corke, H. Physicochemical properties of sweetpotato starch. Starch 2011, 63, 249–259. [Google Scholar] [CrossRef]
- Wang, H.L.; Yang, Q.H.; Ferdinand, U.; Gong, X.W.; Qu, Y.; Gao, W.C.; Ivanistau, A.; Feng, B.L.; Liu, M.H. Isolation and characterization of starch from light yellow, orange, and purple sweet potatoes. Int. J. Biol. Macromol. 2020, 160, 660–668. [Google Scholar] [CrossRef]
- Guo, K.; Zhang, L.; Bian, X.; Cao, Q.; Wei, C. A-, B-and C-type starch granules coexist in root tuber of sweet potato. Food Hydrocoll. 2020, 98, 105279. [Google Scholar] [CrossRef]
- Guo, K.; Lin, L.; Li, E.; Zhong, Y.; Petersen, B.L.; Blennow, A.; Bian, X.; Wei, C. Effects of growth temperature on multi-scale structure of root tuber starch in sweet potato. Carbohydr. Polym. 2022, 298, 120136. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.Y.; Li, J.F.; Zhao, G.H. Physicochemical properties of different-sized fractions of sweet potato starch and their contributions to the quality of sweet potato starch. Food Hydrocoll. 2020, 108, 106023. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Shi, L.; Lin, L.; Cao, Q.; Wei, C. Sizes, components, crystalline structure, and thermal properties of starches from sweet potato varieties originating from different countries. Molecules 2022, 27, 1905. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.D.P.E.; Bento, J.A.C.; Ribeiro, G.O.; Liao, L.M.; Soares, M.S.; Caliar, M. Application potential and technological properties of colored sweet potato starches. Starch 2021, 73, 2000100. [Google Scholar] [CrossRef]
- Dos Santos, T.P.R.; Leonel, M.; De Oliveira, L.A.; Fernandes, A.M.; Leonel, S.; Da Silva Nunes, J.G. Seasonal variations in the starch properties of sweet potato cultivars. Horticulturae 2023, 9, 303. [Google Scholar] [CrossRef]
- Kaufman, R.C.; Wilson, J.D.; Bean, S.R.; Herald, T.J.; Shi, Y.C. Development of a 96-well plate iodine binding assay for amylose content determination. Carbohydr. Polym. 2015, 115, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Jia, X.; Zhao, B.; Zeng, S.; Xiao, J.; Zheng, B. C-type starches and their derivatives: Structure and function. Ann. N. Y. Acad. Sci. 2017, 1398, 47–61. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wei, C. Progress in C-type starches from different plant sources. Food Hydrocoll. 2017, 73, 162–175. [Google Scholar] [CrossRef]
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. [Google Scholar] [CrossRef]
- Yuryev, V.P.; Krivandin, A.V.; Kiseleva, V.I.; Wasserman, L.A.; Genkina, N.K.; Fornal, J.; Blaszczak, W.; Schiraldi, A. Structural parameters of amylopectin clusters and semicrystalline growth rings in wheat starches with different amylose content. Carbohydr. Res. 2004, 339, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Iii, C.H.; Carlson, R. A comparison of rapid visco analyser starch pasting curves and test baking for under-and overdosed dry baked mixes. Cereal Food. World 2016, 61, 29–38. [Google Scholar] [CrossRef]
- Wang, L.Q.; Liu, W.J.; Xu, Y.; He, Y.Q.; Luo, L.J.; Xing, Y.Z.; Xu, C.G.; Zhang, Q. Genetic basis of 17 traits and viscosity parameters characterizing the eating and cooking quality of rice grain. Theor. Appl. Genet. 2007, 115, 463–476. [Google Scholar] [CrossRef]
- Xu, A.; Guo, K.; Liu, T.; Bian, X.; Zhang, L.; Wei, C. Effects of different isolation media on structural and functional properties of starches from root tubers of purple, yellow and white sweet potatoes. Molecules 2018, 23, 2135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Xu, Q.; Kong, Q.; Li, F.; Lu, L.; Xu, Y.; Wei, Y. Synthesis and functions of resistant starch. Adv. Nut. 2023, 14, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Jing, Y.; Cheng, Q.; Zhou, H.; Wang, L.; Gong, W.; Kou, L.; Liu, G.; Meng, X.; Chen, M.; et al. Loss of function of SSIIIa and SSIIIb coordinately confers high RS content in cooked rice. Proc. Natl. Acad. Sci. USA 2023, 120, e2220622120. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiao, Y.; Zhao, W.; Rao, Y.; Shen, H.; Gu, Z.; Fan, X.; Li, Q.; Zhang, C.; Liu, Q. Creating high-resistant starch rice by simultaneous editing of SS3a and SS3b. Plant Biotechnol. J. 2024, 22, 787–789. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch—A review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef]




| Starch Sample | Granule Size | Iodine Absorption Parameters | AC (%) | ||
|---|---|---|---|---|---|
| d(0.5) (μm) | D[4,3] (μm) | OD680 | OD620/550 | ||
| NZS1-100 DAP | 16.610 ± 0.002 b | 16.994 ± 0.003 a | 0.305 ± 0.007 bc | 1.099 ± 0.002 a | 24.3 ± 0.9 de |
| NZS1-120 DAP | 19.439 ± 0.010 j | 19.712 ± 0.011 j | 0.300 ± 0.008 bc | 1.092 ± 0.017 a | 23.3 ± 0.5 cd |
| NZS1-140 DAP | 19.022 ± 0.004 i | 19.058 ± 0.005 h | 0.275 ± 0.002 a | 1.126 ± 0.014 a | 20.1 ± 0.1 a |
| NZS1-160 DAP | 17.671 ± 0.004 e | 18.078 ± 0.006 e | 0.291 ± 0.004 ab | 1.123 ± 0.005 a | 22.0 ± 0.6 bc |
| SS16-100 DAP | 16.256 ± 0.006 a | 17.215 ± 0.006 b | 0.328 ± 0.008 d | 1.140 ± 0.004 a | 26.3 ± 0.5 f |
| SS16-120 DAP | 20.475 ± 0.015 l | 21.093 ± 0.014 l | 0.322 ± 0.003 cd | 1.139 ± 0.018 a | 25.4 ± 0.5 ef |
| SS16-140 DAP | 18.541 ± 0.007 h | 19.470 ± 0.014 i | 0.301 ± 0.008 bc | 1.130 ± 0.010 a | 23.3 ± 0.5 cd |
| SS16-160 DAP | 17.301 ± 0.006 d | 18.276 ± 0.006 f | 0.295 ± 0.002 ab | 1.147 ± 0.037 a | 21.2 ± 0.5 ab |
| SS28-100 DAP | 17.693 ± 0.003 f | 17.840 ± 0.007 d | 0.308 ± 0.007 bcd | 1.143 ± 0.023 a | 24.3 ± 0.5 de |
| SS28-120 DAP | 19.824 ± 0.001 k | 19.859 ± 0.001 k | 0.304 ± 0.010 bc | 1.103 ± 0.020 a | 23.8 ± 0.6 de |
| SS28-140 DAP | 18.196 ± 0.006 g | 18.447 ± 0.008 g | 0.293 ± 0.001 ab | 1.142 ± 0.020 a | 21.9 ± 0.2 bc |
| SS28-160 DAP | 17.111 ± 0.009 c | 17.535 ± 0.010 c | 0.273 ± 0.005 a | 1.151 ± 0.041 a | 20.0 ± 0.3 a |
| Sig. | 0.927 | 0.759 | 0.734 | 0.105 | 0.819 |
| Starch Sample | RC (%) | IR Ratios | Lamellar Structure Parameters | |||
|---|---|---|---|---|---|---|
| IR1045/1022 (cm−1) | IR1022/995 (cm−1) | PI (a.u.) | SMax (Å−1) | D (nm) | ||
| NZS1-100 DAP | 26.7 ± 0.8 c | 0.670 ± 0.015 ab | 1.024 ± 0.028 bcd | 467.8 ± 3.0 f | 0.064 ± 0.000 e | 9.88 ± 0.01 c |
| NZS1-120 DAP | 26.7 ± 0.3 c | 0.656 ± 0.032 ab | 1.044 ± 0.064 cd | 429.9 ± 2.6 e | 0.064 ± 0.000 f | 9.83 ± 0.01 b |
| NZS1-140 DAP | 24.0 ± 0.9 b | 0.702 ± 0.011 b | 0.914 ± 0.006 a | 414.3 ± 0.9 e | 0.065 ± 0.000 g | 9.74 ± 0.00 a |
| NZS1-160 DAP | 26.6 ± 0.6 c | 0.686 ± 0.001 ab | 0.958 ± 0.019 abc | 394.0 ± 10.0 d | 0.064 ± 0.000 g | 9.76 ± 0.00 a |
| SS16-100 DAP | 25.4 ± 0.3 bc | 0.655 ± 0.009 ab | 1.024 ± 0.023 bcd | 425.9 ± 3.1 e | 0.062 ± 0.000 a | 10.10 ± 0.01 g |
| SS16-120 DAP | 25.2 ± 0.8 bc | 0.651 ± 0.015 ab | 1.023 ± 0.013 bcd | 387.2 ± 1.0 d | 0.062 ± 0.000 a | 10.10 ± 0.01 g |
| SS16-140 DAP | 21.6 ± 0.0 a | 0.643 ± 0.009 a | 1.050 ± 0.015 cd | 323.7 ± 8.9 b | 0.062 ± 0.000 a | 10.08 ± 0.01 g |
| SS16-160 DAP | 24.4 ± 1.2 b | 0.655 ± 0.013 ab | 1.056 ± 0.010 d | 293.8 ± 1.4 a | 0.062 ± 0.000 a | 10.08 ± 0.02 g |
| SS28-100 DAP | 24.0 ± 0.2 b | 0.678 ± 0.012 ab | 0.949 ± 0.021 ab | 421.5 ± 0.7 e | 0.063 ± 0.000 d | 9.98 ± 0.00 d |
| SS28-120 DAP | 25.1 ± 0.5 bc | 0.668 ± 0.003 ab | 0.977 ± 0.005 abcd | 390.2 ± 0.6 d | 0.063 ± 0.000 bc | 10.02 ± 0.01 ef |
| SS28-140 DAP | 23.8 ± 0.3 b | 0.663 ± 0.008 ab | 1.007 ± 0.004 bcd | 362.9 ± 6.0 c | 0.063 ± 0.000 b | 10.04 ± 0.00 f |
| SS28-160 DAP | 24.7 ± 0.3 bc | 0.664 ± 0.007 ab | 1.021 ± 0.013 bcd | 324.5 ± 2.5 b | 0.063 ± 0.000 cd | 10.00 ± 0.01 de |
| Sig. | 0.282 | 0.475 | 0.184 | 0.663 | 0.080 | 0.058 |
| Starch Sample | To (°C) | Tp (°C) | Tc (°C) | ∆T (°C) | ∆H (J/g) |
|---|---|---|---|---|---|
| NZS1-100 DAP | 69.8 ± 0.0 g | 75.6 ± 0.1 h | 84.1 ± 0.1 d | 14.3 ± 0.1 bc | 11.84 ± 0.47 d |
| NZS1-120 DAP | 67.7 ± 0.4 f | 74.3 ± 0.2 g | 81.3 ± 0.4 c | 13.6 ± 0.0 ab | 11.10 ± 0.11 cd |
| NZS1-140 DAP | 59.9 ± 0.1 d | 72.9 ± 0.0 ef | 80.4 ± 0.2 bc | 20.5 ± 0.1 d | 10.53 ± 0.44 bcd |
| NZS1-160 DAP | 59.2 ± 0.2 d | 73.7 ± 0.1 fg | 80.9 ± 0.1 c | 21.7 ± 0.1 d | 10.41 ± 0.45 bcd |
| SS16-100 DAP | 68.7 ± 0.1 fg | 74.5 ± 0.0 g | 81.4 ± 0.1 c | 12.8 ± 0.2 a | 11.53 ± 0.13 cd |
| SS16-120 DAP | 64.8 ± 0.2 e | 72.5 ± 0.1 de | 80.1 ± 0.1 abc | 15.4 ± 0.4 c | 9.88 ± 0.53 abc |
| SS16-140 DAP | 56.6 ± 0.3 c | 68.8 ± 0.5 b | 80.4 ± 0.5 bc | 23.8 ± 0.2 e | 9.16 ± 0.54 ab |
| SS16-160 DAP | 53.6 ± 0.3 b | 66.0 ± 0.1 a | 78.4 ± 1.0 ab | 24.8 ± 1.3 e | 10.09 ± 0.90 abc |
| SS28-100 DAP | 67.2 ± 0.3 f | 72.9 ± 0.2 ef | 79.5 ± 0.4 abc | 12.3 ± 0.1 a | 11.15 ± 0.35 cd |
| SS28-120 DAP | 64.9 ± 0.8 e | 71.6 ± 0.1 d | 78.4 ± 0.3 ab | 13.6 ± 0.5 ab | 10.10 ± 0.16 abc |
| SS28-140 DAP | 57.4 ± 0.9 c | 70.4 ± 0.6 c | 78.7 ± 0.9 ab | 21.3 ± 0.0 d | 9.85 ± 0.54 abc |
| SS28-160 DAP | 51.3 ± 1.0 a | 69.7 ± 0.6 c | 78.2 ± 0.9 a | 26.9 ± 0.1 f | 8.45 ± 0.00 a |
| Sig. | 0.409 | 0.587 | 0.199 | 0.096 | 0.937 |
| Starch Sample | PV (mPa s) | HV (mPa s) | BV (mPa s) | FV (mPa s) | SV (mPa s) | PTime (min) | PTemp (°C) |
|---|---|---|---|---|---|---|---|
| NZS1-100 DAP | 3813 ± 6 a | 2365 ± 17 c | 1448 ± 23 a | 3030 ± 14 b | 665 ± 31 ab | 4.37 ± 0.05 cd | 80.38 ± 0.04 g |
| NZS1-120 DAP | 3979 ± 29 bc | 2401 ± 18 cd | 1578 ± 11 bc | 3115 ± 9 bc | 714 ± 9 bc | 4.37 ± 0.05 cd | 79.20 ± 0.57 fg |
| NZS1-140 DAP | 4247 ± 16 fg | 2740 ± 3 f | 1507 ± 13 ab | 3402 ± 31 d | 662 ± 28 ab | 4.40 ± 0.00 d | 76.33 ± 0.04 cd |
| NZS1-160 DAP | 4084 ± 4 cd | 2464 ± 17 de | 1620 ± 13 cd | 3090 ± 42 bc | 626 ± 25 ab | 4.44 ± 0.05 d | 77.58 ± 0.53 de |
| SS16-100 DAP | 3850 ± 57 a | 2056 ± 45 a | 1795 ± 12 f | 2842 ± 23 a | 786 ± 21 c | 4.24 ± 0.05 abc | 79.23 ± 0.60 fg |
| SS16-120 DAP | 4348 ± 95 gh | 2065 ± 50 ab | 2284 ± 45 h | 2761 ± 84 a | 696 ± 34 abc | 4.24 ± 0.05 abc | 77.60 ± 0.57 de |
| SS16-140 DAP | 4377 ± 13 h | 2105 ± 1 ab | 2272 ± 13 h | 2790 ± 14 a | 685 ± 14 ab | 4.33 ± 0.00 bcd | 74.43 ± 0.53 b |
| SS16-160 DAP | 4340 ± 24 gh | 2407 ± 19 cd | 1934 ± 43 g | 3000 ± 28 b | 594 ± 47 a | 4.33 ± 0.00 bcd | 72.35 ± 0.00 a |
| SS28-100 DAP | 3874 ± 18 ab | 2143 ± 18 ab | 1731 ± 1 ef | 2813 ± 11 a | 670 ± 7 ab | 4.17 ± 0.05 a | 77.98 ± 0.04 ef |
| SS28-120 DAP | 4101 ± 33 de | 2150 ± 4 b | 1951 ± 37 g | 2815 ± 49 a | 665 ± 54 ab | 4.17 ± 0.05 a | 76.00 ± 0.49 c |
| SS28-140 DAP | 4389 ± 7 h | 2503 ± 4 e | 1887 ± 11 g | 3180 ± 1 c | 678 ± 4 ab | 4.20 ± 0.00 ab | 74.78 ± 0.04 b |
| SS28-160 DAP | 4213 ± 24 ef | 2529 ± 38 e | 1684 ± 62 de | 3190 ± 14 c | 661 ± 24 ab | 4.33 ± 0.00 bcd | 73.15 ± 0.07 a |
| Sig. | 0.157 | 0.266 | 0.377 | 0.281 | 0.207 | 0.278 | 0.843 |
| Starch Sample | Native (N) Starch | Gelatinized (G) Starch | ||||
|---|---|---|---|---|---|---|
| N-RDS (%) | N-SDS (%) | N-RS (%) | G-RDS (%) | G-SDS (%) | G-RS (%) | |
| NZS1-100 DAP | 2.7 ± 0.1 a | 11.3 ± 0.1 a | 86.1 ± 0.2 d | 76.9 ± 1.7 abcd | 3.0 ± 0.9 bc | 20.0 ± 1.3 bcde |
| NZS1-120 DAP | 3.0 ± 0.2 ab | 10.7 ± 0.2 a | 86.3 ± 0.4 d | 75.2 ± 2.7 ab | 2.7 ± 1.1 abc | 22.1 ± 2.3 de |
| NZS1-140 DAP | 3.4 ± 0.1 b | 11.0 ± 0.4 a | 85.6 ± 0.4 d | 76.1 ± 2.2 abc | 2.2 ± 0.8 abc | 21.7 ± 1.4 cde |
| NZS1-160 DAP | 3.2 ± 0.1 b | 11.8 ± 1.4 ab | 85.0 ± 1.4 d | 74.2 ± 2.9 a | 1.5 ± 0.4 ab | 24.3 ± 2.6 e |
| SS16-100 DAP | 4.9 ± 0.1 cd | 12.7 ± 0.4 bc | 82.5 ± 0.5 c | 84.6 ± 1.4 e | 1.2 ± 0.7 ab | 14.2 ± 1.7 a |
| SS16-120 DAP | 5.0 ± 0.1 d | 13.4 ± 0.1 c | 81.7 ± 0.2 c | 81.6 ± 1.6 cde | 3.7 ± 0.7 c | 14.8 ± 1.3 a |
| SS16-140 DAP | 6.6 ± 0.1 f | 17.4 ± 0.5 d | 76.0 ± 0.6 b | 80.6 ± 1.3 bcde | 2.3 ± 0.2 abc | 17.0 ± 1.4 abcd |
| SS16-160 DAP | 6.8 ± 0.1 f | 18.9 ± 0.4 e | 74.3 ± 0.5 a | 82.6 ± 1.3 de | 1.4 ± 1.0 ab | 16.0 ± 0.8 ab |
| SS28-100 DAP | 4.5 ± 0.2 c | 13.3 ± 0.3 c | 82.2 ± 0.5 c | 80.8 ± 3.0 bcde | 1.4 ± 0.4 ab | 17.8 ± 3.2 abcd |
| SS28-120 DAP | 4.8 ± 0.2 cd | 13.8 ± 0.1 c | 81.4 ± 0.1 c | 80.9 ± 2.2 cde | 2.2 ± 0.7 abc | 16.9 ± 1.5 abc |
| SS28-140 DAP | 6.2 ± 0.1 e | 17.1 ± 0.5 d | 76.7 ± 0.6 b | 80.7 ± 1.9 bcde | 1.3 ± 0.6 ab | 18.0 ± 2.2 abcd |
| SS28-160 DAP | 6.8 ± 0.3 f | 19.6 ± 0.3 e | 73.6 ± 0.6 a | 81.4 ± 0.9 cde | 0.9 ± 0.3 a | 17.7 ± 1.2 abcd |
| Sig. | 0.175 | 0.098 | 0.113 | 0.189 | 0.344 | 0.541 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Feng, Y.; Guo, K.; Shi, L.; Xu, X.; Wei, C. Structural, Thermal, Pasting and Digestion Properties of Starches from Developing Root Tubers of Sweet Potato. Foods 2024, 13, 1103. https://doi.org/10.3390/foods13071103
Wang H, Feng Y, Guo K, Shi L, Xu X, Wei C. Structural, Thermal, Pasting and Digestion Properties of Starches from Developing Root Tubers of Sweet Potato. Foods. 2024; 13(7):1103. https://doi.org/10.3390/foods13071103
Chicago/Turabian StyleWang, Hao, Yuanhao Feng, Ke Guo, Laiquan Shi, Xin Xu, and Cunxu Wei. 2024. "Structural, Thermal, Pasting and Digestion Properties of Starches from Developing Root Tubers of Sweet Potato" Foods 13, no. 7: 1103. https://doi.org/10.3390/foods13071103
APA StyleWang, H., Feng, Y., Guo, K., Shi, L., Xu, X., & Wei, C. (2024). Structural, Thermal, Pasting and Digestion Properties of Starches from Developing Root Tubers of Sweet Potato. Foods, 13(7), 1103. https://doi.org/10.3390/foods13071103






