Potassium Chloride as an Effective Alternative to Sodium Chloride in Delaying the Thermal Aggregation of Liquid Whole Egg
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Samples Elaboration
2.2.1. Preparation of LWE
2.2.2. Heat Treatment of LWE Samples
2.3. Samples Evaluation
2.3.1. Color Characterization
2.3.2. Solubility
2.3.3. Turbidity
2.3.4. Particle Size Distribution (PSD)
2.3.5. Rheological Properties
Apparent Viscosity
Temperature Sweep
2.3.6. Differential Scanning Calorimetry (DSC)
2.3.7. Secondary Structure
2.3.8. Tertiary Structure
2.3.9. Surface Hydrophobicity
2.3.10. Statistical Analysis
3. Results and Discussion
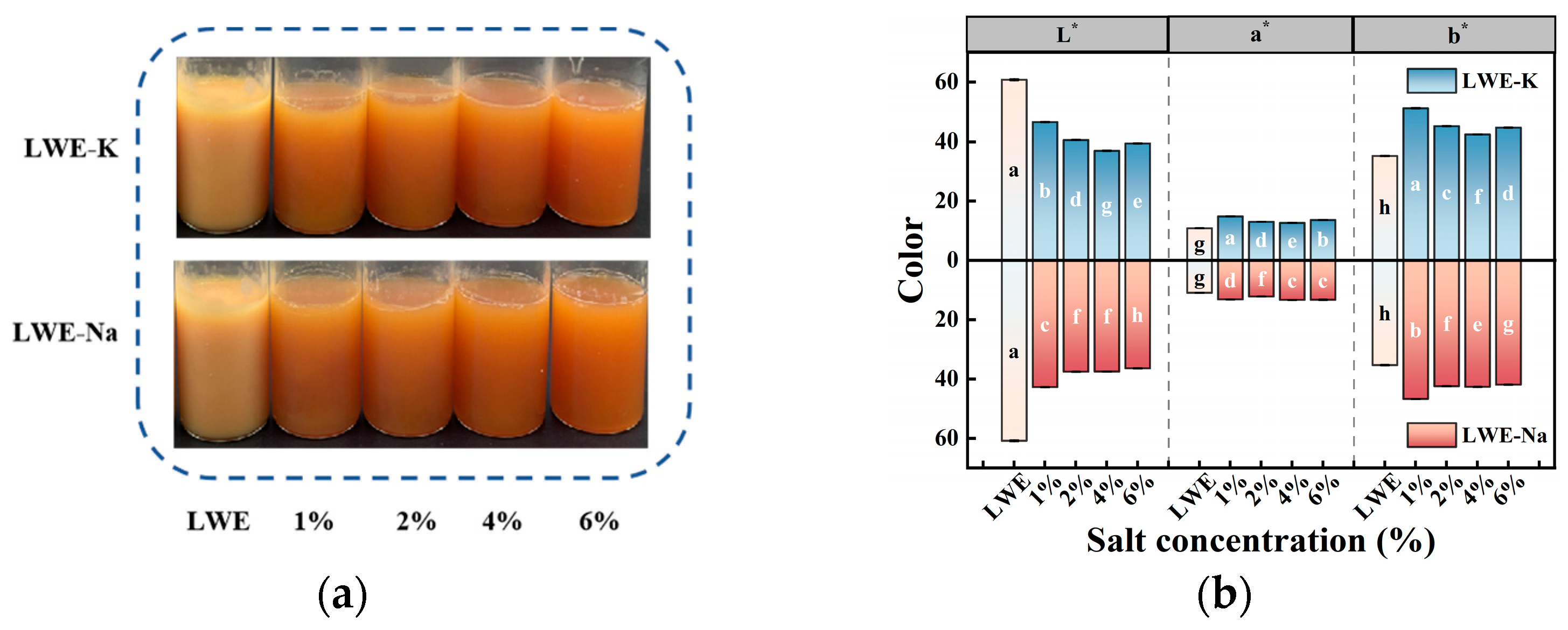
3.1. Effects of KCl and NaCl on the Appearance and Color of LWEs
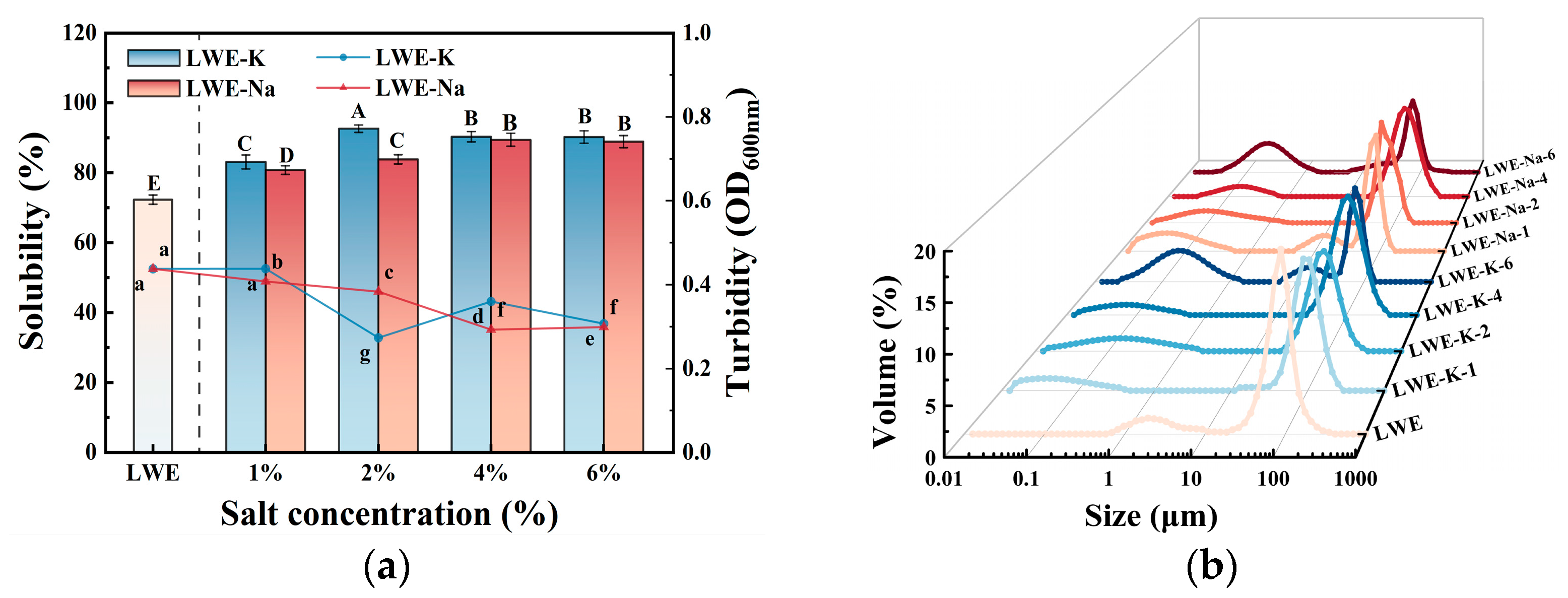
3.2. Effects of KCl and NaCl on LWE Aggregation Behavior
3.3. Rheological Analysis: Apparent Viscosity and Temperature
3.3.1. Apparent Viscosity
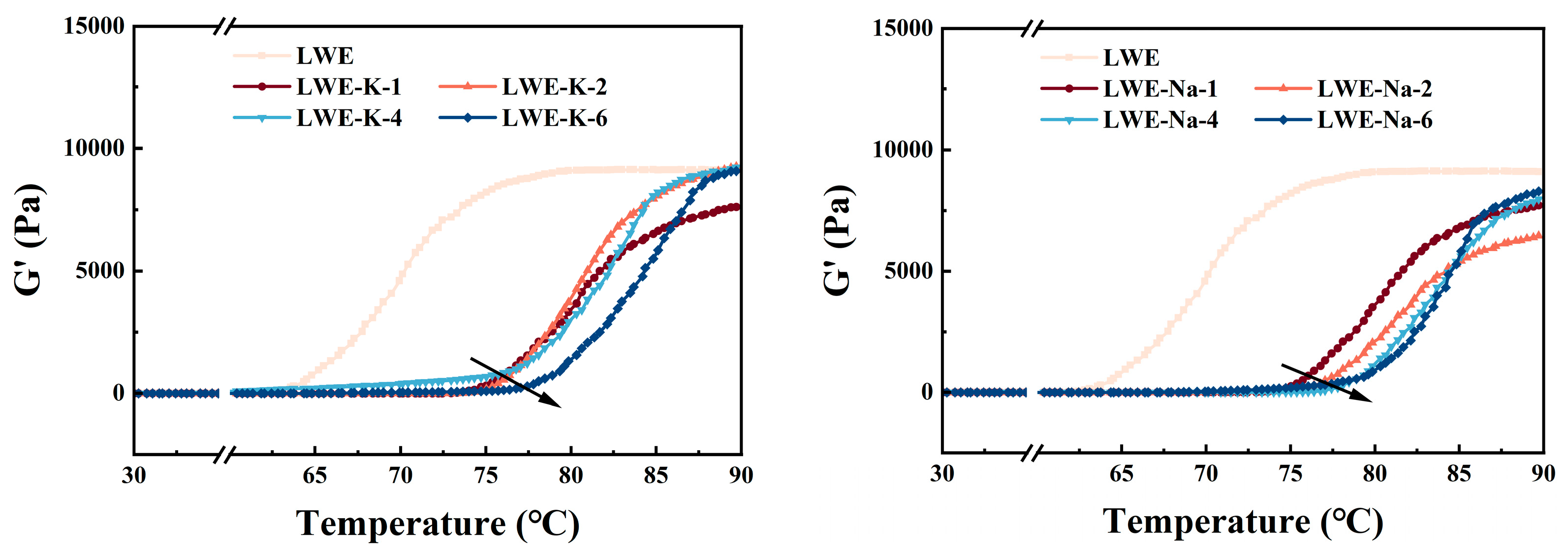
3.3.2. Temperature Scan
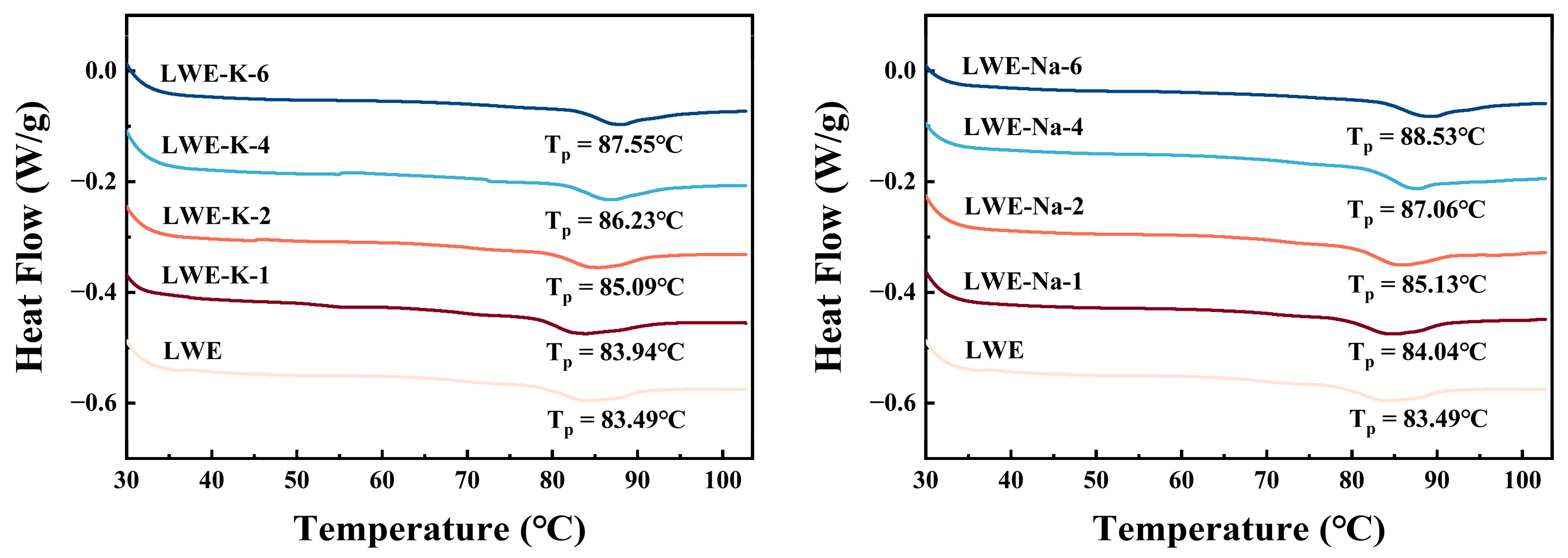
3.4. Effects of KCl and NaCl on the Differential Scanning Calorimetry (DSC) Results of LWE
3.5. Effects of KCl and NaCl on the Secondary Structure of LWE
3.6. Effects of KCl and NaCl on the Tertiary Structure of LWE
3.6.1. Endogenous Fluorescence Spectroscopy
3.6.2. Surface Hydrophobicity
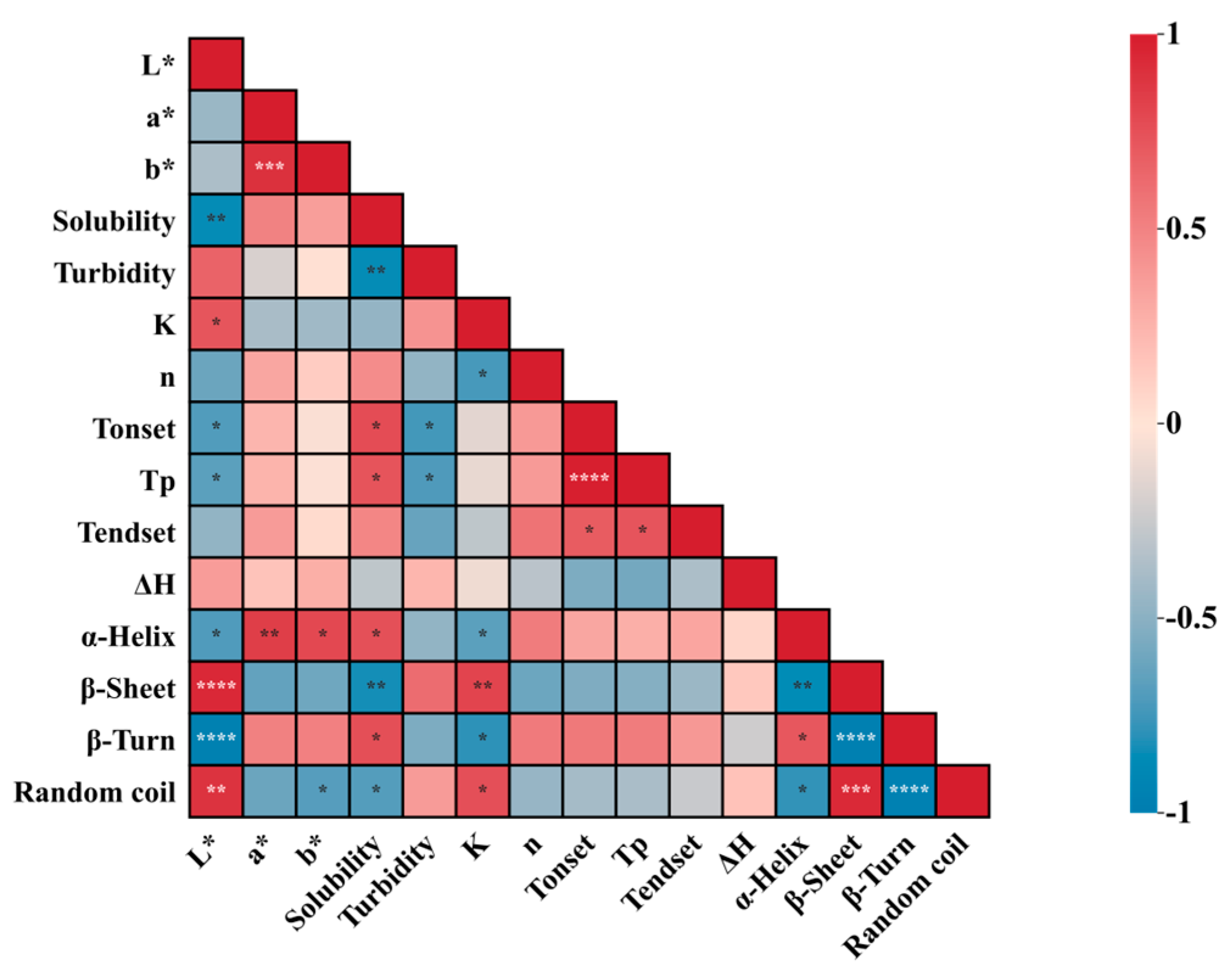
3.7. Relationships among Physical and Chemical Properties, Thermal Properties, and Structural Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Xu, L.; Lv, Y.; Su, Y.; Gu, L.; Chang, C.; Zhang, M.; Yang, Y.; Li, J. To improve the gel properties of liquid whole egg by short-term lactic acid bacteria fermentation. Innov. Food Sci. Emerg. Technol. 2022, 75, 102873. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Esparza, Y.; Temelli, F.; Wu, J. Physicochemical and functional properties of livetins fraction from hen egg yolk. Food Biosci. 2017, 18, 38–45. [Google Scholar] [CrossRef]
- Primacella, M.; Fei, T.; Acevedo, N.; Wang, T. Effect of food additives on egg yolk gelation induced by freezing. Food Chem. 2018, 263, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Nurmilah, S.; Cahyana, Y.; Utama, G.L.; Aït-Kaddour, A. Strategies to Reduce Salt Content and Its Effect on Food Characteristics and Acceptance: A Review. Foods 2022, 11, 3120. [Google Scholar] [CrossRef] [PubMed]
- Guevara, M.; Valdés-Silverio, L.A.; Granda-Albuja, M.G.; Iturralde, G.; Jaramillo-Vivanco, T.; Giampieri, F.; Santos-Buelga, C.; González-Paramás, A.M.; Battino, M.; Álvarez-Suarez, J.M. Pechiche (Vitex cymosa Berteo ex Speng), a Nontraditional Fruit from Ecuador, is a Dietary Source of Phenolic Acids and Nutrient Minerals, in Addition to Efficiently Counteracting the Oxidative-Induced Damage in Human Dermal Fibroblasts. Antioxid. Redox Signal. 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Hu, Y.; Zhang, L.; Wang, Y.; Chen, Q.; Kong, B. Effect of NaCl substitutes on lipid and protein oxidation and flavor development of Harbin dry sausage. Meat Sci. 2019, 156, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Vidal, V.A.S.; Lorenzo, J.M.; Munekata, P.E.S.; Pollonio, M.A.R. Challenges to reduce or replace NaCl by chloride salts in meat products made from whole pieces—A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2194–2206. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Li, S.; Ye, H.; Luo, W.; Huang, Q.; Geng, F. Ovomucin may be the key protein involved in the early formation of egg-white thermal gel. Food Chem. 2022, 366, 130596. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, H.; Zhang, M.; Gong, P.; Yang, M.; Zhang, T.; Liu, X. Ions-regulated aggregation kinetics for egg white protein: A promising formulation with controlled gelation and rheological properties. Int. J. Biol. Macromol. 2022, 200, 263–272. [Google Scholar] [CrossRef]
- Li, T.; Su, H.; Zhu, J.; Fu, Y. Janus effects of NaCl on structure of egg yolk granules. Food Chem. 2022, 371, 131077. [Google Scholar] [CrossRef]
- Llave, Y.; Fukuda, S.; Fukuoka, M.; Shibata-Ishiwatari, N.; Sakai, N. Analysis of color changes in chicken egg yolks and whites based on degree of thermal protein denaturation during ohmic heating and water bath treatment. J. Food Eng. 2018, 222, 151–161. [Google Scholar] [CrossRef]
- Shah, D.D.; You, Y.O.; Cane, D.E. Stereospecific Formation of E- and Z-Disubstituted Double Bonds by Dehydratase Domains from Modules 1 and 2 of the Fostriecin Polyketide Synthase. J. Am. Chem. Soc. 2017, 139, 14322–14330. [Google Scholar]
- Wang, R.; Zhang, L.; Chi, Y.; Chi, Y. Forces involved in freeze-induced egg yolk gelation: Effects of various bond dissociation reagents on gel properties and protein structure changes. Food Chem. 2022, 371, 131190. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, C.; Chang, C.; Jiao, H.; Su, Y.; Gu, L.; Yang, Y.; Yu, H. Changes in stability and in vitro digestion of egg-protein stabilized emulsions and β-carotene gels in the presence of sodium tripolyphosphate. J. Sci. Food Agric. 2021, 101, 5591–5598. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Zhai, J.; Gu, L.; Su, Y.; Chang, C.; Yang, Y. Improving gelling properties of diluted whole hen eggs with sodium chloride and sodium tripolyphosphate: Study on intermolecular forces, water state and microstructure. Food Chem. 2021, 358, 129823. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Liu, J.; Zhang, X.; Zhao, S.; Lv, Y.; Guo, H. Emulsion, gelation, physicochemical properties and microstructure of phosphorylated and succinylated egg yolk. LWT 2020, 131, 109675. [Google Scholar] [CrossRef]
- Ma, Z.; Qing, M.; Zang, J.; Xu, Y.; Gao, X.; Chi, Y.; Chi, Y. Effects of freezing on the gelation behaviors of liquid egg yolks affected by saccharides: Thermal behaviors and rheological and structural changes. Poult. Sci. 2024, 103, 103657. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Chi, Y.; Zhang, H.; Chi, Y.; Ma, Y. Inhibiting effect of dry heat on the heat-induced aggregation of egg white protein. Food Chem. 2022, 387, 132850. [Google Scholar] [CrossRef]
- Tian, Y.; Jin, H.; Guo, S.; Lin, S.; Bao, Z. Effects of different metal ions on the physicochemical properties and microstructure of egg white gel. J. Sci. Food Agric. 2021, 102, 3308–3315. [Google Scholar] [CrossRef]
- Zhang, Q.; Duan, L.; Li, Y. Positive effects and mechanism of ultrasound on chitin preparation from shrimp shells by co-fermentation. Ultrason. Sonochem. 2022, 88, 106066. [Google Scholar] [CrossRef]
- Cui, X.; Liu, X.L.; Shen, G.; Ma, J.; Summons, R.E. Niche expansion for phototrophic sulfur bacteria at the Proterozoic-Phanerozoic transition. Proc. Natl. Acad. Sci. USA 2020, 117, 17599–17606. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Xu, X.; Hu, W.; Pei, H.; Chen, H.; Tong, P.; Gao, J. Effect of L-calcium lactate, zinc lactate, and ferric sodium EDTA on the physicochemical and functional properties of liquid whole egg. J. Food Sci. 2021, 86, 3839–3854. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, L.; Su, Y.; Li, J.; Yang, Y.; Chang, C. Microwave technology as a new strategy to induce structural transition and foaming properties improvement of egg white powder. Food Hydrocoll. 2020, 101, 105530. [Google Scholar] [CrossRef]
- Bravo-Nunez, A.; Garzon, R.; Rosell, C.M.; Gomez, M. Evaluation of Starch(-)Protein Interactions as A Function of pH. Foods 2019, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Chen, J.; Zhang, J.; Sheng, L. Impact of phosphates on heat-induced egg white gel properties: Texture, water state, micro-rheology and microstructure. Food Hydrocoll. 2021, 110, 106200. [Google Scholar] [CrossRef]
- Nasabi, M.; Labbafi, M.; Mousavi, M.E.; Madadlou, A. Effect of salts and nonionic surfactants on thermal characteristics of egg white proteins. Int. J. Biol. Macromol. 2017, 102, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Hidas, K.I.; Németh, C.; Le Nguyen, L.P.; Visy, A.; Tóth, A.; Friedrich, L.; Nyulas-Zeke, I.C. Effect of different salt concentration on the physical properties of frozen thawed egg yolk. Prog. Agric. Eng. Sci. 2021, 17, 29–36. [Google Scholar] [CrossRef]
- Razi, S.M.; Fahim, H.; Amirabadi, S.; Rashidinejad, A. An overview of the functional properties of egg white proteins and their application in the food industry. Food Hydrocoll. 2023, 135, 108183. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Y.; Wang, Q.; Chen, Y.; Liu, H.; Wang, J. Low-sodium salt mediated aggregation behavior of gluten in wheat dough. Int. J. Biol. Macromol. 2022, 205, 231–239. [Google Scholar] [CrossRef]
- Nasabi, M.; Labbafi, M. Thermal aggregation of egg white proteins as affected by saccharides. J. Food Bioprocess Eng. 2020, 3, 110–115. [Google Scholar] [CrossRef]
- Li, T.; Zhong, Q.; Wu, T. Effects of NaCl on the Freezing-Thawing Induced Gelation of Egg Yolk at pH 2.0–8.0. Food Biophys. 2021, 17, 106–113. [Google Scholar] [CrossRef]
- Zhao, W.; Zang, J.; Qing, M.; Wang, H.; Chi, Y.; Chi, Y. The thermal behavior of egg yolk involves lipoprotein instability. J. Food Eng. 2023, 343, 111370. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, Y.; Jiang, Y.; Chi, Y. Effect of dry heating on the aggregation behaviour and aggregate morphologies of ovalbumin. Food Chem. 2019, 285, 296–304. [Google Scholar] [CrossRef]
- Andlinger, D.J.; Schrempel, U.; Hengst, C.; Kulozik, U. Heat-induced aggregation kinetics of potato protein—Investigated by chromatography, calorimetry, and light scattering. Food Chem. 2022, 389, 133114. [Google Scholar] [CrossRef]
- Liu, L.; Bi, J.; Chi, Y.; Chi, Y. Mechanism of amino acid delay on thermal aggregation behavior of liquid egg yolk: Thermal aggregation, water distribution, molecular structure. Food Hydrocoll. 2024, 148, 109453. [Google Scholar] [CrossRef]
- Zhao, W.; Chi, Y.; Chi, Y. Saccharides alleviate the thermal instability behavior of liquid egg yolk: Influence on rheology property, emulsifying property and protein conformation. Food Hydrocoll. 2023, 143, 108853. [Google Scholar] [CrossRef]
- Yang, C.; Hu, G.; Xiang, X.; Wu, D.; Wang, B.; Wang, J.; Geng, F. Translucency mechanism of heat-induced pigeon egg white gel. Int. J. Biol. Macromol. 2023, 253, 126909. [Google Scholar] [CrossRef] [PubMed]
- Razi, S.M.; Motamedzadegan, A.; Shahidi, S.-A.; Rashidinejad, A. Steady and dynamic shear rheology as a toolfor evaluation of the interactions between egg white albumin and basil seed gum. Rheol. Acta 2020, 59, 317–331. [Google Scholar] [CrossRef]
- Ren, L.; Ma, J.; Xu, W.; Lv, Y.; Tong, Q. Stability of low density lipoprotein particles affect the formation of off-flavor in thermal egg yolk. Food Res. Int. 2022, 154, 111029. [Google Scholar] [CrossRef]
- Liu, H.; Feng, F.; Xue, H.; Gao, B.; Han, T.; Li, R.; Hu, X.; Tu, Y.; Zhao, Y. Effects of partial replacement of NaCl by KCl and CaCl(2) on physicochemical properties, microstructure, and textural properties of salted eggs. J. Food Sci. 2022, 87, 795–807. [Google Scholar] [CrossRef]
- Souza, C.J.F.; Garcia-Rojas, E.E. Interpolymeric complexing between egg white proteins and xanthan gum: Effect of salt and protein/polysaccharide ratio. Food Hydrocoll. 2017, 66, 268–275. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Tang, T.; Gu, L.; Su, Y.; Yang, Y.; Chang, C.; Han, Q. Thermal gelation and digestion properties of hen egg white: Study on the effect of neutral and alkaline salts addition. Food Chem. 2023, 409, 135263. [Google Scholar] [CrossRef] [PubMed]
- Ince-Coskun, A.E.; Ozdestan-Ocak, O. Effects of salt ions and heating on the behaviour of whey protein particle dispersions. Food Hydrocoll. 2020, 101, 105433. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Y.; Chi, Y. Effects of Heating Treatment on Functional and Structural Properties of Liquid Whole Egg. Foods 2023, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chi, Y.; Chi, Y. Interventional effect of compound sugar and salt on the thermal instability behavior of liquid egg yolk. J. Food Sci. 2023, 88, 5108–5121. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Fan, Q.; Teng, C.; Xie, W.; Shi, Y.; Su, Y.; Yang, Y. Combination effects of NaOH and NaCl on the rheology and gel characteristics of hen egg white proteins. Food Chem. 2018, 250, 1–6. [Google Scholar] [CrossRef]
- Hamdani, A.M.; Wani, I.A.; Bhat, N.A.; Siddiqi, R.A. Effect of guar gum conjugation on functional, antioxidant and antimicrobial activity of egg white lysozyme. Food Chem. 2018, 240, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, X.; Wu, H.; Tang, J.; Zhang, J. Effects of NaCl Concentration and Potassium Chloride Substitutions on the Thermal Properties and Lipid Oxidation of Dry-Cured Pork. J. Food Sci. 2014, 79, 12504. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, X.; Tang, S.; Mi, S.; Lu, L.; Zeng, Q.; Xia, M.; Cai, Z. Improved effect of ultrasound-assisted enzymolysis on egg yolk powder: Structural properties, hydration properties and stability characteristics. Food Chem. 2022, 382, 132549. [Google Scholar] [CrossRef]
- Li, Q.; Jin, H.; Zhang, X.; Hu, G.; Lei, C.; Sun, H.; Sheng, L.; Jin, Y.; Huang, X.; Lu, L.; et al. Effect of salt penetration and water migration on cooked salted egg yolk gel during storage: Physicochemical properties, structural characteristics and flavor changes. Food Chem. 2023, 404, 134510. [Google Scholar] [CrossRef]
- Gao, X.; Guo, W.; Wu, N.; Yao, Y.; Du, H.; Xu, M.; Zhao, Y.; Tu, Y. Effects of salt and heat treatment on the physicochemical properties, microstructure, secondary structure, and simulated in vitro gastrointestinal digestion of duck egg white. J. Sci. Food Agric. 2021, 101, 6093–6103. [Google Scholar] [CrossRef] [PubMed]


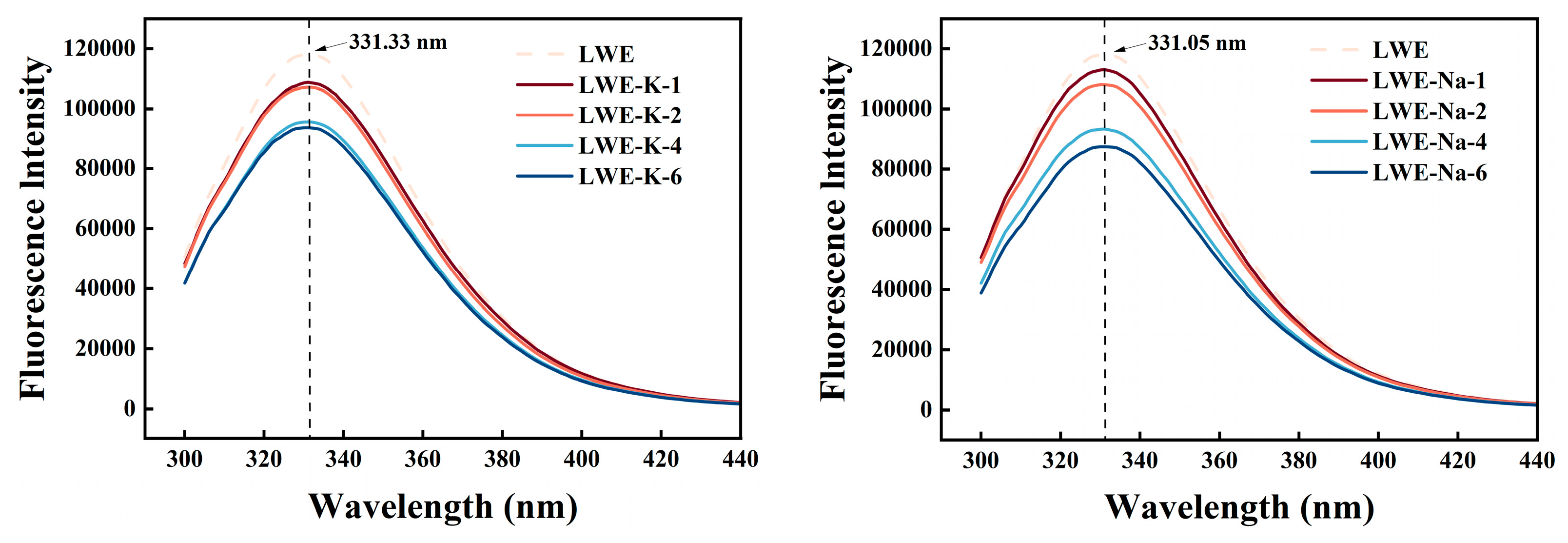

| Samples | Salt Concentration (%) | K/(Pa·s) | n | R2 |
|---|---|---|---|---|
| LWE | 0 | 0.490 ± 0.004 a | 0.520 ± 0.007 e | 0.99 |
| LWE-K | 1 | 0.156 ± 0.003 bc | 0.756 ± 0.009 bc | 0.97 |
| 2 | 0.089 ± 0.003 cd | 0.678 ± 0.014 cd | 0.96 | |
| 4 | 0.227 ± 0.009 b | 0.623 ± 0.020 d | 0.95 | |
| 6 | 0.249 ± 0.010 b | 0.661 ± 0.018 cd | 0.95 | |
| LWE | 0 | 0.406 ± 0.010 a | 0.505 ± 0.021 e | 0.98 |
| LWE-Na | 1 | 0.042 ± 0.000 d | 0.565 ± 0.015 e | 0.98 |
| 2 | 0.023 ± 0.024 d | 0.878 ± 0.004 b | 0.98 | |
| 4 | 0.010 ±0.001 d | 0.955 ± 0.002 a | 0.96 | |
| 6 | 0.011 ± 0.001 d | 0.974 ± 0.001 a | 0.97 |
| Samples | Salt Concentration (%) | Tonset/(°C) | Tp/(°C) | Tendset/(°C) | ΔH/(J/g) |
|---|---|---|---|---|---|
| LWE | 0 | 78.017 ± 0.187 g | 83.487 ± 0.133 g | 90.280 ± 0.436 b | 1.087 ± 0.046 abc |
| LWE-K | 1 | 78.673 ± 0.116 f | 83.940 ± 0.087 f | 90.717 ± 0.421 b | 1.250 ± 0.092 a |
| 2 | 79.770 ± 0.277 e | 85.093 ± 0.029 e | 90.893 ± 0.776 b | 0.835 ± 0.126 c | |
| 4 | 81.620 ± 0.128 c | 86.233 ± 0.231 d | 93.190 ± 0.543 a | 1.009 ± 0.055 abc | |
| 6 | 82.740 ± 0.122 b | 87.553 ± 0.214 b | 92.507 ± 1.243 ab | 1.067 ± 0.147 abc | |
| LWE-Na | 1 | 78.650 ± 0.121 f | 84.037 ± 0.219 f | 90.867 ± 0.834 b | 1.134 ± 0.132 ab |
| 2 | 80.203 ± 0.049 d | 85.130 ± 0.104 e | 90.617 ± 1.094 b | 0.950 ± 0.089 bc | |
| 4 | 82.697 ± 0.150 b | 87.057 ± 0.196 c | 90.543 ± 1.503 b | 1.140 ± 0.15 ab | |
| 6 | 83.460 ± 0.040 a | 88.533 ± 0.040 a | 93.257 ± 0.140 a | 0.875 ± 0.031 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Gao, X.; Chi, Y.; Chi, Y. Potassium Chloride as an Effective Alternative to Sodium Chloride in Delaying the Thermal Aggregation of Liquid Whole Egg. Foods 2024, 13, 1107. https://doi.org/10.3390/foods13071107
Guo J, Gao X, Chi Y, Chi Y. Potassium Chloride as an Effective Alternative to Sodium Chloride in Delaying the Thermal Aggregation of Liquid Whole Egg. Foods. 2024; 13(7):1107. https://doi.org/10.3390/foods13071107
Chicago/Turabian StyleGuo, Jiayu, Xin Gao, Yujie Chi, and Yuan Chi. 2024. "Potassium Chloride as an Effective Alternative to Sodium Chloride in Delaying the Thermal Aggregation of Liquid Whole Egg" Foods 13, no. 7: 1107. https://doi.org/10.3390/foods13071107
APA StyleGuo, J., Gao, X., Chi, Y., & Chi, Y. (2024). Potassium Chloride as an Effective Alternative to Sodium Chloride in Delaying the Thermal Aggregation of Liquid Whole Egg. Foods, 13(7), 1107. https://doi.org/10.3390/foods13071107





