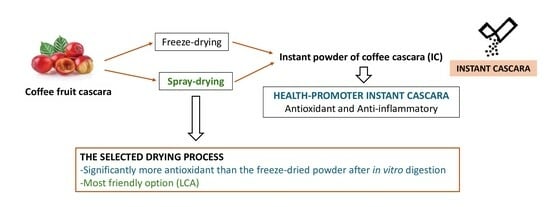
The Impact of the Drying Process on the Antioxidant and Anti-Inflammatory Potential of Dried Ripe Coffee Cherry Pulp Soluble Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Raw Materials
2.2.1. Raw Cascara
2.2.2. Commercial Product (Tabifruit)
2.2.3. Preparation of Dried Ripe Coffee Cherry Pulp Soluble Powders
Product Yield Calculation
2.3. Sensory Analyses
2.4. Physicochemical Parameters
2.4.1. Moisture Content
2.4.2. pH
2.4.3. Soluble Solids Analysis
2.4.4. UV-Vis
2.4.5. Colour
2.4.6. Total Phenolic Content (TPC)
2.4.7. Analysis of Phenolic Compounds by HPLC-QTOF
2.4.8. Analysis of Methylxanthines by HPLC-QTOF
2.5. The Bioactivity of Compounds Present in the Digests
2.5.1. In Vitro Oral–Gastrointestinal Digestion
2.5.2. Antioxidant Capacity
ABTS•+ Scavenging Assay
Determination of Radical Scavenging Capacity against DPPH
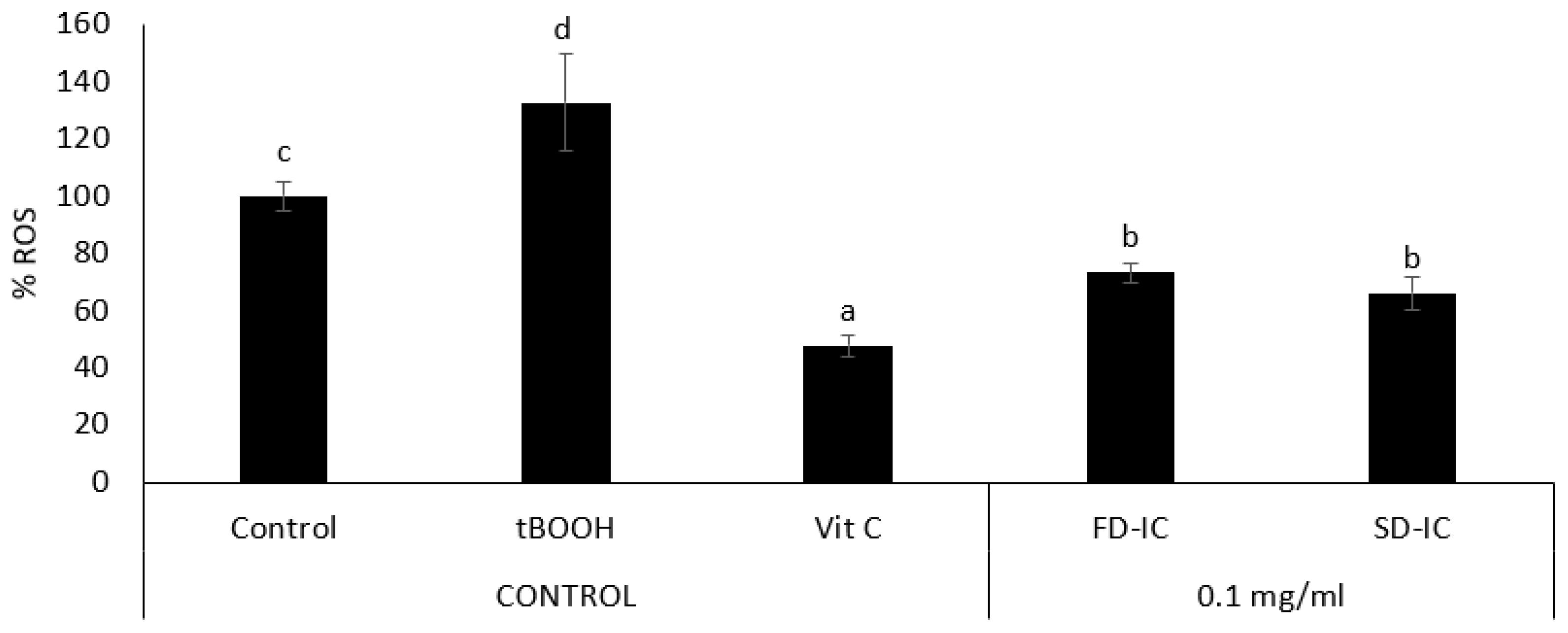
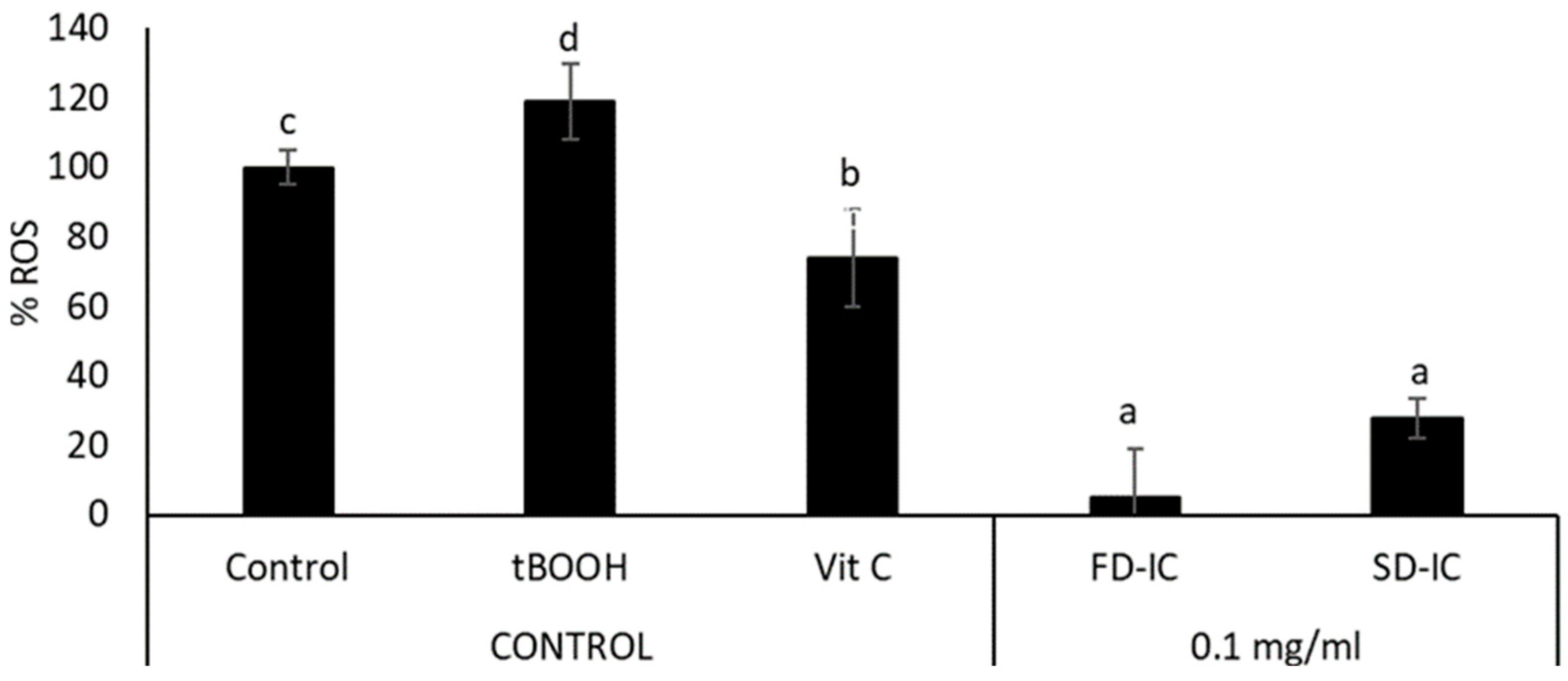
Intracellular Reactive Oxygen Species (ROS) Formation
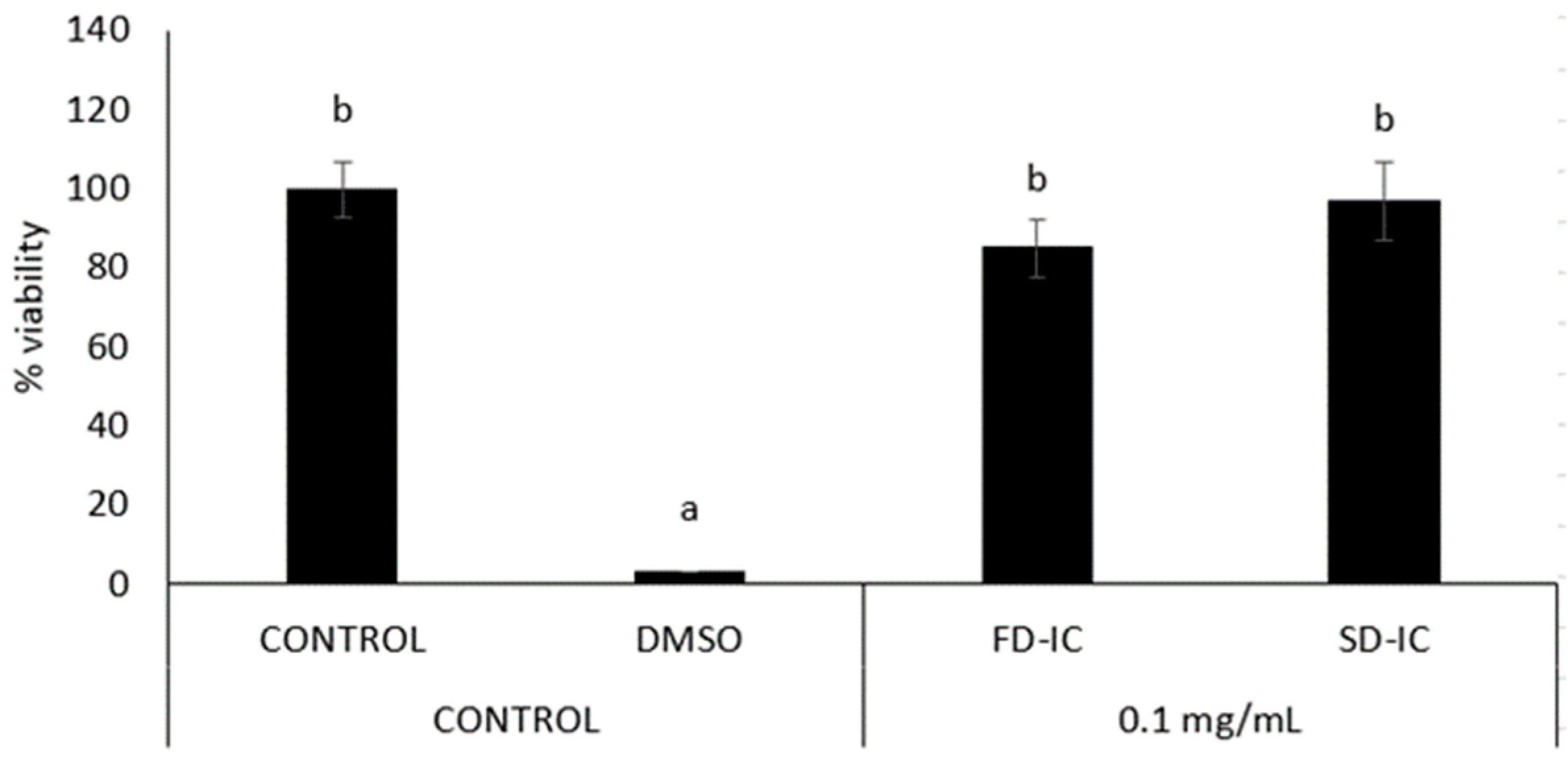
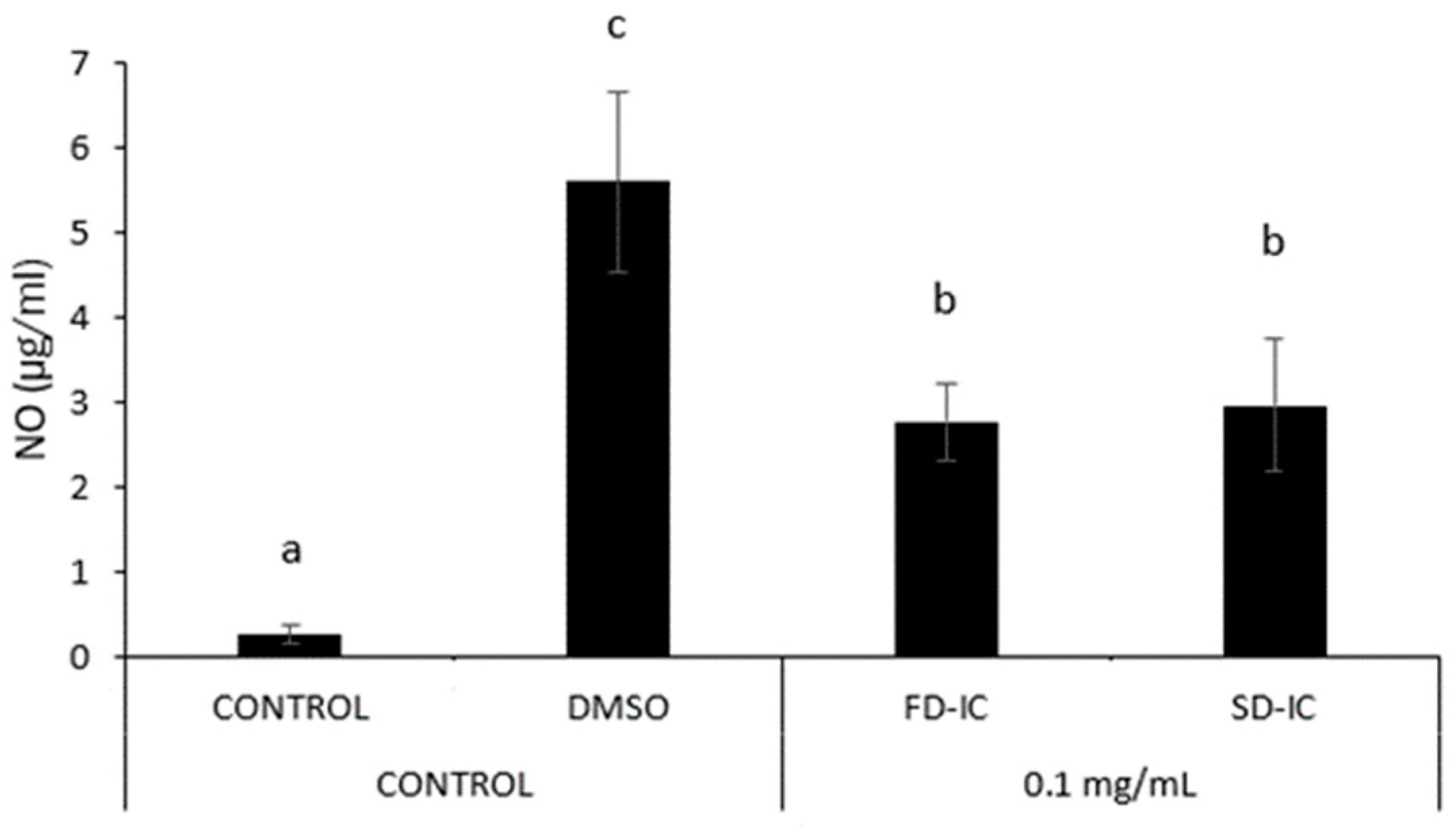
2.5.3. Anti-Inflammatory Properties
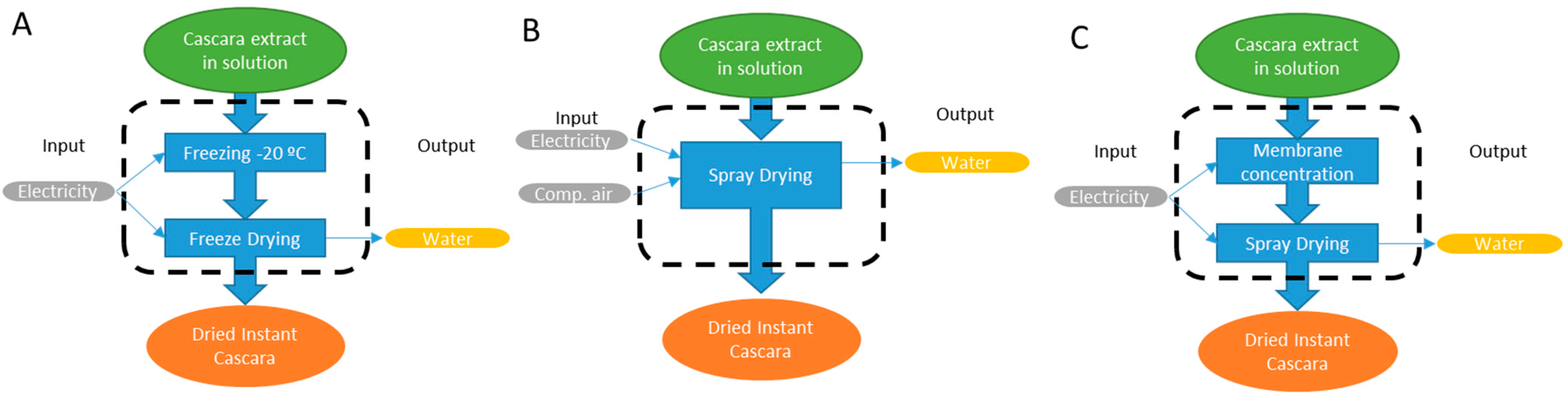
2.6. Environmental Impact Quantification
2.7. Business Model and Operations and Investment Plan
2.8. Ethics Statement
2.9. Statical Data Analysis
3. Results and Discussion
3.1. Sensory Analysis
3.2. Physicochemical Parameters
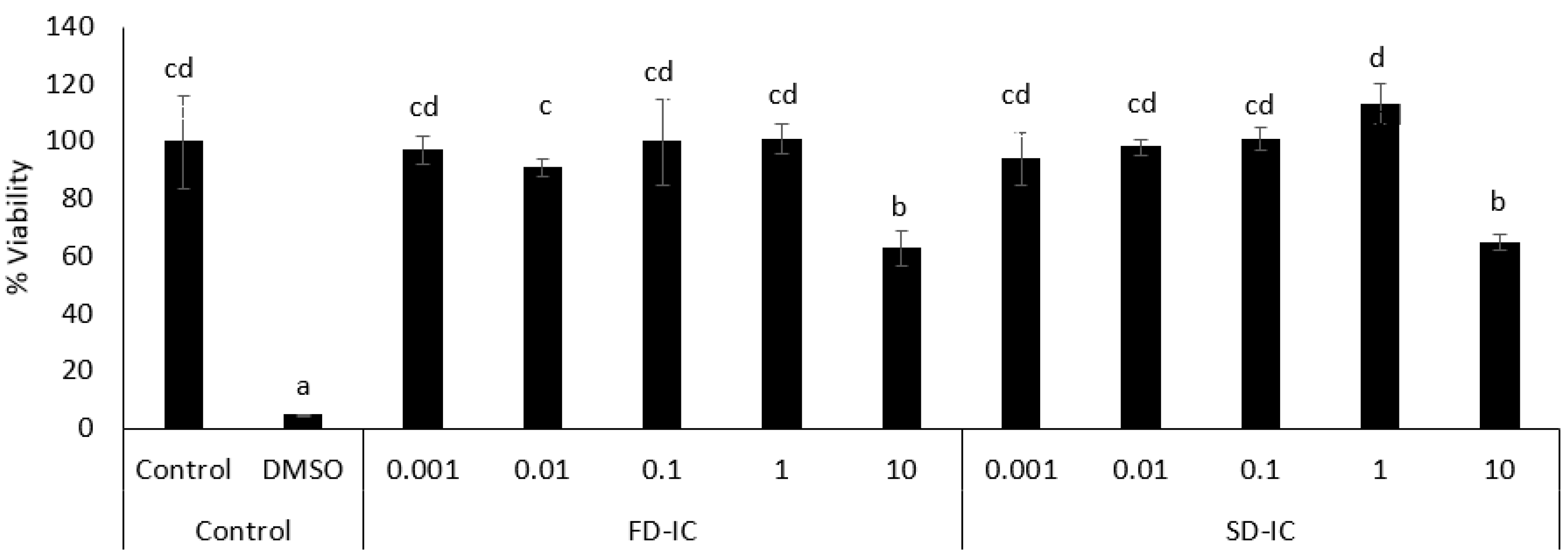
3.3. Bioactivity of Compounds Present in Digests
3.3.1. Analysis of Methylxanthines and Phenolic Compounds by HPLC-QTOF
3.3.2. Overall Antioxidant Capacity
Intracellular Reactive Oxygen Species (ROS) Formation
3.3.3. Anti-Inflammatory Properties
3.4. Environmental Impact Quantification
3.5. A Business Model and an Operations and Investment Plan
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA. Technical Report on the Notification of Dried Cherry Pulp from Coffea arabica L. and Coffea canephora Pierre Ex A. Froehner as a Traditional Food from a Third Country Pursuant to Article 14 of Regulation (EU) 2015/2283. EFSA Support. Publ. 2021, 18, 6808E. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU). 2022/47 of 13 January 2022 Authorising the Placing on the Market of Coffea arabica L. and/or Coffea canephora Pierre Ex A. In Froehner Dried Cherry Pulp and Its Infusion as a Traditional Food from a Third Country under Regulation (EU) 2015/2283 of the European Parliament and of the Council and Amending Commission Implementing Regulation (EU) 2017/2470; EU: Brussels, Belgium, 2022. [Google Scholar]
- Muzaifa, M.; Rahmi, F. Syarifudin Utilization of Coffee By-Products as Profitable Foods-A Mini Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 672, 12077. [Google Scholar] [CrossRef]
- Nestle. NESCAFÉ NATIV Cascara. Available online: https://www.nestle.com.au/en/brands/cascara (accessed on 7 November 2022).
- TEAGLEE. Cascara Tea Blends. Available online: https://www.teaglee.com/collections (accessed on 7 November 2022).
- Sales, A.L.; Iriondo-DeHond, A.; DePaula, J.; Ribeiro, M.; Ferreira, I.M.P.L.V.O.; Miguel, M.A.L.; del Castillo, M.D.; Farah, A. Intracellular Antioxidant and Anti-Inflammatory Effects and Bioactive Profiles of Coffee Cascara and Black Tea Kombucha Beverages. Foods 2023, 12, 1905. [Google Scholar] [CrossRef]
- Bella Vista Coffee KaldiKombucha. Available online: https://kaldikombucha.com/ (accessed on 5 November 2022).
- Supracafe Tabifruit. Available online: https://www.supracafe.com/blogs/news/conoces-la-infusion-de-cascara-de-cafe-supracafe-maximiza-su-sostenibilidad-con-tabifruit (accessed on 5 November 2022).
- Coffeeberry® Cascara is a Patented Extract Derived from Upcycled Coffee Fruit. Available online: https://www.futureceuticals.com/coffeeberry-cascara (accessed on 13 March 2024).
- Iriondo-DeHond, A.; Elizondo, A.S.; Iriondo-DeHond, M.; Ríos, M.B.; Mufari, R.; Mendiola, J.A.; Ibañez, E.; del Castillo, M.D. Assessment of Healthy and Harmful Maillard Reaction Products in a Novel Coffee Cascara Beverage: Melanoidins and Acrylamide. Foods 2020, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jana, A.H.; Chavan, R.S. Functionality of Milk Powders and Milk-Based Powders for End Use Applications—A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 518–528. [Google Scholar] [CrossRef]
- Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Modern Frontiers and Applications of Spray-Freeze-Drying in Design of Food and Biological Supplements. J. Food Process Eng. 2018, 41, e12881. [Google Scholar] [CrossRef]
- Ghirişan, A.; Miclăuş, V. Comparative Study of Spray-Drying and Freeze-Drying on the Soluble Coffee Properties. Stud. Univ. Babes-Bolyai Chem. 2017, 62, 309–316. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of Antioxidant Phenolic Compounds Extracted from Spent Coffee Grounds by Freeze-Drying and Spray-Drying Using Different Coating Materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Ishwarya, S.P.; Anandharamakrishnan, C. Spray-Freeze-Drying Approach for Soluble Coffee Processing and Its Effect on Quality Characteristics. J. Food Eng. 2015, 149, 171–180. [Google Scholar] [CrossRef]
- Darniadi, S.; Ho, P.; Murray, B.S. Comparison of Blueberry Powder Produced via Foam-Mat Freeze-Drying versus Spray-Drying: Evaluation of Foam and Powder Properties. J. Sci. Food Agric. 2018, 98, 2002–2010. [Google Scholar] [CrossRef]
- Sreevalsan, S.; Safe, S. Reactive Oxygen Species and Colorectal Cancer. Curr. Color. Cancer Rep. 2013, 9, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef] [PubMed]
- Tontul, I.; Topuz, A. Spray-Drying of Fruit and Vegetable Juices: Effect of Drying Conditions on the Product Yield and Physical Properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
- Fontes, C.P.M.L.; Silva, J.L.A.; Sampaio-Neta, N.A.; da Costa, J.M.C.; Rodrigues, S. Dehydration of Prebiotic Fruit Drinks by Spray Drying: Operating Conditions and Powder Characterization. Food Bioprocess Technol. 2014, 7, 2942–2950. [Google Scholar] [CrossRef]
- Penner, M. Ultraviolet, Visible, and Fluorescence Spectroscopy. In Food Analysis. Food Science Text Series; Nielsen, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Iriondo-DeHond, A. Assessment of Healthy and Harmful Maillard Reaction Products in a Novel Co Ff Ee Cascara Beverage. Foods 2020, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Contini, M.; Baccelloni, S.; Massantini, R.; Anelli, G. Extraction of Natural Antioxidants from Hazelnut (Corylus avellana L.) Shell and Skin Wastes by Long Maceration at Room Temperature. Food Chem. 2008, 110, 659–669. [Google Scholar] [CrossRef]
- Hollebeeck, S.; Borlon, F.; Schneider, Y.J.; Larondelle, Y.; Rogez, H. Development of a Standardised Human in Vitro Digestion Protocol Based on Macronutrient Digestion Using Response Surface Methodology. Food Chem. 2013, 138, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Saez, N.; Hochkogler, C.M.; Somoza, V.; del Castillo, M.D. Biscuits with No Added Sugar Containing Stevia, Coffee Fibre and Fructooligosaccharides Modifies α-Glucosidase Activity and the Release of GLP-1 from HuTu-80 Cells and Serotonin from Caco-2 Cells after In Vitro Digestion. Nutrients 2017, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Development and Validation of an HPTLC–DPPH Assay and Its Application to the Analysis of Honey. J. Planar Chromatogr. Mod. TLC 2020, 33, 301–311. [Google Scholar] [CrossRef]
- Aljadi, A.M.; Kamaruddin, M.Y. Evaluation of the Phenolic Contents and Antioxidant Capacities of Two Malaysian Floral Honeys. Food Chem. 2004, 85, 513–518. [Google Scholar] [CrossRef]
- Bakondi, E.; Gönczi, M.; Szabó, É.; Bai, P.; Pacher, P.; Gergely, P.; Kovács, L.; Hunyadi, J.; Szabó, C.; Csernoch, L.; et al. Role of Intracellular Calcium Mobilization and Cell-Density-Dependent Signaling in Oxidative-Stress-Induced Cytotoxicity in HaCaT Keratinocytes. J. Investig. Dermatol. 2003, 121, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bedoya-Ramírez, D.; Cilla, A.; Contreras-Calderón, J.; Alegría-Torán, A. Evaluation of the Antioxidant Capacity, Furan Compounds and Cytoprotective/Cytotoxic Effects upon Caco-2 Cells of Commercial Colombian Coffee. Food Chem. 2017, 219, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Ramírez, B.; Escobar, F.V.; del Castillo, M.D. Antioxidant Properties of High Molecular Weight Compounds from Coffee Roasting and Brewing Byproducts. Bioact. Compd. Health Dis. 2019, 2, 48–63. [Google Scholar] [CrossRef]
- Benayad, Z.; Martinez-Villaluenga, C.; Frias, J.; Gomez-Cordoves, C.; Es-Safi, N.E. Phenolic Composition, Antioxidant and Anti-Inflammatory Activities of Extracts from Moroccan Opuntia Ficus-Indica Flowers Obtained by Different Extraction Methods. Ind. Crops Prod. 2014, 62, 412–420. [Google Scholar] [CrossRef]
- Hauschild, M.; Goedkoop, M.; Guinee, J.; Heijungs, R.; Huijbregts, M.; Jolliet, O.; Margni, M.; De Schryver, A.; Pennington, D.; Pant, R.; et al. Recommendations for Life Cycle Impact Assessment in the European Context—Based on Existing Environmental Impact Assessment Models and Factors (International Reference Life Cycle Data System—ILCD Handbook); Publications Office of the European Union: Luxembourg, 2011; ISBN 9789279174513. [Google Scholar]
- Nielsen, C.; Lund, M. A Brief History of the Business Model Concept. In The Basics of Business Models; Ventus: Telluride, CO, USA, 2014; pp. 21–27. [Google Scholar]
- Bocken, N.M.P.; Rana, P.; Short, S.W. Value Mapping for Sustainable Business Thinking. J. Ind. Prod. Eng. 2015, 32, 67–81. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A. Validation of Coffee By-Products as Food Ingredients for a Sustainable Nutrition and Health. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2019. [Google Scholar]
- Schuck, P.; Jeantet, R.; Bhandari, B.; Chen, X.D.; Perrone, Í.T.; de Carvalho, A.F.; Fenelon, M.; Kelly, P. Recent Advances in Spray Drying Relevant to the Dairy Industry: A Comprehensive Critical Review. Dry. Technol. 2016, 34, 1773–1790. [Google Scholar] [CrossRef]
- Pereira, D.C.d.S.; Beres, C.; Gomes, F.d.S.; Tonon, R.V.; Cabral, L.M.C. Spray Drying of Juçara Pulp Aiming to Obtain a “Pure” Powdered Pulp without Using Carrier Agents. Dry. Technol. 2020, 38, 1175–1185. [Google Scholar] [CrossRef]
- Sobulska, M.; Zbicinski, I. Advances in Spray Drying of Sugar-Rich Products. Dry. Technol. 2021, 39, 1774–1799. [Google Scholar] [CrossRef]
- Tantiyani, N.; Othman, A.; Elmi, M.; Mohd, F. Drying of Instant Coffee in a Spray Dryer. J. Kejuruter. 2019, 31, 295–301. [Google Scholar]
- Mennella, J.A. Ontogeny of Taste Preferences: Basic Biology and Implications for Health1-5. Am. J. Clin. Nutr. 2014, 99, 704S–711S. [Google Scholar] [CrossRef] [PubMed]
- Wilkowska, A.; Ambroziak, W.; Czyzowska, A.; Adamiec, J. Effect of Microencapsulation by Spray-Drying and Freeze-Drying Technique on the Antioxidant Properties of Blueberry (Vaccinium myrtillus) Juice Polyphenolic Compounds. Pol. J. Food Nutr. Sci. 2016, 66, 11–16. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Calligaris, S.; Manzocco, L. Shelf-Life Testing of Coffee and Related Products: Uncertainties, Pitfalls, and Perspectives. Food Eng. Rev. 2009, 1, 159–168. [Google Scholar] [CrossRef]
- Forsido, S.F.; Welelaw, E.; Belachew, T.; Hensel, O. Effects of Storage Temperature and Packaging Material on Physico-Chemical, Microbial and Sensory Properties and Shelf Life of Extruded Composite Baby Food Flour. Heliyon 2021, 7, e06821. [Google Scholar] [CrossRef] [PubMed]
- Quek, S.Y.; Chok, N.K.; Swedlund, P. The Physicochemical Properties of Spray-Dried Watermelon Powders. Chem. Eng. Process. Process Intensif. 2007, 46, 386–392. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of Process Conditions on the Physicochemical Properties of Açai (Euterpe oleraceae Mart.) Powder Produced by Spray Drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Germer, S.P.M.; de Aguirre, J.M. Effects of Spray-Drying Conditions on the Physicochemical Properties of Blackberry Powder. Dry. Technol. 2012, 30, 154–163. [Google Scholar] [CrossRef]
- Abdullah, Z.; Taip, F.S.; Kamal, S.M.M.; Abdul Rahman, R.Z. Nonlinear Model-Based Inferential Control of Moisture Content of Spray Dried Coconut Milk. Foods 2020, 9, 1177. [Google Scholar] [CrossRef] [PubMed]
- Maharani, S.; Mustikawati, I.; Nailufhar, L.; Istiqomah, S. The Effect of Brewing Time on PH Values, Polyphenols Content, and Antioxidant Activities of Coffee Husk Tea (Cascara Tea). J. Phys. Conf. Ser. 2021, 1869, 12050. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of PH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Degradation Kinetics of Chlorogenic Acid at Various PH Values and Effects of Ascorbic Acid and Epigallocatechin Gallate on Its Stability under Alkaline Conditions. J. Agric. Food Chem. 2013, 61, 966–972. [Google Scholar] [CrossRef]
- Saarniit, K.; Lang, H.; Kuldjärv, R.; Laaksonen, O.; Rosenvald, S. The Stability of Phenolic Compounds in Fruit, Berry, and Vegetable Purees Based on Accelerated Shelf-Life Testing Methodology. Foods 2023, 12, 1777. [Google Scholar] [CrossRef] [PubMed]
- Deotale, S.M.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Influence of Drying Techniques on Sensory Profile and Chlorogenic Acid Content of Instant Coffee Powders. Meas. Food 2022, 6, 100030. [Google Scholar] [CrossRef]
- Pua, A.; Choo, W.X.D.; Goh, R.M.V.; Liu, S.Q.; Cornuz, M.; Ee, K.H.; Sun, J.; Lassabliere, B.; Yu, B. A Systematic Study of Key Odourants, Non-Volatile Compounds, and Antioxidant Capacity of Cascara (Dried Coffea Arabica Pulp). LWT 2021, 138, 110630. [Google Scholar] [CrossRef]
- Christensen, B.E.; Strand, S.P.; Basset, C.; Kristiansen, K.A.; Ulset, A.S.T.; Ballance, S.; Granum, P.E. Macromolecular Acidic Coating Increases Shelf Life by Inhibition of Bacterial Growth. Int. J. Food Microbiol. 2018, 285, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Murlida, E.; Noviasari, S.; Nilda, C.; Rohaya, S.; Rahmi, F.; Muzaifa, M. Chemical Characteristics of Cascara Tea from Several Varieties of Coffee in Aceh Province. IOP Conf. Ser. Earth Environ. Sci. 2021, 667, 12078. [Google Scholar] [CrossRef]
- Rohaya, S.; Anwar, S.H.; Amhar, A.B.; Sutriana, A.; Muzaifa, M. Antioxidant Activity and Physicochemical Composition of Coffee Pulp Obtained from Three Coffee Varieties in Aceh, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2023, 1182, 12063. [Google Scholar] [CrossRef]
- Frizon, C.N.T.; Nisgoski, S. Color Parameters to Predict Moisture and Tannin Content in Yerba Mate Process. Floresta e Ambiente 2020, 27, e20180098. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of Alkaloids (Indole Alkaloids, Isoquinoline Alkaloids, Tropane Alkaloids); Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128164556. [Google Scholar]
- Monteiro, J.P.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Structure-Bioactivity Relationships of Methylxanthines: Trying to Make Sense of All the Promises and the Drawbacks. Molecules 2016, 21, 974. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Frankowski, R.; Zgoła-Grześkowiak, A. Comparison of Methylxantines, Trigonelline, Nicotinic Acid and Nicotinamide Contents in Brews of Green and Processed Arabica and Robusta Coffee Beans—Influence of Steaming, Decaffeination and Roasting Processes on Coffee Beans. LWT 2020, 125, 109344. [Google Scholar] [CrossRef]
- Touil, A.; Peczalski, R.; Timoumi, S.; Zagrouba, F. Influence of Air Temperature and Humidity on Dehydration Equilibria and Kinetics of Theophylline. J. Pharm. 2013, 2013, 892632. [Google Scholar] [CrossRef] [PubMed]
- Jokić, S.; Gagić, T.; Knez, E.; Ubarić, D.; Kerget, M. Separation of Active Compounds from Food By-Product (Cocoa Shell) Using Subcritical Water Extraction. Molecules 2018, 23, 1408. [Google Scholar] [CrossRef] [PubMed]
- Mohdaly, A.A.A.; Roby, M.H.H.; Sultan, S.A.R.; Groß, E.; Smetanska, I. Potential of Low Cost Agro-Industrial Wastes as a Natural Antioxidant on Carcinogenic Acrylamide Formation in Potato Fried Chips. Molecules 2022, 27, 7516. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Sadhukhan, P.; Sil, P.C. Mangiferin: A Xanthonoid with Multipotent Anti-Inflammatory Potential. BioFactors 2016, 42, 459–474. [Google Scholar] [CrossRef]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; De Rezende, T.R.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Majerska, J.; Lech, K.; Brzezowska, J. Qualitative and Quantitative Evaluation of Heat-Induced Changes in Polyphenols and Antioxidant Capacity in Prunus domestica L. By-Products. Molecules 2019, 24, 3008. [Google Scholar] [CrossRef]
- Prata, E.R.B.A.; Oliveira, L.S. Fresh Coffee Husks as Potential Sources of Anthocyanins. LWT 2007, 40, 1555–1560. [Google Scholar] [CrossRef]
- Nur Fitriani, U.A.; Yusuf, M.; Ilyas, F.S. Spray Drying of Rosella (Hibiscus sabdariffa L.) Powder: Effect of Shelf Life on Physicochemical Properties and Cyanidin 3-O-Glucoside. IOP Conf. Ser. Earth Environ. Sci. 2021, 755, 12002. [Google Scholar] [CrossRef]
- Soares, M.J.; Sampaio, G.R.; Guizellini, G.M.; Figueira, M.S.; Pinaffi, A.C.d.C.; Soares Freitas, R.A.M.; Shahidi, F.; de Camargo, A.C.; Torres, E.A.F.d.S. Regular and Decaffeinated Espresso Coffee Capsules: Unravelling the Bioaccessibility of Phenolic Compounds and Their Antioxidant Properties in Milk Model System upon in Vitro Digestion. LWT 2021, 135, 110255. [Google Scholar] [CrossRef]
- Iriondo-Dehond, A.; Uranga, J.A.; Del Castillo, M.D.; Abalo, R. Effects of Coffee and Its Components on the Gastrointestinal Tract and the Brain–Gut Axis. Nutrients 2021, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.; Coimbra, M.A.; del Castillo, M.D.; Coreta-Gomes, F. Mechanisms of Action of Coffee Bioactive Compounds—A Key to Unveil the Coffee Paradox. Crit. Rev. Food Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Depaula, J.; Farah, A. Caffeine Consumption through Coffee: Content in the Beverage, Metabolism, Health Benefits and Risks. Beverages 2019, 5, 37. [Google Scholar] [CrossRef]
- Graham, T.E. Caffeine and Exercise: Metabolism, Endurance and Performance. Cafeine et Exercice: Metabolisme, Endurance et Performance. Sports Med. 2001, 31, 785–807. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Dried Coffee Husk (Cascara) from Coffea arabica L. as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, e07185. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, P.; Viñas, M.; Steingass, C.B.; Gruschwitz, M.; Guevara, E.; Carle, R.; Schweiggert, R.M.; Jiménez, V.M. Coffee (Coffea arabica L.) by-Products as a Source of Carotenoids and Phenolic Compounds—Evaluation of Varieties With Different Peel Color. Front. Sustain. Food Syst. 2020, 4, 1–13. [Google Scholar] [CrossRef]
- Cha, K.H.; Song, D.G.; Kim, S.M.; Pan, C.H. Inhibition of Gastrointestinal Lipolysis by Green Tea, Coffee, and Gomchui (Ligularia fischeri) Tea Polyphenols during Simulated Digestion. J. Agric. Food Chem. 2012, 60, 7152–7157. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Del Bo′, C.; Tucci, M.; Venturi, S.; Mantegazza, G.; Taverniti, V.; Møller, P.; Riso, P.; Porrini, M. A Mix of Chlorogenic and Caffeic Acid Reduces C/EBPß and PPAR-Γ1 Levels and Counteracts Lipid Accumulation in Macrophages. Eur. J. Nutr. 2022, 61, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, A.A.; Oliveira, A.; Jesus, D.; Rodrigues, C.; Figueira, C.; Gomes, A.; Pintado, M. Chlorogenic Acids Composition and the Impact of in Vitro Gastrointestinal Digestion on Espresso Coffee from Single-Dose Capsule. Food Res. Int. 2020, 134, 109223. [Google Scholar] [CrossRef]
- Kim, I.; Moon, J.K.; Hur, S.J.; Lee, J. Structural Changes in Mulberry (Morus microphylla. Buckl) and Chokeberry (Aronia melanocarpa) Anthocyanins during Simulated in Vitro Human Digestion. Food Chem. 2020, 318, 126449. [Google Scholar] [CrossRef]
- Liang, L.; Wu, X.; Zhao, T.; Zhao, J.; Li, F.; Zou, Y.; Mao, G.; Yang, L. In Vitro Bioaccessibility and Antioxidant Activity of Anthocyanins from Mulberry (Morus atropurpurea Roxb.) Following Simulated Gastro-Intestinal Digestion. Food Res. Int. 2012, 46, 76–82. [Google Scholar] [CrossRef]
- Khochapong, W.; Ketnawa, S.; Ogawa, Y.; Punbusayakul, N. Effect of in Vitro Digestion on Bioactive Compounds, Antioxidant and Antimicrobial Activities of Coffee (Coffea arabica L.) Pulp Aqueous Extract. Food Chem. 2021, 348, 129094. [Google Scholar] [CrossRef]
- Abdelazim Mohdaly, A.A.; Ramadan, M.F. Characteristics, Composition and Functional Properties of Seeds, Seed Cake and Seed Oil from Different Brassica Carinata Genotypes. Food Biosci. 2022, 48, 100752. [Google Scholar] [CrossRef]
- Alves, R.C.; Rodrigues, F.; Nunes, M.A.A.; Vinha, A.F.; Oliveira, M.B.P.P. State of the Art in Coffee Processing By-Products; Galanakis, C., Ed.; Academic Press-Elsevier: Burlington, MA, USA, 2017; ISBN 978-0-12-811290-8. [Google Scholar]
- Sorolla, M.A.; Hidalgo, I.; Sorolla, A.; Montal, R.; Pallisé, O.; Salud, A.; Parisi, E. Microenvironmental Reactive Oxygen Species in Colorectal Cancer: Involved Processes and Therapeutic Opportunities. Cancers 2021, 13, 5037. [Google Scholar] [CrossRef] [PubMed]
- Condello, M.; Meschini, S. Role of Natural Antioxidant Products in Colorectal Cancer Disease: A Focus on a Natural Compound Derived from Prunus spinosa, Trigno Ecotype. Cells 2021, 10, 3326. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell Longev. 2020, 2019, 6175804. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; de Mejia, E.G. Relationship of the Phytochemicals from Coffee and Cocoa By-Products with Their Potential to Modulate Biomarkers of Metabolic Syndrome in Vitro. Antioxidants 2019, 8, 279. [Google Scholar] [CrossRef]
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of Coffee Cherry Pulp and Its Utilisation for Production of Cascara Beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Phenolic Compounds from Coffee By-Products Modulate Adipogenesis-Related Inflammation, Mitochondrial Dysfunction, and Insulin Resistance in Adipocytes, via Insulin/PI3K/AKT Signaling Pathways. Food Chem. Toxicol. 2019, 132, 110672. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, S.Y.; Park, Y.L.; Myung, D.S.; Rew, J.S.; Joo, Y.E. Chlorogenic Acid Suppresses Lipopolysaccharide-Induced Nitric Oxide and Interleukin-1β Expression by Inhibiting JAK2/STAT3 Activation in RAW264.7 Cells. Mol. Med. Rep. 2017, 16, 9224–9232. [Google Scholar] [CrossRef]
- Mehaya, F.M.; Mohammad, A.A. Thermostability of Bioactive Compounds during Roasting Process of Coffee Beans. Heliyon 2020, 6, e05508. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Nebesny, E.; Oracz, J. Correlation between the Stability of Chlorogenic Acids, Antioxidant Activity and Acrylamide Content in Coffee Beans Roasted in Different Conditions. Int. J. Food Prop. 2015, 18, 290–302. [Google Scholar] [CrossRef]
- Gallego-Barceló, P.; Bagues, A.; Benítez-Álvarez, D.; López-Tofiño, Y.; Gálvez-Robleño, C.; López-Gómez, L.; del Castillo, M.D.; Abalo, R. Evaluation of the Effects of Instant Cascara Beverage on the Brain-Gut Axis of Healthy Male and Female Rats. Nutrients 2023, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Moreno, O.A.V.; Swarr, T.E.; Asselin, A.C.; Milà i Canals, L.; Colley, T.; Valdivia, S. Implementation of Life Cycle Management Practices in a Cluster of Companies in Bogota, Colombia. Int. J. Life Cycle Assess. 2015, 20, 723–730. [Google Scholar] [CrossRef]
- De Marco, I.; Riemma, S.; Iannone, R. Life Cycle Assessment of Supercritical CO2 Extraction of Caffeine from Coffee Beans. J. Supercrit. Fluids 2018, 133, 393–400. [Google Scholar] [CrossRef]
- Giraldi-Díaz, M.R.; De Medina-Salas, L.; Castillo-González, E.; León-Lira, R. Environmental Impact Associated with the Supply Chain and Production of Grounding and Roasting Coffee through Life Cycle Analysis. Sustainability 2018, 10, 4598. [Google Scholar] [CrossRef]
- Brommer, E.; Stratmann, B.; Quack, D. Environmental Impacts of Different Methods of Coffee Preparation. Int. J. Consum. Stud. 2011, 35, 212–220. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Kannah, R.Y.; Kumar, M.D.; Preethi; Kavitha, S.; Gunasekaran, M.; Zhen, G.; Awasthi, M.K.; Kumar, G. Spent Coffee Grounds Based Circular Bioeconomy: Technoeconomic and Commercialization Aspects. Renew. Sustain. Energy Rev. 2021, 152, 111721. [Google Scholar] [CrossRef]
- Overturf, E.; Pezzutto, S.; Boschiero, M.; Ravasio, N.; Monegato, A. The Circo (Circular Coffee) Project: A Case Study on Valorization of Coffee Silverskin in the Context of Circular Economy in Italy. Sustainability 2021, 13, 9069. [Google Scholar] [CrossRef]
- Ministerio de Industria y Turismo C. y T. Niveles de Madurez de la Tecnología Technology Readiness Levels. TRLs. Una introducción. Available online: https://www.mincotur.gob.es/publicaciones/publicacionesperiodicas/economiaindustrial/revistaeconomiaindustrial/393/notas.pdf (accessed on 8 March 2023).
- Bozzola, M.; Charles, S.; Ferretti, T.; Gerakari, E.; Manson, H.; Rosser, N.; von der Goltz, P. The Coffee Guide; International Trade Centre: Geneva, Switzerland, 2021; ISBN 9789291373949. [Google Scholar]
- Iriondo-DeHond, A.; Iriondo-DeHond, M.; Del Castillo, M.D. Applications of compounds from coffee processing by-products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef]



| Attributes | Adults | Adolescents |
|---|---|---|
| Tabifruit | ||
| Overall liking | 5.12 ± 1.81 | 4.92 ± 2.31 b |
| Visual appearance | 6.12 ± 2.29 | 4.94 ± 2.69 |
| Smell | 5.00 ± 2.20 | 4.40 ± 2.67 |
| Taste | 4.23 ± 2.17 | 3.45 ± 2.16 |
| IC 4 mg/mL | ||
| Overall liking | 5.23 ± 1.84 | 5.00 ± 1.99 b |
| Visual appearance | 5.88 ± 2.49 | 4.73 ± 2.59 |
| Smell | 4.73 ± 2.17 | 4.48 ± 7.75 |
| Taste | 4.38 ± 2.18 | 3.61 ± 2.41 |
| IC 10 mg/mL | ||
| Overall liking | 5.16 ± 2.08 | 3.95 ± 1.98 a |
| Visual appearance | 6.08 ± 2.42 | 4.52 ± 2.47 |
| Smell | 4.88 ± 2.43 | 4.47 ± 2.64 |
| Taste | 4.36 ± 2.41 | 2.71 ± 2.06 |
| Parameters | Freeze Drying | Spray Drying | p < 0.05 | ||
|---|---|---|---|---|---|
| Appearance | FD-IC | FD-IC beverage | SD-IC | SD-IC beverage | |
| Moisture (%) | 5.32 ± 0.31 | 3.71 ± 0.34 | * | ||
| pH | 3.44 ± 0.11 | 4.31 ± 0.04 | * | ||
| °Brix | 1 | 1 | |||
| Colour (lightness parameter) | FD-IC 85.89 ± 0.49 | FD-IC beverage 144.73 ± 0.29 | SD-IC 90.92 ± 0.77 | SD-IC beverage 102.28 ± 1.07 | * |
| UV-VIS |  | 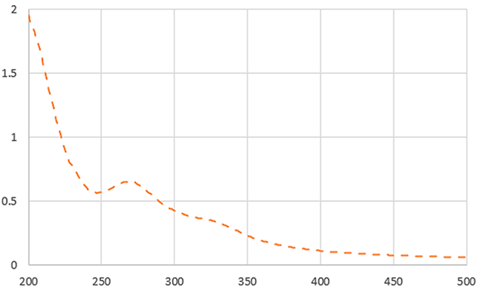 | |||
| Compounds Studied | Freeze Drying | Spray Drying | p < 0.05 |
|---|---|---|---|
| Methylxanthines | |||
| Caffeine (mg/g of dried sample) | 12.33 ± 2.43 | 35.47 ± 0.44 | * |
| Theobromine (µg/g of dried sample) | 72.20 ± 4.79 | 31.72 ± 3.49 | * |
| Theophylline (µg/g of dried sample) | 117.84 ± 1.25 | 25.47 ± 2.81 | * |
| Total (mg/g of dried sample) | 12.52 | 35.52 | * |
| Phenolic compounds | |||
| Isoflavones | |||
| Epicatechin (µg/g of dried sample) | 1.32 ± 0.22 | 3.08 ± 0.33 | * |
| Mangiferin (µg/g of dried sample) | 176.55 ± 29.24 | 662.84 ± 38.83 | * |
| Rutin (µg/g of dried sample) | 88.14 ± 19.80 | 132.20 ± 2.19 | |
| Total (mg/g of dried sample) | 0.26 | 0.79 | * |
| Hydroxycinnamic acids | |||
| p-coumaroylquinic acid (µg/g of dried sample) | 242.45 ± 45.82 | 162.43 ± 5.48 | * |
| Caffeoylquinic acid (µg/g of dried sample) | 890.41 ± 284.47 | 1191.70 ± 448.53 | |
| 3-O-caffeoylquinic acid (chlorogenic acid) (µg/g of dried sample) | 2851.58 ± 526.78 | 2986.58 ± 54.55 | |
| 4-O-feruloylquinic acid (µg/g of dried sample) | 21.81 ± 2.12 | 16.44 ± 4.76 | |
| 5-O-feruloylquinic acid (µg/g of dried sample) | 350.08 ± 70.27 | 478.13 ± 14.33 | * |
| 3,4-di-O-caffeoylquinic acid (µg/g of dried sample) | 69.67 ± 17.93 | 236.98 ± 62.79 | |
| 4,5-di-O-caffeoylquinic acid (µg/g of dried sample) | 32.54 ± 4.40 | 88.84 ± 6.43 | * |
| Total (mg/g of dried sample) | 4.45 | 5.16 | |
| Anthocyanins | |||
| Cyanidin-3-O-glucoside (µg/g of dried sample) | 1.32 ± 0.23 | N.D. | |
| Cyanidin-3-O-rutinoside (µg/g of dried sample) | 5.25 ± 1.40 | N.D. | |
| Total (mg/g of dried sample) | 0.0065 | N.D. | |
| TOTAL (mg/g of dried sample) | 17.23 | 41.47 | * |
| Compounds Studied (µg eq./mL of Digest) | FD-IC Digested | SD-IC Digested | p < 0.05 |
|---|---|---|---|
| Methylxanthines | |||
| Caffeine | 867.55 ± 5.12 | 663.27 ± 3.21 | * |
| Theobromine | 1.76 ± 0.04 | 1.76 ± 0.06 | |
| Theophylline | 0.91 ± 0.05 | 1.02 ± 0.06 | |
| Total | 870.22 | 666.05 | * |
| Phenolic compounds | |||
| Isoflavones | |||
| Catechin hexoside | 0.35 ± 0.01 | 0.44 ± 0.03 | * |
| Mangiferin | 4.52 ± 0.31 | 4.68 ± 0.44 | |
| Rutin | 0.25 ± 0.02 | 0.23 ± 0.04 | |
| Total | 5.12 | 5.35 | |
| Hydroxycinnamic acids | |||
| p-coumaroylquinic acid | 0.80 ± 0.03 | 1.01 ± 0.07 | * |
| Caffeoylquinic acid | 23.38 ± 11.69 | 19.12 ± 9.56 | |
| 3-O-caffeoylquinic acid (chlorogenic acid) | 23.08 ± 1.83 | 27.88 ± 2.27 | * |
| 4-O-feruloylquinic acid | 1.24 ± 0.10 | 0.85 ± 0.07 | * |
| 5-O-feruloylquinic acid | 4.78 ± 0.38 | 4.88 ± 0.47 | |
| 3,4-di-O-caffeoylquinic acid | 0.61 ± 0.30 | 0.45 ± 0.23 | |
| 4,5-di-O-caffeoylquinic acid | 0.56 ± 0.06 | 0.37 ± 0.04 | * |
| Total | 54.45 | 54.56 | |
| TOTAL | 929.79 | 725.96 | * |
| Assay | Freeze Drying | Spray Drying |
|---|---|---|
| TPC | 92.64 ± 6.75 | 99.56 ± 3.45 |
| DPPH | 62.29 ± 1.60 | 78.56 ± 29.18 |
| ABTS | 242.92 ± 8.66 | 249.25 ± 7.06 |
| Assay (mmol eq. CGA/l of Digest) | Freeze Drying | Spray Drying | p < 0.05 |
|---|---|---|---|
| ABTS | 24.2 ± 1.83 | 30.59 ± 1.89 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Parra, M.B.; Gómez-Domínguez, I.; Iriondo-DeHond, M.; Villamediana Merino, E.; Sánchez-Martín, V.; Mendiola, J.A.; Iriondo-DeHond, A.; del Castillo, M.D. The Impact of the Drying Process on the Antioxidant and Anti-Inflammatory Potential of Dried Ripe Coffee Cherry Pulp Soluble Powder. Foods 2024, 13, 1114. https://doi.org/10.3390/foods13071114
López-Parra MB, Gómez-Domínguez I, Iriondo-DeHond M, Villamediana Merino E, Sánchez-Martín V, Mendiola JA, Iriondo-DeHond A, del Castillo MD. The Impact of the Drying Process on the Antioxidant and Anti-Inflammatory Potential of Dried Ripe Coffee Cherry Pulp Soluble Powder. Foods. 2024; 13(7):1114. https://doi.org/10.3390/foods13071114
Chicago/Turabian StyleLópez-Parra, Marta B., Irene Gómez-Domínguez, Maite Iriondo-DeHond, Esther Villamediana Merino, Vanesa Sánchez-Martín, Jose A. Mendiola, Amaia Iriondo-DeHond, and Maria Dolores del Castillo. 2024. "The Impact of the Drying Process on the Antioxidant and Anti-Inflammatory Potential of Dried Ripe Coffee Cherry Pulp Soluble Powder" Foods 13, no. 7: 1114. https://doi.org/10.3390/foods13071114
APA StyleLópez-Parra, M. B., Gómez-Domínguez, I., Iriondo-DeHond, M., Villamediana Merino, E., Sánchez-Martín, V., Mendiola, J. A., Iriondo-DeHond, A., & del Castillo, M. D. (2024). The Impact of the Drying Process on the Antioxidant and Anti-Inflammatory Potential of Dried Ripe Coffee Cherry Pulp Soluble Powder. Foods, 13(7), 1114. https://doi.org/10.3390/foods13071114